Abstract
Parkinson’s disease (PD) is the most common neurodegenerative motor disease. Pathologically, PD is characterized by Lewy body deposition and subsequent death of dopamine neurons in the substantia nigra pars compacta. PD also consistently features degeneration of the locus ceruleus, the main source of norepinephrine in the central nervous system. We have previously reported a mouse model of dopaminergic neurodegeneration based on reduced expression of the vesicular monoamine transporter (VMAT2 LO). To determine if reduced vesicular storage can also cause noradrenergic degeneration, we examined indices of damage to the catecholaminergic systems in brain and cardiac tissue of VMAT2 LO mice. At two months of age, neurochemical analyses revealed substantial reductions in striatal dopamine (94%), cortical dopamine (57%) and norepinephrine (54%), as well as cardiac norepinephrine (97%). These losses were accompanied by increased conversion of dopamine and norepinephrine to their deaminated metabolites. VMAT2 LO mice exhibited loss of noradrenergic innervation in the cortex, as determined by norepinephrine transporter immunoreactivity and 3H-nisoxetine binding. Using unbiased stereological techniques, we observed progressive degeneration in the locus ceruleus that preceded degeneration of the substantia nigra pars compacta. In contrast, the ventral tegmental area, which is spared in human PD, remained unaffected. The coordinate loss of dopamine and norepinephrine neurons in VMAT2-LO mice parallels the pattern of neurodegeneration that occurs in human PD, and demonstrates that insufficient catecholamine storage can cause spontaneous degeneration in susceptible neurons, underscoring cytosolic catecholamine catabolism as a determinant of neuronal susceptibility in PD.
Keywords: VMAT2, norepinephrine, Parkinson’s disease, locus ceruleus
1. Introduction
Parkinson’s disease (PD) is primarily characterized by motor deficits due to the loss of dopamine (DA) producing cells of the substantia nigra pars compacta (SNpc). However, PD is not restricted to the SNpc, as there is significant neurodegeneration in the locus ceruleus (LC), which is the primary source of brain norepinephrine (NE). The degree and rate of LC loss have been reported to be similar to, if not greater than, that of the SNpc (Braak et al., 2003; Zarow et al., 2003). Noradrenergic degeneration in PD has been implicated in parkinsonian motor deficits, neuropsychiatric symptoms (including depression, dementia, and psychosis), and sleep disturbances (Del Tredici and Braak, 2012; Gesi et al., 2000; Paulus and Jellinger, 1991). Noradrenergic deficits in PD also extend into the periphery; cardiac sympathetic denervation occurs in most PD cases, and can manifest in the pre-motor phases of the disease (Goldstein et al., 2007). Loss of sympathetic noradrenergic nerves in the myocardium may be as profound as the loss of dopaminergic terminals in the striatum (Amino et al., 2005).
Each of these affected neuronal populations is of the catecholaminergic phenotype. The shared vulnerability of catecholamine neurons in PD can be ascribed in part to ongoing cytoplasmic neurotransmitter breakdown. Due to ongoing synthesis, plasmalemmal uptake, and vesicular leak, catecholamines are continuously present in the cytosol. The vesicular monoamine transporter 2 (Slc18a2; VMAT2) transports catecholamines from the cytosol into acidic synaptic vesicles, where they are stored for exocytic release. In noradrenergic neurons, NE is synthesized from DA via dopamine-β-hydroxylase inside the vesicle. Products of cytoplasmic catecholamine breakdown by autoxidation and enzymatic deamination are toxic (Burke et al., 2004; Caudle et al., 2007; Cubells et al., 1994). This burden is characteristic of catecholamine neurons and may be a critical determinant of their susceptibility to neurotoxicity in PD (Caudle et al., 2008; Sulzer and Surmeier, 2012).
Because VMAT2 is essential for vesicular catecholamine storage, its dysregulation can be deleterious to neuronal health. In mice, genetic ablation of VMAT2 is fatal, and while heterozygote knockout animals develop normally, they are more susceptible than wild-type mice to MPTP- and amphetamine- induced neurotoxicity (Guillot et al., 2008; Takahashi et al., 1997; Wang et al., 1997). We previously reported that VMAT2 LO mice, which have a 95% reduction in VMAT2 expression, exhibit progressive nigrostriatal degeneration, highly dysregulated DA homeostasis, α-synuclein accumulation, L-DOPA-responsive motor deficits, and nonmotor features of PD, including depressive and anxiety-like behaviors (Caudle et al., 2007; Taylor et al., 2009).
In this study, we tested whether VMAT2 LO mice exhibit noradrenergic degeneration. We assayed concentrations of DA, NE, and their metabolites in the cortex, striatum, and heart. Changes in noradrenergic innervation were assessed by immunochemistry for the norepinephrine transporter (NET) and by 3H-nisoxetine binding. To quantify the extent of neuronal loss, we performed stereological analyses of catecholaminergic cell bodies in the LC and SNpc as well as in the ventral tegmental area (VTA), a dopaminergic nucleus less affected in PD.
2. Materials and Methods
Animals
Male and female VMAT2 LO mice were generated as previously described (Caudle et al., 2007; Taylor et al., 2009). Briefly, the laboratory of Piers Emson (Mooslehner et al., 2001) generated a VMAT2 hypomorphic mouse on a C57BL/6 background. It was later revealed that the C57BL/6 subpopulation from which these mice were derived contained a spontaneous deletion spanning the α-synuclein gene locus (Specht and Schoepfer, 2001). Through outbreeding to mice of the 129 strain, we reintroduced the deleted locus while maintaining the hypomorphic VMAT2 allele. These mice (VMAT2 LO) have been maintained on a C57/BL6 and 129 mixed background. The genotypes of all mice were confirmed by PCR of genomic DNA extracted from tail snips. This report represents the fourth set of data on VMAT2 LO mice with a normal α-synuclein background (Caudle et al., 2007; Guillot et al., 2008; Taylor et al., 2009). All procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Emory University.
HPLC determination of catecholamines and metabolites
Mice were decapitated and their brains and hearts immediately dissected. From the brain, we collected the isocortex and ventral and dorsal striata between Bregma coordinates 2.0 mm and −1.9 mm. Tissues were frozen in liquid nitrogen, and catechol levels were measured by liquid chromatography with electrochemical detection after batch alumina extraction at the Clinical Neurochemistry Laboratory of the Clinical Neurocardiology Section in intramural NINDS (Goldstein et al., 2011b; Holmes et al., 1994). Catechols of interest included the catecholamines, the catecholamine precursor L-DOPA, dihydroxyphenylacetic acid (DOPAC, the main neuronal metabolite of DA), and dihydroxyphenylglycol (DHPG, the main neuronal metabolite of NE). Mice of two months of age were used to determine catechol levels prior to the onset of neurodegeneration.
3H-Nisoxetine binding to quantify norepinephrine transporter expression
3H-Nisoxetine binding in brain homogenates was performed as previously described (Tejani-Butt, 1992), with minor modifications. Briefly, mice were decapitated and their brains rapidly removed and dissected (described above). Cortical tissue was homogenized in 0.32 M sucrose, 10 mM HEPES at 1000 RPM with a Teflon homogenizer and Wheaton glass tube and centrifuged at 48,000×g. The resulting pellet was resuspended, homogenized, and centrifuged again. The final pellet was resuspended in 500 µl assay buffer (50 mM Tris, 300mM NaCl, 5mM KCl, pH 6.8). 200–400 µg (post-hoc determination) of sample was incubated in a 400 µL reaction mixture of 5 nM 3H-nisoxetine (American Radiolabeled Chemicals, St. Louis, MO) for total binding, and with 10 µM desipramine (Sigma-Aldrich, St. Louis, MO) to determine nonspecific binding. Hearts were processed similarly, with the addition of first homogenizing cardiac tissue in ice cold 0.1M phosphate-buffered with a Tissue-Tearor tissue homogenizer (Bio Spec Products, Bartlesville, OK). Mice of 12 months of age were used to examine NET expression at the start of LC degeneration.
Immunohistochemical analysis of tyrosine hydroxylase and norepinephrine transporter
Tissue staining was completed as previously described (Caudle et al., 2006; Miller et al., 1999; Miller et al., 1997). Briefly, we transcardially perfused phosphate buffered saline and 4% paraformaldehyde into mice. Tissue was removed and fixed in 4% paraformaldehyde for 24 h, followed by cryoprotectection in 30% sucrose for 48 h. The brains were sliced to a thickness of 40 µm on a freezing microtome (Microm, Kalamazoo, MI). For fluorescent immunohistochemistry, sections were incubated with mouse monoclonal anti-TH (1:1,000; Millipore) and rabbit polyclonal anti-VMAT2 (1:15,000, custom-made). Rabbit antisera was raised against the C-terminal VMAT2 peptide sequence CTQNNVQPYPVGDDEESESD (immunizations and sera collection performed by Covance, Princeton, NJ). Sections were then incubated with fluorophore-linked secondary antibodies against rabbit and mouse IgG, raised in goats (1:800, Life Technologies, Grand Island, NY). For DAB-peroxidase staining, free-floating sections were incubated with either rabbit polyclonal anti-tyrosine hydroxylase (TH) (1:2000; Millipore), or mouse monoclonal anti-NET (1:1,000, MAb Technologies, Stone Mountain, GA) overnight, followed by incubation in a biotinylated goat anti-rabbit secondary antibody (TH) or biotinylated goat anti-mouse (1:200; Jackson Immunoresearch) for 1 h at room temperature. NET detection was optimized with Citra citrate antigen retrieval kit (Biogenex, Fremont, CA). Visualization was performed using 3, 3´-diaminobenzidine (DAB, St. Louis, MO) for 2 min at room temperature. After DAB, all sections were counterstained in hematoxylin, mounted on slides, dehydrated, and coverslipped using Permount (Fisher Scientific, Waltham, MA) with sections viewed using a light microscope (Olympus, San Jose, CA).
Stereological analysis of cell bodies
Stereological sampling (West et al., 1991) was performed using the Stereo Investigator software (MicroBrightField, Colchester, VT). Tissue staining was performed as described previously (McCormack et al., 2002; Reveron et al., 2002). Whole brains were removed and processed for frozen sections as described above, and serially sectioned at 50 µm (final mounted thickness of 26 µm) for systematic analysis of randomly placed counting frames (size, 50 × 50 µm) on a counting grid (size of 120 × 160 µm) and sampled using an 22 µm optical dissector with 2 µm upper and lower guard zones. The boundaries of SNpc, LC, and VTA were outlined under magnification of the 4X objective as per the atlas of Paxinos and Franklin (2001). Cells were counted with a 40X objective (1.3 numerical aperture) using an Eclipse e800 microscope (Nikon, Melville, NY). Guard zones of 2 µm ensured the exclusion of lost profiles on the top and bottom of the section sampled. A dopaminergic or noradrenergic neuron was defined as an in-focus TH-immunoreactive (TH-IR) cell body with a TH-negative nucleus within the counting frame. For the SNpc and VTA, every other section was processed for TH-IR, resulting in 20–25 sections sampled per mouse. For the LC, every section was stained for TH-IR and counterstained with hematoxylin, resulting in 15 sections sampled per mouse. The number of neurons in the SNpc, VTA, and LC was estimated using the optical fractionator method, which is unaffected by changes in the volume of reference of the structure sampled. Between 100 and 250 objects were counted-to generate the stereological estimates. Gundersen (m=1) coefficients of error were less than 0.1.
Statistics
Statistical analyses were performed using GraphPad Prism 5.0 (La Jolla, CA) Experiments with two factors (HPLC analysis of genotype and brain region, stereological analyses between genotypes) were analyzed by two-way ANOVA, followed by Bonferroni post-hoc tests for pairwise comparisons. One-factor experiments (HPLC of cardiac tissue, radioligand binding) were analyzed with t-tests for independent means. Results were reported as mean ± SEM, and statistical significance set at α=0.05. In figures, *p<0.05, **p<0.01, ***p<0.001.
3. Results
3.1 Immunohistochemical assessment of VMAT2 loss in SNpc and LC neurons
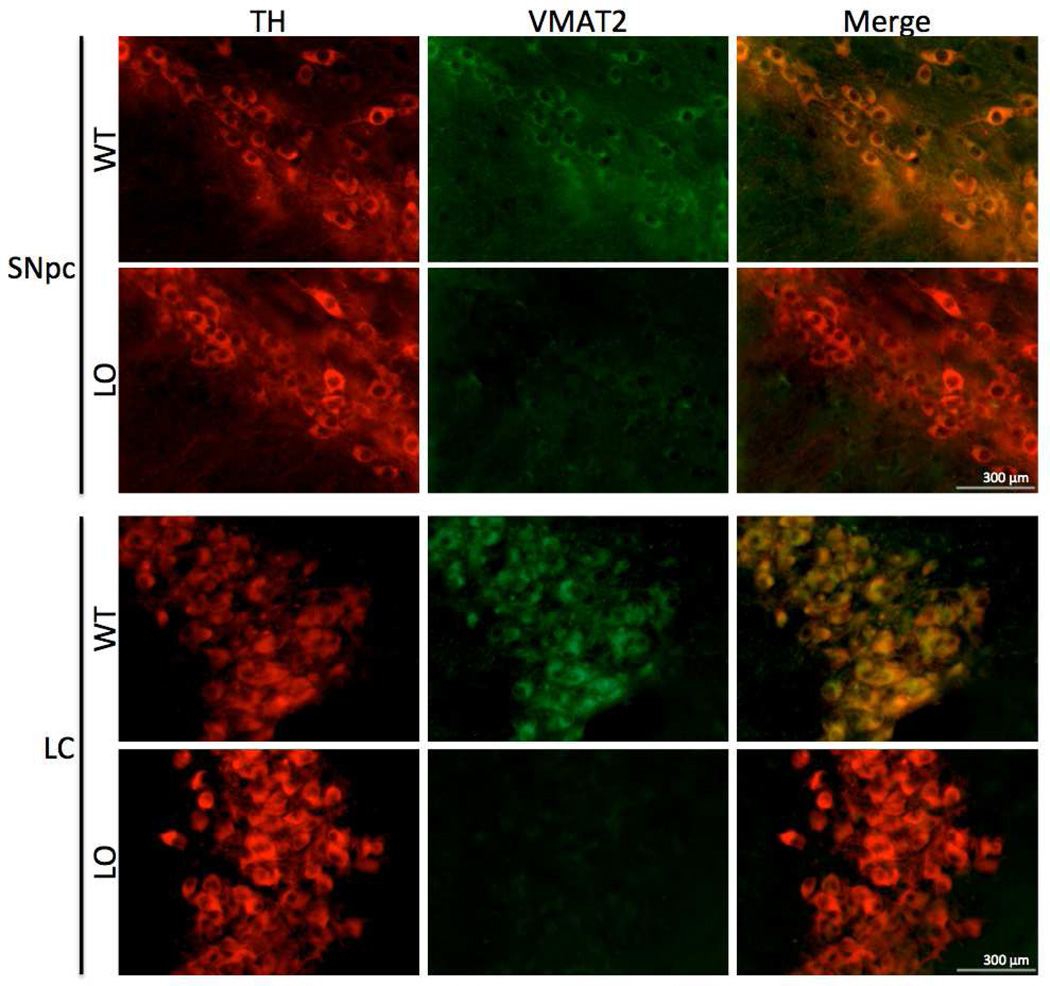
In this study, we sought to compare the effects of very low VMAT2 expression on the dopaminergic and noradrenergic systems. We thus evaluated VMAT2 expression in the SNpc and LC of VMAT2 LO mice using immunohistochemistry to confirm comparable reductions of the protein (Figure 1). Wildtype animals exhibited a cytosolic distribution of VMAT2 immunoreactivity in TH+ neurons of the SNpc and LC. Consistent with an earlier report (Mooslehner et al., 2001), VMAT2 immunoreactivity in the SNpc of VMAT2 LO brains was essentially absent, save for trace somatic staining. In the LC, TH+ neurons were also nearly devoid of VMAT2 immunoreactivity, indicating very low Slc18a2 gene product in both regions.
Figure 1. Immunohistochemical analysis of VMAT2 expression in the substantia nigra and locus ceruleus.
Widefield immunofluorescent images of TH and VMAT2 expression in the SNpc and LC. Top panels, WT mice exhibited normal TH (red) and VMAT2 (green) immunoreactivity in the SNpc, while VMAT2 LO mice had normal TH immunoreactivity, but trace somatic VMAT2 immunoreactivity. Lower panels, WT mice had marked TH and VMAT2 immunoreactivity in the LC, while VMAT2 LO mice exhibited normal TH immunoreactivity and trace VMAT2 immunoreactivity, as in the SNpc. Scale bars represent 300 µm.
3.2. VMAT2 LO mice have markedly reduced tissue catecholamine contents and increased relative deamination of catecholamines
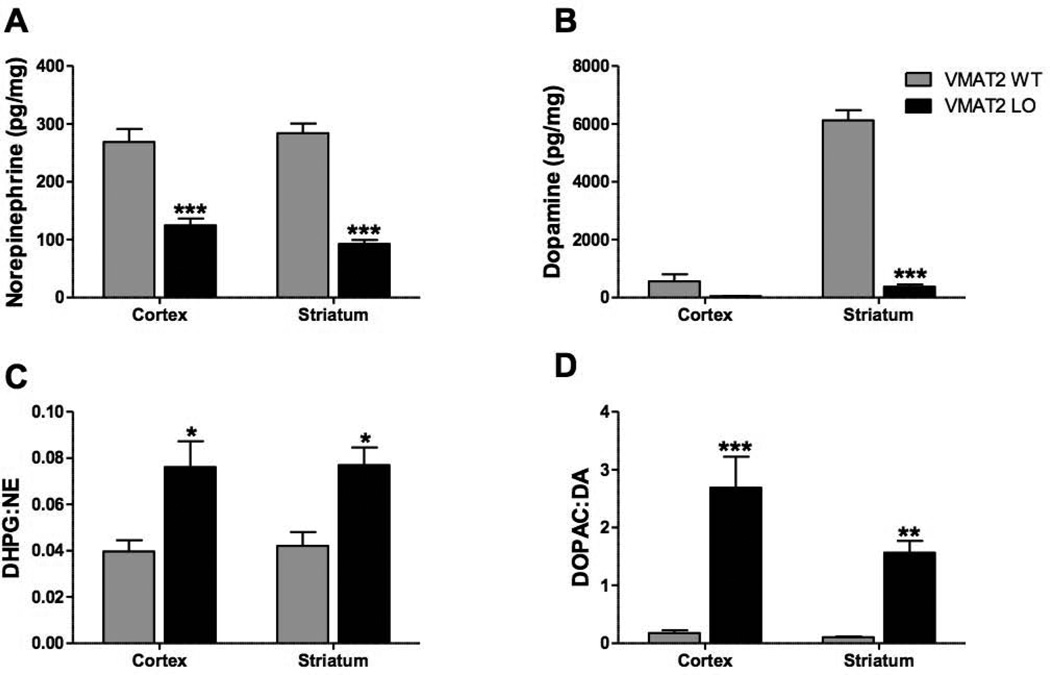
Cortical and striatal tissues of 2-month old VMAT2 LO animals had reduced levels of both NE and DA (Figure 2A, B). ANOVA revealed an effect of genotype on NE (F(1,18)=110.3, p<0.0001) and DA (F(1,18)=171.8, p<0.0001). In the cortex, we observed a 54% decrease in NE (wildtype: 268.8 ± 22.9 pg/mg, VMAT2 LO: 124.8 ± 11.81 pg/mg, p<0.001) and a tendency toward decrease in DA (wildtype: 557.5 ± 243.3 pg/mg, VMAT2 LO: 50.0 ± 9.9 pg/mg, p>0.05). In the striatum, we observed a 67% decrease in NE (wildtype: 284.2 ± 16.7 pg/mg, VMAT2 LO: 92.6 ± 6.9 pg/mg, p<0.001) and 94% reduction in DA (wildtype: 6125.8 ± 349.2, VMAT2 LO: 379.2 ± 70.7, p<0.001).
Figure 2. VMAT2 LO mice have reduced brain catecholamines and increased catecholamine turnover at 2 months of age.
A and B: NE and DA content varied with respect to genotype (n=4). A, Brain NE was reduced in the cortex and striatum of VMAT2 LO mice. B, DA was reduced in the striatum of VMAT2 LO animals; DA content in the cortex trended toward reduction. C and D, In all brain regions surveyed, VMAT2 LO mice exhibited increased relative conversion of NE (C) and DA (D) to their metabolites DHPG and DOPAC, respectively. Results represent the mean ± SEM for 4 animals per genotype at each age. *p<0.05, **p<0.01, ***p<0.001.
In both the cortex and striatum of 2-month old VMAT2 LO mice, we observed a genotype-dependent increase in tissue DHPG:NE ratios (Figure 2C) (F(2,18)=23.73, p<0.0001). There were significant increases in both tissues (cortex: p<0.05, striatum: p<0.05). These mice also had genotype-dependent increase in DOPAC:DA (Figure 2D) (F(2,18)=47.4, p<0.0001) in the cortex (p<0.001) and striatum (p<0.01). L-DOPA levels were unchanged between genotypes (F(1,18)=0.02, p=0.9; striatum, wildtype: 20.3 ± 10.8, VMAT2 LO: 19.4 ± 4.4; cortex, wildtype: 12.8 ± 0.9, VMAT2 LO: 12.1 ± 1.9).
3.3. VMAT2 LO mice have decreased cortical expression of NET
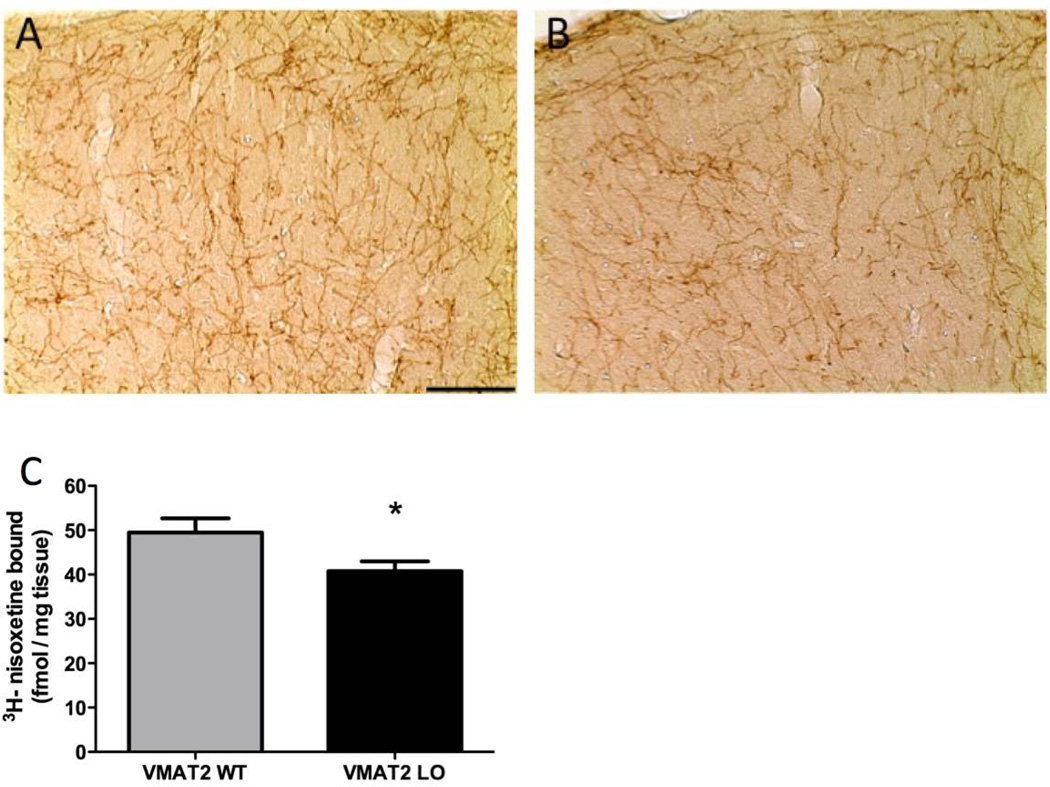
We examined NET expression using immunohistochemistry in the somatomotor cortex of 12-month old wildtype and VMAT2 LO mice (Figure 3A, B) and observed a reduction in NET immunoreactivity. We quantified the reduction of NET expression in the cortex with 3H-nisoxetine binding (Figure 3C), VMAT2 LO animals had a 20% reduction in 3H-nisoxetine binding, compared to wild type (40.8 ± 2.2 vs. 49.5 ± 3.2 fmol/mg, p<0.05).
Figure 3. VMAT2 LO mice have reduced expression of the norepinephrine transporter.
A and B, Immunohistochemical labeling in the somatomotor cortex. At 12 months of age, VMAT2 LO mice showed a reduction in NET immunoreactivity throughout the cortex, compared to wildtype (representative images of somatomotor area shown at 20× magnification). C, 3H-nisoxetine-binding was performed to quantify changes in NET abundance. C, There was a reduction (20%) in nisoxetine binding sites in 12-month VMAT2 LO cortices (n=6). Results represent the mean ± SEM for 6 animals per genotype at each age. *p<0.05.
3.4. VMAT2 LO mice have age-related catecholaminergic degeneration in the LC and SNpc, but not in the VTA
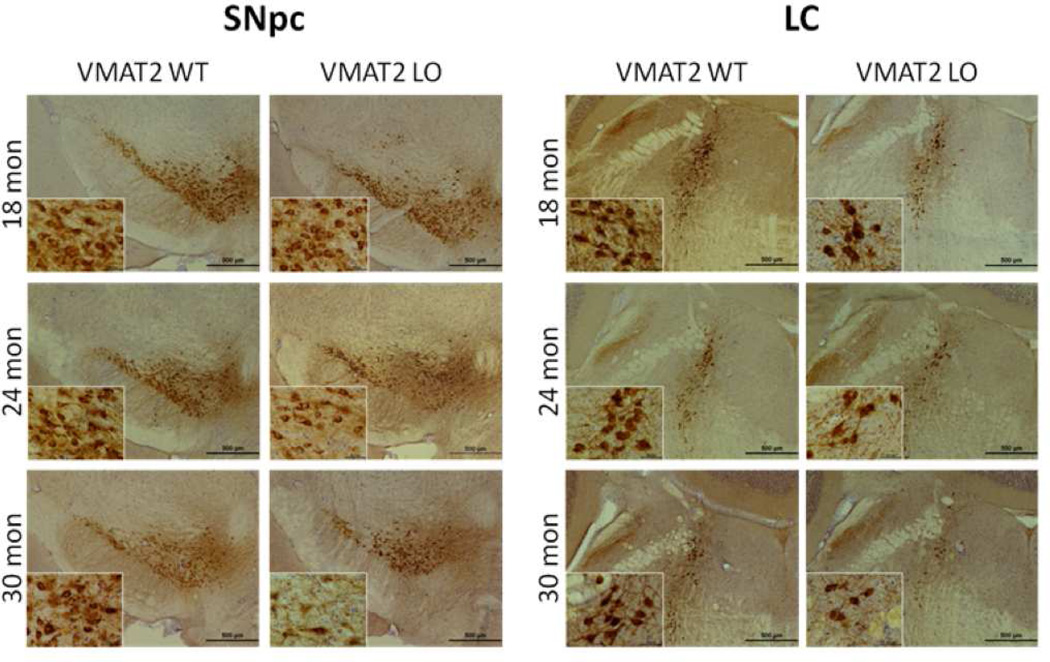
At 12 months of age, VMAT2 LO mice did not differ from age-matched controls in TH expression or evidence of neurodegeneration, as measured by silver deposition, in the SNpc or LC (data not shown). At 18 months of age, the VMAT2 LO group had mildly reduced TH staining in the SNpc. The extent of abnormality progressed with age (Figure 4). Larger reductions in TH staining were observed in the LC than the SNpc at 18, 24, and 30 months of age.
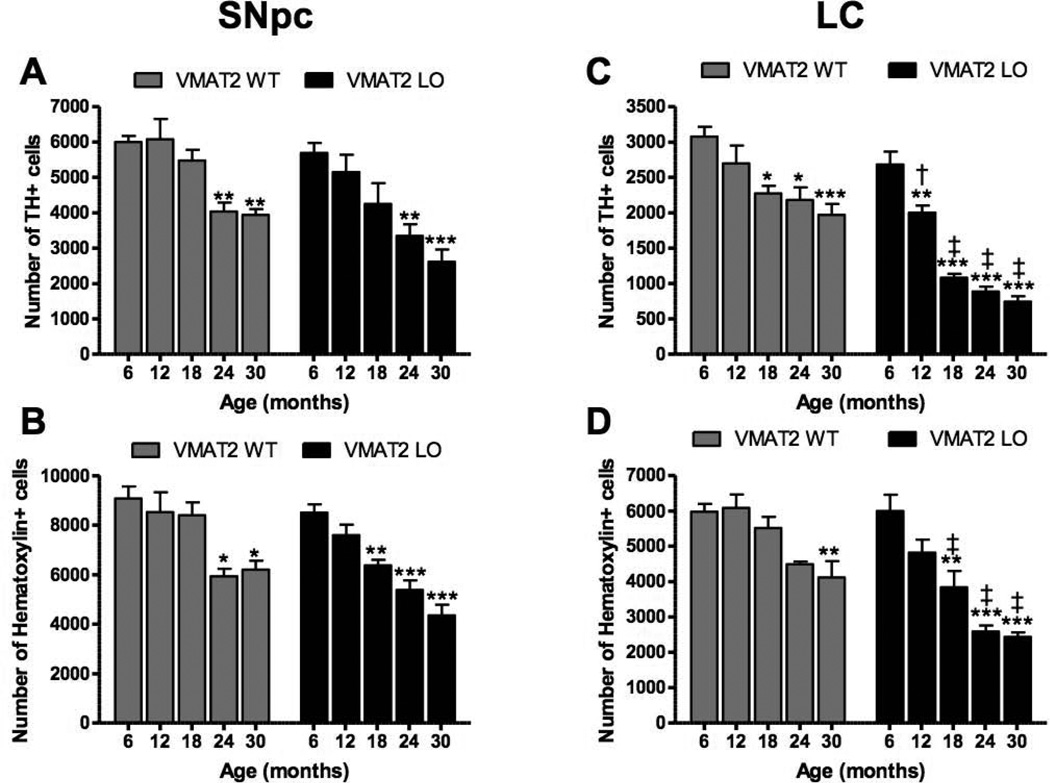
Figure 4. VMAT2 LO animals undergo progressive catecholaminergic cell loss.
Immunohistochemical analysis of tyrosine hydroxylase in locus ceruleus of aged VMAT2 LO mice. TH immunoreactivity was substantially reduced in VMAT2 LO mice at 18, 24, and 30 months of age, preceding loss observed in the substantia nigra. Analysis was performed on 5 animals per genotype at each age. Representative sections are shown. Scale bars, 500 µm.
To quantify the extent of neuronal loss in the SNpc and LC in the VMAT2 LO mice, unbiased stereological cell counts were performed on TH− and hematoxylin-stained cells. In the SNpc, two-way ANOVA revealed an effect of genotype (F(1,35)=44.14, p<0.001) and age (F(4,35)=120.6, p<0.0001) on the number of TH+ neurons (Figure 5A), although there was no interaction (F(4,35)=4.219, p=0.6). Hematoxylin+ cells followed the same pattern. At all ages, VMAT2 LO brains had fewer TH+ SNpc neurons than did wildtype littermates, though Bonferroni post-hoc tests did not reveal significance between genotypes at any single time point. Within genotypes, wildtype and VMAT2 LO mice underwent significant SNpc degeneration at 24 months of age, with respect to counts at 6 months (wildtype, 6 months: 5999 ± 176, wildtype, 24 months: 4039 ± 252, p<0.001; VMAT2 LO, 6 months: 5691 ± 286, 24 months: 3347 ± 331, p<0.001). At 30 months, wildtype mice (30 months: 3943 ± 159) had lost 34% of TH+ SNpc neurons, while VMAT2 LO mice (2614 ± 344) lost 54% of TH+ neurons with respect to the corresponding 6 month value for each groups.
Figure 5. Quantitative analysis of catecholaminergic cell loss reveals degeneration of LC precedes that of SNpc.
TH and hematoxylin cell counts in the SNpc and LC of 6, 12 18, 24, and 30 month wildtype and VMAT2 LO mice. A, Significant reductions in TH cell counts in the SNpc were observed beginning at 24 months in wildtype and VMAT2 LO mice, with VMAT2 LO mice having fewer TH cells overall. B, Furthermore, a similar age-dependent reduction in hematoxylin cells in the SNpc was seen in wildtype and VMAT2 LO mice. C, Wildtype and VMAT2 LO mice underwent progressive loss of TH cells in the LC, with VMAT2 LO mice having accelerated loss compared to wildtype, beginning at 12 months of age. D, A significant reduction was also seen in hematoxylin cells in the LC at 18, 24, and 30 months of age in VMAT2 LO mice compared to wildtype in the LC. Results represent the mean ± SEM for 5 animals per genotype at each age. *p<0.05, **p<0.01; ***p<0.001 (with respect to 6 month mice within genotype) †<0.05, ‡<0.001 (between genotypes).
Within the LC, neuronal survival was an effect of age (F(4,37)=44.14, p<0.0001) and genotype (F(1,37)=120.6, p<0.0001). There was an interaction between these factors (F(4,37)=4.219, p<0.01). Hematoxylin+ cell loss followed the same pattern as TH+ neuron loss. Bonferroni post-hoc tests revealed that degeneration of TH+ LC neurons was significantly greater in VMAT2 LO mice than in wildtype littermates beginning at 12 months (wildtype: 2695 ± 255, VMAT2 LO: 2001 ± 104, p<0.05) proceeding through 30 months (wildtype: 1969 ± 155, VMAT2 LO: 744 ± 76, p<0.001). Within genotype, LC degeneration was first significant in wildtype mice at 18 months of age (wildtype, 6 months: 3079 ± 137, wildtype, 18 months: 2274 ± 107, p<0.05) and first significant in VMAT2 LO mice at 12 months of age (VMAT2 LO, 6 months: 2683 ± 182, VMAT2 LO, 12 months: 2001 ± 104). At 30 months, wildtype mice lost 36% (p<0.001) of TH+ LC neurons, while VMAT2 LO mice lost 72% (p<0.001) of TH+ LC neurons with respect to the corresponding 6 month value for each group.
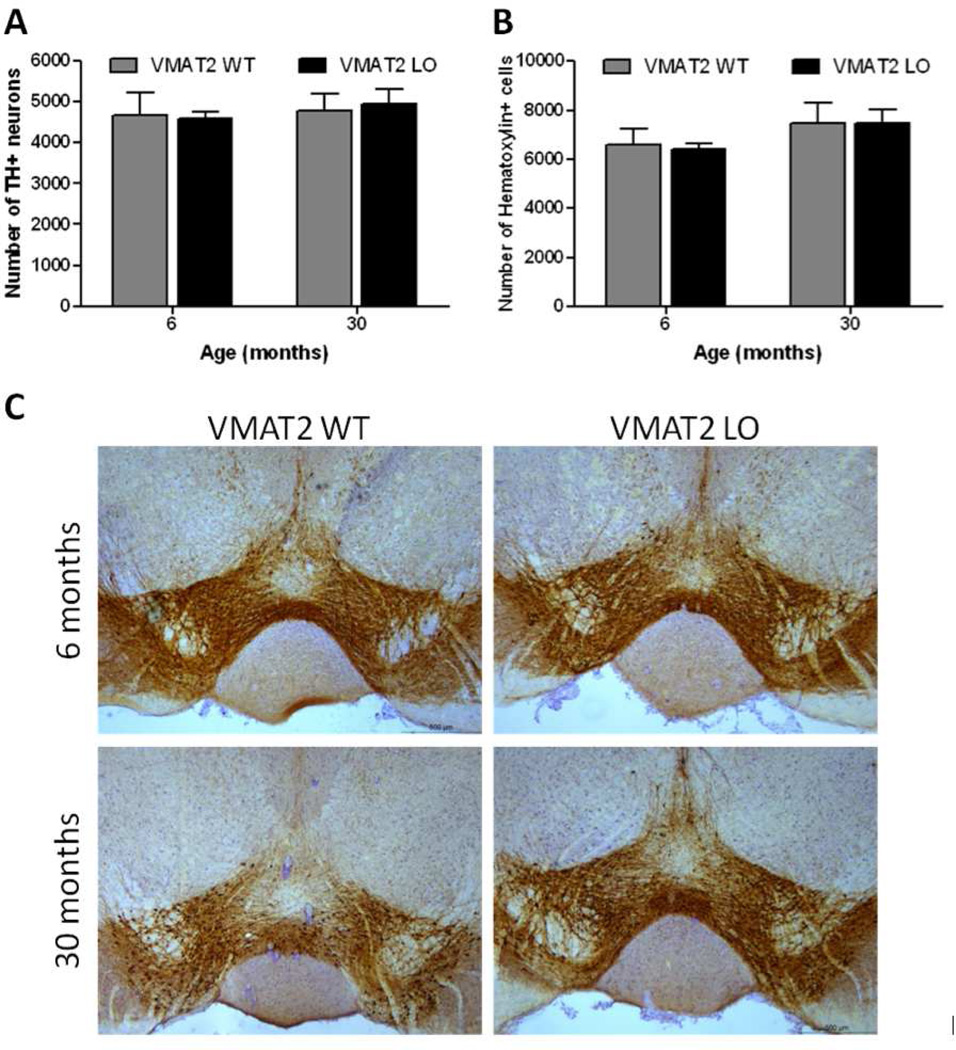
When the VTA was examined for evidence of neuronal loss, no change was seen in wildtype or VMAT2 LO animals at 6 or 30 months of age (Figure 6). At 6 months of age, wildtype and VMAT2 LO animals displayed similar numbers of TH-positive (wildtype: 4684 ± 544, VMAT2 LO: 4590 ± 159; p>0.05) and hematoxylin-positive neurons (wildtype: 6594 ± 694, VMAT LO: 6413 ± 236; p>0.05). Moreover, at 30 months of age, we observed no difference in the quantity of TH+ neurons in the VTA between wildtype and VMAT2 LO animals (wildtype: 4784 ± 429, VMAT2 LO: 4948 ± 349, p>0.05; hematoxylin-positive: wildtype: 7470 ± 813, VMAT2 LO: 7461 ± 567, p>0.05).
Figure 6. VMAT2 LO mice do not display neuronal loss in the VTA.
TH and hematoxylin cell counts in the VTA of 6 and 30 month wildtype and VMAT2 LO animals. A, No differences in TH-positive neurons were seen in 6 or 30 month VMAT2 LO mice when compared with age-matched WT controls (p>0.05). B, Similarly, no changes were seen in the number of hematoxylin stained cells in the VTA at either age. Results represent the mean ± SEM for 5 animals per genotype at each age. C, Immunohistochemical analysis of tyrosine hydroxylase in the VTA demonstrates that TH immunoreactivity is not significantly changed in VMAT2 WT or LO animals from 6 to 30 months of age. Representative sections are shown. Scale bars, 500 µm.
3.5. VMAT2 LO mice have reduced cardiac noradrenergic innervation
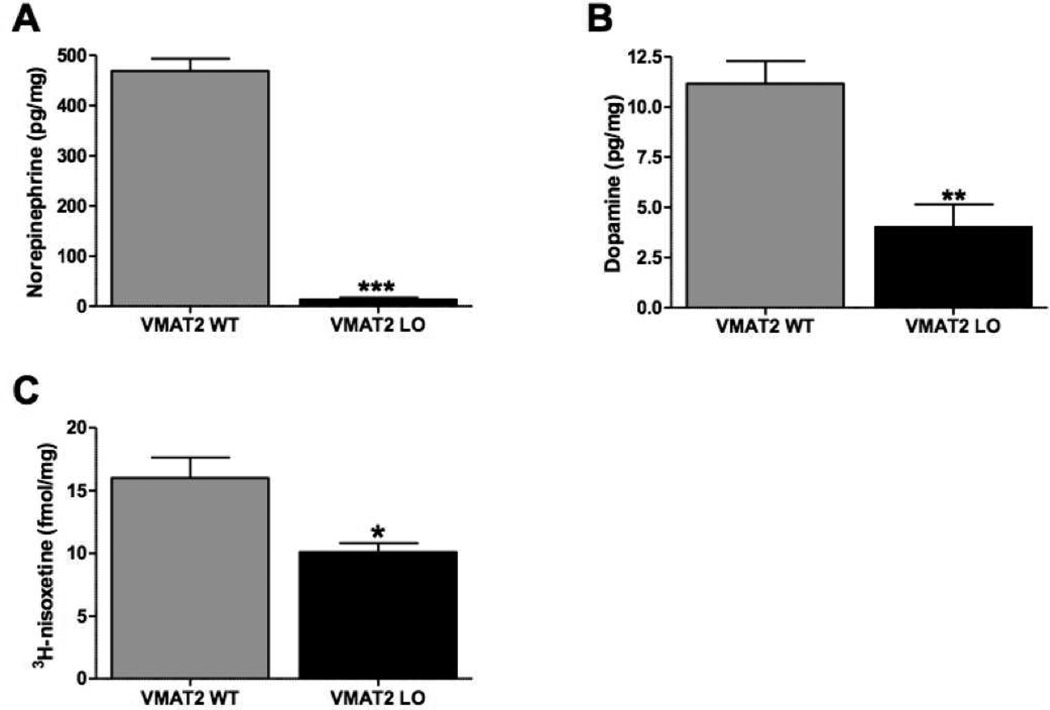
We assayed cardiac tissue for neurochemical aberrations in 2-month VMAT2 LO hearts (Figure 7). At this time point, we observed a 97% reduction in cardiac NE (Figure 6A, wildtype: 469.1 ± 24.5 pg/mg, VMAT2 LO: 13.73 ± 13.6 pg/mg, p<0.0001). VMAT2 LO mice also showed reductions in cardiac DA (Figure 7B, wildtype: 11.15 ± 1.1, VMAT2 LO: 4.03 ± 1.1, p<0.01). To ascertain if reduced catecholamine storage affected the integrity of sympathetic terminals over time, we performed 3H-nisoxetine-binding in cardiomyocyte homogenates in 12-month old mice (Figure 7C). VMAT2 LO animals had substantially reduced (38%) 3H-nisoxetine binding in contrast to their wildtype littermates (wildtype: 16.00 ± 1.64 fmol bound/mg tissue, VMAT2 LO: 10.09 ± 0.73, p<0.05).
Figure 7. VMAT2 LO mice have reduced cardiac noradrenergic innervation.
HPLC analysis of cardiac catecholamine and metabolite content. A, Young (2 month) VMAT2 LO mice had a baseline reduction (97%) in cardiac NE (n=4). B, VMAT2 LO mice also had reduced levels of cardiac DA. C, 3H-nisoxetine binding in cardiomyocyte homogenates revealed that VMAT2 LO mice had reduced specific 3H-nisoxetine binding sites at 12 months. Results represent the mean ± SEM for 4 animals per genotype at each age. *p<0.05, **p<0.01, ***p<0.001.
4. Discussion
Our findings demonstrate that dramatically reduced VMAT2 expression augments the age-related loss of noradrenergic neurons in the locus ceruleus. We observed that genetic VMAT2 deficiency in vivo drives catecholamine depletion in the central nervous system and heart and increases relative cytosolic catecholamine catabolism by MAO. These findings in transgenic mice bear particular relevance to the pathology of PD where vesicular function may be compromised as a consequence of exogenous insult (Corrigan et al., 1998; Hatcher-Martin et al., 2012), genetic polymorphism (Brighina et al., 2013; Glatt et al., 2006; Uhl et al., 2000), or a cytosolic milieu that favors an oxidative state, especially with mitochondrial dysfunction (Schapira, 2011) and aging (Cruz-Muros et al., 2008). Indeed, post-mortem analysis of putamen tissue has revealed that PD patients exhibit neurochemical indices of reduced vesicular uptake of DA and buildup of DOPAL, a toxic MAO metabolite of DA (Goldstein et al., 2013).
In young (2 months) VMAT2 LO mice, we observed baseline deficits in striatal and cortical NE and DA, a direct result of insufficient vesicular catecholamine storage (Figure 2). Cytosolic catecholamines are subject to enzymatic deamination and autoxidation. Cytosolic autoxidation of catecholamines and their metabolites generates reactive quinone species and other oxidative metabolites (Sulzer and Zecca, 1999). We previously reported that the striata of VMAT2 LO mice showed elevated indices of cytosolic DA oxidation, in the form of cysteinyl-catechol adducts, as well as relatively increased generation of DOPAC (Caudle et al., 2007). Here, we have additionally reported that the cortices and striata of VMAT2 LO mice have increased conversion of NE to DHPG. These neurochemical aberrations are life-long in VMAT2 LO mice, and set the stage for the eventual age-related neuronal loss we observed in the LC and SNpc (Figure 5).
We observed that the LC of VMAT2 LO mice degenerated earlier (12 months) and to a greater extent than did the SNpc (24 months) (Figure 5). Clinically, the LC consistently degenerates in PD patients (Chan-Palay and Asan, 1989; Del Tredici and Braak, 2012; Forno, 1996) with severe decreases (as great as 78%) in neuronal number, possibly contributing to the neuropsychiatric symptoms of PD (Mann, 1983; Zarow et al., 2003). It has been theorized that the trophic signaling from the LC compensates for the debilitating effects of progressive nigrostriatal death and delays the onset of clinical signs until greater DA depletion has occurred (Antelman and Caggiula, 1977). Moreover, it has been demonstrated that noradrenergic neurons of the LC are responsible for dopaminergic signaling within the mouse hippocampus (Smith and Greene, 2012); this may occur elsewhere, especially in a setting of dopaminergic degeneration. The relationship between noradrenergic and dopaminergic degeneration established in this study suggests that NE loss could play a significant role in parkinsonian manifestations ranging from olfactory disturbances observed at 4 months of age in VMAT2 LO mice to depressive-like behaviors seen at 12–15 months (Taylor et al., 2009). Degeneration of the noradrenergic LC and other non-dopaminergic centers have been inferred in post-mortem PD brains. These monoaminergic centers are not directly related to the motor phenotype, yet contribute significantly to disease progression (Braak et al., 2003; Del Tredici and Braak, 2012; Gesi et al., 2000; Halliday et al., 1990; Murai et al., 2001; Rommelfanger and Weinshenker, 2007). Braak and colleagues propose that PD begins in the dorsal motor and olfactory nuclei of the brainstem and progresses topographically through susceptible regions (Braak et al., 2002; Braak et al., 2003). Motor disturbances do not present clinically until approximately 70–80% of striatal dopaminergic terminals have been lost; however, other non-motor symptoms are evident before the onset of motor disturbances. In the VMAT2 LO mice, it has been observed that the major motor deficits (e.g. shortened stride length) do not appear until 28 months of age, coinciding with the most severe SNpc cell loss (Figure 5A) (Taylor et al., 2011). The preceding phenotypes seen in the VMAT2 LO mice may be attributed to extra-dopaminergic changes (Taylor et al., 2009).
An intriguing finding in this study is that while the LC and SNpc of VMAT2 LO mice degenerated, the dopaminergic VTA remained unaffected (Figure 6). The VTA is less affected or spared in human PD and is resistant to degeneration in toxicant models (Dauer and Przedborski, 2003; German et al., 1992). Physiological differences between the VTA and SNpc may explain their disparate susceptibilities to neurotoxicity. The VTA is phenotypically distinct from the neighboring SNpc in its expression of calbindin, a calcium buffering protein, and unlike the SNpc it is not reliant on calcium influx for autonomous pacemaking (Surmeier et al., 2005). It has been demonstrated that cytosolic calcium increases conversion of L-DOPA to DA by amino acid decarboxylase, which occurs to a greater extent in SNpc neurons than in the VTA; this mechanism was shown to be responsible for the selective susceptibility of the SNpc over the VTA to L-DOPA-induced neurotoxicity in mice (Mosharov et al., 2009; Surmeier, 2009).
PD also affects the autonomic nervous system. Cardiac sympathetic denervation is a common comorbid event in the pathophysiology of PD, with 30% of patients meeting the diagnostic criteria for orthostatic hypotension (Velseboer et al., 2011). Loss of VMAT2 function either contributes to, or is associated with, this pathology. PD patients with orthostatic hypotension exhibit reduced VMAT2 function as measured by peripheral vesicular sequestration of 18F-labeled catecholamine analogs (Goldstein et al., 2011a). By design, VMAT2 LO mice are unable to sequester cytosolic catecholamines and consequently bear a striking 97% reduction in cardiac NE is seen at 2 months (Figure 7A). The magnitude of cardiac NE loss in contrast to the more modest reductions in the striatum and cortex may reflect variability between neuron types with respect to synthesis and release patterns, extraneuronal milieu, and expression of catecholamine metabolizing enzymes. Patients with cardiac sympathetic denervation have reduced binding of high-affinity PET ligands for NET in heart (Dae, 1994; Goldstein, 2003). Similarly, hearts of 12-month VMAT2 LO mice have substantial (40%) reduction of NET, revealed by 3H-nisoxetine binding (Figure 7C).
While the focus of this work examined the neurotoxic effects of cytoplasmic catecholamines, we should not overlook the role of α-synuclein in this pathology. VMAT2 LO mice do not undergo spontaneous neurodegeneration in the absence of endogenous α-synuclein (Colebrooke et al., 2006). Similarly, VMAT2 deficiency potentiates the toxic effects of lentiviral α-synuclein overexpression in mice (Ulusoy et al., 2012). Lastly, knockout of α–synuclein dramatically abrogates the effects of L-DOPA- and calcium-induced neurotoxicity in midbrain DA neurons (Mosharov et al., 2009). This interplay is supported by in vitro experiments which have demonstrated that products of cytoplasmic dopamine breakdown oligomerize α-synuclein (Burke et al., 2008) and stabilize subsequent protofibril formation (Conway et al., 2001); it is hypothesized that this mechanism could drive Lewy body pathology in vivo (Sulzer, 2001). Collectively, these findings indicate that cytoplasmic catecholamine catabolism and α-synuclein may synergistically participate in PD pathogenesis.
5. Conclusions
Our findings in VMAT2 LO mice demonstrate that insufficient catecholamine storage is capable of driving noradrenergic neurodegeneration in an in vivo setting. Despite the deleterious effects of reduced VMAT2 expression on the SNpc and LC, the VTA remained unaffected, likely due to physiological differences that make this region resilient in animal models and human PD. The combination of LC and SNpc degeneration and the absence of VTA degeneration is strikingly consistent with human PD and underscores the importance of proper catecholamine sequestration to neuronal health. VMAT2 activity, with its role in catecholamine homeostasis, may be a critical determinant in noradrenergic and dopaminergic susceptibility to neurotoxicity.
Highlights.
-
**
VMAT2 LO mice have drastic reductions in VMAT2 expression in SNpc and LC neurons.
-
**
VMAT2 LO mice have baseline deficits in brain and cardiac catecholamines.
-
**
VMAT2 LO neurons have increased cytosolic catecholamine turnover.
-
**
VMAT2 LO mice exhibit reduced NET expression in the brain and heart.
-
**
VMAT2 LO mice have age-dependent SNpc and LC degeneration, but VTA is spared.
Acknowledgements
This work was supported by funding from F31 ES017247 (Taylor), T32 ES012870, T32 GM008602 (Alter), and P01 ES01673 (Miller). The authors are grateful for the technical assistance provided by Patricia Sullivan in acquisition of HPLC data. We would like to thank Dr. Alison Bernstein for generating and characterizing the VMAT2 antibody.
Abbreviations
- DA
dopamine
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- DOPEGAL
3-4-dihydroxyphenylglycolaldehyde
- LC
locus ceruleus
- MAO
monoamine oxidase
- NE
norepinephrine
- NET
norepinephrine transporter
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter 2
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amino T, et al. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195:646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–III/5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brighina L, et al. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson's disease. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathologica. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Burke WJ, et al. Neurotoxicity of MAO Metabolites of Catecholamine Neurotransmitters: Role in Neurodegenerative Diseases. NeuroToxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Caudle WM, et al. Altered vesicular dopamine storage in Parkinson's disease: a premature demise. Trends in Neurosciences. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Caudle WM, et al. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Caudle WM, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol. 1989;287:357–372. doi: 10.1002/cne.902870307. [DOI] [PubMed] [Google Scholar]
- Colebrooke RE, et al. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson's disease. Eur J Neurosci. 2006;24:2622–2630. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- Conway KA, et al. Kinetic Stabilization of the Alpha-Synuclein Protofibril by a Dopamine-Alpha-Synuclein Adduct. Science. 2001;294:1346. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, et al. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Cruz-Muros I, et al. Deglycosylation and subcellular redistribution of VMAT2 in the mesostriatal system during normal aging. Neurobiol Aging. 2008;29:1702–1711. doi: 10.1016/j.neurobiolaging.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Cubells J, et al. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. The Journal of Neuroscience. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dae M. Imaging of myocardial sympathetic innervation with metaiodobenzylguanidine. Journal of Nuclear Cardiology. 1994;1:S23–S30. doi: 10.1007/BF02940065. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Dysfunction of the locus coeruleus–norepinephrine system and related circuitry in Parkinson's disease-related dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2012 doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- German DC, et al. Midbrain Dopaminergic Cell Loss in Parkinson's Disease and MPTP-Induced Parkinsonism: Sparing of Calbindin-D25k—Containing Cells. Annals of the New York Academy of Sciences. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- Gesi M, et al. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Glatt CE, et al. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Human Molecular Genetics. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Dysautonomia in Parkinson's disease: neurocardiological abnormalities. The Lancet Neurology. 2003;2:669–676. doi: 10.1016/s1474-4422(03)00555-6. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, et al. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. The Journal of Clinical Investigation. 2011a;121:3320–3330. doi: 10.1172/JCI45803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, et al. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res. 2007;17:118–121. doi: 10.1007/s10286-007-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, et al. Catechols in post-mortem brain of patients with Parkinson disease. European Journal of Neurology. 2011b;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, et al. Determinants of Buildup of the Toxic Dopamine Metabolite DOPAL in Parkinson Disease. J Neurochem. 2013 doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, et al. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. Journal of Neurochemistry. 2008;106:2205–2217. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, et al. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson's disease. Brain Res. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- Hatcher-Martin JM, et al. Association between polychlorinated biphenyls and Parkinson's disease neuropathology. NeuroToxicology. 2012;33:1298–1304. doi: 10.1016/j.neuro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, et al. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. Journal of Chromatography B: Biomedical Sciences and Applications. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mech Ageing Dev. 1983;23:73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Miller GW, et al. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson's disease. Exp Neurol. 1999;156:138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Miller GW, et al. Immunochemical analysis of dopamine transporter protein in Parkinson's disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, et al. Mice with Very Low Expression of the Vesicular Monoamine Transporter 2 Gene Survive into Adulthood: Potential Mouse Model for Parkinsonism. Molecular and Cellular Biology. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, et al. Interplay between Cytosolic Dopamine, Calcium, and α-Synuclein Causes Selective Death of Substantia Nigra Neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, et al. In vivo evidence for differential association of striatal dopamine and midbrain serotonin systems with neuropsychiatric symptoms in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2001;13:222–228. doi: 10.1176/jnp.13.2.222. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The Neuropathologic Basis of Different Clinical Subgroups of Parkinsons-Disease. Journal of Neuropathology and Experimental Neurology. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial pathology in Parkinson's disease. Mt Sinai J Med. 2011;78:872–881. doi: 10.1002/msj.20303. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS Dopamine Transmission Mediated by Noradrenergic Innervation. The Journal of Neuroscience. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. [alpha]-synuclein and cytosolic dopamine: Stabilizing a bad situation. Nat Med. 2001;7:1280–1282. doi: 10.1038/nm1201-1280. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Movement Disorders. 2012 doi: 10.1002/mds.25187. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotoxicity Research. 1999;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ. A Lethal Convergence of Dopamine and Calcium. Neuron. 2009;62:163–164. doi: 10.1016/j.neuron.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, et al. Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway? Curr Opin Neurobiol. 2005;15:312–318. doi: 10.1016/j.conb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Takahashi N, et al. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proceedings of the National Academy of Sciences. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, et al. VMAT2-Deficient Mice Display Nigral and Extranigral Pathology and Motor and Nonmotor Symptoms of Parkinson's Disease. Parkinsons Dis. 2011;2011:124165. doi: 10.4061/2011/124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, et al. Nonmotor Symptoms of Parkinson's Disease Revealed in an Animal Model with Reduced Monoamine Storage Capacity. The Journal of Neuroscience. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. Journal of Pharmacology and Experimental Therapeutics. 1992;260:427–436. [PubMed] [Google Scholar]
- Uhl GR, et al. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14:2459–2465. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, et al. Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiology of Disease. 2012;47:367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Velseboer DC, et al. Prevalence of orthostatic hypotension in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- West MJ, et al. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zarow C, et al. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]