Abstract
Objective
Therapeutic arteriogenesis, i.e., expansive remodeling of pre-existing collaterals, using single-action factor therapies has not been as successful as anticipated. Modulation of factors that act as a master switch for relevant gene programs may prove more effective. Transcriptional co-activator P300/CBP-associated factor (PCAF) has histone acetylating activity and promotes transcription of multiple inflammatory genes. Because arteriogenesis is an inflammation-driven process, we hypothesized that PCAF acts as multifactorial regulator of arteriogenesis.
Approach and Results
After induction of hind limb ischemia, blood flow recovery was impaired in both PCAF−/− mice and healthy wild type mice treated with the pharmacological PCAF inhibitor Garcinol, demonstrating an important role for PCAF in arteriogenesis. PCAF deficiency reduced the in vitro inflammatory response in leukocytes and vascular cells involved in arteriogenesis. In vivo gene expression profiling revealed that PCAF deficiency results in differential expression of 3505 genes during arteriogenesis and, more specifically, in impaired induction of multiple pro-inflammatory genes. Additionally, recruitment from the bone marrow of inflammatory cells, in particular “pro-inflammatory” Ly6Chi monocytes, was severely impaired in PCAF−/− mice.
Conclusions
These findings indicate that PCAF acts as master switch in the inflammatory processes required for effective arteriogenesis.
Keywords: arteriogenesis, inflammation, monocyte subtypes, PCAF
Introduction
Peripheral arterial occlusive disease is a leading cause of morbidity and mortality. Blood flow to ischemic tissues in the affected limb can be restored by distinct processes1, namely vasculogenesis, angiogenesis and arteriogenesis, of which arteriogenesis, the remodeling of pre-existing collateral arterioles into larger arteries, has the greatest impact2.
Effective arteriogenesis requires coordination of multiple events. Arteriogenesis is triggered by an increase in fluid shear stress across pre-existing collaterals cross-connecting adjacent arterial trees, which is caused by a pressure gradient created by occlusion or atherosclerotic stenosis of one of the trees. This leads to activation of the endothelial cells and adjacent vascular smooth muscle cells (VSMCs) of the collateral wall. Induction of adhesion molecules, cytokines and chemokines then follows as the first step of an inflammatory cascade essential for arteriogenesis. Recruitment of leukocytes from blood and bone marrow follows, in particular monocytes3-6 but also CD4+, CD8+ and regulatory T cells and natural killer cells7-11. These cells infiltrate into the perivascular space around collaterals and release additional paracrine signaling molecules and growth factors. Subsequent degradation and reorganization of the extracellular matrix by released matrix metalloproteases (MMPs), including MMP2 and MMP9, creates space required for expansive remodeling of the pre-existing collaterals. Proliferation of collateral endothelial cells, VSMCs and fibroblasts is stimulated, resulting in an increased anatomic lumen diameter. All of the steps described above underline the crucial role of inflammation in effective arteriogenesis.
Although stimulation of collateral remodeling is regarded as a promising therapeutic alternative to surgical interventions, clinical trials aimed at modulating individual growth factors or cytokines have thus far not been as successful as anticipated12. We now know that the coordinated inflammatory and immune modulatory processes driving collateral growth are multifactorial and too complex to be modulated by therapeutics that target a single gene or pathway. In contrast, modulation of a factor that acts as a master switch for multiple relevant gene programs may be a more effective strategy to augment arteriogenesis.
A protein with such master switch potential is P300/CBP-Associated Factor (PCAF), a transcriptional co-activator with intrinsic histone acetyltransferase activity. PCAF acetylates histones H3 and H4, but there is also increasing evidence that PCAF modulates non-histone proteins13-16, including hypoxia-inducible factor 1α (Hif-1α)17 and Notch18. Furthermore, the histone acetylating activity of PCAF is essential for NF-κB-mediated gene transcription19 and facilitates inflammatory gene regulation20. Since arteriogenesis is an inflammatory-like process, we hypothesized that PCAF acts as master switch that stimulates multiple inflammatory processes important for collateral remodeling.
Recently, it was shown in a large patient population study (>3000 individuals)21 that a variation in the promoter region of PCAF is associated with coronary heart disease-related mortality22. In support of this observation, we recently demonstrated a role for PCAF in vascular remodeling in a mouse model for reactive stenosis. However, whether PCAF participates in arteriogenesis has not yet been investigated.
In the present study, we investigated the contribution of PCAF to post-ischemic neovascularization in a hind limb ischemia (HLI) model23, using PCAF deficient (PCAF−/−) mice. When studying arteriogenesis in a knockout model, it is possible that the gene deletion may affect vascular development in the embryo, including collaterogenesis, thus affecting the number of collaterals available for remodeling after an occlusive event in the adult. To investigate whether observed effects were caused by differences in arteriogenesis, in the native collateral circulation or a combination of both, we examined the pre-existing collateral density as well as the effect of administration of the PCAF inhibitor Garcinol to wild type (WT) mice after induction of HLI. We also studied gene expression and leukocyte recruitment in PCAF−/− and WT mice after induction of HLI to examine potential mechanisms by which PCAF regulates arteriogenesis.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
PCAF contributes to collateral remodeling
PCAF−/− mice showed impaired blood flow recovery after HLI (Figure 1A-B). Postoperative blood flow was decreased to approximately 6% of blood flow in the contralateral limb in both groups, with a trend towards reduced blood flow in PCAF−/−mice compared to WT mice (Figure 1C, p=0.07). Thereafter, blood flow recovery in PCAF−/− mice was reduced and did not recover completely before termination at 28 days. Moreover, PCAF−/− mice showed significantly more necrotic toe nails than WT mice (PCAF−/− 2.9 ±0.6 vs WT 0.45±0.2, p<0.001) (Figure 1D). No auto-amputation of hind limb digits was observed in either group. The reduced blood flow recovery in PCAF−/−mice was confirmed by quantification of smooth muscle α-actin positive (αSMA+) vessels in the adductor muscle group, 28 days after HLI (Figure 1E). Both the number of αSMA+ vessels (Figure 1F) and the diameter of αSMA+ vessels (Figure 1G-H) in PCAF−/− mice were significantly reduced, resulting in a reduced blood flow. The mean lumen area per αSMA+ vessel (PCAF−/− 139±15μm2 vs WT 297±26μm2, p<0.001) and total lumen area per section (PCAF−/− 447±46μm2 vs WT 1253±117μm2, p<0.001) were severely reduced in PCAF−/− mice (Figure 1G-H). Thus, PCAF deficiency leads to reduced arteriogenesis after induction of HLI.
Figure 1.
Arteriogenesis in PCAF−/− mice. (A) Representative LDPI images (laser Doppler perfusion imaging) of paws from PCAF−/− and WT mice directly and 7 days after induction of HLI in the left limb, by double electrocoagulation of the femoral artery. High blood flow is displayed in red. (B) Quantification of LDPI measurements of PCAF−/− and WT mice over time. Data are calculated as the ratio of ligated over non-ligated paw. (C) Quantification of LDPI measurements of PCAF−/− and WT mice directly after induction of HLI. Data are calculated as the ratio of ligated over non-ligated paw. (D) Quantification of necrotic toe nails of the ligated limb in PCAF−/− and WT mice counted 28 days after HLI. (E). Immunohistochemical staining of paraffin-embedded adductor muscle group of PCAF−/− and WT mice 28 days after HLI using anti-αSMA (red) antibodies. Lumen diameter of αSMA+ vessels is indicated by black bars. Scale bars = 50 μm. (F-H) Number, mean lumen area (μm2) and total lumen area per section (μm2 / section) of αSMA+ vessels, measured at the center of the adductor muscle group in ligated and non-ligated limbs of PCAF−/− and WT mice. Data are calculated as the ratio of ligated over non-ligated paw. All values are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
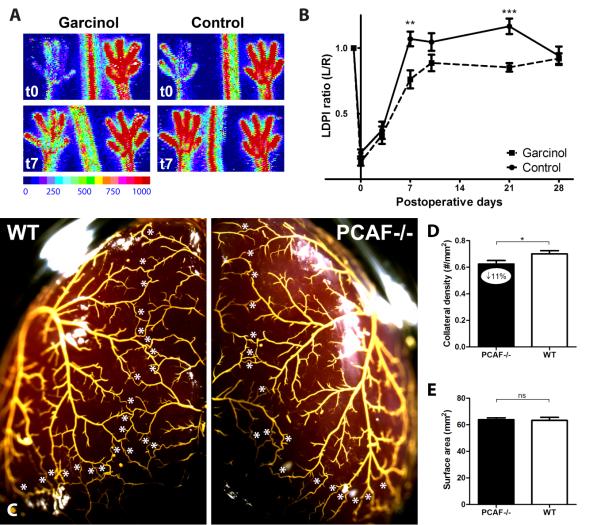
To assess whether the reduction in blood flow recovery in PCAF−/− mice was caused by reduced collateral remodeling or by fewer pre-existing collaterals, we performed two additional experiments. First, we inhibited PCAF by pharmacological intervention with Garcinol to rule out any effects on number of pre-existing collaterals in the PCAF−/− mice. In WT mice, local PCAF inhibition by Garcinol resulted in reduced blood flow restoration compared to the empty pluronic gel control group (Figure 2A-B). Second, the preexisting vascular bed of PCAF−/− and WT mice was assessed in the pial circulation using an arterial vascular casting (Figure 2C). Pial collateral density in PCAF−/− mice was reduced by 11% compared to WT mice, reflecting a moderate but significant contribution of PCAF in determining the abundance of the native collateral circulation (Figure 2D-E, p=0.02). This was in agreement with the trend towards decreased blood flow perfusion in PCAF−/− mice directly after HLI.
Figure 2.
Arteriogenesis after pharmacological inhibition of PCAF and assessment of the pre-existing collateral bed in PCAF−/− mice. (A) Representative LDPI images of paws directly and 7 days after induction of HLI in the left limb, by single electrocoagulation of the femoral artery. In WT mice, pluronic gel with or without 25 mg/ml Garcinol was applied topically to the adductor muscle before skin closure. High blood flow is displayed in red. (B) Quantification of LDPI measurements of WT mice treated with Garcinol or control over time. (C) Representative images of the pial circulation in PCAF−/− and WT mice. White asterisks indicate collateral arteries between anterior, middle and posterior cerebral arteries (ACA, MCA and PCA, respectively). Following exsanguination and maximal dilation of the dorsal cerebral circulation, Microfil™ was used as a casting agent, after which the whole brain was fixated in 4% PFA. (D) Pial collateral density was calculated in PCAF−/− and WT mice by dividing the sum of ACA to MCA, ACA to PCA and MCA to PCA by the surface area of the cerebral hemispheres. (E) Region of the brain utilized for calculation of pial density. Areas were excluded when they were damaged, had poor filling with Microfil™, or were otherwise uncountable. NS = non-significant. All values are presented as the mean ± SEM. *P < 0.05, PCAF−/− versus WT.
PCAF is required for in vitro inflammatory response
We investigated the role of PCAF in the inflammatory response of multiple cell types, given the above evidence for decreased collateral remodeling and the known involvement of these cells in arteriogenesis. Analysis of circulating cells in a whole blood LPS stimulation assay showed dose-dependent increase of TNFα in blood from WT mice, which was significantly reduced in blood from PCAF−/− mice (Figure 3A). Next, the splenic cell reservoir was subjected to LPS stimulation and pharmacological PCAF inhibition with Garcinol. LPS (300 ng/ml)-stimulated MCP-1 levels of splenocytes from both PCAF−/− mice (63±32pg/ml) and WT splenocytes treated with 20 μM Garcinol (195±35 pg/ml) were both significantly reduced in comparison to WT splenocytes (372±13 pg/ml, p=0.005 and p=0.04 respectively) (Figure 3B). Also the inflammatory phenotype of PCAF−/− VSMCs was assessed. Similar to the splenocyte stimulation, MCP-1 levels were markedly reduced after LPS (0.1 ng/ml) stimulation of PCAF−/− VSMCs (689±49 pg/ml) and WT VSMCs when exposed to 15μM Garcinol (3087±284 pg/ml) compared with untreated WT VSMCs (4175±264 pg/ml, p<0.001 and p=0.049 respectively) (Figure 3C). In addition, upregulation of MCP-1 mRNA was significantly reduced by 53% in PCAF−/− VSMCs (Figure 3D, p=0.01). To exclude non-specific effects of Garcinol, these experiments were repeated in WT VSMCs treated with siRNAs against PCAF mRNA instead of Garcinol. Transfection with siRNAs targeting PCAF mRNA efficiently decreased PCAF mRNA expression by 61% and, like Garcinol, inhibited MCP-1 production (Supplemental Figure IA-C).
Figure 3.
The role of PCAF in in vitro inflammatory response. (A) Inflammatory response of whole blood from PCAF−/− and WT mice was evaluated. Blood from tail vein was collected, diluted (1:25) and incubated 24 h with LPS (0-500 ng/ml). TNFα (pg/ml) level in cell-free supernatant was measured by ELISA. ND = non-detectable. (B) Splenocytes of PCAF−/− and WT mice were cultured and incubated for 24 h with LPS (300 ng/ml) or control. Splenocytes of WT mice were also incubated with Garcinol (20 μM) in combination with LPS (300 ng/ml) or control. Cell-free supernatant MCP-1 (pg/ml) level was measured by ELISA. (C) VSMCs of PCAF−/− and WT mice were cultured and incubated for 24 h with LPS (0.1 and 1 ng/ml) or control. VSMCs of WT mice were also incubated with Garcinol (15 μM) in combination with LPS (0.1 and 1 ng/ml) or control. Cell-free supernatant MCP-1 (pg/ml) level was measured by ELISA. (D) Vascular smooth muscle cells (VSMCs) of PCAF−/− and WT mice were cultured and incubated for 24 h with LPS (1 ng/ml) or control. MCP-1 mRNA expression was measured by real-time quantitative PCR. Cts were normalized against Cts of HPRT1. All values are presented as the mean ± SEM of triplicates. *P < 0.05, **P < 0.01, ***P < 0.001.
PCAF modulates post-ischemic gene regulation
PCAF staining showed enhanced expression in cells of large developing collaterals in the adductor muscle group compared to surrounding skeletal muscle (Figure 4A). To study differential gene expression after HLI between PCAF−/− and WT mice, total RNA isolated from the adductor muscle group was used in a whole-genome expression analysis using Illumina Beadchips. Statistical analysis by SAM on t1/t0avg ratios identified 1963 genes with a significant lower ratio and 1542 genes with a higher ratio in PCAF−/−relative to WT mice (q<5%), indicating that PCAF exhibits a large effect on gene transcription after HLI (Figure 4B).
Figure 4.
Gene regulation in PCAF−/− mice after HLI. (A) Immunohistochemical staining on fresh frozen sections of WT adductor muscle 1 day after HLI, using anti-αSMA (red) and anti-PCAF (green) antibodies. Cell nuclei were stained with DAPI (blue). Scale bars = 50 μm. (B) Heatmap of differentially regulated genes in whole-genome expression analysis, comparing PCAF−/− and WT mice. Included are genes that were significantly different between PCAF−/− and WT mice (q-value < 5). Data are presented as the fold change in expression between day 1 (t1) and average preoperative baseline levels (t0), generating t1/t0avg ratios. Red indicates increased and green indicates reduced expression relative to average baseline levels. The pie graph illustrates a significant decrease of 1963 genes (green) and increase of 1542 (red) genes in PCAF−/− relative to WT mice. (C-G) Microarray validation by real-time quantitative PCR of a selection of relevant regulated inflammatory factors MMP9, TNFα, CCL9, IRF7, CXCL12 and its receptor CXCR4 (H). The height of the bars represents the ratio of expression in PCAF−/−(black bars) and WT (white bars) mice at day 1 over day 0. Cts were normalized against Cts of HPRT1. All values are presented as the mean ± SEM. *P < 0.05, **P < 0.01, PCAF−/− versus WT.
Supplemental Table I shows the top 50 genes with impaired upregulation in PCAF−/− mice compared to WT mice, including MMP9, critical in matrix degradation required for collateral artery expansion. Since PCAF has been shown to regulate inflammatory gene transcription, we selected inflammatory genes that were significantly regulated (Supplemental Table II and Supplemental Figure II). Among the inflammatory genes showing a more pronounced induction in WT mice compared to PCAF−/− mice were genes encoding cytokines CXCL12, CCL9 and TNFα, chemokine receptor CXCR1, transcription factor IRF7, TNF receptor associated factors TRAF2 and TRAF3, TNF receptor associated protein TRAP1 and members of the TNF receptor superfamily TNFRSF19 and TNFRSF11a (also known as RANK). The total of inflammatory genes with greater induction in PCAF−/− mice was much smaller than the number of genes more strongly induced in WT, and included inhibitors of the NF-κB pathway like NFKBIA and NKIRAS1. Aberrant regulation of several relevant regulated factors (MMP9, TNFα, CCL9, CXCL12, IRF7) were confirmed using real-time quantitative PCR (Figure 4C-G).
PCAF deficiency alters leukocyte recruitment
We quantified leukocyte subtypes that are involved in arteriogenesis, including T cells (helper CD4+, cytotoxic CD8+ and regulatory T cells) and natural killer cells, and subtypes which have not been previously implicated in arteriogenesis, including B cells and dendritic cells. Blood samples from before (t0) and 1 day after (t1) HLI, were analyzed by FACS. PCAF deficiency had effects on most of the leukocyte subtypes examined. Following HLI, circulatory T cells were significantly decreased in PCAF−/− mice compared to WT mice. This difference was caused mainly by a reduction in CD4+ T cells, especially by the fraction of activated CD4+ T cells, defined by the loss of CD62L (L-selectin), and regulatory T cells (CD4+CD25+FoxP3+ T cells). The number of circulatory CD8+ T cells did not differ between WT and PCAF−/− mice. Also counts of other leukocyte subtypes, including B cells and natural killer cells were decreased by PCAF deficiency.
To investigate the migratory behavior of the leukocyte subtypes, the spleen, bone marrow and lymph nodes were harvested from both mouse strains before (t0) and 1 day after (t1) HLI. Compared to WT mice, we observed reduced numbers of dendritic cells in the draining inguinal lymph nodes of PCAF−/− mice after HLI. Accordingly, the fraction of dendritic cells expressing the co-stimulatory molecule CD86+ was smaller in the draining lymph nodes of PCAF−/− mice. Furthermore, nearly all tested leukocytes subtypes were increased in the bone marrow of PCAF−/− mice compared to WT mice, including CD4+ and CD8+ T cells, natural killer cells and dendritic cells, suggesting that these subpopulations are retained in the bone marrow of PCAF−/− mice during recovery after HLI (Supplemental Figure IIIA-I).
Because monocytes play a key role in arteriogenesis and are among the first leukocytes recruited to remodeling collaterals, we evaluated different monocyte populations in blood, spleen and bone marrow. After HLI, the absolute number of circulating monocytes in WT mice was equal to baseline numbers, but monocytes in PCAF−/− mice significantly decreased compared to baseline (PCAF−/− 0.13±0.05 vs WT 0.37±0.02×106/mL, p=0.002) (Figure 5A). In WT mice, the monocyte population increased in the spleen and decreased in the bone marrow after HLI. In contrast, bone marrow monocytes in PCAF−/−mice increased compared to baseline and were significantly higher after HLI compared to WT mice (Figure 5B-C). The differences in monocyte numbers were caused mainly by the specific subtype of “pro-inflammatory” Ly6Chi monocytes (Figure 5D-F). The activation state of total and Ly6Chi monocytes did not differ between the two strains, measured by mean fluorescent intensity of the adhesion molecule CD11b (Figure 5G-H).
Figure 5.
Monocyte recruitment in PCAF−/− mice after HLI. (A-C) Flow cytometry analysis of monocytes before (t0) and 1 day after (t1) HLI in PCAF−/− and WT mice. Values are presented as total monocyte counts in blood (nx106/mL), spleen (% of total cells) and bone marrow (% of total cells). (D-F) Flow cytometry analysis of “pro-inflammatory” Ly6Chi monocytes after HLI in PCAF−/− and WT mice. Values are presented as total Ly6Chi monocyte counts in blood (nx106/mL), spleen (% of total cells) and bone marrow (% of total cells). (G-H) Activation state of monocytes and Ly6Chi monocytes measured by mean fluorescence intensity (MFI) of CD11b. *P < 0.05, **P < 0.01, ***P < 0.001.
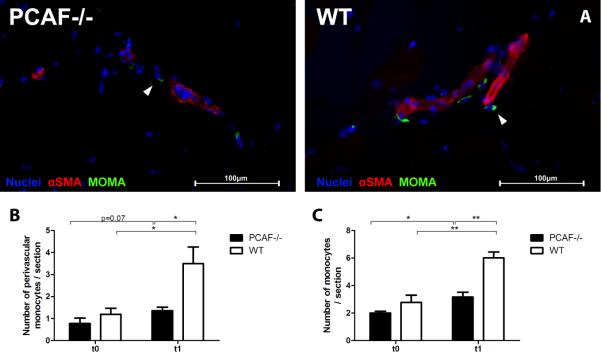
Finally, we assessed the number of monocytes/macrophages in the adductor muscle group by fluorescent staining with antibodies against MOMA-2 and smooth muscle α-actin (Figure 6A). Although PCAF−/− mice showed a significant increase in MOMA-2-positive cells 24 hours after HLI, the increase in WT mice was significantly higher (PCAF−/− 3.2±0.35 vs WT 6.0±0.43 / section, p=0.001) (Figure 6B). Differences were most evident in the perivascular space of remodeling collaterals (PCAF−/− 1.4±0.16 vs WT 3.5±0.76 / section, p=0.01) (Figure 6C).
Figure 6.
Monocyte recruitment to collateral arteries in PCAF−/− mice after HLI. (A) Immunohistochemical staining on fresh frozen sections of the adductor muscle group from PCAF−/− and WT mice 1 day after HLI, using anti-αSMA (red) and anti-MOMA-2 (green) antibodies. Cell nuclei were labeled with DAPI (blue). Scale bars = 100 μm. (B C) Quantification of MOMA-2 positive cells in the adductor muscle group of PCAF−/− and WT before (t0) and 1 day after (t1) HLI. Monocytes were quantified from at least six consecutive sections per mouse and expressed as the number of MOMA-2 positive cells per section and as the number of MOMA-2 positive cells in the perivascular space of αSMA+ vessels per section. *P < 0.05, **P < 0.01.
Discussion
We demonstrate that blood flow recovery after induction of HLI is strongly impaired in PCAF−/− mice, in association with reduced expansive remodeling of collaterals. Furthermore, local PCAF inhibition by Garcinol in WT mice also reduces recovery, indicating that PCAF is directly required for normal arteriogenesis. PCAF gene deficiency results in a repressed in vitro inflammatory response in many cell types known to be involved in arteriogenesis. One day after induction of HLI, 3505 genes are differentially regulated in the adductor muscle group of PCAF−/− mice compared to WT mice. Additionally, recruitment of different pro-arteriogenic leukocyte subtypes in PCAF−/−mice, in particular “inflammatory” monocytes, is significantly impaired at this time. Our data therefore demonstrate that PCAF plays a key role in post-ischemic arteriogenesis.
Compared to WT mice, PCAF−/− mice showed an impaired blood flow recovery after HLI. Our findings suggest that two deficiencies caused by a lack of PCAF are involved. First, the expansive remodeling of αSMA+ arterioles at the center of the adductor muscle group, of which most are collaterals, was reduced by 53% in PCAF−/− mice compared to WT mice. Correspondingly, local application of PCAF inhibitor Garcinol in healthy WT mice also resulted in impaired blood flow recovery compared to control animals. Hence, PCAF has a major impact on arteriogenesis. Second, we observed that the density of native pre-existing collaterals in the pial circulation of PCAF−/− mice was reduced by 11%. Even changes of this magnitude have significant effects on collateral-dependent perfusion of tissue downstream from an arterial obstruction24-26. Previous studies have shown that genetic-dependent variation in collateral number in the cerebral pial circulation is shared, at least qualitatively, by similar differences in collateral density in other tissues24-26. Accordingly, we also observed a trend towards a decrease in blood flow directly after induction of HLI in PCAF−/− mice. In mice, the density of the native collaterals in tissues varies widely among strains from differences in genetic background24-26. Hence, besides collateral remodeling, genetic PCAF deficiency also contributes to reduced formation of the collateral circulation which occurs during embryonic development.
In a clinical setting, the outcome after an ischemic event varies among individuals, and differences in abundance of the native collateral circulation have also been reported in patients27, 28. Moreover, a previous study found that the −2481G allele in the promoter region of the PCAF gene associates with an increased risk of mortality in patients with coronary heart disease22, which further supports our findings that PCAF deficiency impairs collateral function.
In order for PCAF to serve as a master switch in collateral remodeling, it needs to impact multiple critical phases in the process, namely activation of the endothelium and vessel wall, leukocyte recruitment, matrix degradation and arteriolar expansion. We examined gene expression in the adductor muscle group containing remodeling collaterals in the initial phase after HLI. Over 3500 genes were differentially regulated between PCAF−/−and WT mice. This suggests that PCAF impacts expression of a large number of genes activated in this setting. More specifically, PCAF−/− mice showed impaired induction of multiple pro-arteriogenic and pro-inflammatory genes, including matrix metalloproteinase 9 (MMP9) and chemokines CXCL12 (SDF1) and CCL9.
MMP9 is critical in degradation and remodeling of the extracellular matrix allowing cell migration and outward expansion of the collaterals and thus effective arteriogenesis29. CXCL12 is elevated in ischemic skeletal muscle of patients with critical limb ischemia30 and acts as chemoattractant for CXCR4+ cells, including leukocytes and progenitor cells. CXCL12-mediated recruitment of bone marrow-derived cells to ischemic tissues results in enhanced neovascularization31, 32. Also CCL9, which is a strong chemoattractant for bone marrow derived cells33, is upregulated after muscle injury34.
In addition, PCAF−/− mice showed impaired induction of multiple factors related to the pro-inflammatory TNFα pathway35. TNFα−/− mice have reduced collateral artery perfusion36 and anti-TNFα therapy attenuates arteriogenesis37. Thus, reduced TNFα expression in PCAF−/− mice likely contributes to the impaired arteriogenesis in these mice. Our data suggest that PCAF regulates many factors that have previously been described to play an important role in both inflammation and arteriogenesis38.
It should be noted that RNA was isolated from the adductor muscle group as a whole38 and not from the embedded collateral arteries alone, as was described previously39. In that report, a whole-genome microarray analysis was performed on collaterals microdissected from the gracilis muscle 24 hours after HLI. Here we found exceedingly more differentially expressed genes, then the 404 genes that were found upregulated in gracilis collaterals of WT mice39. Using the entire adductor muscle group for microarray analysis, not only the collaterals but also infiltrating leukocytes and surrounding nonvascular tissues were included in these analyses.
As discussed in the introduction, an inflammatory-like process plays a role in all stages of arteriogenesis. To investigate the impact of PCAF on the inflammatory response of the different cell types involved in arteriogenesis, we studied circulating cells in whole blood, splenic leukocytes and VSMCs in vitro. PCAF is critical for the regulation of transcription factor NF-κB, that consists of a p65 and p50 subunit bound to inhibitory proteins in the cytoplasm. Upon stimulation NF-κB is translocated to the nucleus and regulates the expression of multiple genes, including TNFα and MCP-120, 40. PCAF binds to the NF-κB p65 subunit and activates NF-κB-related inflammatory gene expression19,20. We clearly demonstrate that PCAF deficiency results in decreased production of pro-inflammatory cytokines by multiple cell types after stimulation with LPS. LPS stimulated whole blood from PCAF−/− mice produced less TNFα than blood from WT mice, indicating a reduced inflammatory phenotype of circulating cells. Also PCAF−/− cells isolated from the spleen, one of the major leukocyte reservoirs, showed a reduced inflammatory response compared to splenocytes from WT mice. PCAF−/− VSMCs produced less MCP-1 than WT VSMCs in response to LPS, which would favor reduced monocyte recruitment and therefore reduced VSMC proliferation, which is essential for collateral remodeling. We obtained similar results using either Garcinol or PCAF-specific siRNA knockdown in WT VSMCs, thus excluding effects of any pre-existing differences in PCAF deficient cells. Our data correspond with a report that TNFα-induced NF-κB activity increases in human airway smooth muscle cells overexpressing PCAF41 and provide strong evidence for a wide effect of PCAF on inflammatory gene transcription.
The p65 subunit of NF-κB recruits co-activator PCAF and activates NF-κB-mediated gene transcription. In contrast, the NF-κB p50 subunit lacks the transcriptional activation domain and inhibits gene transcription42. Mice deficient of the NF-κB p50 subunit showed enhanced blood flow recovery after HLI as the result of increased monocyte recruitment to the perivascular space of collaterals43. Whereas arteriogenesis and monocyte recruitment is enhanced by NF-κB activation in NF-κB p50−/− mice, reduced regulation of the NF-κB p65 subunit in PCAF−/− mice could likely explain the impaired arteriogenesis by inhibition of monocyte recruitment. In WT mice, the monocyte population increased in the spleen and decreased in the bone marrow after HLI. This is in line with earlier reports that monocytes are mobilized from the bone marrow after HLI44. In that report, the pro-arteriogenic potential of monocytes was described to originate from a specific “pro-inflammatory” subtype, which is characterized by high expression of Ly6C. These Ly6Chi monocytes are recruited in the early stage of collateral remodeling44, 45 and our data confirm that they are mobilized from the bone marrow in WT mice. In contrast, recruitment of monocytes proved to be severely impaired in PCAF−/− mice. PCAF−/− mice showed reduced numbers of circulating monocytes following HLI, particularly reduced numbers of Ly6Chi monocytes. Whereas monocytes migrated away from the bone marrow in WT mice, PCAF−/− mice showed an increase in bone marrow monocytes, suggesting a defect in monocyte mobilization. Concomitantly, 24 hours after HLI fewer monocytes were recruited to the collaterals in PCAF−/− mice. Monocytes stimulate arteriogenesis by secretion of growth factors and degradation of extracellular matrix at the site of collateral remodeling. Therefore, the lack of monocyte accumulation along collaterals likely contributes to the impaired arteriogenesis in PCAF−/− mice.
Besides monocytes, PCAF also affected numerous other leukocyte subtypes. In PCAF−/−mice, we demonstrated decreased numbers of circulating leukocytes involved in arteriogenesis, like T cells46 (predominantly activated CD4+ T cells7, 11), natural killer cells11 and regulatory T cells9, 10, and also in those cells that have not previously been implicated in arteriogenesis, including B cells. Furthermore, fewer dendritic cells were found in draining inguinal lymph nodes compared to WT mice, where the interaction between antigen-presenting dendritic cells and T cells takes place. Interestingly, nearly all subtypes were increased in the bone marrow of PCAF−/− mice, after HLI. This indicates that PCAF deficiency interferes with recruitment of pro-arteriogenic leukocytes from the bone marrow reservoir47.
In conclusion, PCAF−/− mice demonstrated impaired collateral remodeling after HLI, together with a reduction in the number of native pre-existing collaterals present before arterial obstruction. PCAF deficiency resulted in altered expression of a large number of genes, including those in immune and inflammatory pathways, and an attenuated inflammatory response in multiple cell types involved in arteriogenesis. These findings indicate that PCAF is a key regulator in post-ischemic blood flow recovery by impacting the inflammatory processes required for robust arteriogenesis.
Supplementary Material
Significance.
Although the induction of arteriogenesis is a promising therapeutic alternative to surgical interventions for patients with severe peripheral arterial disease, clinical trials aimed at modulating individual growth factors or cytokines have thus far not been as successful as anticipated. Master switches for multiple relevant gene programs will likely be a more effective strategy to stimulate arteriogenesis. The transcriptional co-activator P300/CBP-associated factor (PCAF) acts as such a master switch, as it induces multiple inflammatory processes crucial to arteriogenesis. Here, we show that PCAF is an essential factor in arteriogenesis in a model for peripheral arterial disease. Our findings clearly demonstrate that PCAF plays a key role in the formation of native pre-existing collateral arterioles and post-ischemic arteriogenesis. PCAF alters the expression of over 3500 genes and impacts inflammatory processes required for effective arteriogenesis, signifying PCAF as an unanticipated target gene that yields new mechanistic insight in arteriogenesis.
Acknowledgments
We thank Rob C.M. de Jong and Annemarie M. van Oeveren-Rietdijk for their technical assistance.
Sources of Funding This study was performed with financial support from BioMedical Materials, Dutch Ministry of Economic Affairs, Agriculture and Innovation (BMM-PENT; P1.03), the Netherlands Organization for Scientific Research (NWO) (Veni 916.12.041) and by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908).
Abbreviations
- HLI
(hind limb ischemia)
- LDPI
(laser Doppler perfusion imaging)
- PCAF
(P300/CBP-associated factor)
Footnotes
Disclosures The authors declare that they have no conflict of interest.
References
- 1.van Oostrom MC, van Oostrom O, Quax PH, Verhaar MC, Hoefer IE. Insights into mechanisms behind arteriogenesis: what does the future hold? J Leukoc Biol. 2008;84:1379–91. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- 2.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–58. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 3.Schaper J, Konig R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Histol. 1976;370:193–205. doi: 10.1007/BF00427580. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 5.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 6.Voskuil M, Hoefer IE, van Royen N, Hua J, de GS, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–92. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 7.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–10. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 8.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118–24. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 9.Hellingman AA, van der Vlugt LE, Lijkwan MA, Bastiaansen AJ, Sparwasser T, Smits HH, Hamming JF, Quax PH. A limited role for regulatory T cells in post-ischemic neovascularization. J Cell Mol Med. 2012;16:328–36. doi: 10.1111/j.1582-4934.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Recalde A, Duriez M, Levy BI, Lutgens E, Mallat Z, Silvestre JS. Regulatory T cells modulate postischemic neovascularization. Circulation. 2009;120:1415–25. doi: 10.1161/CIRCULATIONAHA.109.875583. [DOI] [PubMed] [Google Scholar]
- 11.van Weel V, Toes RE, Seghers L, Deckers MM, de Vries MR, Eilers PH, Sipkens J, Schepers A, Eefting D, van Hinsbergh V, van Bockel JH, Quax PH. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–8. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 12.Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–7. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 13.Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–92. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–9. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang JY, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–34. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 16.Itoh S, Ericsson J, Nishikawa J, Heldin CH, ten Dijke P. The transcriptional co-activator P/CAF potentiates TGF-beta/Smad signaling. Nucleic Acids Res. 2000;28:4291–8. doi: 10.1093/nar/28.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–78. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Guarani V, Deflorian G, Franco CA, Kruger M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R, Schmidt MH, Zimmermann B, Brandes RP, Mione M, Westphal CH, Braun T, Zeiher AM, Gerhardt H, Dimmeler S, Potente M. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. 2011;473:234–8. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–78. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–7. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 21.Monraats PS, Rana JS, Zwinderman AH, de Maat MP, Kastelein JP, Agema WR, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J, Frants RR, van der Laarse A, van der Wall EE, Jukema JW. -455G/A polymorphism and preprocedural plasma levels of fibrinogen show no association with the risk of clinical restenosis in patients with coronary stent placement. Thromb Haemost. 2005;93:564–9. doi: 10.1160/TH04-11-0708. [DOI] [PubMed] [Google Scholar]
- 22.Pons D, Trompet S, de Craen AJ, Thijssen PE, Quax PH, de Vries MR, Wierda RJ, van den Elsen PJ, Monraats PS, Ewing MM, Heijmans BT, Slagboom PE, Zwinderman AH, Doevendans PA, Tio RA, de Winter RJ, de Maat MP, Iakoubova OA, Sattar N, Shepherd J, Westendorp RG, Jukema JW. Genetic variation in PCAF, a key mediator in epigenetics, is associated with reduced vascular morbidity and mortality: evidence for a new concept from three independent prospective studies. Heart. 2011;97:143–50. doi: 10.1136/hrt.2010.199927. [DOI] [PubMed] [Google Scholar]
- 23.Hellingman AA, Bastiaansen AJ, de Vries MR, Seghers L, Lijkwan MA, Lowik CW, Hamming JF, Quax PH. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010;40:796–803. doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;107:558–68. doi: 10.1161/CIRCRESAHA.110.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–34. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 2010;42:469–79. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 28.Menon BK, Bal S, Modi J, Sohn SI, Watson TW, Hill MD, Demchuk AM, Goyal M. Anterior temporal artery sign in CT angiography predicts reduced fatal brain edema and mortality in acute M1 middle cerebral artery occlusions. J Neuroimaging. 2012;22:145–8. doi: 10.1111/j.1552-6569.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, Lo WY, Wu TC, Sata M, Chen JW, Lin SJ. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1179–84. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 30.Ho TK, Tsui J, Xu S, Leoni P, Abraham DJ, Baker DM. Angiogenic effects of stromal cell-derived factor-1 (SDF-1/CXCL12) variants in vitro and the in vivo expressions of CXCL12 variants and CXCR4 in human critical leg ischemia. J Vasc Surg. 2010;51:689–99. doi: 10.1016/j.jvs.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 32.Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–84. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Odgren PR. Molecular cloning and characterization of rat CCL9 (MIP-1gamma), the ortholog of mouse CCL9. Cytokine. 2005;31:94–102. doi: 10.1016/j.cyto.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silke J, Brink R. Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ. 2010;17:35–45. doi: 10.1038/cdd.2009.114. [DOI] [PubMed] [Google Scholar]
- 36.Hoefer IE, van Royen N, Rectenwald JE, Bray EJ, Abouhamze Z, Moldawer LL, Voskuil M, Piek JJ, Buschmann IR, Ozaki CK. Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation. 2002;105:1639–41. doi: 10.1161/01.cir.0000014987.32865.8e. [DOI] [PubMed] [Google Scholar]
- 37.Grundmann S, Hoefer I, Ulusans S, van Royen N, Schirmer SH, Ozaki CK, Bode C, Piek JJ, Buschmann I. Anti-tumor necrosis factor-{alpha} therapies attenuate adaptive arteriogenesis in the rabbit. Am J Physiol Heart Circ Physiol. 2005;289:H1497–H1505. doi: 10.1152/ajpheart.00959.2004. [DOI] [PubMed] [Google Scholar]
- 38.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–82. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–81. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenardo MJ, Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–9. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 41.Clarke DL, Sutcliffe A, Deacon K, Bradbury D, Corbett L, Knox AJ. PKCbetaII augments NF-kappaB-dependent transcription at the CCL11 promoter via p300/CBP-associated factor recruitment and histone H4 acetylation. J Immunol. 2008;181:3503–14. doi: 10.4049/jimmunol.181.5.3503. [DOI] [PubMed] [Google Scholar]
- 42.Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Groot D, Haverslag RT, Pasterkamp G, de Kleijn DP, Hoefer IE. Targeted deletion of the inhibitory NF-kappaB p50 subunit in bone marrow-derived cells improves collateral growth after arterial occlusion. Cardiovasc Res. 2010;88:179–85. doi: 10.1093/cvr/cvq150. [DOI] [PubMed] [Google Scholar]
- 44.Cochain C, Rodero MP, Vilar J, Recalde A, Richart AL, Loinard C, Zouggari Y, Guerin C, Duriez M, Combadiere B, Poupel L, Levy BI, Mallat Z, Combadiere C, Silvestre JS. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc Res. 2010;88:186–95. doi: 10.1093/cvr/cvq153. [DOI] [PubMed] [Google Scholar]
- 45.Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–8. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. 1999;99:3188–98. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 47.Meisner JK, Price RJ. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: regulation of the endogenous response and therapeutic implications. Microcirculation. 2010;17:583–99. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.