Abstract
Rationale
Postsynaptic density-95 (PSD95) is a scaffolding protein that associates with voltage-gated, Shaker-type K+ (KV1) channels and promotes the expression of KV1 channels in vascular smooth muscle cells (cVSMCs) of the cerebral circulation. However, the physiological role of PSD95 in mediating molecular signaling in cVSMCs is unknown.
Objective
We explored whether a specific interaction between PSD95 and KV1 channels enables PKA phosphorylation of KV1 channels in cVSMCs to promote vasodilation
Methods and Results
Rat cerebral arteries (CA) were used for analyses. A membrane-permeable peptide (KV1-C peptide) corresponding to the PDZ binding motif in the C-terminus of KV1.2α was designed as a dominant negative peptide to disrupt the association of KV1 channels with PSD95. Application of KV1-C peptide to cannulated, pressurized CA rapidly induced vasoconstriction and depolarized cVSMCs. These events corresponded to reduced co-immunoprecipitation of the PSD95 and KV1 proteins without altering surface expression. Middle cerebral arterioles imaged in situ through cranial window also constricted rapidly in response to local application of KV1-C peptide. Patch-clamp recordings confirmed that KV1-C peptide attenuates KV1 channel blocker (Psora4)-sensitive current in cVSMCs. Western blots employing a phospho-PKA substrate antibody revealed CA exposed to KV1-C peptide showed markedly less phosphorylation of KV1.2α subunits. Finally, phosphatase inhibitors blunted both KV1-C peptide-mediated and PKA inhibitor peptide-mediated vasoconstriction.
Conclusions
These findings provide initial evidence that PKA phosphorylation of KV1 channels is enabled by a dynamic association with PSD95 in CA, and suggest that a disruption of such association may compromise cerebral vasodilation and blood flow.
Keywords: cerebral arteries, PDZ domains, potassium channels, vascular smooth muscle
INTRODUCTION
Shaker-type voltage-gated K+ (KV1) channels composed of KV1.2 and KV1.5 α-subunits are expressed in cerebral vascular smooth muscle cells (cVSMCs), where they contribute to the resting diameter and vasodilation of cerebral arteries (CA).1, 2 KV1 channels are multi-protein structures composed of four KVα pore-forming subunits co-assembled with intracellular KVβ subunits which may affect channel trafficking and kinetics.3–8 In addition, post-translational modifications such as glycosylation and protein kinase A (PKA)-mediated phosphorylation of the KVα subunits may increase protein expression and activity of KV1 channels.9–15
Recently we reported the expression of a scaffolding protein, postsynaptic density protein-95 (PSD95), in rat CA.16 Previously PSD95 was studied primarily in neurons, where it provides an assembly platform at the plasma membrane for macromolecular signaling complexes including ion channels.17–22 However, we reported that PSD95 serves as a molecular scaffold for KV1 channels in cVSMCs, and this interaction is required for the proper expression of KV1 channels that exerts a tonic vasodilator influence.16 Accordingly, antisense-mediated knockdown of PSD95 in rat CA resulted in a loss of KV1 channel expression and caused vasoconstriction, inferring that PSD95 promotes the expression of KV1 channels in cVSMCs.16
Notably, the C-terminus of the KV1.2α subunit contains a structural motif that permits the channel to interact with PSD95.16–20 Collectively, the interactions of signaling proteins with PSD95 are enabled by three PDZ (post synaptic density-95, discs large, zonula occludens-1) domains, which act as docking sites for signaling molecules and show preference for distinct binding partners (Figure 1A). PDZ1 and PDZ2 preferentially bind to the C-terminus of the KV1.2α subunit via an association that is intrinsically unstable, thereby permitting a dynamic and reversible interaction.17, 19, 23, 24 Src-homology (SH3) and guanylate kinase (GK) domains also interact with other scaffolding proteins such as guanylate kinase associated protein or A-kinase anchoring protein (AKAP), providing a platform for macromolecular complexes.21, 25, 26
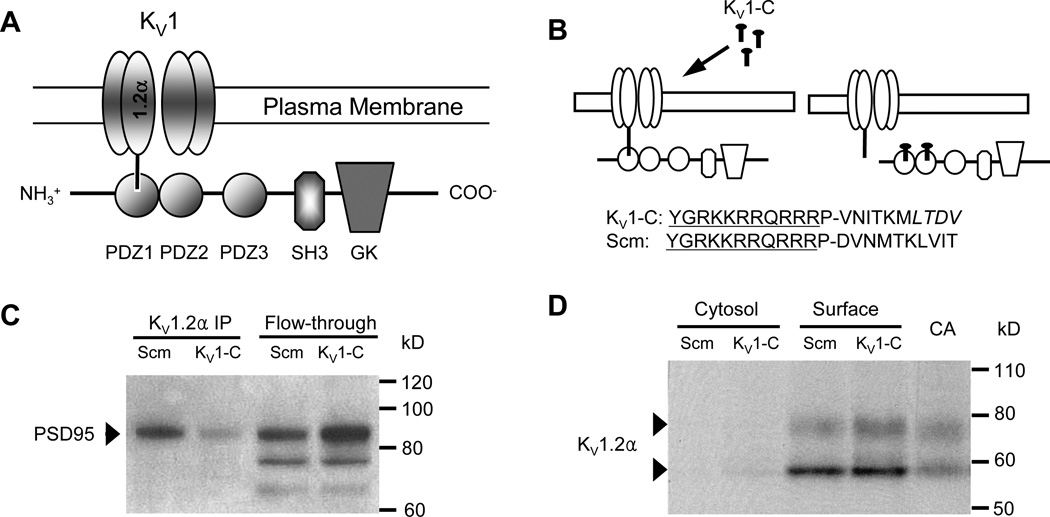
Figure 1. KV1-C peptide disrupts association of PSD95 and KV1.2α.
A) Schematic of the association of KV1.2α channels with the PSD95 scaffolding protein via the PDZ1 binding domain. PSD95 contains three PDZ binding domains (PDZ1-3), Src-homology (SH3) and guanylate kinase (GK) domains. B) The KV1-C dominant negative peptide was designed to compete for the PDZ binding domain on PSD95. The last 10 amino acids of the KV1.2α C-terminus were conjugated to HIV-tat (YGRKKRRQRRR) to confer cell-permeability. P is a spacer. LTDV is a class-1 PDZ binding motif on KV1.2α. A peptide with same amino acid composition but in a scrambled order (Scm) was used as control. C) Immunoprecipitation using anti-KV1.2α of CA lysate treated with Scm or KV1-C peptide for 30 min. Elution (KV1.2α IP) and column flow-through (Flow-through) were probed for PSD95 on a Western blot. Depicted is a representative scan from three similar experiments. D) Biotinylation of CA treated with Scm or KV1-C peptide for 30 min. Cytosolic and surface fractions were probed for KV1.2α. Control lysate from freshly isolated CA was loaded for size comparison. Depicted is a representative blot from five similar experiments.
Since the three PDZ domains of PSD95 can form interactions with several signaling molecules, the design of interfering peptides that disrupt the interaction between PSD95 and a specific molecular partner has emerged as an important strategy to pinpoint the physiological impact of a single scaffolding interaction.27, 28 In this approach, a dominant negative peptide of identical sequence to the PDZ binding motif of a molecular partner is overexpressed to disrupt this PDZ interaction only. The importance of PSD95 scaffolding of N-methyl-D-aspartate receptors (NMDAR) and neuronal nitric oxide synthase (nNOS) in neurons was revealed using this strategy.27, 28 A similar dominant-negative peptide was administered to rodents and non-human primates in vivo to reduce neuronal damage after experimental stroke by disrupting PSD95-dependent excitotoxic signaling between NMDAR and nNOS. In order to achieve optimal cell penetration in these studies, an HIV-tat sequence was coupled to the C-terminus peptide sequence of the NMDAR-NR2B subunit that binds to PDZ domains.29–32
In the present study, we adopted this general strategy to evaluate if association with PSD95 is required for the proper function of KV1 channels in rat CA, and to identify other components in the PSD95 complex that also may be required to confer cerebral vasodilation. We designed a cell-permeable dominant negative peptide corresponding to the C-terminus PDZ motif of the KV1.2α-subunit (KV1-C peptide) to disrupt KV1 scaffolding by PSD95. Our findings draw attention to PSD95 as a key scaffolding protein in cVSMCs that enables the basal phosphorylation and opening of KV1 channels to contribute to the resting diameter of CA, and infer that conditions that interrupt the PSD95 complex may compromise cerebral vasodilation and blood flow.
METHODS
Cerebral arteries were isolated from ten- to fourteen-week-old male Sprague–Dawley rats as approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. A dominant-negative peptide (KV1-C) was used to disrupt the association of KV1 and PSD95 (Figure 1B). KV1-C consists of the final 10 amino acids of the C-terminus of KV1.2α attached to an N-terminus HIV-tat sequence (NH3-YGRKKRRQRRR) to confer membrane permeability. An N-terminus fluorescein label was attached to some peptides for visualization. Two scrambled variations of the peptide were used as negative controls (21st Century Biochemicals). Peptide disruption of KV1 channel-PSD95 association was determined by co-immunoprecipitation.16 Protein surface expression was determined by biotinylation.33 CA diameter was measured using a pressure myograph and software (Danish Myo Technology). In situ response of middle cerebral arterioles to local application of peptides was measured by suffused cranial window imaging using a Sony HDR-PJ580 camera and an automated IPLab script. Membrane potential was measured by glass microelectrodes connected to a preamplifier (DAGAN) and analyzed by WinDaq Lite software (DATAQ). Whole-cell cVSMC patch-clamp was performed with an EPC 7 amplifier (HEKA) and pCLAMP 6 software (Molecular Devices).16 Non-permeable peptides (NP) without the HIV-tat (GenScript) were used for patch-clamp experiments. Images were obtained using a confocal microscope.16 Data are presented as mean ± SEM. P<0.05 was considered statistically significant. An expanded Methods section is available in the Online Data Supplement.
RESULTS
Cell-permeable KV1-C peptide disrupts the interaction of KV1.2α and PSD95 in cVSMCs
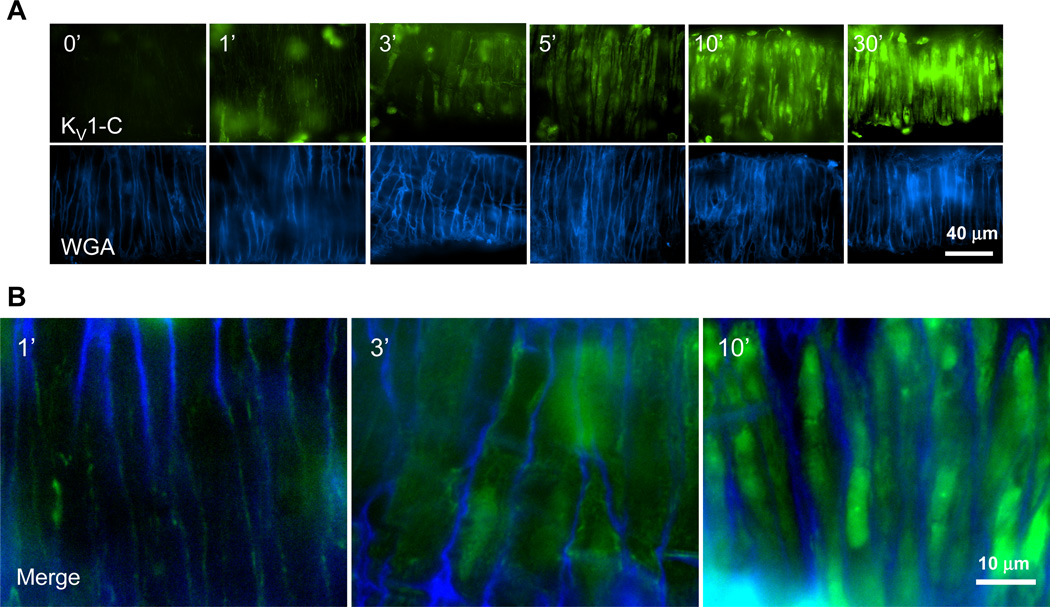
KV1 channel and PSD95 association (Figure 1A) was targeted for disruption using a peptide (KV1-C), which couples an N-terminus HIV-tat sequence to the last 10 amino acid sequence of the KV1.2α C-terminus that contains a class I PDZ binding motif, LTDV (Figure 1B). To ensure specificity of the KV1-C peptide, a scrambled peptide (Scm) sharing the same amino acid composition as KV1-C but randomly ordered was used as control (Figure 1B). In isolated CA, incubation for 30 min with KV1-C peptide disrupted the KV1.2α-PSD95 interaction as evidenced by a loss of immunoreactive signal for PSD95 in anti-KV1.2α immunoprecipitate (IP) compared to arteries incubated with Scm peptide (Figure 1C, lanes 1-2). A corresponding increase in the PSD95 band was observed in the flow-through fraction that did not bind to KV1.2α antibody (Figure 1C, lanes 3-4). As previously reported,16 only the full form of PSD95 (upper band at 95 kD) associates with the KV1.2α subunit. In order to examine the effect of KV1-C peptide on the plasma membrane expression of KV1 channels, isolated arteries were treated with Scm or KV1-C peptides, biotinylated and analyzed by Western blot.33 In the resulting Western blot, the bulk of KV1.2α–containing channels were found in the biotinylated surface fraction compared to the non-biotinylated cytosolic fraction (Figure 1D). The surface expression of KV1.2α subunits was not different between arteries exposed to KV1-C compared to Scm, indicating that KV1-C did not cause a loss of surface KV1 channels (Figure 1D). We also confirmed that KV1-C peptide penetrated cVSMCs of intact arteries using confocal imaging (Figure 2). After 1 min of incubation, fluorescein-labeled KV1-C peptide appeared at the cell surface and a strong fluorescent signal was observed intracellularly by 3 min that persisted until 30 min. Similar penetration patterns were observed for fluorescein-labeled Scm peptide (Online Figures I and II). Compared to smooth muscle cells, neurons in the adventitia and the endothelial cells absorbed the peptides sooner, within the first minute (Online Figure I). Collectively, these findings indicate that our cell-permeable dominant-negative peptide successfully competes with the native KV1.2α subunit to prevent its binding to the PDZ domains of PSD95, and it provides a tool to disrupt the association between KV1 channels and PSD95 in cVSMCs of intact arteries.
Figure 2. KV1-C peptide rapidly penetrates cVSMCs in CA.
A) Confocal images of isolated rat CA incubated with fluorescein-labeled KV1-C peptide (top row) for 0, 1, 3, 5, 10, and 30 min at 37°C. Alexa350-labeled wheat germ agglutinin (bottom row, WGA) was used as a cell surface marker for cVSMCs. Individual cVSMCs are visible vertically wrapping around the CA circumferentially, since the artery was placed horizontally for imaging. The brightness settings for the green channel in 10- and 30-minute treatments were reduced in order to display individual cells. Representative images from three similar experiments. B) Merged images from 1, 3, and 10-minute time points.
KV1-C peptide constricts and depolarizes isolated CA
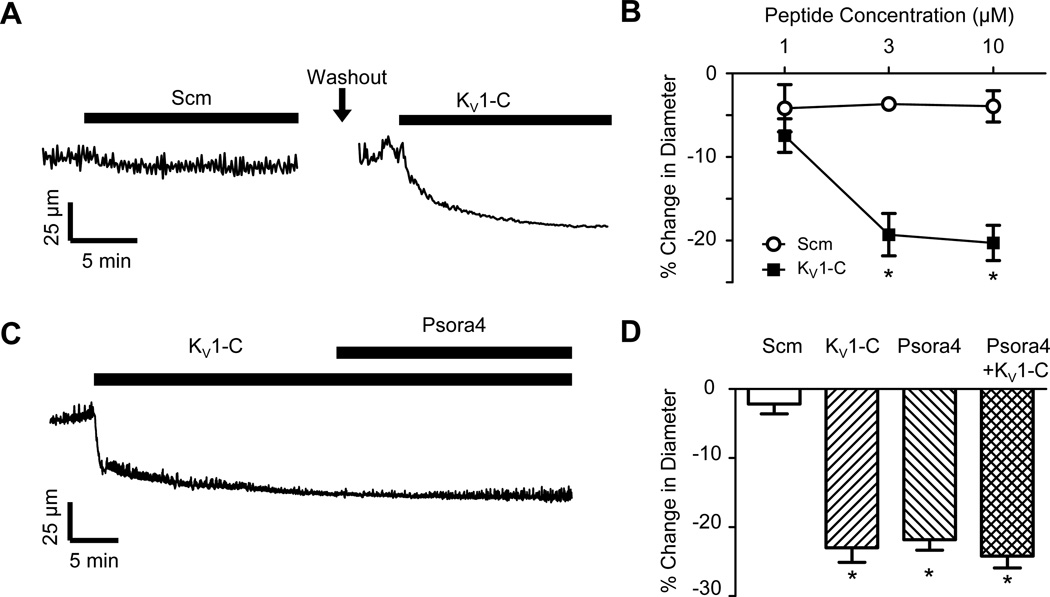
Diameter recordings of isolated, pressurized rat CA exposed to KV1-C peptide revealed a rapid constriction of CA in response to disruption of the KV1 channel-PSD95 complex, whereas Scm peptide did not affect vessel diameter (Figure 3A). Increasing concentrations of 1, 3 and 10 µmol/L KV1-C peptide progressively reduced CA diameter by −7.4 ± 2.0%, −19.3 ± 2.5% and −20.3 ± 2.1% (Figure 3B). The corresponding values for Scm peptide were −4.2 ± 2.8%, −3.7 ± 0.6% and −3.9 ± 1.9%. A second scrambled control peptide (Scm2) also did not significantly constrict CA (Online Figure III). Bath application of the KV1 channel blocker, 5-(4-phenylalkoxypsoralen) (Psora4)2, 16, 34, 35 did not further reduce the diameter of arteries already constricted by KV1-C peptide (Figures 3C-D). Notably, the constrictor response to 100 nmol/L Psora4 was not different from the constriction caused by 10 µmol/L KV1-C peptide (Figure 3D) or the diameter change induced by combined Psora4 and KV1-C peptide (Figure 3D). Absolute diameter values are provided in Online Figure IV. The constriction induced by KV1-C peptide was not significantly altered by the presence of 100 µmol/L Nω-Nitro-L-arginine methyl ester (L-NAME) and 10 µmol/L indomethacin in the bath solution to block nitric oxide synthase and cyclooxygenase, respectively (Online Figure V). Constrictor responses to 1 µmol/L linopirdine, a KV7 channel blocker, or 30 µmol/L BaCl2, a KIR channel blocker, were not significantly altered by the presence of 3 µmol/L KV1-C peptide (Online Figure VI). Additionally, 1 µmol/L glibenclamide, a KATP channel blocker, or 30 nmol/L stromatoxin, a KV2.1 channel blocker, did not constrict pressurized CA significantly (n=5 each, data not shown).
Figure 3. KV1-C peptide disrupts association of PSD95 and KV1.2 and constricts CA.
A) Representative recording of outer diameter in a cannulated, pressurized (80 mmHg) rat CA. The artery was initially exposed to 10 µmol/L Scm peptide, and then after extensive washes, exposed to 10 µmol/L KV1-C peptide. B) CA constricted in response to KV1-C peptide in a concentration-dependent manner, but did not constrict to Scm peptide (n=5 each). * indicates significant difference from Scm, P<0.05. C) Diameter recordings from a CA exposed sequentially to 10 µmol/L KV1-C peptide and the specific KV1 channel antagonist, 100 nmol/L Psora4. D) Percent change in diameter from baseline in response to 10 µmol/L KV1-C peptide and 100 nmol/L Psora4 (n=6 each). *: significant difference from Scm, P<0.05.
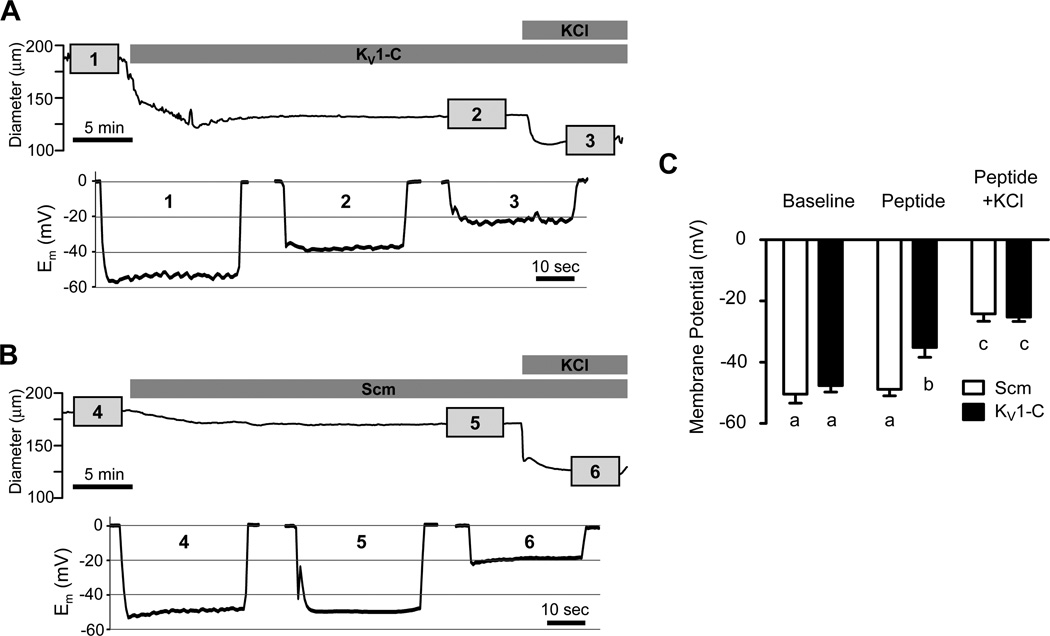
Importantly, microelectrode recordings indicated that cVSMCs of pressurized CA depolarized from a resting membrane potential (Em) of −47.6 ± 2.1 mV to −35.2 ± 3.2 mV in response to 10 µmol/L KV1-C peptide (Figures 4A and 4C), a finding consistent with a loss of hyperpolarizing K+ current. In contrast, cVSMCs in arteries treated with Scm peptide showed no significant change in resting Em (Figures 4B-C). The addition of 60 mmol/L KCl to bath solutions already containing KV1-C or Scm peptide further depolarized arteries of both groups to similar values (Figure 4). These findings indicate that the specific disruption of KV1.2 channel-PSD95 association in pressurized CA leads to depolarization and constriction from the resting tone. These effects appear to correspond selectively to the blockade of KV1.2 channels and imply a loss of function of KV1.2 channels in cVSMCs when dissociated from the PSD95 complex.
Figure 4. KV1-C peptide causes depolarization of in situ cVSMCs.
A, B) Recordings of CA diameter and membrane potential (Em) in rat CA exposed to KV1-C (A) or Scm (B) peptide. After recording baseline Em (1, 4), 10 µmol/L KV1-C (2) or Scm (5) peptide was added to the bath followed by 60 mmol/L KCl (3, 6) to elicit maximal depolarization. Em was recorded in each step for ~5 min as indicated by the numbered boxes in the diameter trace. C) Average Em values in the presence of KV1-C peptide or Scm peptide only, and after the further addition of 60 mmol/L KCl (n=6 each). a,b,c: significant difference, P <0.05.
KV1-C peptide constricts CA in vivo
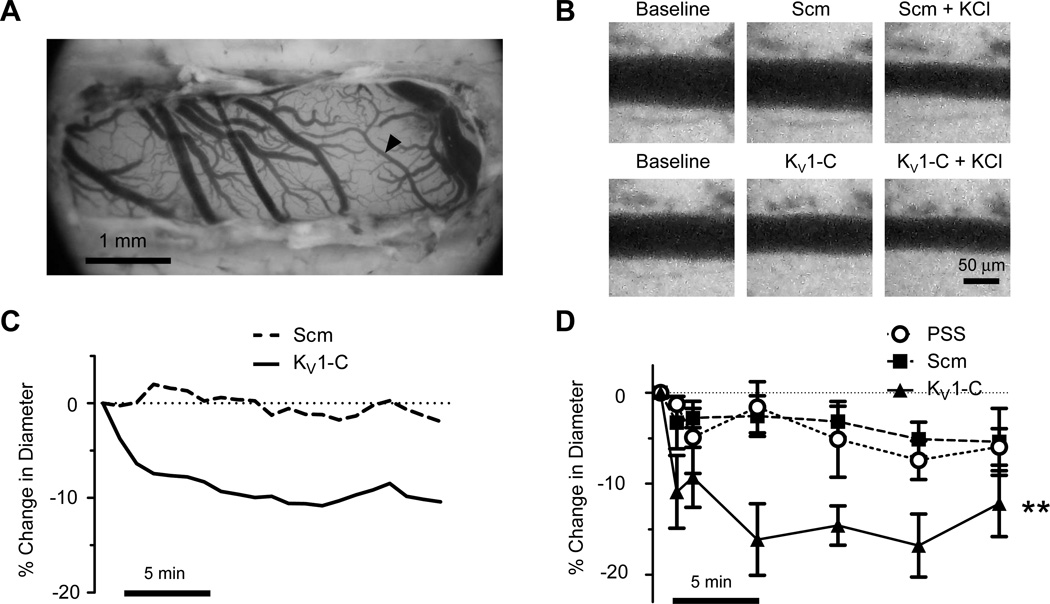
After confirming the selective disruption of KV1-PSD95 interaction ex vivo by KV1-C peptide, we evaluated the impact of KV1-C peptide on CA in vivo. A partial craniectomy of the right parietal plate of anesthetized rats immobilized in a stereotaxic frame, followed by mounting of a cranial window, exposed branches of the middle cerebral arteries for imaging and topical peptide treatment (Figure 5A). Suffusion of the cranial window with 30 µmol/L KV1-C peptide resulted in a rapid constriction of CA, which was sustained for at least 20 min (Figures 5B-C). Average values indicated that middle cerebral arteries treated with KV1-C peptide showed a significant reduction in diameter of −16.1 ± 3.9% by 5 min with maximum constriction at 15 min averaging −16.8 ± 3.5% (Figure 5D). In contrast, the same concentration of Scm peptide did not affect vessel diameter (Figures 5B-C), although the same arteries responded to 60 mmol/L KCl by strongly constricting as evidence of viability (Figure 5B). Arteries in physiological salt solution (PSS) or Scm peptide showed a small decrease in diameter by 15 min, which were not statistically significant from each other (Figure 5D). These results provide initial evidence that KV1 channels in PSD95 signaling complexes contribute to the resting diameter of cerebral resistance arteries in vivo and disruption of such association may have a profound effect on cerebral blood flow.
Figure 5. KV1-C peptide constricts rat CA in vivo.
A) An overview of the cranial window. A branch of middle cerebral artery (arrowhead) is analyzed for diameter changes. B) Representative images reveal the constrictor response of a middle cerebral artery branch to 30 µmol/L KV1-C peptide, but not to the same concentration of Scm peptide. C) Diameter responses of single arterial branches to Scm or KV1-C peptide. D) Percent change in diameter from baseline in response to 30 µmol/L Scm or KV1-C peptides or physiological salt solution (PSS). Scale bar, 5 min. **: Significant difference from PSS and Scm, P<0.01, n=5–7.
KV1-C peptide suppresses Psora4-sensitive K+ currents in cVSMCs
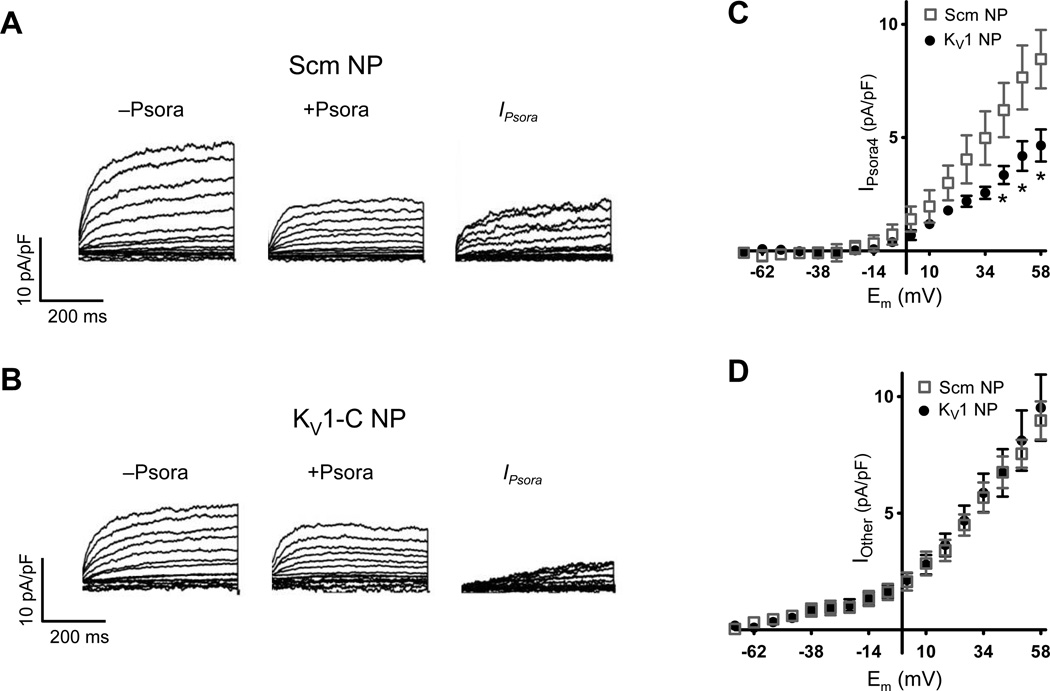
Next we used patch-clamp techniques to confirm that KV1-C peptide directly reduces KV1 channel function in cVSMCs. In these studies, non-permeable (NP) peptides were added to the dialyzing pipette solution after extensive attempts to add permeable HIV-tat containing peptides extracellularly resulted in disruption of high resistance gigaohm seals. Accordingly, control K+ currents were recorded from cVSMCs dialyzed with either 3 µmol/L Scm NP (Figure 6A) or 3 µmol/L KV1-C NP (Figure 6B) before addition of the selective KV1 channel blocker, 100 nmol/L Psora4.2, 16, 34, 35 Digital subtraction of post-Psora4 (+Psora4) from pre-Psora4 (−Psora4) recordings provided an estimation of Psora4-sensitive KV1 current (Figure 6C, IPsora). The residual currents were regarded as Psora4-insensitive (Figure 6D, Iother). The peak IPsora density at +58 mV was 8.47 ± 1.29 pA/pF for Scm NP-treated cells. This value decreased by 45% to 4.65 ± 0.71 pA/pF for cells dialyzed with KV1-C NP (Figure 6C). In contrast, the peak density of residual Psora4-insensitive current (Iother) was not significantly different between Scm and KV1-C treated cells (Figure 6D). These findings concur with reports that only KV1α-subunits have PDZ binding motifs to enable PSD95 interaction.16, 17 In this regard, Shab-type KV2 channels and KCNQ (Kv7) channels likely contribute to the Psora4-insensitive residual current. Stromatoxin-sensitive KV2 channels are not blocked by Psora4 in cVSMCs16, 35 and Psora4 does not compromise vasoconstrictor responses of CA to the KV7 channel blocker, linopirdine (Online Figure VI).
Figure 6. KV1-C peptide reduces Psora4 -sensitive K+ current.
A, B) Representative whole-cell K+ currents recorded with 3 µmol/L Scm (A) or KV1-C peptide (B) in the pipette solution. Current densities before (−Psora) and after (+Psora) the bath addition of 100 nmol/L Psora4 in are plotted. Psora4-sensitive current density IPsora was calculated by digitally subtracting post-Psora4 (+Psora) from pre-Psora4 (−Psora). C, D) Psora4-sensitive (C) and Psora4-insensitive (D) current densities (n=6 each). * indicates significant difference from Scm, P<0.05.
KV1-C peptide reduces PKA phosphorylation of KV1 channels
After demonstrating that PSD95 is the critical scaffold required for KV1 channel function and dilation of CA, we searched for the molecular component(s) of the PSD95 signaling complex responsible for the basal opening of KV1 channels. One candidate binding partner of PSD95 is AKAP150, which is known to act as a focal point in multi-protein signaling complexes to facilitate PKA-dependent phosphorylation of target proteins.26 Huang et al first reported that direct application of PKA catalytic subunits lead to the activation of cloned cardiac KV1 channels in oocytes.10 Later, PKA-dependent phosphorylation and opening of KV1 channels were reported in native smooth muscle cells from rabbit portal vein11 and canine colon.12 However, many later studies using cAMP analogues or forskolin on recombinant KV1.2 channels in heterologous cells only revealed an increase in KV1.2 protein levels by PKA phosphorylation with small to no increase in gating of channels.13–15, 36 Therefore we examined if the association of KV1 channels with PSD95 signaling complex could explain this apparent discrepancy.
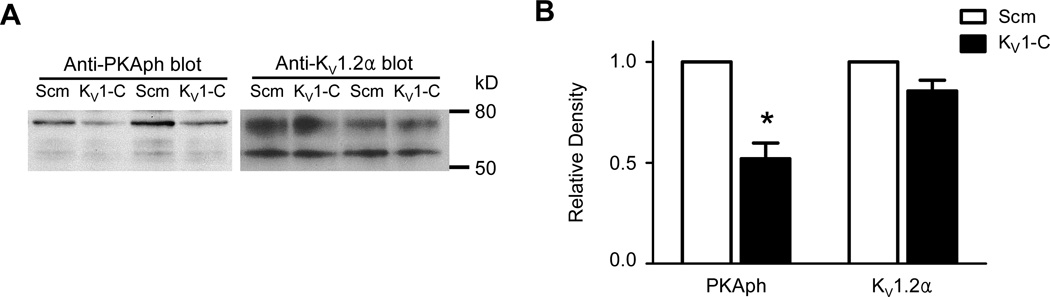
Previously, Connors et al14 successfully used an antibody that detects PKA-phosphorylation of serine or threonine residues to identify PKA phosphorylation of rat KV1.2α subunits overexpressed in a heterologous expression system. We used the same antibody to explore if KV1.2α subunit-PSD95 interaction is required for PKA-phosphorylation of KV1 channels in cVSMCs, thereby inferring co-localization of KV1 channels with AKAP150 in the vascular PSD95 signaling complex. CA treated with 10 µmol/L Scm or KV1-C peptide for 30 min were lysed and loaded equally (30 µg) into lanes of a Western blot probed with anti-phospho PKA substrate.14 Compared to CA treated with Scm peptide, KV1-C treated CA showed a marked loss in the phosphorylation of the ~75 kD band (Figure 7A, left blot), which is the fully-glycosylated form of KV1.2α1 required for PKA phosphorylation.9 Average densitometric values from five similar preparations demonstrated a ~50% reduction in PKA phosphorylation of KV1.2α, whereas the expression level of total KV1.2α protein did not change significantly (Figures 7A, left panel and 7B). These results suggest that PSD95 scaffolding promotes PKA-mediated phosphorylation of KV1 channels, an event associated with increased KV1 channel opening.
Figure 7. PKA phosphorylation of KV1.2α is reduced in arteries exposed to KV1-C peptide.
A) Western blot using a phospho-PKA substrate antibody (PKAph, left blot) detected a ~75 kD protein corresponding to KV1.2α in CA protein lysates. CA from age-matched rats were incubated in either Scm or KV1-C peptide, and then lysates (30 µg) were loaded into adjacent lanes. Lanes 1-2 and lanes 3-4 represent separate preparations. The blot was stripped and reprobed with anti-KV1.2α antibody (KV1.2α, right blot). B) Densitometry measurement of PKAph and KV1.2α bands relative to Scm treatment (n=5). *: significant difference from Scm, P<0.05.
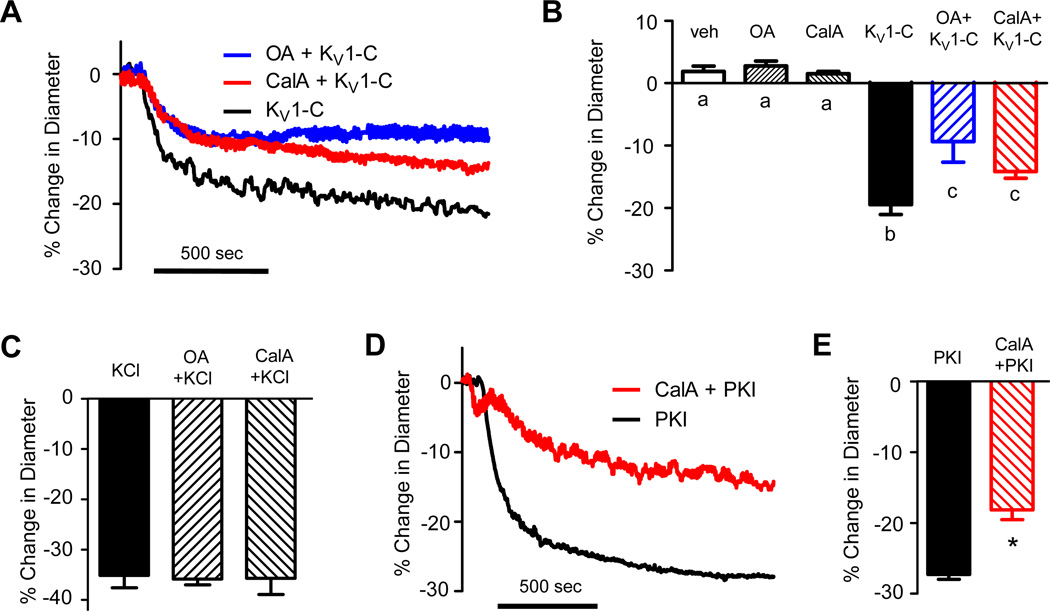
In order to confirm that reduced PKA-mediated phosphorylation of KV1 channels contributed to the constriction of CA by KV1-C peptide, we intervened to preserve channel phosphorylation using low concentrations of the protein serine/threonine phosphatase inhibitors, okadaic acid (OA, 300 nmol/L) and calyculin-A (CalA, 100 nmol/L). Neither phosphatase inhibitor significantly altered the resting arterial diameter of isolated CA (Figure 8B), but both significantly blunted the constrictor response to KV1-C peptide (Figures 8A-B). In drug-free PSS, KV1-C peptide reduced the diameter of CA by −19.5 ± 1.6% compared to −9.3 ± 3.3% and −14.1 ± 1.1% in the presence of OA and CalA, respectively (Figure 8B). Constriction in response to KCl was not altered by the presence of OA or CalA (Figure 8C) indicating that the ability of CA to constrict was not nonspecifically reduced by the presence of low concentrations of phosphatase inhibitors. In a final set of experiments we verified that a loss of PKA activity results in constriction of rat CA, since earlier studies defining mechanisms of PKA modulation of KV1 channel properties were mostly performed in cell systems.11, 12 Indeed, 1 µmol/L PKI peptide rapidly constricted isolated rat CA by −27.3 ± 0.7% and this constrictor response was significantly reduced in the presence of 100 nmol/L CalA (Figures 8D-E). These findings highlight that PKA phosphorylation of KV1 channels is an important contributor to the basal dilation of CA and that a dynamic association with the PSD95 signaling complex is critically required for PKA phosphorylation of KV1 channels in CA.
Figure 8. Phosphatase inhibitors blunt the vasoconstriction caused by KV1-C.
A) Representative traces show that the phosphatase inhibitors, 300 nmol/L okadaic acid (OA) and 100 nmol/L calyculin A (CalA), blunt the vasoconstrictor response of rat CA to KV1–C peptide. B) Average % change in diameter in response to vehicle (veh), OA, CalA, 3 µmol/L KV1–C peptide, or KV1–C peptide in the presence of veh, OA, or CalA (n=5-10). The phosphatase inhibitors did not affect the resting diameter of CA, but blunted the vasoconstrictor response to KV1-C peptide. a, b, c: statistical significance, P<0.05. C) Average % change in diameter caused by 60 mmol/L KCl in the presence and absence of a phosphatase inhibitors (n=4-10). No significant difference between groups. D) Representative traces show that 100 nmol/L CalA blunts the vasoconstrictor response to 1 µmol/L PKA inhibitor peptide (PKI). E) Average % change in diameter caused by 1 µmol/L PKI in the absence or presence of CalA (n=5). *: significantly different from PKI alone, P<0.05.
DISCUSSION
PSD95 was originally regarded as an exclusive feature of the post-synaptic density in neurons, and it was only rarely detected in nonneural tissues.37, 38 However, two recent reports identified PSD95 as a potentially important scaffold of KV1 channels in lymphocytes and in cVSMCs.16, 39 First, mutation of the KV1.3 subunit PDZ binding motif or knockdown of PSD95 in T lymphocytes inhibited the recruitment of KV1.3 into contact points on the cell surface, implying an important role of PSD95-KV1 channel interaction in T-cell activation.39 Second, we demonstrated in cVSMCs that PSD95 is expressed and antisense knockdown of PSD95 in rat CA for 72 hours results in a concomitant loss of KV1 channel protein and vasodilator function. These findings suggested a critical role of PSD95 in maintaining the expression of KV1 channels in the cerebral circulation.16 However, because KV1 channels were down-regulated by PSD95 knockdown in our previous study, the impact of PSD95 signaling complex on KV1 channel function could not be assessed. Furthermore, knockdown of PSD95 effectively limits interaction with all of its binding partners, potentially disrupting multiple signaling pathways in addition to KV1 channels involved in regulating the diameter of CA. For example, β1 adrenergic receptors40, serotonin receptors41, inward-rectifying potassium channels42, and nNOS43 are known binding partners of PSD95 in neurons that could similarly interact with PSD95 in arteries to alter vascular tone. Thus, the present study designed a dominant negative peptide to specifically disrupt the scaffolding interaction between KV1 channels and PSD95 in cVSMCs. Our results provide initial evidence that the PSD95 signaling complex is critically required for KV1 channel dilator function in rat CA in situ and further suggest that PSD95 provides a platform for interaction between PKA and KV1 channels that enables PKA–mediated phosphorylation and opening of KV1 channels to promote hyperpolarization and relaxation of cVSMCs.
Our initial experiments explored whether a dynamic association with PSD95 was required for the proper vasodilator function of KV1 channels in CA. After directly disrupting the KV1-PSD95 association by application of KV1-C peptide, we observed a rapid and profound vasoconstriction ex vivo and in vivo. Ex vivo KV1-C peptide-induced constriction was equivalent to constriction caused by the KV1 channel blocker Psora4 and there were no additive effects of concomitant treatment whereas vasoconstriction caused by KV7 channel blocker linopirdine or KIR channel blocker BaCl2 was not altered by KV1-C peptide. Psora4 is a highly selective blocker of KV1 channels and does not significantly block other voltage-gated K+ channels or large-conductance, Ca2+-activated K+ channels.2, 16, 34, 35 A recent report identified a secondary binding site in the unique side pocket of KV1 channels that strengthens the selectivity of Psora4.35 In our patch clamp study KV1-C peptide caused a significant reduction in Psora4-sensitive K+ currents while Psroa4-insensitive K+ currents that may contain KV2.1 or KV7 currents remained identical to Scm-treated cells. Considering the diversity of K+ channels in CA, the effects caused by disruption by KV1-C peptide is remarkably specific to the blockade of KV1 channels and strengthens the idea of selective PDZ binding of KV1 channels to the PSD95 complex.
Application of KV1-C peptide to isolated CA also depolarized the cVSMC membrane without an apparent change in KV1 channel expression, implying that basal KV1 channel opening relies on PSD95 scaffolding to mediate hyperpolarizing K+ efflux. The rapid onset of KV1-C peptide-induced effects was consistent with the idea that the intrinsic instability of the C-terminus of the KV1.2α subunit allows for a dynamic interaction with the PDZ binding domains of PSD95.24 In general, PDZ binding appears to be reversible and similar competing peptides have been applied to cultured neuronal cells28 and to neurons in vivo30–32 to disrupt the binding of NMDAR and PSD95 and break the excitotoxic signaling pathway mediated by PSD95. The rapid intracellular delivery of peptides conferred by the HIV-tat sequence has been reported in many cell types44 and now successfully used in the present study to explore ion channel signaling pathways in arteries. Considering our finding that an HIV-tat conjugated-peptide targeting PSD95 can markedly alter cerebral vascular tone, it is possible that the vasoconstrictor effects of an HIV-tat peptide delivered intravenously by Bach et al30 and Cook et al31 to interrupt NMDAR-PSD95 interaction in neurons and ameliorate stroke may have a direct action on cVSMCs as an unexpected side effect. Since KV1.2α and NMDAR share the PDZ1 and PDZ2 binding domains of PSD9530, C-terminus peptides designed for NMDAR can potentially cross-compete with KV1 channels for binding to PSD95. Considering this therapy is already in Phase 1 and 2 clinical trials in Canada and the U.S., the need to fully define the physiological role of PSD95 in CA is even more urgent. In this respect, the present study provides initial proof for mechanism by which disruption of PSD95 scaffolding could adversely affect cerebral circulation.
We also observed that KV1-C peptide reduced PKA phosphorylation of KV1 channels without altering cell surface expression. Our study indicates that association with the PSD95 complex is required for the direct PKA-mediated phosphorylation of KV1.2α subunit and increased channel function independent of expression level. Our observation may explain the dichotomy between early reports of PKA-induced increase in KV1.2 channel activity10–12 and the lack of such increase in KV1.2 channel activity in many later studies in heterologous expression systems.13–15 This apparent discrepancy may have resulted from the lack of PSD95 signaling complex in heterologous cells which is necessary to hold PKA in proximity to KV1.2 channels for proper phosphorylation. In seeming contrast to the present study, we previously reported that antisense-knockdown of PSD95 in rat CA for 72 hours results in marked loss of the KV1.2α protein.16 However, in the present study, CA were only exposed to the KV1-C peptide for 30 min, a time frame that apparently was too short to cause a loss of KV1 channels and only their vasodilator function was significantly blunted. The finding that PSD95 is required for PKA–mediated phosphorylation of the KV1.2α subunit in cVSMCs raises the possibility that PSD95-AKAP interaction exists as unrecognized scaffolding machinery in the vasculature. Colledge et al26 reported that PSD95-AKAP interaction is required for PKA-dependent phosphorylation of AMPA receptors in neurons, and our data suggest that the PSD95-AKAP complex also may be required for PKA-dependent phosphorylation of vascular KV1 channels. Dissociation of KV1.2α–containing channels from this complex by the KV1-C peptide may reduce subunit phosphorylation leading to phosphatase-dependent dephosphorylation, less channel opening, depolarization of the cell, and constriction of CA.
Several limitations of our study should be acknowledged. First, we have used non-permeable peptides in patch clamp studies to verify that KV1-C peptide disruption of PSD95-KV1 interaction reduces KV1 channel–mediated K+ current. Standard whole-cell and perforated-patch studies were attempted with the cell-permeable peptides, but application of the HIV-tat peptide even in low concentrations consistently disrupted gigaohm seals. Thus, apparently the HIV-tat sequence that contains several positive charges interferes with seal integrity. For this reason, we also carried out membrane potential measurements to provide strong evidence that K+ efflux through KV1 channels is attenuated by KV1-C peptide disruption of the KV1 channel-PSD95 complex. Second, we used a higher concentration of KV1-C peptide for in vivo than ex vivo studies of vascular reactivity. Isolated CA constricted maximally to 10 µmol/L KV1-C peptide, but 30 µmol/L KV1-C was required to elicit sustained constriction in vivo (Online Figure VII). In contrast to isolated perfused arteries, a reduction in peptide potency and efficacy may be expected in vivo, since the cranial window preparation unavoidably has adventitia and neural tissue as barriers or sinks of peptide, potential peptide loss through cerebrospinal fluid or metabolism may occur, and compensatory mechanisms may exist to normalize arterial diameter in response to disruption of normal homeostatic mechanisms. Third, we did not identify which serine or threonine residues of KV1.2α subunit are targets of PSD95-mediated PKA phosphorylation in cVSMCs and left this line of inquiry for future studies. Finally, KV1-C peptide may have unidentified non-specific effects in CA. Our KV1-C peptide corresponding to a PDZ-binding motif on the KV1.2α subunit was designed to act as a dominant negative peptide to compete for the PDZ1 and PDZ2 domains on PSD95. Of the known voltage-gated K+ channel subunits in CA, KV1.2α is uniquely equipped with a PDZ binding motif16, which may enable the KV1 channels to be downstream targets of PKA-dependent signaling on the PSD95 scaffold. However, the PDZ domains of PSD95 also could bind unidentified vasoactive proteins in CA that contain a PDZ binding motif. In addition to KV1.2α, these proteins also could be displaced by the KV1-C peptide, thereby potentially modifying arterial diameter.
In summary, we propose that the scaffolding of KV1 channels by PSD95 in CA is a dynamic interaction required for the efficient vasodilator function of KV1 channels through PKA phosphorylation. Dissociation of KV1 channels from this complex by a dominant negative peptide causes rapid vasoconstriction of CA ex vivo and in vivo. Our findings emphasize that therapeutic targeting of PSD95 to reduce cytotoxic injury caused by stroke or other ischemic insults should consider that manipulation of PSD95 also may critically alter cerebral arterial diameter and blood flow independently of neuronal function. Similarly disease-based alterations of PSD95 structure or function may potentially contribute to cerebral blood flow abnormalities by disrupting KV1 channel dilator function or interfering with the roles of other PSD95 binding partners in vascular smooth muscle cells.
Supplementary Material
Novelty and Significance.
What Is Known?
Postsynaptic density-95 (PSD95), once considered a neuronal marker, is a scaffolding protein that assembles ion channels and signaling molecules at the plasma membrane of several cell types.
PSD95 is expressed and co-assembles with Shaker-type potassium (KV1) channels in the vascular smooth muscle cells (cVSMCs) of cerebral arteries (CA).
The opening of KV1 channels contributes to the resting diameter of CA, which determines the level of blood flow to distal neurons.
What New Information Does This Article Contribute?
Selective disruption of the association between PSD95 and KV1 channels results in a severe constriction of rat CA ex vivo and in vivo.
Protein kinase A (PKA)-phosphorylation and opening of KV1 channels in CA requires the PSD95 scaffolding function.
PKA-mediated opening of KV1 channels enabled by PSD95 maintains the resting diameter of CA and prevents abnormal vasoconstriction.
PSD95, a scaffolding protein, is expressed near the plasma membrane of cVSMCs and interacts with KV1 channels in rat CA. However, the physiological function of PSD95 in cVSMCs is unknown. Here, we demonstrate that the PSD95 scaffold is required for PKA-mediated phosphorylation and opening of KV1 channels, which in turn, significantly contribute to the resting diameter of rat CA. Our results reveal a vasodilator signaling complex on the PSD95 scaffold in cVSMCs, which regulate cerebral perfusion. Our results suggest that conditions that disrupt the PSD95 scaffolding of KV1 channels in cVSMCs may cause a loss of vasodilator function and impaired cerebral blood flow. Further studies are warranted to investigate the role of the PSD95 complex in pathological conditions such as hypertension, a disease in which vasodilator function is compromised.
ACKNOWLEDGEMENTS
We thank Dr. Charles Leffler and Mr. Alex Fedinec at the University of Tennessee Health Sciences Center for demonstration and advice on the cranial window preparation. We thank Ms. Hillary Hanvey for expert technical assistance.
SOURCES OF FUNDING
This work was supported in part by American Heart Association predoctoral grant 13PRE17070035 (CLM) and NIH R01 HL097107 (SWR).
Nonstandard Abbreviations and Acronyms
- AKAP
A-kinase anchoring protein
- CA
cerebral arteries
- CalA
calyculin-A
- cVSMCs
cerebral vascular smooth muscle cells
- Em
membrane potential
- GK
guanylate kinase
- IP
immunoprecipitate
- KV1
Shaker-type voltage-gated K+ channel
- KV1-C
membrane-permeable peptide mimicking KV1 channel C-terminus
- L-NAME
Nω-Nitro-L-arginine methyl ester
- NMDAR
N-methyl-D-aspartate receptor
- nNOS
neuronal nitric oxide synthase
- NP
non-permeable
- OA
okadaic acid
- PDZ
post synaptic density-95, discs large, zonula occludens-1
- PKA
protein kinase A
- PKI
myristoylated protein kinase A inhibitor peptide
- Psora4
5-(4-phenylalkoxypsoralen)
- PSD95
postsynaptic density protein-95
- Scm
scrambled control peptide
- SH3
Src homology 3
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular Shaker-type K+ channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297:H293–H303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong A, Dedman AM, Xu SZ, Beech DJ. KVa1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281:H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 4.Coppock EA, Tamkun MM. Differential expression of KV channel alpha- and beta-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1350–L1360. doi: 10.1152/ajplung.2001.281.6.L1350. [DOI] [PubMed] [Google Scholar]
- 5.Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric KV1.2-KV1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 6.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric KV1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96:216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- 7.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 8.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe I, Zhu J, Sutachan JJ, Gottschalk A, Recio-Pinto E, Thornhill WB. The glycosylation state of Kv1.2 potassium channels affects trafficking, gating, and simulated action potentials. Brain Res. 2007;1144:1–18. doi: 10.1016/j.brainres.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 10.Huang XY, Morielli AD, Peralta EG. Molecular basis of cardiac potassium channel stimulation by protein kinase A. Proc Natl Acad Sci U S A. 1994;91:624–628. doi: 10.1073/pnas.91.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 12.Koh SD, Sanders KM, Carl A. Regulation of smooth muscle delayed rectifier K+ channels by protein kinase A. Pflugers Arch. 1996;432:401–412. doi: 10.1007/s004240050151. [DOI] [PubMed] [Google Scholar]
- 13.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104:20055–20060. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connors EC, Ballif BA, Morielli AD. Homeostatic regulation of KV1.2 potassium channel trafficking by cyclic AMP. J Biol Chem. 2008;283:3445–3453. doi: 10.1074/jbc.M708875200. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and Functional Characterization of Protein Kinase A-catalyzed Phosphorylation of Potassium Channel KV1.2 at Serine 449. J Biol Chem. 2009;284:16562–16574. doi: 10.1074/jbc.M109.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph BK, Thakali KM, Pathan AR, Kang E, Rusch NJ, Rhee SW. Postsynaptic density-95 scaffolding of Shaker-type K+ channels in smooth muscle cells regulates the diameter of cerebral arteries. J Physiol. 2011;589:5143–5152. doi: 10.1113/jphysiol.2011.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JM. Cell signalling: MAGUK magic. Curr Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 19.Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol. 1999;113:71–80. doi: 10.1085/jgp.113.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 22.Gardoni F, Marcello E, Di Luca M. Postsynaptic density-membrane associated guanylate kinase proteins (PSD-MAGUKs) and their role in CNS disorders. Neuroscience. 2009;158:324–333. doi: 10.1016/j.neuroscience.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 23.Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K+ channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magidovich E, Orr I, Fass D, Abdu U, Yifrach O. Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K+ channel modulates its interaction with scaffold proteins. Proc Natl Acad Sci U S A. 2007;104:13022–13027. doi: 10.1073/pnas.0704059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 27.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 28.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 29.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 30.Bach A, Clausen BH, Moller M, Vestergaard B, Chi CN, Round A, Sorensen PL, Nissen KB, Kastrup JS, Gajhede M, Jemth P, Kristensen AS, Lundstrom P, Lambertsen KL, Stromgaard K. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1-2 and protects against ischemic brain damage. Proc Natl Acad Sci U S A. 2012;109:3317–3322. doi: 10.1073/pnas.1113761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 32.Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, Ryan CL, Bernard PB, Lau A, Forder JP, Salter MW, Wang YT, Tasker RA, Tymianski M. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–2553. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- 33.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 35.Marzian S, Stansfeld PJ, Rapedius M, Rinne S, Nematian-Ardestani E, Abbruzzese JL, Steinmeyer K, Sansom MS, Sanguinetti MC, Baukrowitz T, Decher N. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat Chem Biol. 2013;9:507–513. doi: 10.1038/nchembio.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett. 2010;486:60–67. doi: 10.1016/j.neulet.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stathakis DG, Hoover KB, You Z, Bryant PJ. Human postsynaptic density-95 (PSD95): location of the gene (DLG4) and possible function in nonneural as well as in neural tissues. Genomics. 1997;44:71–82. doi: 10.1006/geno.1997.4848. [DOI] [PubMed] [Google Scholar]
- 38.Godreau D, Vranckx R, Maguy A, Rucker-Martin C, Goyenvalle C, Abdelshafy S, Tessier S, Couetil JP, Hatem SN. Expression, regulation and role of the MAGUK protein SAP-97 in human atrial myocardium. Cardiovasc Res. 2002;56:433–442. doi: 10.1016/s0008-6363(02)00602-8. [DOI] [PubMed] [Google Scholar]
- 39.Szilagyi O, Boratko A, Panyi G, Hajdu P. The role of PSD-95 in the rearrangement of KV1.3 channels to the immunological synapse. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1256-6. [DOI] [PubMed] [Google Scholar]
- 40.Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. Beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 41.Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- 42.Nehring RB, Wischmeyer E, Doring F, Veh RW, Sheng M, Karschin A. Neuronal inwardly rectifying K+ channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J Neurosci. 2000;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 44.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.