ABSTRACT
The only tuberculosis (TB) vaccine in use today, bacillus Calmette-Guérin (BCG), provides insufficient protection and can cause adverse events in immunocompromised individuals, such as BCGosis in HIV+ newborns. We previously reported improved preclinical efficacy and safety of the recombinant vaccine candidate BCG ΔureC::hly, which secretes the pore-forming listeriolysin O of Listeria monocytogenes. Here, we evaluate a second-generation construct, BCG ΔureC::hly Δpdx1, which is deficient in pyridoxine synthase, an enzyme that is required for biosynthesis of the essential cofactor vitamin B6. This candidate was auxotrophic for vitamin B6 in a concentration-dependent manner, as was its survival in vivo. BCG ΔureC::hly Δpdx1 showed markedly restricted dissemination in subcutaneously vaccinated mice, which was ameliorated by dietary supplementation with vitamin B6. The construct was safer in severe combined immunodeficiency mice than the parental BCG ΔureC::hly. A prompt innate immune response to vaccination, measured by secretion of interleukin-6, granulocyte colony-stimulating factor, keratinocyte cytokine, and macrophage inflammatory protein-1α, remained independent of vitamin B6 administration, while acquired immunity, notably stimulation of antigen-specific CD4 T cells, B cells, and memory T cells, was contingent on vitamin B6 administration. The early protection provided by BCG ΔureC::hly Δpdx1 in a murine Mycobacterium tuberculosis aerosol challenge model consistently depended on vitamin B6 supplementation. Prime-boost vaccination increased protection against the canonical M. tuberculosis H37Rv laboratory strain and a clinical isolate of the Beijing/W lineage. We demonstrate that the efficacy of a profoundly attenuated recombinant BCG vaccine construct can be modulated by external administration of a small molecule. This principle fosters the development of safer vaccines required for immunocompromised individuals, notably HIV+ infants.
IMPORTANCE
Mycobacterium tuberculosis can synthesize the essential cofactor vitamin B6, while humans depend on dietary supplementation. Unlike the lipophilic vitamins A, D, and E, water-soluble vitamin B6 is well tolerated at high doses. We generated a vitamin B6 auxotroph of the phase II clinical tuberculosis vaccine candidate bacillus Calmette-Guérin ΔureC::hly. The next-generation candidate was profoundly attenuated compared to the parental strain. Adaptive immunity and protection in mice consistently depended on increased dietary vitamin B6 above the daily required dose. Control of vaccine efficacy via food supplements such as vitamin B6 could provide a fast track toward improved safety. Safer vaccines are urgently needed for HIV-infected individuals at high risk of adverse events in response to live vaccines.
INTRODUCTION
Vaccination is the most cost-efficient measure for controlling infectious diseases (1), and yet, we lack efficient vaccines for the major infectious diseases AIDS, malaria, and tuberculosis (TB). The only TB vaccine in use today, bacillus Calmette-Guérin (BCG), was introduced into clinical practice more than 90 years ago (2). It resulted from repeated passage of virulent Mycobacterium bovis. Although the protection provided by BCG against pulmonary TB is incomplete, it significantly reduces TB-related childhood morbidity and mortality and is relatively safe in immunocompetent individuals (3). The global AIDS pandemic has raised new challenges: human immunodeficiency virus (HIV)-Mycobacterium tuberculosis coinfection accounts for one-fourth of the 1.3 million deaths due to TB annually (4), and BCG vaccination of HIV-infected newborns can result in a disseminated disease with TB-like symptoms named BCGosis (3). Therefore, a major focus of live TB vaccine research and development is improving vaccine safety in immunocompromised individuals.
Novel genetic tools for effective mutagenesis of the mycobacterial genome have paved the way for the development of recombinant live TB vaccines based on attenuated mycobacteria (5, 6). Auxotrophic mutants have been explored as potential vaccine candidates for more than a decade (7, 8). Although the auxotroph M. tuberculosis ΔpanCD, which fails to synthesize vitamin B5 (pantothenic acid), was markedly attenuated, subcutaneous (s.c.) vaccination of mice provided short-term (28 days) protection against M. tuberculosis challenge comparable to that afforded by BCG (9). To address safety concerns, notably reversion of a single mutation to full virulence, a second, independent attenuating mutation (leuD gene) was included (10). The protection induced by the double auxotroph M. tuberculosis ΔleuD ΔpanCD was comparable to that of BCG in guinea pigs, but booster immunization with the same construct did not improve efficacy in this model (10). Several other auxotrophic mutants have been tested as TB vaccines either alone (11, 12) or in combination (13, 14). Although auxotrophic constructs have shown improved safety in preclinical models, they collectively failed to provide better protection than canonical BCG.
Vitamin B6 is a water-soluble essential cofactor for humans, which in humans must be supplied by dietary intake. In contrast, plants, fungi, and bacteria, including M. tuberculosis, synthesize pyridoxal-5′-phosphate (PLP), the bioactive form of vitamin B6, from glutamine and derivatives of the carbohydrate metabolism in a two-step reaction catalyzed by Pdx1 (Rv2606c) and Pdx2 (Rv2604c) (15). Both proteins form a functional class I glutamine amidotransferase (16, 17). The protein sequences and crystal structures of Pdx1 orthologs from Bacillus subtilis, Plasmodium species, Saccharomyces cerevisiae, and M. tuberculosis are highly similar, suggesting evolutionary conservation (18–21). Deletion of the pdx1 gene renders M. tuberculosis auxotrophic for vitamin B6 and markedly compromises persistence in mice (15).
We previously engineered BCG to secrete pore-forming listeriolysin O (Hly) of L. monocytogenes (22). BCG ΔureC::hly (VPM1002) showed superior protection and safety compared to the results for BCG in preclinical models and proved to be safe and immunogenic in humans (22, 23). VPM1002 has successfully completed phase IIa clinical assessment in newborns (http://ClinicalTrials.gov identifier NCT01479972). Here, we deleted pdx1 in the genetic background of BCG ΔureC::hly to further improve its safety. The immunogenicity, protective capacity, and safety of the new construct, BCG ΔureC::hly Δpdx1, were evaluated in mice that received either a normal or vitamin B6-enriched diet. Our studies provide an innovative basis for novel vaccination strategies in immunocompromised individuals.
RESULTS
Disruption of pdx1 renders BCG auxotrophic for vitamin B6.
The pdx1 loss-of-function mutant of M. tuberculosis was shown to be fully auxotrophic for vitamin B6 (15). The operon consisting of pdx1 (also called snzP, Rv2606c, and BCG2631c), tesB2 (also called Rv2605c and BCG2630c), and pdx2 (also called snoP, Rv2604c, and BCG2629c) encodes the functional vitamin B6 synthase complex composed of Pdx1 and Pdx2 and is identical in M. tuberculosis and BCG (see Fig. S1 in the supplemental material) (24, 25). Thus, we expected a phenotype for a pdx1 knockout in BCG similar to that described for M. tuberculosis (15). Indeed, disruption of pdx1 by allelic exchange prevented the growth of BCG ΔureC::hly in vitamin B6-free culture medium (Fig. 1A). Replication of bacilli was fully restored when cultures were supplemented with 5 µM B6 vitamer pyridoxine. Lower pyridoxine concentrations resulted in suboptimal growth rates of the pdx1 mutant, demonstrating concentration-dependent complementation. Moreover, genetic complementation of the mutant with a functional copy of pdx1 under the control of its native promoter completely reversed the observed growth defect in minimal medium (Fig. 1B). Altogether, deletion of pdx1 rendered BCG auxotrophic for vitamin B6, and this phenotype could be reverted by chemical and genetic means.
FIG 1 .
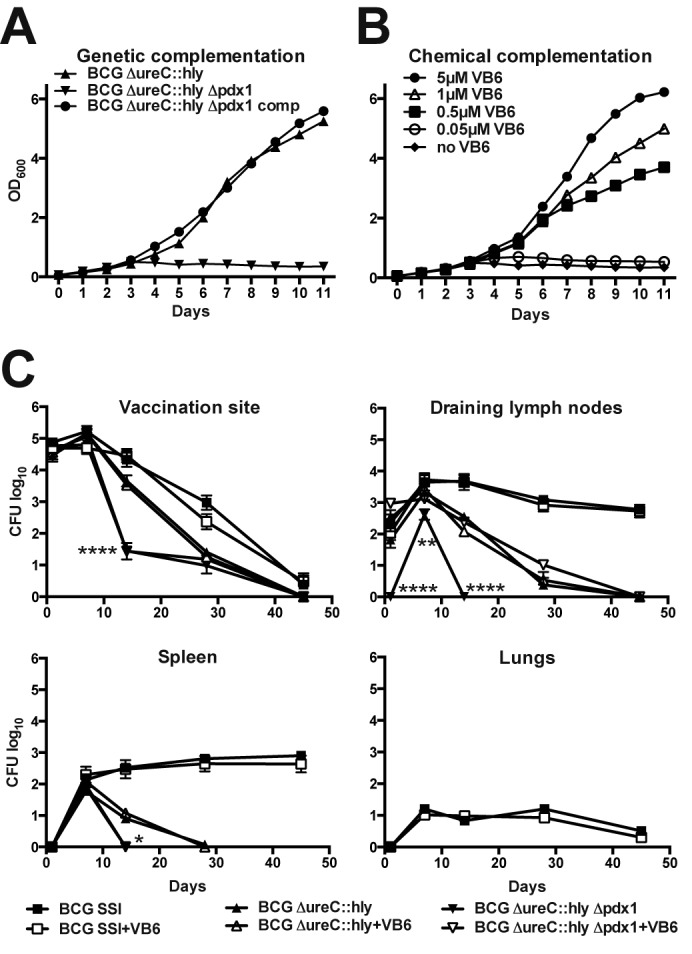
Vitamin B6 controls growth in vitro and partially controls survival in vivo of the BCG ΔureC::hly Δpdx1 auxotroph. Genetic complementation with pdx1 (A) and chemical complementation by supplementation of Sauton’s minimal medium with vitamin B6 (VB6) (B) restored the growth defect of BCG ΔureC::hly Δpdx1 in vitro. (C) Dissemination and clearance of BCG, BCG ΔureC::hly, and BCG ΔureC::hly Δpdx1 in mice on a normal diet or vitamin B6-enriched nutrition. Vaccines (106 CFU) were administered s.c. at the tail base. Independent from vitamin B6 administration, BCG ΔureC::hly and BCG ΔureC::hly Δpdx1 were not detected in lungs. Shown are means ± standard deviations (SD) (n = 5) analyzed using two-way analysis of variance (ANOVA) and Tukey’s post hoc test. Significant statistical differences between BCG ΔureC::hly and BCG ΔureC::hly Δpdx1 groups are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Experiments were performed twice.
Dissemination and survival of BCG ΔureC::hly Δpdx1 in mice is profoundly compromised.
The spread and survival of live vaccines in mice provide key information for safety assessment. We examined the bacterial loads at the site of vaccination and in draining lymph nodes (dLNs), spleen, and lungs over 90 days in s.c.-vaccinated mice that received either a standard or vitamin B6-enriched diet. BCG disseminated from the injection site to dLNs and spleen, where the bacterial counts remained relatively stable until the end of the experiment, in contrast to BCG ΔureC::hly, which was cleared from these organs after 45 or 28 days, respectively (Fig. 1C). These observations are in line with previous experiments where BCG persisted for up to 120 days in dLNs and 90 days in the spleen, while at 60 days postvaccination, BCG ΔureC::hly was undetectable (26). BCG was the only vaccine tested that disseminated to lungs (Fig. 1C). The survival of BCG and BCG ΔureC::hly in mice remained independent of dietary vitamin B6 levels. Intriguingly, BCG ΔureC::hly Δpdx1 did not persist beyond 45 days (vaccination site) or 14 days (dLNs and spleen) (Fig. 1C), suggesting that its attenuation is determined by the pdx1 deletion rather than ureC disruption or Hly expression. Vitamin B6 supplementation restored the persistence of the auxotrophic construct to that of the parental BCG ΔureC::hly in dLNs but not at the site of vaccination or in the spleen (Fig. 1C). We conclude that knockout of pdx1 profoundly attenuated BCG constructs in mice. Elevated vitamin B6 administration could partially compensate for reduced persistence but did not achieve complete chemical complementation in vivo.
BCG ΔureC::hly Δpdx1 is safer than parental BCG ΔureC::hly in immunodeficient mice.
Having demonstrated that the deletion of pdx1 profoundly attenuated BCG in immunocompetent mice (Fig. 1), we went on to determine the safety of BCG ΔureC::hly Δpdx1 in mice with severe combined immunodeficiency (SCID). Groups of animals were s.c. vaccinated with 106 CFU of the constructs under investigation. The SCID mice receiving the parental BCG ΔureC::hly survived significantly longer than the BCG-vaccinated group, confirming the superior safety of the recombinant construct (Fig. 2) (22). When the experiment was terminated 500 days postvaccination, the survival of groups that received BCG ΔureC::hly Δpdx1 (with or without vitamin B6 supplementation) or phosphate-buffered saline (PBS) was significantly improved over that of mice receiving BCG ΔureC::hly, demonstrating profound attenuation of the auxotrophic construct in immunodeficient mice (Fig. 2).
FIG 2 .
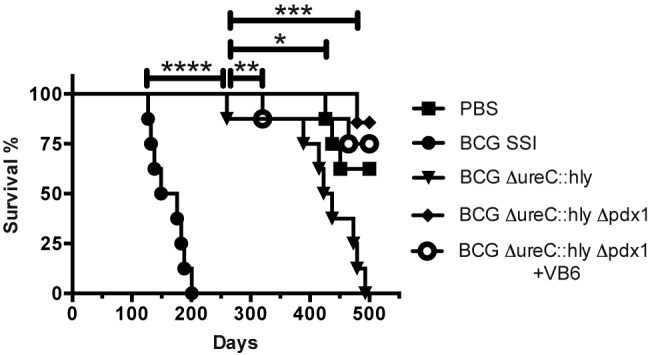
Deletion of pdx1 improved the superior safety profile of parental BCG ΔureC::hly over that of canonical BCG in SCID mice. Survival of SCID mice s.c. vaccinated with 106 CFU of indicated strains was monitored over time. Selected groups received vitamin B6 (VB6)-enriched diet from 2 weeks prior to vaccination to the end of the experiment. Median times of survival were 162.5 days (BCG) and 430 days (BCG ΔureC::hly). Data shown were analyzed using the Mantel-Cox log-rank test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Experiment was performed twice.
Vitamin B6 controls acquired but not innate immunity induced by BCG ΔureC::hly Δpdx1.
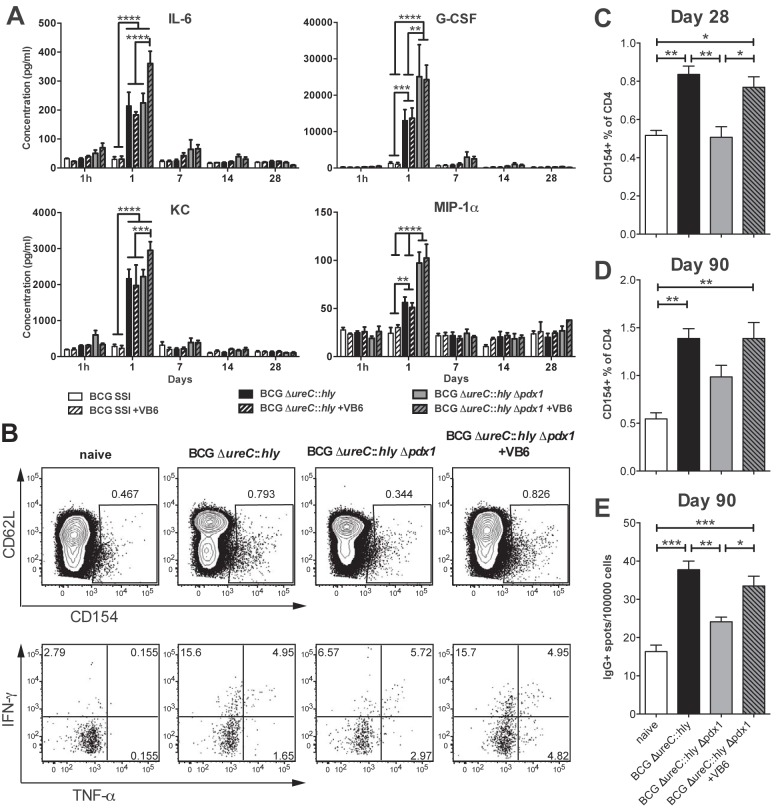
The systemic innate immune responses measured by cytokine secretion were independent of dietary vitamin B6 levels (Fig. 3A). However, 24 h following inoculation, BCG ΔureC::hly Δpdx1 showed a more pronounced stimulation of the inflammatory mediators interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), keratinocyte cytokine (KC), and macrophage inflammatory protein-1α (MIP-1α) than BCG SSI 1331 and BCG ΔureC::hly, which depended on pdx1 deletion (G-CSF and MIP-1α) or vitamin B6 supplementation (IL-6 and KC) (Fig. 3A). In order to detect mycobacterium-specific T cells, we stimulated splenocytes with antigen ex vivo, in the presence of a CD40-blocking monoclonal antibody. Antigen-specific CD4 T cells, which consequently upregulated CD154 under these conditions, were then enumerated by flow cytometry and compared to those from naive controls (27). Twenty-eight days postvaccination, the absolute numbers and proportions of antigen-specific CD4 T cells were increased in mice vaccinated with BCG ΔureC::hly, as well as in mice vaccinated with BCG ΔureC::hly Δpdx1 under vitamin B6 supplementation (Fig. 3B and C). The proportion of CD4 T cells secreting gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) was also increased in mice vaccinated with BCG ΔureC::hly and in the supplemented auxotrophic-strain-vaccinated groups compared to the proportion in naive mice (Fig. 3B). This immune response persisted for 3 months postvaccination. We conclude that the improved survival of the auxotrophic BCG ΔureC::hly Δpdx1 strain due to the administration of vitamin B6 supported the generation of memory T cells, which persist after clearance of the vaccine strain (Fig. 1C and 3D). Vaccination also generated long-lived, IgG-secreting plasma cells, which homed to bone marrow. These cells were detected 3 months postvaccination with either BCG ΔureC::hly or vitamin B6-supplemented BCG ΔureC::hly Δpdx1 (Fig. 3E). Thus, adaptive immunity, particularly the generation of antigen-specific CD4 T cells and B cells in response to mycobacterial antigens, depended on the persistence of the vaccine strains, and the persistence of the auxotrophic strain could be regulated by exogenous administration of vitamin B6.
FIG 3 .
Dietary vitamin B6 supplementation restores adaptive immune responses in BCG ΔureC::hly Δpdx1-vaccinated mice to levels in parental BCG ΔureC::hly-vaccinated mice. (A) Influence of vitamin B6 (VB6) on serum cytokine responses following immunization with indicated constructs. Significant differences were only observed 1 day postvaccination. Shown are means ± standard errors of the means (SEM) (n = 5) analyzed using two-way ANOVA and Tukey’s posttest. (B) Representative flow cytometry results showing percentages of antigen-specific CD154+ T cells among CD3+ CD4+ T cells and levels of intracellular IFN-γ and TNF-α in spleen after in vitro culture with M. tuberculosis lysate 28 days postvaccination. (C and D) Graphs showing proportions of antigen-specific CD154+ T cells among total splenic CD3+ CD4+ cultured as in B, at 28 or 90 days postvaccination. (E) The proportion of IgG-secreting cells in bone marrow measured by ELISPOT 3 months postvaccination. One-way ANOVA followed by Bonferroni’s posttest was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; n = 3 or 4. Representative data from two independent experiments are shown.
Vitamin B6 controls early protection against M. tuberculosis in BCG ΔureC::hly Δpdx1-vaccinated mice.
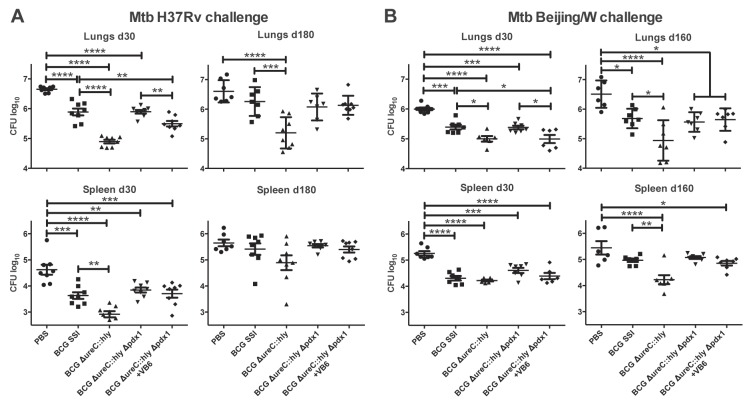
In order to assess the protective capacity of BCG ΔureC::hly Δpdx1, we aerosol challenged mice with the M. tuberculosis laboratory strain H37Rv 90 days postvaccination. The bacterial burdens in lungs and spleens were determined at 30 and 180 days postinfection (Fig. 4A). As in previous experiments (23, 26), BCG ΔureC::hly consistently induced better protection than BCG (Fig. 4B). The early protection provided by BCG ΔureC::hly Δpdx1 depended on vitamin B6 supplementation and was comparable to that induced by BCG but was completely lost at 180 days postinfection (Fig. 4B). Presumably, the vitamin B6-dependent persistence of the construct in dLNs and spleen (Fig. 1C) was not sufficient to induce long-lasting protection. We conclude that a single immunization with BCG ΔureC::hly Δpdx1 does not suffice for long-term protection against aerogenic M. tuberculosis infection in mice.
FIG 4 .
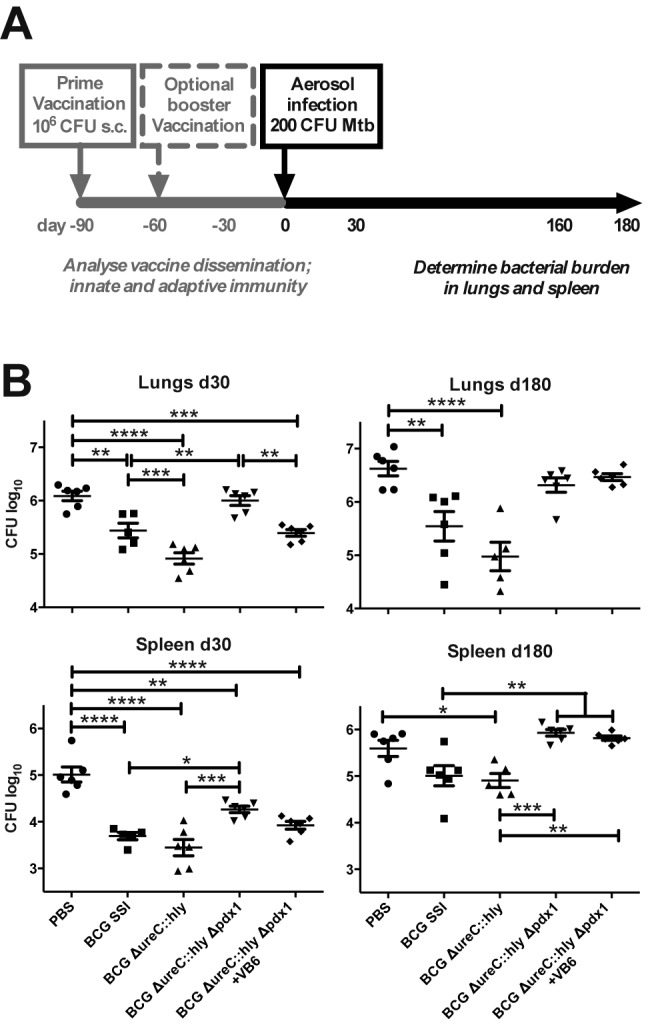
Early protection induced by primary BCG ΔureC::hly Δpdx1 vaccination depends on vitamin B6 supplementation of mice. (A) Schematic design of protection studies. (B) Impact of vitamin B6 on bacterial burdens in lungs and spleen after M. tuberculosis aerosol infection of mice that received primary vaccination only. Groups of mice received vitamin B6 (VB6)-enriched diet as indicated starting 2 weeks prior to vaccination until M. tuberculosis challenge. Shown are means ± SEM (n = 5 or 6) analyzed using one-way ANOVA and Tukey’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Data from one experiment representative of two independent biological replicates are shown.
Prime-boost vaccination improves the efficacy of BCG ΔureC::hly Δpdx1.
The fast clearance of the auxotrophic vaccine construct from mouse organs (Fig. 1C) led us to hypothesize that the protective capacity of BCG ΔureC::hly Δpdx1 could be improved by homologous prime-boost immunization. After vaccination, mice were aerosol challenged with the M. tuberculosis laboratory strain H37Rv or bacilli of the clinically relevant Beijing/W lineage (28). Mice vaccinated with BCG ΔureC::hly Δpdx1 and fed a normal diet showed bacterial burdens in lungs equal to those of animals vaccinated with BCG (Fig. 5A and B). The early protection (day 30) in lungs provided by the auxotrophic strain consistently depended on vitamin B6-enriched nutrition and, for Beijing/W-infected groups, was equal to the protection provided by the parental BCG ΔureC::hly and significantly better than that of BCG (Fig. 5A). Homologous prime-boost vaccination with BCG ΔureC::hly Δpdx1 improved the long-term efficacy against M. tuberculosis H37Rv to the level afforded by BCG and performed significantly better than the mock-vaccinated control group against Beijing/W infection (Fig. 5A and B). The protection of the lung at late time points (180 days for M. tuberculosis H37Rv and 160 days for M. tuberculosis Beijing/W) remained independent from vitamin B6 supplementation (Fig. 5A and B). The results obtained from the spleen were less pronounced and often lacked statistical significance. Taken together, homologous prime-boost immunization improved the efficacy afforded by BCG ΔureC::hly Δpdx1 compared to that of single prime vaccination and consistently depended on a dietary vitamin B6 supply at early time points following M. tuberculosis challenge.
FIG 5 .
Homologous prime-boost vaccination with BCG ΔureC::hly Δpdx1 improves protection and maintains early vitamin B6 dependence. Mice vaccinated with the auxotrophic construct received a homologous booster immunization after 30 days (Fig. 4A). Animals were aerosol infected with the M. tuberculosis laboratory strain H37Rv (A) or a clinical isolate of the Beijing/W lineage (B). Groups of mice received vitamin B6 (VB6)-enriched diet as indicated starting 2 weeks prior to vaccination until M. tuberculosis challenge. Bacterial burdens of lungs and spleen were assessed 30 and 180 (or 160) days postchallenge. Shown are means ± SEM (n = 6-8) analyzed using one-way ANOVA and Tukey’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Data from one experiment representative of two independent biological replicates are shown.
DISCUSSION
The increasing realization that the current BCG vaccine provides insufficient protection against TB, even in newborns, prompted us to develop a recombinant BCG, BCG ΔureC::hly, which had improved efficacy and safety in preclinical models and is currently undergoing phase II clinical assessment (29). The increasing proportion of immunocompromised vaccinees has now led us to further modify this construct toward a superior safety profile by rendering it auxotrophic for vitamin B6. Several auxotrophic mycobacterial mutants have been described (15, 30). Due to their inability to synthesize certain amino acids or cofactors, such mutants rely on uptake of the missing factor in order to survive. During infection, mycobacteria reside in the phagosome of antigen-presenting cells, notably, macrophages (31). The phagosome is an organelle specialized in the eradication of microbial intruders and, therefore, poor in nutrients but rich in reactive radicals and hydrogen ions (31). Pathogenic mycobacteria can survive and replicate in this hostile environment and eventually egress to the host cell cytosol (32, 33). In contrast, auxotrophic mutants of M. tuberculosis are severely attenuated in vivo, demonstrating that infected host cells do not provide sufficient concentrations of the respective factors to overcome deficient persistence (8, 9, 11, 15).
The cytosolic concentration of PLP, the bioactive form of vitamin B6, in eukaryotic cells is regulated by “metabolic trapping,” a process in which the uncharged vitamers become phosphorylated upon entering cells by passive diffusion (34). As a result, leakage of the negatively charged B6 vitamer phosphoesters through biological membranes is prevented. Indeed, PLP could not restore the growth of M. tuberculosis Δpdx1 in cultures, confirming the absence of a dedicated uptake system (15). As a corollary, mycobacterial pdx1 mutants are attenuated in mice (Fig. 1C) (15). Intriguingly, when we fed mice an ~10-fold daily dose of vitamin B6—more than 10,000-fold below the 50% lethal dose reported for mice (35)—the persistence of BCG ΔureC::hly Δpdx1 in dLNs improved and the vaccine candidate was as immunogenic as its parental BCG ΔureC::hly and superior to BCG (Fig. 1C). Clearance of the auxotrophic strain at the site of vaccination and in the spleen was independent of vitamin B6 supplementation, suggesting that the availability of the cofactor could not be raised by elevated uptake into these organs. Intriguingly, the superiority of the safety profile of BCG ΔureC::hly over that of canonical BCG in SCID mice that serve as an immunodeficiency model was further improved by pdx1 deletion and remained independent of vitamin B6 supplementation (Fig. 2).
The assessment of blood serum cytokine concentrations as indicators of innate immunity suggests a proinflammatory phenotype for BCG ΔureC::hly Δpdx1 compared to that of its parental strain (Fig. 3A). While the IL-6 and KC release induced by the auxotrophic strain depended on a vitamin B6-enriched diet, the increased levels of G-CSF and MIP-1α were related to the pdx1 mutation. Vitamin B6 supplementation left these innate immune parameters in response to BCG or BCG ΔureC::hly unaffected (Fig. 3A), thus linking our observations to the persistence of the auxotrophic construct. While inflammatory mediators of innate immunity were stimulated by the attenuated strain, long-lasting antigen-specific T cell responses were only induced when bacterial persistence had been re-established by vitamin B6 supplementation. This is particularly relevant as CD4 T cell responses are thought to be an important component of protection against pulmonary TB (36) and are frequently harnessed as biomarkers of vaccine efficacy (37, 38). Although the role of antibodies in protection remains controversial, the ability of vitamin B6 supplementation to induce long-lived plasma cells supports the importance of vaccine persistence for effective stimulation of adaptive immune responses.
The blood serum concentrations of B6 vitamers (pyridoxal 5-phosphate, 5 to 111 nM; 4 to pyridoxic acid, 6 to 93 nM; and pyridoxal, 3 nM) vary considerably in humans on a normal diet but stabilize (pyridoxal 5-phosphate, ~350 nM; 4-pyridoxic acid, ~1,000 nM; and pyridoxal, ~700 nM) 3 days after daily intake of 40 mg of vitamin B6 (39). Approximately 70 to 80% of overall vitamin B6 is found bound to glycogen phosphorylase in muscles (40). The upper tolerated dose of the vitamin in adults is 100 mg/day (41). Vitamin B6 deficiency affects innate and acquired immune responses, but physiological functions can be restored by standard supplementation with the cofactor, while larger doses were not more beneficial (42). Accordingly, increasing dietary vitamin B6 supplementation beyond sufficient doses did not influence either the protective efficacy of BCG and BCG ΔureC::hly in a murine challenge model or the course of M. tuberculosis infection per se in naive mice (see Fig. S2 and S3 in the supplemental material). Therefore, the early protection induced by BCG ΔureC::hly Δpdx1 consistently depended on the persistence of the construct under the control of vitamin B6 supplementation (Fig. 4B). Mice immunized with the auxotrophic strain in a homologous prime-boost vaccination scheme (Fig. 4A) maintained the vitamin B6-related differences but revealed improved protection, to a degree similar to that of parental BCG ΔureC::hly 30 days postchallenge with M. tuberculosis Beijing/W (Fig. 5A and B). Whether protection could be further improved by a second booster vaccination remains the topic of future studies.
Altogether, we demonstrate for the first time that a nutritional supplement, namely, the small-molecule vitamin B6, controls the persistence of a recombinant BCG auxotroph in vivo. Most importantly, this translated into vitamin B6 dependency of acquired early immune protection in response to the auxotrophic construct. This principle will enable the development of safer BCG vaccines that are urgently needed for the growing proportion of immunocompromised individuals among vaccinees.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
BCG (BCG SSI 1331; ATCC 35733; American Type Culture Collection), BCG ΔureC::hly (BCG Prague background) (22, 23), M. tuberculosis H37Rv (ATCC 27294), and Beijing/W (RIVM 17919, isolated in Mongolia) were maintained in Middlebrook 7H9 medium (Becton, Dickinson) supplemented with 0.2% glycerol, 0.05% Tween 80, 10% albumin-dextrose-catalase supplement (Becton, Dickinson), in Sauton’s minimal medium (0.5 g/liter KH2PO4, 0.5 MgSO4, 2 g/liter citric acid, 0.05 g/liter ferric ammonium citrate, 4 g/liter asparagine, 6% glycerol, 0.05% Tween 80, pH 6.8), or on Middlebrook 7H11 agar (Becton, Dickinson) containing 10% (vol/vol) oleic acid-albumin-dextrose-catalase enrichment (Becton, Dickinson) and 0.2% glycerol. Mycobacterial cultures were grown to mid-log phase in 1-liter roller bottles (450 cm2) at 37°C and 2 rpm. For in vitro growth studies, mycobacteria were grown in Sauton’s minimal medium supplemented with 5 µM pyridoxine. Cultures were pelleted at 3,200 rpm and washed three times prior to resuspension in fresh Sauton’s medium with or without pyridoxine. The optical density at 600 nm (OD600) was determined daily. For vaccine stock preparations, bacilli were collected by centrifugation at 3,200 rpm, washed with phosphate-buffered saline (PBS), and stored at −80°C as a suspension in PBS--10% glycerol. Prior to vaccination, vials were thawed and cells harvested and resuspended in an appropriate volume of PBS. For CFU enumeration, serial dilutions were performed in PBS--0.05% Tween 80 (PBST) and plated on Middlebrook 7H11 agar. The plates were incubated at 37°C for 3 to 4 weeks prior to CFU counting.
Generation of BCG ΔureC::hly Δpdx1 and genetic complementation.
The pdx1 gene of BCG ΔureC::hly was disrupted as described earlier for M. tuberculosis (15). Briefly, 1-kb fragments flanking pdx1 were amplified by PCR using the specific oligonucleotides ko5′pdx1.fwd (5′ TACTTAAGCGGGTCAGCGGGCATTCC 3′)/ko5′pdx1.rev (5′ ATTCTAGACCGGGGTGACAACGTCCATGAT 3′) and ko3′pdx1.fwd (5′ TAAAGCTTTGTGCTGGCCAAGGTGTCG 3′)/ko3′pdx1.rev (5′ ATACTAGTCGACCCGTGGAACGCTCACAG 3′) (restriction sites are underlined) and inserted into pYUB854 (43). The knockout plasmid was then electroporated into BCG ΔureC::hly, and transformants were selected on Middlebrook 7H11 agar (contains vitamin B6) supplemented with 80 µg/ml hygromycin B (Roche). The hygromycin resistance cassette was subsequently removed by standard methods described previously (44). Site-directed mutagenesis and selection marker removal were confirmed by automated sequencing of the pdx1 region. For genetic complementation, pdx1, including its putative promoter, was amplified by PCR (comppdx1.fwd [5′ GCTGGTACCAGGGAAAGGTTGCCGATG 3′] and comppdx1.rev [5′ GCTGGTACCAGGGAAAGGTTGCCGATG 3′]) and inserted into the integrative vector pMV306 (45). BCG ΔureC::hly Δpdx1 was then electroporated with the complementing vector, and transformants were selected on Middlebrook 7H11 agar containing 25 µg/ml kanamycin (Sigma-Aldrich).
Multiplex cytokine assays.
The Bio-Rad mouse cytokine 23-plex panel was used for analysis of cytokines in sera of vaccinated mice. In all multiplex assays, the volume of the coupled beads, detection antibodies, and streptavidin-phycoerythrin (PE) conjugate was halved and topped up with the appropriate buffer. The assays were otherwise performed according to the manufacturer’s instructions. The assay plates were read using a Bio-Plex 200 instrument (Bio-Rad).
Flow cytometry.
Cytokine-secreting antigen-specific T cells were enumerated as described previously (27). In brief, single-cell suspensions of splenocytes were cultured in complete RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 100 U/ml penicillin, 100 U/ml streptomycin, 2 mM l-glutamine, and 50 µM 2-mercaptoethanol (Sigma) for 14 h along with 10 µg/ml M. tuberculosis H37Rv whole-cell lysate in the presence of 2 µg/ml anti-CD40 blocking antibody (HM40-3; BioLegend) and 0.5 µg/ml PE-conjugated anti-CD154 (clone MR1; BioLegend). Ten micrograms/milliliter Brefeldin A (eBioscience) was added for the final 3 h of culture. After stimulation, cells were washed and stained on ice in PBS--2% FCS with fluorescent antibodies against cell surface markers CD4, CD3, CD8 (BD Biosciences), and CD62L (BioLegend), followed by fixation and permeabilization with the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Intracellular cytokines were detected with antibodies against IFN-γ and TNF-α (BD Biosciences). Cells were analyzed on an LSRII cytometer using Diva (Becton, Dickinson) and FlowJo software (Tree Star).
Enzyme-linked immunosorbent spot assay (ELISPOT).
Ninety-six-well multiscreen filtration plates (Millipore) were soaked with 30% ethanol and then rinsed and coated overnight at 4°C with 5 µg/ml polyclonal goat anti-mouse IgM+IgG+IgA (Millipore). The following day, the plates were washed and blocked with PBS--3% bovine serum albumin for 2 h at 37°C. Serial dilutions of single-cell suspensions of bone marrow were cultured on plates for 3 h at 37°C in medium prepared as described above. Cells were washed off, and the plates incubated with anti-mouse IgG-alkaline phosphatase (SouthernBiotech) for 1 h at 37°C. Finally, plates were washed with water before detection of IgG+ spots with 1-Step NBT-BCIP (nitro-blue tetrazolium chloride--5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt) substrate (Thermo Scientific).
Mouse strains and housing conditions.
Mice were 9 to 10 weeks old at the beginning of experiments. Female BALB/c (Janvier) were housed in groups of 5 or 6 in individually ventilated cages. Drinking water and food pellets (Ssniff R/M-H autoclavable, 0.032 mg pyridoxine per g) were offered ad libitum. Experimental groups that were maintained with vitamin B6-enriched nutrition received drinking water with 0.1 mg/ml pyridoxine hydrochloride (Sigma-Aldrich). Female SCID mice (CB-17/Icr-PrkdcSCID/Rj; Janvier) were kept under sterile conditions. All experimental procedures involving mice were approved by the State Office for Health and Social Services, Berlin, Germany (Landesamt für Gesundheit und Soziales Berlin, LAGeSo). Mice were sacrificed by cervical dislocation, and all efforts were made to minimize suffering and pain.
Dissemination, protective efficacy, and safety of BCG and derivatives in mice.
Mice were s.c. immunized in the tail base with 106 CFU BCG or recombinant derivative strains. At designated time points postvaccination, mice were sacrificed, and then a 1-cm2 skin portion at the side of injection and the dLNs, spleen, and lungs of each animal were aseptically removed and homogenized in PBS--0.05% Tween 80 prior to CFU enumeration. For protective efficacy studies, mice were aerosol challenged 90 days postvaccination with 100 to 200 CFU of M. tuberculosis. At designated time points, lungs and spleens were aseptically removed, homogenized in PBS--0.05% Tween 80, and plated in serial dilutions onto Middlebrook 7H11 agar for CFU enumeration. After vaccination, SCID mice did not receive further experimental manipulations. Weight was monitored weekly, and mice were euthanized when weight loss exceeded 20%.
SUPPLEMENTAL MATERIAL
Vitamin B6 biosynthesis operons of M. tuberculosis and BCG are identical. The genes pdx1 and pdx2 encode subunits of functional vitamin B6 synthase in mycobacteria. In the presence of free ammonia, Pdx1 alone has limited capacity to synthesize vitamin B6. The acyl-coenzyme A (CoA) thioesterase II (TesB2) has no direct association with vitamin B6 biosynthesis. Download
Vitamin B6 supplementation does not influence vaccine efficacy of BCG and BCG ΔureC::hly in mice. Effects of vitamin B6 on bacterial burdens in lungs (A) and spleen (B) after M. tuberculosis aerosol infection of mice that received only primary vaccination are shown. Experimental design is shown in Fig. 4A. Groups of mice received vitamin B6 (VB6)-enriched diet as indicated starting 2 weeks before vaccination until M. tuberculosis challenge. Shown are means ± SEM (n = 5 or 6) analyzed using one-way ANOVA and Tukey’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The experiment was performed twice. Download
Vitamin B6 supplementation does not influence M. tuberculosis infection in immunocompetent mice. Indicated groups received vitamin B6-enriched diet from 2 weeks prior to aerosol challenge of mice with 200 CFU M. tuberculosis H37Rv until the end of the experiment. Bacterial burdens of lungs and spleen were assessed over the course of TB infection. Differences between experimental groups were analyzed by Student’s t test (n = 5). A P value of >0.05 was considered statistically insignificant. Download
ACKNOWLEDGMENTS
We thank Mary Louise Grossman for excellent editorial support and Ulrike Zedler for technical assistance.
This work was funded by the European Research Council’s 7th Framework Program (FP7), NEWTBVAC (grant no. HEALTH-F3-2009-241745).
Footnotes
Citation Gengenbacher M, Vogelzang A, Schuerer S, Lazar D, Kaiser P, Kaufmann SHE. 2014. Dietary pyridoxine controls efficacy of vitamin B6-auxotrophic tuberculosis vaccine bacillus Calmette-Guérin ΔureC::hly Δpdx1 in mice. mBio 5(3):e01262-14. doi:10.1128/mBio.01262-14.
REFERENCES
- 1. Kaufmann SH. 2007. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat. Rev. Microbiol. 5:491–504. 10.1038/nrmicro1688 [DOI] [PubMed] [Google Scholar]
- 2. Calmette A, Guérin C, Boquet A, Négre L. 1927. La vaccination préventive contre la tuberculose par le “BCG.” Masson, Paris, France [Google Scholar]
- 3. Kaufmann SH, Gengenbacher M. 2012. Recombinant live vaccine candidates against tuberculosis. Curr. Opin. Biotechnol. 23:900–907. 10.1016/j.copbio.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 4. WHO 2013. Global tuberculosis report 2013. WHO, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 5. Bardarov S, Kriakov J, Carriere C, Yu S, Vaamonde C, McAdam RA, Bloom BR, Hatfull GF, Jacobs WR., Jr. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10961–10966. 10.1073/pnas.94.20.10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelicic V, Jackson M, Reyrat JM, Jacobs WR, Jr, Gicquel B, Guilhot C. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10955–10960. 10.1073/pnas.94.20.10955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guleria I, Teitelbaum R, McAdam RA, Kalpana G, Jacobs WR, Jr, Bloom BR. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334–337. 10.1038/nm0396-334 [DOI] [PubMed] [Google Scholar]
- 8. Jackson M, Phalen SW, Lagranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray DN, Gicquel B, Guilhot C. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR., Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171–1174. 10.1038/nm765 [DOI] [PubMed] [Google Scholar]
- 10. Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Jr, Bloom BR, Hondalus MK. 2004. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect. Immun. 72:3031–3037. 10.1128/IAI.72.5.3031-3037.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR, Jr, Bloom BR. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888–2898. 10.1128/IAI.68.5.2888-2898.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pavelka MS, Jr, Chen B, Kelley CL, Collins FM, Jacobs WR., Jr. 2003. Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 71:4190–4192. 10.1128/IAI.71.7.4190-4192.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR., Jr. 2005. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect. Immun. 73:1196–1203. 10.1128/IAI.73.2.1196-1203.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sampson SL, Mansfield KG, Carville A, Magee DM, Quitugua T, Howerth EW, Bloom BR, Hondalus MK. 2011. Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate. Vaccine 29:4839–4847. 10.1016/j.vaccine.2011.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dick T, Manjunatha U, Kappes B, Gengenbacher M. 2010. Vitamin B6 biosynthesis is essential for survival and virulence of Mycobacterium tuberculosis. Mol. Microbiol. 78:980–988. 10.1111/j.1365-2958.2010.07381.x [DOI] [PubMed] [Google Scholar]
- 16. Gengenbacher M, Fitzpatrick TB, Raschle T, Flicker K, Sinning I, Müller S, Macheroux P, Tews I, Kappes B. 2006. Vitamin B6 biosynthesis by the malaria parasite Plasmodium falciparum: biochemical and structural insights. J. Biol. Chem. 281:3633–3641. 10.1074/jbc.M508696200 [DOI] [PubMed] [Google Scholar]
- 17. Burns KE, Xiang Y, Kinsland CL, McLafferty FW, Begley TP. 2005. Reconstitution and biochemical characterization of a new pyridoxal-5'-phosphate biosynthetic pathway. J. Am. Chem. Soc. 127:3682–3683. 10.1021/ja042792t [DOI] [PubMed] [Google Scholar]
- 18. Kim S, Kim KJ. 2013. Crystal structure of Mycobacterium tuberculosis Rv2606c: a pyridoxal biosynthesis lyase. Biochem. Biophys. Res. Commun. 435:255–259. 10.1016/j.bbrc.2013.04.068 [DOI] [PubMed] [Google Scholar]
- 19. Neuwirth M, Strohmeier M, Windeisen V, Wallner S, Deller S, Rippe K, Sinning I, Macheroux P, Tews I. 2009. X-ray crystal structure of Saccharomyces cerevisiae Pdx1 provides insights into the oligomeric nature of PLP synthases. FEBS Lett. 583:2179–2186. 10.1016/j.febslet.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 20. Strohmeier M, Raschle T, Mazurkiewicz J, Rippe K, Sinning I, Fitzpatrick TB, Tews I. 2006. Structure of a bacterial pyridoxal 5′-phosphate synthase complex. Proc. Natl. Acad. Sci. U. S. A. 103:19284–19289. 10.1073/pnas.0604950103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guedez G, Hipp K, Windeisen V, Derrer B, Gengenbacher M, Bottcher B, Sinning I, Kappes B, Tews I. 2012. Assembly of the eukaryotic PLP-synthase complex from Plasmodium and activation of the Pdx1 enzyme. Structure 20:172–184. 10.1016/j.str.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 22. Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, Bancroft GJ, Reyrat JM, van Soolingen D, Raupach B, Kaufmann SH. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J. Clin. Invest. 115:2472–2479. 10.1172/JCI24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grode L, Ganoza CA, Brohm C, Weiner J, III, Eisele B, Kaufmann SH. 2013. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 31:1340–1348. 10.1016/j.vaccine.2012.12.053 [DOI] [PubMed] [Google Scholar]
- 24. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 25. Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C, Inwald JK, Golby P, Garcia JN, Hewinson RG, Behr MA, Quail MA, Churcher C, Barrell BG, Parkhill J, Cole ST. 2007. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. U. S. A. 104:5596–5601. 10.1073/pnas.0700869104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rao M, Vogelzang A, Kaiser P, Schuerer S, Kaufmann SH, Gengenbacher M. 2013. The tuberculosis vaccine candidate bacillus Calmette-Guerin ΔureC::hly coexpressing human interleukin-7 or -18 enhances antigen-specific T cell responses in mice. PLoS One 8:e78966. 10.1371/journal.pone.0078966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirchhoff D, Frentsch M, Leclerk P, Bumann D, Rausch S, Hartmann S, Thiel A, Scheffold A. 2007. Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur. J. Immunol. 37:2370–2377. 10.1002/eji.200737322 [DOI] [PubMed] [Google Scholar]
- 28. Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 8:843–849. 10.3201/eid0805.020002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufmann SH, Cotton MF, Eisele B, Gengenbacher M, Grode L, Hesseling AC, Walzl G. 2014. The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev. Vaccines. 13:613–630. 10.1586/14760584.2014.905746 [DOI] [PubMed] [Google Scholar]
- 30. Hingley-Wilson SM, Sambandamurthy VK, Jacobs WR., Jr. 2003. Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 4:949–955. 10.1038/ni981 [DOI] [PubMed] [Google Scholar]
- 31. Gengenbacher M, Kaufmann SH. 2012. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol. Rev. 36:514–532. 10.1111/j.1574-6976.2012.00331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houben D, Demangel C, van Ingen J, Perez J, Baldeon L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, Peters PJ. 2012. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 14:1287–1298. 10.1111/j.1462-5822.2012.01799.x [DOI] [PubMed] [Google Scholar]
- 33. van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298. 10.1016/j.cell.2007.05.059 [DOI] [PubMed] [Google Scholar]
- 34. Berdanier CD. 1998. Vitamin B6, p 99–105 In Advanced nutrition: micronutrients. CRC Press, Boca Raton, FL [Google Scholar]
- 35. Kleeman A, Engel J, Kutscher B, Reichert D. 2001. Pharmaceutical substances—syntheses, patents, applications. Thieme Medical Publishers, Stuttgart, Germany [Google Scholar]
- 36. Cooper AM. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393–422. 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersen P, Smedegaard B. 2000. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect. Immun. 68:621–629. 10.1128/IAI.68.2.621-629.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 39. Bor MV, Refsum H, Bisp MR, Bleie O, Schneede J, Nordrehaug JE, Ueland PM, Nygard OK, Nexø E. 2003. Plasma vitamin B6 vitamers before and after oral vitamin B6 treatment: a randomized placebo-controlled study. Clin. Chem. 49:155–161. 10.1373/49.1.155 [DOI] [PubMed] [Google Scholar]
- 40. Coburn SP, Ziegler PJ, Costill DL, Mahuren JD, Fink WJ, Schaltenbrand WE, Pauly TA, Pearson DR, Conn PS, Guilarte TR. 1991. Response of vitamin B6 content of muscle to changes in vitamin B6 intake in men. Am. J. Clin. Nutr. 53:1436–1442 [DOI] [PubMed] [Google Scholar]
- 41. Institute of Medicine 1998. Vitamin B6, p 150–195 In Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline.National Acadamies Press, Washington, DC: http://www.nap.edu/catalog.php?record_id=6015 [PubMed] [Google Scholar]
- 42. Rall LC, Meydani SN. 1993. Vitamin B6 and immune competence. Nutr. Rev. 51:217–225 [DOI] [PubMed] [Google Scholar]
- 43. Bardarov S, Bardarov S, Jr, Pavelka MS, Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR., Jr 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007–3017 [DOI] [PubMed] [Google Scholar]
- 44. Malaga W, Perez E, Guilhot C. 2003. Production of unmarked mutations in mycobacteria using site-specific recombination. FEMS Microbiol. Lett. 219:261–268. 10.1016/S0378-1097(03)00003-X [DOI] [PubMed] [Google Scholar]
- 45. Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs WR, Jr, Bloom BR. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460. 10.1038/351456a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vitamin B6 biosynthesis operons of M. tuberculosis and BCG are identical. The genes pdx1 and pdx2 encode subunits of functional vitamin B6 synthase in mycobacteria. In the presence of free ammonia, Pdx1 alone has limited capacity to synthesize vitamin B6. The acyl-coenzyme A (CoA) thioesterase II (TesB2) has no direct association with vitamin B6 biosynthesis. Download
Vitamin B6 supplementation does not influence vaccine efficacy of BCG and BCG ΔureC::hly in mice. Effects of vitamin B6 on bacterial burdens in lungs (A) and spleen (B) after M. tuberculosis aerosol infection of mice that received only primary vaccination are shown. Experimental design is shown in Fig. 4A. Groups of mice received vitamin B6 (VB6)-enriched diet as indicated starting 2 weeks before vaccination until M. tuberculosis challenge. Shown are means ± SEM (n = 5 or 6) analyzed using one-way ANOVA and Tukey’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The experiment was performed twice. Download
Vitamin B6 supplementation does not influence M. tuberculosis infection in immunocompetent mice. Indicated groups received vitamin B6-enriched diet from 2 weeks prior to aerosol challenge of mice with 200 CFU M. tuberculosis H37Rv until the end of the experiment. Bacterial burdens of lungs and spleen were assessed over the course of TB infection. Differences between experimental groups were analyzed by Student’s t test (n = 5). A P value of >0.05 was considered statistically insignificant. Download