Abstract
The human immunodeficiency virus type-1 (HIV-1) integrase enzyme has recently emerged as a primary alternative target to block viral replication, and integrase strand transfer inhibitors (INSTIs) are now considered an alternative ‘third agent’ class of antiretroviral (ARV) drugs. Dolutegravir is the first next-generation INSTI showing some novel and intriguing characteristics: it has a favorable pharmacokinetic profile with a prolonged intracellular halflife, rendering feasible a once daily dosing without the need for pharmacokinetic boosting. Secondly, it is largely metabolized via uridine diphosphate glucuronosyltransferase-1A1 with a minor component of cytochrome P450 isoforms, thus allowing a low grade of drug–drug interactions, so that its metabolic profile consents co-administration with the majority of the other ARV drugs without dose adjustments. Lastly, but no less important, virological studies have clearly demonstrated that dolutegravir has a significant activity against HIV-1 isolates showing raltegravir and/or elvitegravir associated resistance mutations. The attributes of once daily administration and the potential to treat INSTI-resistant viruses make dolutegravir an interesting and promising new agent in the treatment of both naïve and experienced HIV-1 subjects. In this review, the main concerns on dolutegravir efficacy are focused through the analysis of the currently available data from clinical studies in naïve and experienced patients, evaluating its possible place within the anti-HIV-1 drug armamentarium. The development of newer once daily, single tablet coformulations improved drug adherence and maximized the success of ARV therapy. Pharmacokinetic studies and dose-ranging trials suggested that dolutegravir is a good candidate for a single tablet regimen in one or more new coformulated pills that will be available in the near future.
Keywords: antiretroviral drugs, dolutegravir, HIV-1, integrase inhibitors, once-daily dosing
Introduction
The significant advances in the treatment of human immunodeficiency virus type-1 (HIV-1) infection during the past 15 years have led to a dramatic reduction in HIV-1 related morbidity and mortality. The potency, tolerability and convenience of the latest antiretroviral (ARV) agents have considerably improved and made easier the lifelong treatment of HIV-1. Despite the success of existing therapies in controlling viral replication and preventing disease progression, ARV therapies are not curative, thus remaining a permanent commitment for HIV-1 infected patients [Palella et al. 1998; Antiretroviral Therapy Cohort Collaboration 2008; van Sighem et al. 2010]. Moreover, long-term positive effects of combined ARV treatments (cART) are often complicated by the occurrence of drug resistance (mainly in nonadherent subjects) and/or drug-related side effects and metabolic toxicities. There is a need for simplified regimens that provide a lower pill burden, a reduced dose frequency and a more favorable safety profile [Juday et al. 2011].
There are five classes of drugs that fight against HIV-1 infection (Table 1). Each class has a name that comes from the mechanism of action against the virus: nucleos(t)ide reverse transcriptase inhibitors [N(t)RTIs]; non-nucleoside reverse transcriptase inhibitors (NNRTIs); protease inhibitors (PIs); entry inhibitors and antagonists of the CCR5 chemokine receptor; and integrase strand transfer inhibitors (INSTIs). The standard of care for treatment of HIV-1 infection involves the use of a combination of at least three ART drugs belonging to different classes [Panel on Antiretroviral Guidelines for Adults and Adolescents, 2013; EACS, 2013]. Coformulated options, and even more, once-daily single tablet regimens represent the best cART simplification achieved so far (Table 2). They include drugs with favorable pharmacokinetics that allow once-daily administration, that do not need dose adjustments, have no additional toxicities, and do not require dissimilar intake conditions [Llibre and Clotet, 2012].
Table 1.
List of the currently used antiretroviral drugs and marketed coformulations.
| NRTIs | NNRTIs | PIs | INSTIs | Entry inhibitors | Coformulated options |
|---|---|---|---|---|---|
| Zidovudine, ZDV | Efavirenz, EFV | Indinavir, IDV | Raltegravir, RAL | Enfuvirtide (T-20) | ZDV/3TC |
| Lamivudine, 3TC | Nevirapine, NVP | Saquinavir, SQV | Elvitegravir, EVG | Maraviroc, MVC | ZDV/3TC/ABC |
| Abacavir, ABC | Etravirine etR | Nelfinavir, NFV | (as coformulation only) | LPV/r | |
| Tenofovir, TDF | Rilpivirine, RPV | Fosamprenavir, FPV | Dolutegravir, DTG | ABC/3TC | |
| Emtricitabine, FTC | Delavirdine, DLV | Lopinavir, LPV | TDF/FTC | ||
| Didanosine, ddI | (no longer used) | Atazanavir, ATV | TDF/FTC/EFV | ||
| Stavudine, d4T | Darunavir, DRV | TDF/FTC/RPV | |||
| Zalcitabine, ddC | Ritonavir, RTV | TDF/FTC/EVG/COBI | |||
| (no longer used) | (as booster only) |
COBI, cobicistat; INSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleos(t)ide reverse transcriptase inhibitors; PIs, protease inhibitors.
Table 2.
Recommended first-line regimens: DHHS and EACS guidelines update, October 2013.
| DHHS |
EACS |
||
|---|---|---|---|
| Preferred regimens | Alternative regimens | Recommended regimens | |
| NNRTI | EFV/TDF/FTC | EFV + ABC/3TC | EFV or RPV + ABC/3TC or TDF/FTC |
| RPV/TDF/FTC or | |||
| RPV + ABC/3TC | |||
| Boosted PI | ATV/r + TDF/FTC | ATV/r + ABC/3TC | ATV/r or DRV/r + ABC/3TC or TDF/FTC |
| DRV/r + TDF/FTC | DRV/r + ABC/3TC | ||
| FPV/r + (TDF/FTC or ABC/3TC) | |||
| LPV/r + (TDF/FTC or ABC/3TC) | |||
| INSTI | RAL + TDF/FTC | RAL + ABC/3TC | RAL + TDF/FTC or ABC/3TC |
| EVG/COBI/TDF/FTC | |||
| DTG + ABC/3TC | |||
| DTG + TDF/FTC | |||
3TC, lamivudine; ABC, abacavir; ATV, Atazanavir; COBI, cobicistat; DHHS, Department of Health and Human Services; DTG, dolutegravir; DRV, darunavir; EACS, European AIDS Conference Society; EFV, efavirenz; EVG, elvitegravir; FPV, fosamprenavir; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; NNRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; r, ritonavir; RPV, rilpivirine; LPV, lopinavir; RAL, raltegravir; TDF, tenofovir di-fumarate.
N(t)RTIs have traditionally been an important ‘backbone’ of almost all ART regimens. However, concerns about long-term toxicities and a crossresistance pattern within the N(t)RTI class combined with the continuing development of newer, apparently safer agents of different classes has led to an increasing interest in the potential use of feasible, innovative and more appealing N(t)RTI-sparing options [Achhra and Boyd, 2013]. PIs constitute an important component of cART in the light of their potency and higher genetic barrier that they impose against the selection of drug resistance variants [Kempf et al. 1997; De Meyer et al. 2005]. PIs are the only class of ARV drugs that have been used as monotherapy and shown to be not inferior to cART regimens in maintaining suppression of viral replication [Bierman et al. 2009; Perez-Valero and Arribas, 2011].
INSTIs are a new class of ARV drugs designed to block the action of the integrase viral enzyme, which catalyzes several key steps in the HIV-1 lifecycle and is responsible for insertion of the viral genome into the DNA of the host. Because integration is a crucial step in retrovirus replication machinery, the viral enzyme has become an attractive molecule for the treatment of HIV-1 infected patents [Reinke et al. 2002; Pommier et al. 2005]. Inhibitors of this enzyme represent the new class of ARV agents available in our armamentarium to treat HIV-1 infection [Hazuda et al. 2000].
Raltegravir (RAL) was the first drug of the INSTI class approved by the US Food and Drug Administration (FDA) in 2007; it is a potent and well-tolerated antiviral agent. When combined with other active agents, it has demonstrated similar virological efficacy up to 240 weeks to the combination of efavirenz (EFV), tenofovir (TDF) and emtricitabine (FTC) in treatment-naïve patients [Markowitz et al. 2011; Rockstroh et al. 2013]. However, RAL has the limitations of twice-daily dosing and a relatively modest genetic barrier to the development of resistance. Another first-generation INSTI is elvitegravir (EVG), available in a single tablet regimen and dosed once daily when administered with ritonavir (RTV) or the pharmacokinetic booster cobicistat (COBI), a potent CYP3A4 inhibitor that can lead to clinically significant drug–drug interactions. Also this drug shows a low genetic barrier as RAL, with an overlapping resistance profile. Following the results of larger studies comparing a fixed-dose formulation consisting of EVG/COBI/FTC/TDF versus a EFV/TDF/FTC single tablet regimen or a once-daily RTV-boosted atazanavir (ATZ) plus FTC/TDF, the new single tablet EVG/COBI/FTC/TDF (Stribild®) is available in several countries for the once-daily treatment of HIV-1 infection in ARV therapy-naïve adults [Perry, 2014].
Both RAL and EVG are now guideline-preferred agents as part of an ARV regimen for treatment-naïve patients. However, the above-mentioned proprieties of RAL and EVG have prompted the search for new agents with once-daily dosing, a high genetic barrier and a resistance profile of limited overlap with the respect of the first-generation INSTIs [Karmon and Markowitz, 2013].
Dolutegravir (DTG, S/GSK1349572) is a new (next-generation) drug in this class that offers some novel and intriguing characteristics: it has a favorable pharmacokinetic profile with a prolonged intracellular halflife, rendering feasible a once-daily dosing without needs of pharmacokinetic boosting and without regard to meal. It also offers a favorable resistance profile showing a higher genetic barrier to resistance compared to the other INSTIs. Table 3 summarizes the main characteristics of the currently available INSTIs.
Table 3.
Main characteristics of INSTIs currently used in clinical practice.
| Recommended dose | Metabolism | Advantages | Disadvantages | |
|---|---|---|---|---|
| RAL | 400 mg BID | UGT1A1-mediated glucuronidation |
|
|
| EVG | 150 mg QD + booster (100 mg ritonavir or cobicistat) to be taken with meals | Predominantly cytochrome P450 (CYP3A4) metabolized, minor pathways via UGT1A1/3 glucuronidation and oxidative metabolism |
|
|
| DTG | 50 mg QD in INSTI-naïve patients, 50 mg BD in INSTI-experienced patients | Predominantly UGT1A1-mediated glucuronidation, cytochrome P450 (CYP3A4) metabolisation as minor pathway |
|
|
BID, twice daily; CNS, central nervous system; COBI, cobicistat; DTG, dolutegravir; eGFR, estimated glomerular filtration rate; EVG, elvitegravir; FDC, fixed dose combination; INSTI, integrase strand transfer inhibitor; QD, once daily; RAL, raltegravir; UGT, uridine diphosphate glucuronosyl-transferase.
The primary route of DTG metabolism is its glucuronidation via uridine diphosphate (UDP) glucuronosyl-transferase (UGT) 1A1, without a significant induction or inhibition of cytochrome P450 (CYP) isoenzymes [Min et al. 2010]. DTG has a terminal elimination halflife of 13–14 h and maintains concentrations over the in vitro, protein-adjusted IC90 for more than 30 h following a single dose. DTG exhibits rapid absorption, with a median time to the maximum plasma concentration (tmax) ranging from 0.5 to 2 h. DTG also displays extensive protein binding, with 99% of the DTG blood plasma concentrations being bound to albumin and α1-acid glycoprotein (AAG) [Cottrell et al. 2013; Underwood et al. 2012; Canducci et al. 2011]. DTG exhibits lower intersubject pharmacokinetic variability than other integrase inhibitors. DTG is a substrate for the transporters P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP), but does not demonstrate inhibition or induction of the transporters Pgp, BCRP, organic anion transporter (OAT)-P1B1, OATP1B3, multidrug resistance protein (MRP)-2 or cation transporter transporter (OCT)-1 at clinically relevant concentrations. DTG potently inhibits the renal organic OCT2 at concentrations below the peak concentrations demonstrated in clinical trials. DTG use over 48 weeks of therapy does not appear to impact renal function, although the long-term effects of DTG on renal function are still unknown [Reese et al. 2013]. DTG absorption is modestly affected by the fat content of a meal. Phase II and III investigations to date have not employed food restrictions for DTG dosing. Only a small proportion of the drug dose (<1%) is excreted unchanged in the urine and, therefore, DTG is not expected to require dose adjustments in subjects with renal impairment [Min et al. 2011].
INSTI resistance pattern
Drug resistance mutations have been reported for all currently approved anti-HIV drugs, including the latest INSTIs. Resistance to INSTIs occurs with a single point mutation within the integrase gene. The three most common RAL mutations (N155H, Q148H/K/R and Y143C/H/R) are associated with virological failure and reduced susceptibility to RAL [Cooper et al. 2008]. If the virus harbors one or more of these mutations, crossresistance between RAL and EVG can occur.
The next-generation INSTI DTG shows a more robust resistance profile than RAL and EVG. An in vitro study demonstrated that the highest genetic barrier of DTG may be attributed to its significantly slower rate of dissociation from the integrase enzyme in viruses that are either wildtype or contain the N155, Q148 or Y143 mutations [Hightower et al. 2011]. Furthermore, DTG may undergo slight conformational change at the active site to overcome the physical barrier created by these single point mutations [Hare et al. 2011]. These properties are probably responsible for the effectiveness of DTG against most RAL/EVG resistant strains, although viruses containing E138K, G140S or Q148H mutations had lower susceptibility [Quashie et al. 2012b]. Exposure to DTG in selection studies can cause changes in the viral genome at positions E92, L101, T124, S153 and G193 [Kobayashi et al. 2011]. However, susceptibility fold changes are moderate (<2.5) for all these substitutions. Although no major resistance mutations against DTG have been identified thus far, the accumulation of multiple mutations is required to result in a fold change >10. In vitro selection studies revealed R263K, followed by H51Y, as the most common mutation to emerge. Further analyses showed that R263K did confer low-level resistance to DTG in culture, with an approximate 20–30% loss in viral replication fitness. H51Y alone did not significantly affect either strand transfer activity or resistance. The presence of both mutations increased levels of resistance to DTG, but this combination rarely emerged due to severe attenuation of both viral replicative capacity and integrase strand transfer activity compared with the presence of R263K alone [Quashie et al. 2012a; Mesplede et al. 2012]. Recently, biochemical and structural data reported that the G118R substitution caused low-level resistance to DTG (3.1-fold), and the addition of H51Y to G118R did not significantly increase the level of resistance (3.4-fold). The combinations of G118R together with multiple other substitutions might result in an enzyme that most likely would be catalytically defective [Quashie et al. 2013]. Furthermore, DTG-resistant viruses containing either the R263K or G118R and/or H51Y mutations were unable to develop further resistance mutations against several reverse transcriptase inhibitors during in vitro selection (i.e. nevirapine and lamivudine). These findings may explain the fact that no individual has yet progressed to virological failure with DTG resistance mutations in clinical trials in which patients received the drug together with an optimized background regimen [Oliveira et al. 2014]. In cell cultures, the emergence of the resistance mutation R263K followed by the polymorphic substitution M50I has been observed. This polymorphism has also been described in 10–25% of patients naïve to INSTIs and in one subject (in combination with the R263K mutation) failing a RAL-based regimen. The M50I polymorphism in combination with R263K increases resistance to DTG in tissue culture and in biochemical assays, but does not restore the diminished viral fitness associated with the R263K mutation. This combination results in a virus with limited crossresistance, so the R263K resistance pathway may represent an evolutionary dead end. According to these in vitro findings, it may be more advantageous to use DTG in a first-line strategy rather than in a more advanced setting [Wares et al. 2014].
Clinical efficacy of DTG
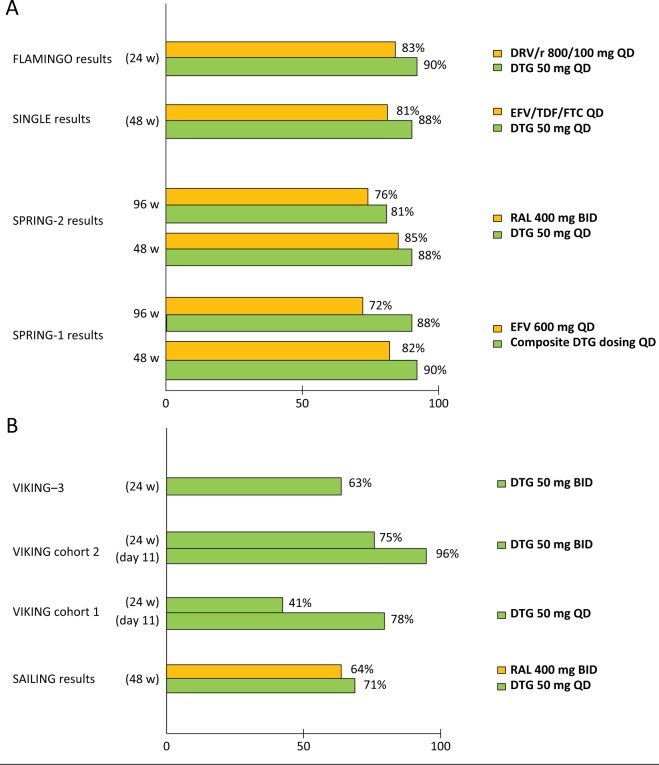
The virological outcomes of the main DTG studies reported are detailed in Figure 1.
Figure 1.
Virological response rates from DTG studies in naïve (A) and experienced (B) HIV-1 patients.
BID, twice daily; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; QD, once daily; RAL, raltegravir; TDF, tenofovir.
Results from studies in naïve subjects
SPRING-1 was a 96-week, randomized, partially blinded, phase IIb dose-ranging study to evaluate the efficacy and safety/tolerability of DTG. Treatment-naïve HIV-1 infected patients were randomly assigned to receive DTG 10, 25, or 50 mg once daily or EFV 600 mg once daily (control arm) combined with investigator-selected dual NRTI backbone regimen (TDF/FTC or ABC/3TC). The primary endpoint was the proportion of subjects with plasma HIV-1 RNA <50 copies/ml, based on time to loss of virological response at 16 weeks (conducted for the purpose of phase III dosing selection), with a planned analysis at 96 weeks. Briefly, 205 patients received study drug and the rate of plasma HIV-1 RNA <50 copies/ml at week 96 was 79%, 78%, and 88% for subjects on DTG (10, 25, and 50 mg, respectively), compared with 72% of those on EFV. The median increase from baseline in CD4+ T cells was 338 cells/µl with DTG (all treatment groups combined) compared with 301 cells/µl in EFV group. No clinically significant dose-related trends in adverse events were observed and fewer participants who received DTG withdrew because of adverse events (3%) compared with EFV group (10%). Nausea and headache occurred more frequently with DTG, whereas dizziness, rash, insomnia and fatigue were more common in EFV arm. Consistent with the findings observed, DTG demonstrated slightly more rapid and sustained virological suppression when compared with EFV [van Lunzen et al. 2012; Stellbrink et al. 2013].
In another study (SPRING-2) DTG was compared to RAL in a 96 week, phase III, randomized, double-blind, active-controlled, noninferiority study in treatment-naïve adults with HIV-1 RNA levels ≥1000 copies/ml; enrolled subjects were randomly assigned (1:1) to receive either DTG (50 mg once daily) or RAL (400 mg twice daily). Randomization was stratified by screening HIV-1 RNA plasma levels (> or ≤100,000 copies/ml) and NRTI backbone [TDF/FTC or abacavir (ABC)/lamivudine (3TC)]. A total of 822 subjects received at least one dose of the study drug (411 patients in each group). At week 96, 332 (81%) patients in the DTG group and 314 (76%) patients in the RAL group had HIV-1 RNA <50 copies/ml, thus confirming the DTG noninferiority versus RAL. Virological nonresponse occurred less frequently in the DTG group [22 (5%) patients for DTG versus 43 ([10%)] patients for RAL]. Within treatment groups, virological nonresponse was similar for ABC/3TC and TDF/FTC. Median increases in CD4+ T cell count from baseline were similar between groups (276 cells/μl for DTG and 264 cells/μl for RAL). At virological failure, no additional resistance to INIs or NRTIs was detected since week 48 or in any patient receiving DTG [Raffi et al. 2013b]. The tolerability and safety of DTG and RAL were similar in terms of frequency and nature of adverse events through 96 weeks. Patients receiving DTG had small mean increases in serum creatinine (grade 1–2) that were evident by week 2 and remained stable through week 96 [Raffi et al. 2013a]. Results from the SPRING-1 and 2 studies clearly show that once-daily DTG-based therapy is an attractive treatment option for HIV-1-infected treatment-naïve patients.
SINGLE was a 48-week, randomized, double-blind, phase III study on HIV-1 infected ART-naïve adults who had an HIV-1 RNA level ≥1000 copies/ml. Participants were randomly assigned to DTG 50 mg plus ABC/3TC once daily or fixed-dose combination therapy with EFV/TDF/FTC (Atripla®). The primary endpoint was the proportion of participants achieving HIV-1 RNA levels <50 copies/ml at week 48 [intention-to-treat analysis (ITT)]. Secondary endpoints included the time to viral suppression, the change from baseline in CD4+ T cell count, safety profile and viral resistance pattern. A total of 833 subjects received at least one dose of study drug. At week 48, the proportion of participants with an HIV-1 RNA level <50 copies/ml was significantly higher in the DTG plus ABC/3TC group than in the EFV/TDF/FTC group (88% versus 81%, p = 0.003), thus meeting the criterion for superiority. Overall differences in response were due primarily to discontinuations because of adverse events (2% in the DTG plus ABC/3TC group versus 10% in the EFV/TDF/FTC group). The difference in the treatment response in favor of DTG plus ABC/3TC was observed among participants indifferently from the baseline HIV-1 RNA levels (> or ≤100,000 copies/ml); treatment differences were also maintained across key demographic characteristic subgroups. Moreover, the DTG plus ABC/3TC arm presented a shorter median time to viral suppression than did the EFV/TDF/FTC group (28 versus 84 days, p < 0.001), as well as a greater increase in CD4+ T cell count (267 versus 208 cells/µl, p < 0.001). No subject in the DTG arm had detectable resistance mutations; one TDF-associated mutation and four EFV-associated mutations were detected in patients with virological failure in the EFV group [Walmsley et al. 2013].
Recently the FLAMINGO study results were reported. This is a randomized, multicenter, open-label, noninferiority study on HIV-1 infected ART-naïve adults with HIV-1 RNA ≥1000 copies/ml and no N(t)RTIs or PIs resistance mutations. Patients were randomized 1:1 to receive DTG 50 mg once daily or darunavir/ritonavir (DRV/r) (800/100 mg once daily with an investigator-selected fixed dose combination backbone (TDF/FTC or ABC/3TC). Randomization was stratified by HIV-1 RNA plasma levels (≤ or >100.000 copies/ml) and NRTI backbone. The primary endpoint was the proportion of patients reaching HIV-1 RNA <50 copies/ml through week 48 by FDA snapshot analysis. A total of 484 patients (242 in each arm) were treated. At week 48, 90% of DTG and 83% of DRV/r patients had HIV-1 RNA <50 copies/ml, thus demonstrating the superiority (p = 0.025) of once daily DTG according to a prespecified testing procedure. A more pronounced treatment difference occurred in individuals with higher baseline viral loads. In the DTG arm, no statistically significant differences were observed in terms of virological response according to the background dual NRTI strata. No treatment emergent genotypic resistance occurred in either arm at the time of virological failure. In terms of laboratory abnormalities, the serum creatinine in DTG recipients increased 0.1–0.2 mg/dl, attributable to DTG’s inhibition of renal tubular secretion of creatinine via OCT2. No other laboratory abnormalities of clinical significance were reported. Increases in fasting LDL were somewhat higher in the DRV/r group [Feinberg et al. 2013; Clotet et al. 2013].
These preliminary results reinforce the role of DTG as a new first-line option in the treatment of HIV-1 infected subjects. In all studies in which DTG has been compared with the currently approved standards of care (EFV, DRV/r and RAL), results have demonstrated virological efficacy rates comparable with each of these gold standard agents, with comparably low rates of adverse effects and (remarkably) no emergence of integrase resistance at failure reported.
Results from studies in experienced subjects
To assess whether DTG retained activity in the face of ARV resistance, different studies have been conducted: the SAILING study in ARV-experienced, INSTI-naïve subjects (versus RAL); and the VIKING studies in ARV-experienced subjects harboring a virus with RAL/EVG resistance patterns.
The SAILING study was a 48-week, phase III, randomized, double-blind, active-controlled, noninferiority study. Eligible patients were ARV-experienced and INI-naïve, had two consecutive plasma HIV-1 RNA assessments ≥400 copies/ml (unless those with ≥1000 copies/ml at screening), resistance to two or more classes of ARV drugs, but at least one to two fully active drugs for background therapy. Patients were randomly assigned (1:1) to once-daily DTG 50 mg or twice-daily RAL 400 mg, with investigator-selected background therapy. Analysis included 715 patients (354 in the DTG group and 361 in the RAL group). At week 48, 71% of patients on DTG arm had HIV-1 RNA <50 copies/ml versus 64% of those on RAL arm, with a significant virological superiority of DTG versus RAL (p = 0.03). Fewer patients had virological failure with treatment-emergent INSTI resistance on DTG (four versus 17 patients; p = 0.003). Once-daily DTG, in combination with up to two other ARV drugs, was well tolerated with a greater virological effect compared with twice-daily RAL in this selected treatment-experienced, INSTI-naïve patient group [Cahn et al. 2013].
The VIKING study (including cohorts 1 and 2) was a single-arm phase II trial that analyzed the feasibility of an INSTI salvage therapy by replacing RAL 400 mg twice daily with DTG 50 mg once or twice daily in two cohorts of HIV-1 infected patients failing their current ARV therapy due to the development of a RAL-resistant virus; 27 and 24 subjects were enrolled, with CD4+ T cell count <200 cells/μl and Centers for Disease Control and Prevention (CDC) Class C staging in 60%. The primary efficacy endpoint was the proportion of subjects on day 11 in whom the plasma HIV-1 RNA load decreased by ≥0.7 log10 copies/ml from baseline or was <400 copies/ml. VIKING participants in the first cohort began DTG 50 mg once daily for 10 days without other active drugs. After this period, the background regimens were optimized to include active drugs. A total of 78% of subjects achieved a viral load <400 copies/ml; the average decrease of HIV-1 RNA was 1.45 log10. The second VIKING cohort enrolled 24 subjects; after 10 days of DTG 50 mg twice daily, the background therapy was optimized including at least one active drug. The results showed that 96% of subjects in this second cohort had a viral load decrease to <400 copies/ml or a reduction of at least 0.7 log10 from their baseline values. At week 24, 41% and 75% of subjects had an HIV-1 RNA load of <50 copies/ml in cohorts I and II, respectively. Further integrase genotypic evolution was uncommon. DTG had a good, similar safety profile with each dosing regimen. These data are the first clinical demonstration of the activity of DTG in subjects with HIV-1 resistant to RAL. Based on these findings, DTG 50 mg twice-daily dose has been chosen for the phase III trials in HIV-1 experienced INI-resistant subjects [Eron et al. 2013].
VIKING-3 was a multicenter, open-label, single-arm study assessing the antiviral activity and safety of DTG 50 mg twice daily for 24 weeks in 183 ARV-experienced adults with historical or current evidence of resistance to RAL or EVG, with a HIV-1 RNA level >500 copies/ml. After 7 days of open-label DTG, subjects received an optimized background therapy along with the study drug. At baseline, 124 patients had current resistance to INSTIs and 59 showed historical resistance to the class; median CD4+ T cell count was 140 cells/μl, 13 years of prior ARV therapy exposure, and CDC Class C staging in 56%, 79% had >2 N(t)RTI, 75% >1 NNRTI and 70% >2 PI resistance-associated mutations. Non-R5 tropic virus was detected in 61% of patients. HIV-1 RNA load declined by a mean of 1.4 log10 copies/ml at day 8, whereas the proportion of subjects with HIV-1 RNA <50 copies/ml at week 24 by FDA snapshot analysis was 63%. Virological response varied according to the genotype pathway of INSTI resistance. In subjects with Q148 pathway mutations, the virological response decreased with increasing number of secondary mutations. Overall background susceptibility score (number of active drugs in the optimized background therapy) was not associated with week 24 response. DTG 50 mg twice daily had a low (3%) discontinuation rate due to adverse events, similar to INSTI-naïve subjects receiving DTG 50 mg once daily. DTG 50 mg twice daily was shown to be highly effective in this heavy treatment-experienced population with INSTI-resistant virus [Castagna et al. 2014].
VIKING-4 was a phase III, randomized, double-blind, placebo-controlled, superiority study. A total of 30 ART-experienced adults, with a screening resistance to RAL/EVG and to two or more other ART classes, were randomized to DTG 50 mg twice daily or placebo, while continuing their failing regimen (without RAL/EVG) up to day 7. From day 8, all patients received open-label DTG with an optimized background regimen. The day 8 antiviral activity and safety/tolerability data were presented at the European AIDS Clinical Society (EACS) meeting held in Brussels, Belgium, on 16–19 October 2013. Patients enrolled in this study were highly ARV-experienced with a 17 years’ prior median cART duration, comprising a median of 14 prior different cARTs. The proportion of Q148 viruses at baseline was higher in VIKING-4 DTG arm as well as plasma HIV-1 RNA load compared to those present in the placebo arm. The DTG activity reported was consistent with that observed in previous studies. The day 8 mean response was best for viruses without Q148 mutation: –1.43 log10 copies/ml (n = 5). Response for viruses with ‘Q148 + 1’ (9/14; 64%) was better than that in the VIKING-3 study (57/183; 31%). The DTG arm day 8 HIV-1 RNA mean change from baseline in subjects with ‘Q148+ ≥2’ mutations was lower (-0.9 log10 copies/ml). The most frequent adverse events were diarrhea, nausea and headache; five subjects developed a serious adverse event, all considered unrelated to study drug. Superior day 8 antiviral effect of DTG versus placebo confirms that antiviral activity was attributable to DTG and not to the failing regimen. DTG antiviral activity was consistent with the larger VIKING-3 pivotal phase III study results [Akil et al. 2013].
In all phase II and III trials, DTG has always demonstrated a favorable safety profile. Table 4 summarizes the more frequent adverse events reported. The majority of patients in each clinical trial experienced some adverse event during the course of treatment, with event rates ranging from 57 to 89%. Nausea, headache, diarrhea and sleep disturbances were among the most commonly reported adverse events, ranging from 5 to 23% of subjects. No dose-related patterns were identified. The majority of treatment-emergent adverse events were mild or moderate in nature. The frequencies of graded laboratory abnormalities were similar between all DTG treatment and comparator arms. Laboratory abnormalities reported in 1–5% of subjects included increased cholesterol, lipase, bilirubin, alanine aminotransferase/aspartate transaminase (ALT/AST), creatine phosphokinase (CPK) and prothrombin time, as well as decreased phosphorous and neutrophil count [Cottrell et al. 2013].
Table 4.
Summary of adverse events reported in phase II/III clinical trials of dolutegravir.
| Adverse event | SPRING-1: Composite DTG treatment groups, n = 155 (n, %) | SPRING-2: DTG arm, n = 411 (n, %) | SINGLE: DTG arm, n = 414 (n, %) | VIKING: cohorts I and II, DTG arm, n = 51 (n, %) | SAILING: DTG arm, III, n = 357 (n, %) | FLAMINGO: DTG arm, IIIb, n = 242 (n, %) |
|---|---|---|---|---|---|---|
| Nausea | 19 (12) | 59 (14) | 59 (14) | NR | 29 (8) | 39 (16) |
| Headache | 10 (6) | 51 (12) | 55 (13) | NR | 33 (9) | 37 (15) |
| Diarrhea | 12 (8) | 47 (11) | 72 (17) | 3 (6) | 71 (20) | 41 (17) |
| Nasopharyngitis | NR | 46 (11) | 62 (15) | NR | 23 (6) | NR |
| Dizziness | 5 (3) | 23 (6) | 37 (9) | NR | NR | NR |
| Sleep disturbances (insomnia, abnormal dreams, etc.) | 3 (2) | 21 (5) | 94 (23) | 3 (6) | NR | NR |
| Fatigue | 5(3) | 20 (5) | 54 (13) | NR | 15(4) | NR |
| Upper respiratory tract infection | NR | 26 (6) | 36 (9) | NR | 38(11) | NR |
| Pyrexia | NR | 20 (5) | NR | NR | NR | NR |
| Depression | NR | 21 (5) | 23 (6) | NR | NR | NR |
| Pharyngitis | NR | 14 (3) | NR | NR | NR | NR |
| Bronchitis | NR | 19 (5) | NR | 3(6) | NR | NR |
| Anxiety | NR | 14 (3) | 14 (3) | NR | NR | NR |
| Cough | NR | NR | NR | 3 (6) | 33 (9) | NR |
| Rash | 2 (1) | NR | 14 (3) | NR | 19 (5) | NR |
| Asthenia | 4 (3) | NR | NR | NR | NR | NR |
DTG, dolutegravir; NR, not reported.
Data from SPRING-2, SINGLE and VIKING-3 were published in 2012 and data from SAILING were announced in 2013: these four studies formed the basis of the registration package leading to the FDA approval of DTG (Tivicay®) on 12 August 2013. With a similar registration package, DTG was approved in Europe on 21 January 2014 by the European Medicines Agency (EMA). The drug joins RAL and EVG as a guideline-preferred agent for the management of HIV-1 infected treatment-naïve patients [Shah et al. 2013].
Perspectives
Over the past 15 years, knowledge of cART side effects has improved and new, convenient, supposedly less toxic, and more tolerable molecules have become available, both in the oldest and in the new ARV classes. However, there is a need for simplified regimens that provide a lower pill burden, a reduced dose frequency and a more favorable safety profile, all combined with higher genetic barrier against viral resistance. Coformulated options, and even more, once-daily single tablet regimens represent the best cART simplification achieved so far. The first single pill once-daily option for HIV-1 therapy was approved in 2006 (TDF/FTC/EFV, Atripla®). The second one followed in 2011 (TDF/FTC/RPV, Complera®). Another single pill once-daily regimen (EVG/COBI/TDF/FTC, Stribild®) was recently approved by the FDA (20 August 2012) [Johnson and Saravolatz, 2014].
During the last years, INSTIs entered into clinical practice. Like the previously summarized issues, what is the role of INSTIs, and in particular that of DTG, in these scenarios?
There are now several INSTIs available for initial therapy of HIV-1 infected persons. All are potent and well tolerated, with favorable metabolic profiles. RAL and DTG have relatively fewer drug–drug interactions compared with EVG. Transmitted resistance to INSTIs is currently low. Resistance seems to be less common with DTG. All drugs belonging to the INSTI class share an impressive, rapid virus load decline in both treatment-naïve and experienced patients; however, this characteristic does not seem to be associated with greater chance of long-term virological control when compared with other cART strategies, probably due to the relatively short period of use of INSTIs. DTG is the newest ARV drug and also the newest second-generation INSTI. The phase III clinical trials whose results are actually available compared DTG as first-line therapy in HIV-1 naïve subjects with the anchor drugs in the preferred regimens in each of the three classes. The SPRING-2 study was a double-blinded head-to-head comparison of DTG with RAL; the SINGLE study was a head-to-head double-blind study versus EFV; and the FLAMINGO study was an open-label head-to-head comparison with DRV/r. In all trials DTG has been always confirmed as not inferior (and sometimes superior) compared with the standards of care, both in terms of virological and immunological efficacy as well as in the safety profile.
Taken together, these studies suggest that once-daily 50 mg DTG, both in combination with either TDF/FTC or ABC/3TC and in the presence of HIV-1 RNA levels < or ≥ 100,000 copies/ml, is well tolerated and has sustained antiviral efficacy as initial therapy for the treatment of adults with HIV-1 infection. The drug is a valid alternative to the twice-daily RAL regimens. Furthermore, RAL and EVG regimens were not convenient since RAL required twice-daily dosing and EVG required food intake and pharmacological boosting. Studies in ARV-experienced patients, and in those harboring RAL/EVG resistant viruses, also show a high virological activity in this kind of challenging patient. In combination with up to two other ARV drugs, DTG was well tolerated with greater virological effects than RAL. All these findings may represent innovative changes in clinical practice and most likely in the near future may determine different approaches in the treatment of HIV-1 infected patients.
According with these studies, the main advantages of DTG are its safety and efficacy in both treatment-naïve and experienced patients, and its pharmacokinetic characteristics that allow once-daily administration independent from food, without boosting and with a low grade of drug–drug interactions. Furthermore, DTG exhibits an interesting resistance profile, probably due to the higher binding to the IN enzyme, when compared with RAL and EVG. As yet, in treatment-naïve patients we have not seen the emergence of resistance at virological failure, and the results of the VIKING studies confirm its high genetic barrier against RAL/EVG resistant virus, showing an impressive virological efficacy in the setting of ARV-experienced subjects. Some DTG disadvantages: it is not yet available as part of a fixed-dose combination and there is a creatinine effect with its use that should be monitored. DTG dosing, like COBI, is associated with a rise in serum creatinine of about 0.1–0.2 mg/dl in the first 2–4 weeks of treatment and tends to be stable over time. It is also due to inhibition of tubular secretion of creatinine, although the drug inhibits a different transporter than COBI. The overall effect however is similar. Pharmacokinetic studies and dose-ranging trials suggest DTG as a good candidate for a single-tablet regimen in a new coformulated pill. This possibility is actually under evaluation in a trial designed to explore the bioavailability of a fixed-dose pill containing DTG 50 mg/ABC 600 mg/3TC 300 mg [ClinicalTrials.gov identifier: NCT01366547].
The more rapid rate of viral attenuation seen with DTG may warrant consideration of this agent in clinical scenarios requiring rapid virological suppression, such as HIV-1 infected patients presenting with AIDS-defining illness or women presenting late in pregnancy. Furthermore, DTG characteristics make it a promising option in the treatment of HIV-1 infected organ transplant recipients [Waki and Sugawara, 2011]. The main aspects of a cART regimen in a HIV-1 infected transplant recipient should meet at least the following requirements: be potent with a high resistance barrier; have low toxicity profile and lack of interactions with immunosuppressive agents; no (or low) impact on graft function; and, finally, an easy dosing. DTG responds to all these conditions [Fantauzzi et al. 2013].
With the availability of DTG in anti-HIV-1 management, new cART strategies may require to be explored with ‘ad hoc’ trials to address several emerging issues. What is the potential for potent, tolerable and safe combination ARV regimens that do not need the inclusion of N(t)RTIs or pharmacokinetic boosting with RTV or COBI? Could dual combination therapies such as INSTIs (RAL or DTG) and NNRTIs or PIs, or other dual strategies, offer an effective, safe and durable alternative at the current ARV regimens? Several dual ARV combinations have been studied or are actually under investigation (i.e. LPV/r + RAL, boosted PIs + 3TC, DRV/r + RAL, DRV/r + ETR, DTG + RPV, etc.) in different clinical settings – as first-line or simplification/maintenance strategies as well as rescue approaches in multi-experienced patients. The results of these trials will probably change in the near future the therapeutic approach to HIV-1infected persons [Burgos et al. 2012; Calin et al. 2012; Di Giambenedetto et al. 2013; Katlama et al. 2010; Reynes et al. 2013]. With its high barrier to resistance, as emerged in the VIKING studies, in which DTG functional monotherapy acted as did DRV/r in the POWER trials [Clotet et al. 2007], what about DTG monotherapy? DTG has the potential to have a major effect in low-income and middle-income countries, where most HIV-1 infections exist. In these settings, the World Health Organization (WHO) now recommends for naïve patients a preferred single-tablet cART with EFV/TDF/3TC [WHO, 2013]. ARV combinations with newer drugs conferring proven efficacy at lower doses might provide not only attractive and more affordable alternatives but also greater tolerability and safety.
New ARV therapeutic strategies are in development. In this perspective, long-acting ARV drugs may improve adherence and extend opportunities for therapeutic or prophylactic interventions in different patient populations. Investigational long-acting injectable nanoformulations of RPV and GSK1265744 (a long-acting DTG analogue) are clinical stage development candidates. At present, phase I studies of the pharmacokinetics and safety of each long-acting formulations, alone and in combination, indicate that a monthly dosing regimen is possible for HIV-1 treatment. An ongoing phase IIb trial of oral GSK1265744 and oral RPV is evaluating this two-drug regimen for maintenance of virological suppression. Moreover, additional preclinical and clinical trials indicate a potential use of each agent for HIV-1 pre-exposure prophylaxis [Spreen et al. 2013].
In conclusion, DTG represents an interesting molecule, with the potential to improve the adherence of HIV-1 infected patients and increase the long-term tolerability of cART. With its potent activity, tolerability, ease of dosing and minimal drug interaction profile, DTG is poised to become one of the key components in the treatment of HIV-1 infection.
Acknowledgments
The work was supported by an unrestricted educational grant from ViiV Healthcare.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Alessandra Fantauzzi, Department of Clinical Medicine, Sapienza – University of Rome, Rome, Italy.
Ivano Mezzaroma, Department of Clinical Medicine, Sapienza – University of Rome, Viale dell’Università 37, Rome, 00185, Italy.
References
- Achhra A., Boyd M. (2013) Antiretroviral regimens sparing agents from the nucleoside(tide) reverse transcriptase inhibitor class: a review of the recent literature. AIDS Res Ther 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil B., Blick G., Hagins D., Ramgopal M., Richmond G., Samuel R. (2013) Activity of dolutegravir (DTG) 50mg BID versus placebo (PBO) over 7 days of functional monotherapy in patients harbouring raltegravir and/or elvitegravir resistance virus: primary endpoint results of the VIKING-4 study (ING116529) In: Programme and Abstracts of the 14th European AIDS Conference, October, Brussels, abstract PE7/3. [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman W., van Agtmael M., Nijhuis M., Danner S., Boucher C. (2009) HIV monotherapy with ritonavir-boosted protease inhibitors: a systematic review. AIDS 10: 279–291 [DOI] [PubMed] [Google Scholar]
- Burgos J., Crespo M., Falcó V., Curran A., Navarro J., Imaz A., et al. (2012) Simplification to dual antiretroviral therapy including a ritonavir-boosted protease inhibitor in treatment-experienced HIV-1-infected patients. J Antimicrob Chemother 67: 2479–2486 [DOI] [PubMed] [Google Scholar]
- Cahn P., Pozniak A., Mingrone H., Shuldyakov A., Brites C., Andrade-Villanueva J., et al. (2013) Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naïve adults with HIV: week 48 results from the randomised double-blind, non-inferiority SAILING study. Lancet 382: 700–708 [DOI] [PubMed] [Google Scholar]
- Calin R., Paris L., Simon A., Peytavin G., Wirden M., Schneider L., et al. (2012) Dual raltegravir/etravirine combination in virologically suppressed HIV-1-infected patients on antiretroviral therapy. Antivir Ther 17: 1601–1604 [DOI] [PubMed] [Google Scholar]
- Canducci F., Ceresola E., Boeri E., Spagnuolo V., Cossarini F., Castagna A., et al. (2011) Cross-resistance profile of the novel integrase inhibitor dolutegravir (S/GSK1349572) using clonal viral variants selected in patients failing raltegravir. J Infect Dis 204: 1811–1815 [DOI] [PubMed] [Google Scholar]
- Castagna A., Maggiolo F., Penco G., Wright D., Mills A., Grossberg R., et al. (2014) Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 10.1093/infdis/jiu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet B., Bellos N., Molina J., Cooper D., Goffard J., Lazzarin A., et al. (2007) Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 369: 1169–1178 [DOI] [PubMed] [Google Scholar]
- Clotet B., Khuong M., Antinori A., van Lunzen J., Dumitru I., Pokrovskiy V., et al. (2013) Once-daily dolutegravir versus darunavir/ritonavir in antiretroviral naïve subjects: 48 week subgroup analyses from FLAMINGO. In: Programme and Abstracts of the 14th European AIDS Conference, October, Brussels, abstract LBPS4/6 p.15 [Google Scholar]
- Cooper D., Steigbigel R., Gatell J., Rockstroh J., Katlama C., Yeni P., et al. (2008) Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 359: 355–365 [DOI] [PubMed] [Google Scholar]
- Cottrell M., Hadzic T., Kashuba A. (2013) Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 52: 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer S., Azijn H., Surleraux D., Jochmans D., Tahri A., Pauwels R., et al. (2005) TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. J Antimicrob Chemother 10: 2314–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Fabbiani M., Colafigli M., Ciccarelli N., Farina S., Sidella L., et al. (2013) Safety and feasibility of treatment simplification to atazanavir/ritonavir + lamivudine in HIV-infected patients on stable treatment with two nucleos(t)ide reverse transcriptase inhibitors + atazanavir/ritonavir with virological suppression (Atazanavir and Lamivudine for treatment Simplification, AtLaS pilot study). J Antimicrob Chemother 68: 1364–1372 [DOI] [PubMed] [Google Scholar]
- EACS (2013) Guidelines for the clinical management and treatment of HIV-infected adults in Europe, October 2013 Version 7.0. Brussel: European AIDS Clinical Society; Available at http://www.eacsociety.org (accessed 24 January 2014). [Google Scholar]
- Eron J, Clotet B., Durant J., Katlama C., Kumar P., Lazzarin A., et al. (2013) Safety and efficacy of dolutegravir (DTG) in HIV-1 treatment-experienced subjects with raltegravir-resistant virus: 24-week results of the VIKING Study. J Infect Dis 207: 740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantauzzi A., Turriziani O., Mezzaroma I. (2013) Potential benefit of dolutegravir once daily: efficacy and safety. HIV AIDS 5: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg J., Clotet B., Khuong M, Antinori A., van Lunzen J., Dumitru I., et al. (2013) Once-daily dolutegravir is superior to darunavir/ritonavir in antiretroviral naïve adults: 48 week results from FLAMINGO (ING114915). In: Programme and Abstracts of the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, September, Denver, CO, abstract H-1464a. [Google Scholar]
- Hare S., Smith S., Métifiot M., Jaxa-Chamiec A., Pommier Y, Hughes S., et al. (2011) Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol 80: 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D., Felock P., Witmer M., Wolfe A., Stillmock K., Grobler J., et al. (2000) Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287: 646–650 [DOI] [PubMed] [Google Scholar]
- Hightower K., Wang R., Deanda F., Johns B., Weaver K., Shen Y., et al. (2011) Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother 55: 4552–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Saravolatz L. (2014) The quad pill, a once-daily combination therapy for HIV infection. Clin Infect Dis 58: 93–98 [DOI] [PubMed] [Google Scholar]
- Juday T., Gupta S., Grimm K., Wagner S., Kim E. (2011) Factors associated with complete adherence to HIV combination antiretroviral therapy. HIV Clin Trial 12: 71–78 [DOI] [PubMed] [Google Scholar]
- Karmon S., Markowitz M. (2013) Next-generation integrase inhibitors: where to after raltegravir? Drugs Mar 73: 213–228 [DOI] [PubMed] [Google Scholar]
- Katlama C., Clotet B., Mills A., Trottier B., Molina J., Grinsztejn B., et al. (2010) Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther 15: 1045–1052 [DOI] [PubMed] [Google Scholar]
- Kempf D., Marsh K., Kumar G., Rodrigues A., Denissen J., McDonald E., et al. (1997) Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. J Antimicrob Chemother 10: 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yoshinaga T., Seki T., Wakasa-Morimoto C., Brown K., Ferris R., et al. (2011) In vitro antiretroviral properties of S/GSK1349572, a next generations HIV integrase inhibitor. Antimicrob Agents Chemother 55: 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llibre J., Clotet B. (2012) Once-daily single-tablet regimens: a long and winding road to excellence in antiretroviral treatment. AIDS Rev 14: 168–178 [PubMed] [Google Scholar]
- Markowitz M., Nguyen B., Gotuzzo E., Mendo F., Ratanasuwan W., Kovacs C., et al. (2007) Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naïve patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 46: 125–133 [DOI] [PubMed] [Google Scholar]
- Mesplede T., Quashie P., Oliveira M., Underwood M., Wang R., Fujiwara T., et al. (2012) Selection in culture of HIV resistance to dolutegravir by mutations at integrase positions R263K and H51Y that diminish viral replication fitness. Abstracts of the Eleventh International Congress on Drug Therapy in HIV Infection. J Int AIDS Soc 15(Suppl. 4): 18113 [Google Scholar]
- Min S., Sloan L., DeJesus E., Hawkins T., McCurdy L., Song I., et al. (2011) Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 25: 1737–1745 [DOI] [PubMed] [Google Scholar]
- Min S., Song I., Borland J., Chen S., Lou Y., Fujiwara T., et al. (2010) Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother 54: 254–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M., Mesplède T., Quashie P., Moïsi D., Wainberg M. (2014) Resistance mutations against dolutegravir in HIV integrase impair the emergence of resistance against reverse transcriptase inhibitors. AIDS 28: 813–819 [DOI] [PubMed] [Google Scholar]
- Palella F., Jr., Delaney K., Moorman A., Loveless M., Fuhrer J., Satten G., et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853–860 [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents (2013) Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, 12 February 2013. Washington DC: Department of Health and Human Services; Available at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0 (accessed 24 January 2014). [Google Scholar]
- Perez-Valero I., Arribas J. (2011) Protease inhibitor monotherapy. Curr Opinion Infect Dis 10: 7–11 [DOI] [PubMed] [Google Scholar]
- Perry C. (2014) Elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Stribild(®): a review of its use in the management of HIV-1 infection in adults. Drugs 74: 75–97 [DOI] [PubMed] [Google Scholar]
- Pommier Y., Johnson A., Marchand C. (2005) Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 4: 236–248 [DOI] [PubMed] [Google Scholar]
- Quashie P., Mesplède T., Han Y., Oliveira M., Singhroy D., Fujiwara T., et al. (2012a) Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol 86: 2696–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quashie P., Mesplède T., Han Y., Veres T., Osman N., Hassounah S., et al. (2013) Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase. Antimicrob Agents Chemother 57: 6223–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quashie P., Sloan R., Wainberg M. (2012b) Novel therapeutic strategies targeting HIV integrase. BMC Med 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi F., Jaeger H., Quiros-Roldan E., Albrecht H., Belonosova E., Gatell J., et al. (2013a) Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naïve adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 13: 927–935 [DOI] [PubMed] [Google Scholar]
- Raffi F., Rachlis A., Stellbrink H., Hardy W., Torti C., Orkin C., et al. (2013b) Once-daily dolutegravir versus raltegravir in antiretroviral-naïve adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 381: 735–743 [DOI] [PubMed] [Google Scholar]
- Reese M., Savina P., Generaux G., Tracey H., Humphreys J., Kanaoka E., et al. (2013) In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 41: 353–361 [DOI] [PubMed] [Google Scholar]
- Reinke R., Lee D., Robinson W. (2002) Inhibition of human immunodeficiency virus type 1 isolates by the integrase inhibitor L-731,988, a diketo acid. Antimicrob Agents Chemother 46: 3301–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes J., Trinh R., Pulido F., Soto-Malave R., Gathe J., Qaqish R., et al. (2013) Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naïve subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses 29: 256–265 [DOI] [PubMed] [Google Scholar]
- Rockstroh J., DeJesus E., Lennox J., Yazdanpanah Y., Saag M., Wan H., et al. (2013) Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 63: 77–85 [DOI] [PubMed] [Google Scholar]
- Shah B., Schafer J., Desimone J., Jr. (2013) Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy 10.1002/phar.1386 [DOI] [PubMed] [Google Scholar]
- Spreen W., Margolis D., Pottage J., Jr. (2013) Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 8: 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellbrink H., Reynes J., Lazzarin A., Voronin E., Pulido F., Felizarta F., et al. (2013) Dolutegravir in antiretroviral-naïve adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS 27: 1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood M., Johns B., Sato A., Martin J, Deeks S., Fujiwara T. (2012) The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr 61: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunzen J., Maggiolo F., Arribas J., Rakhmanova A., Yeni P., Young B., et al. (2012) Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 12: 111–118 [DOI] [PubMed] [Google Scholar]
- van Sighem A., Gras L., Reiss P., Brinkman K., de Wolf F., for the ATHENA national observational cohort study (2010) Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24: 1527–1535 [DOI] [PubMed] [Google Scholar]
- Waki K., Sugawara Y. (2011) Implications of integrase inhibitors for HIV-infected transplantation recipients: raltegravir and dolutegravir (S/GSK 1349572) Biosci Trends 5: 189–191 [DOI] [PubMed] [Google Scholar]
- Walmsley S., Antela A., Clumeck N., Duiculescu D., Eberhard A., Gutiérrez F., et al. (2013) Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 369: 1807–1818 [DOI] [PubMed] [Google Scholar]
- Wares M., Mesplède T., Quashie P., Osman N., Han Y., Wainberg M. (2014) The M50I polymorphic substitution in association with the R263K mutation in HIV-1 subtype B integrase increases drug resistance but does not restore viral replicative fitness. Retrovirology 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available at: www.who.int/hiv/pub/guidelines/arv2013/download/en/ [PubMed]