Abstract
Vitamin A deficiency (VAD) is a leading cause of pediatric morbidity and mortality due to infectious diseases. Recent pre-clinical studies have revealed that VAD impairs mucosal IgA-producing antibody forming cell (AFC) responses toward a paramyxovirus vaccine in the upper respiratory tract (URT), thus impeding a first line of defense at the pathogen's point-of-entry. The studies described here tested the hypothesis that VAD may also impair immune responses after FluMist vaccinations. Results show that (i) IgA-producing antibody forming cells (AFCs) are significantly reduced following FluMist vaccination in VAD mice, and (ii) oral doses of either retinyl palmitate or retinoic acid administered on days 0, 3, and 7 relative to vaccination rescue the response. Data encourage the conduct of clinical studies to identify FluMist vaccine weaknesses in human VAD populations and to test corrective supplementation strategies. Improvements in vaccine efficacy may ultimately reduce the morbidity and mortality caused by influenza virus worldwide.
Keywords: FluMist, respiratory tract, mucosal IgA, oral, retinyl palmitate, retinoic acid, vitamin A, antibody forming cells
Introduction
Vitamin A deficiencies (VAD) and insufficiencies have been long-standing problems in both developed and developing countries, particularly in conditions of low socioeconomic status, prematurity and disease. VAD is best known for its contribution to xerophthalmia, while its contribution to infectious diseases is less well appreciated. Individuals with low serum levels of retinol or retinol binding protein are especially vulnerable to morbidity and mortality caused by gastrointestinal and respiratory viral infections [1-3].
To prevent infectious diseases of the respiratory tract, we are developing Sendai virus (SeV) as a candidate vaccine for human parainfluenza virus type 1 and as a recombinant vaccine for respiratory syncytial virus (RSV [4]). Each of these vaccines is administered intranasally (i.n.), as is FluMist, a vaccine licensed by MedImmune (Gaithersburg, MD) and produced each year in response to drifting and shifting influenza virus variants. FluMist comprises a cocktail of reassortant, replication-competent, cold-adapted influenza viruses that express internal proteins from a master donor virus, and hemagglutinin and neuraminidase membrane proteins from circulating wildtype influenza viruses. FluMist is recommended for vaccination of individuals between the ages of 2 and 49 years of age by i.n. spray. The vaccine is not recommended for use in very young children, the elderly, and individuals with asthma, due in part to potential adverse events including hospitalization and wheezing. The i.n. administration of vaccines ensures the induction of a robust IgA antibody forming cell (AFC) response in the upper respiratory tract (URT)[5], a first line of defense against virus at its point-of-entry, and a correlate of protection against viral disease [6-9].
Using a mouse model for VAD, we previously demonstrated that SeV-specific mucosal IgA responses were impaired in the URT of VAD animals [10]. We therefore asked if VAD would also affect responses toward FluMist. Results in this report show that the IgA-AFC response in the URT was indeed impaired toward FluMist in VAD animals. However, the administration of vitamin A supplements at the time of vaccination was sufficient to reverse the defect and induce healthy levels of FluMist-specific IgA AFCs. Results encourage clinical evaluation of FluMist responses in children in the context of VAD, and the testing of vitamin A supplements to improve FluMist vaccine efficacy in VAD populations.
Materials and Methods
VAD mice and vaccinations
Pregnant female C57BL/6 mice were from Jackson Laboratories (Bar Harbor, Maine). Animals were housed in a Biosafety Level 2+ area as specified by AAALAC and approved by the IACUC. To establish VAD mice, day 4-5 estrus C57BL/6 females were placed on VAD or control diets (the latter diet was supplemented with 15 IU Vitamin A palmitate/gram; Harlan Laboratories, Madison, WI) upon arrival at St. Jude Children's Research Hospital, as described previously [10;11]. The standard rodent breeder diet at St. Jude contains 30 IU/gram vitamin A (Autoclavable Rodent Breeder Diet #5013; LabDiet, Brentwood, MO). Sera were spot-checked for retinol by submission to the Colorado State Veterinary Laboratory for retinol testing at the Texas Veterinary Diagnostic Laboratory (Fort Collins).
Viral infections and vitamin supplementation
Infections of adult mice involved anesthesia followed by i.n. inoculations with FluMist (30 μl undiluted FluMist; seasons 2011-12 or 2012-13). This high dose was used, because the licensed, human vaccine had not been mouse-adapted. VAD animals were grouped to receive oral supplements on days 0, 3 and 7 (relative to vaccination) by gavage with either retinoic acid (300 μg/mouse; Sigma Cat#R2625) in 100 μl canola oil or retinyl palmitate (600 IU/mouse; Nutrisorb A, Interplexus Inc., Kent, WA) in 100 μl PBS. The numbers of mice per group are stated in figure legends. All immunizations and downstream analyses were repeated to ensure reproducibility.
Preparation of samples
Animals were sacrificed one month after vaccinations with avertin and exsanguination. Nasal wash samples were collected by exposing the trachea and washing the upper trachea and nasal cavity with 200 µl of PBS. Mice were perfused with PBS injected through the retro-orbital sinus. Diffuse nasal-associated lymphoid tissues(d-NALT) were harvested as described previously [10;11].
ELISA
The influenza virus-specific ELISA was conducted by plating Fluzone (Sanofi Pasteur) matched by season with vaccine on 96-well plates. Prior to plating, Fluzone was mixed with disruption buffer (500 μl Fluzone + 40 μl PBS + 60 μl disruption buffer (0.05% TritonX-100, 60 mM KCl, 10 mM Tris pH7.8)) and brought to a final concentration of 1 μg/ml in PBS. After blocking plates, serially diluted test samples were applied for 1h incubation at 37°C. Plates were washed and developed with alkaline phosphatase-conjugated goat anti-mouse IgA (Cat #1040-04, Southern Biotechnology, Birmingham, AL) followed by p-nitrophenyl phosphate (Sigma Aldrich Cat#N2640) for reading at OD 405 nm.
AFC ELISPOT
ELISPOT plates (multiscreen™-IP, Millipore, Cat#MAIPS4510, Billerica, MA) were coated with Fluzone (Sanofi Pasteur) as described previously [5] and incubated overnight at 4°C. Wells were washed with PBS and blocked with medium containing 10% FCS. Cells were then applied to wells (1×105 cells/well) usually in triplicate per sample and incubated for 3 hours at 37°C. Plates were washed and incubated with 100 μl of alkaline phosphatase-conjugated goat anti-mouse IgA (Cat #1040-04) in PBS-Tween 20 with 1% BSA. After overnight incubation at 4°C, antibodies were removed and plates were developed with BCIP/NBT substrate (Sigma Aldrich, Cat#B5655) and rinsed with water prior to spot counting.
Statistics
Unpaired T and Fisher's exact tests were used to compare groups (GraphPad Prism, San Diego, CA).
Results and Discussion
VAD impairs URT mucosal IgA responses toward FluMist vaccinations
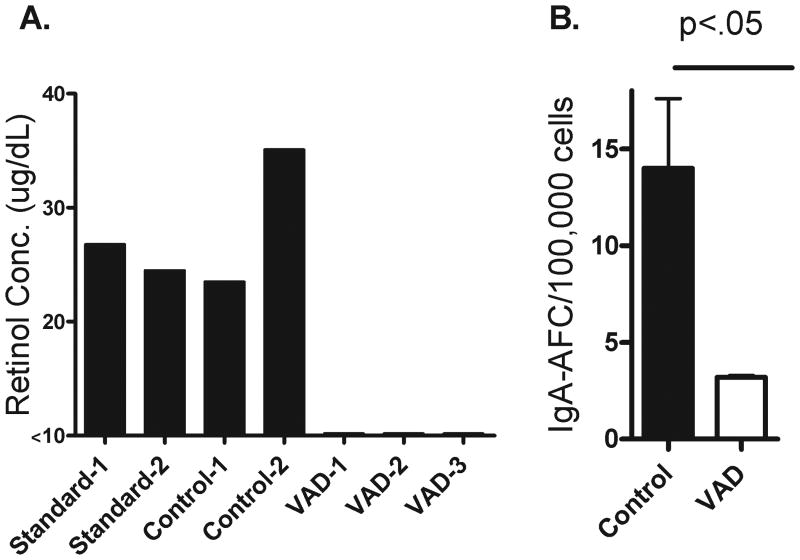
Based on our previous results showing that mucosal IgA responses toward a candidate parainfluenza virus vaccine (SeV) were impaired in a VAD mouse model, we questioned whether IgA responses toward FluMist might also be poor. To initiate the study, we placed pregnant females on VAD or control diets. The control diet matched the VAD diet precisely except for the addition of 15 IU/gram vitamin A palmitate. Progeny were also maintained on VAD or control diets until adulthood when experiments were conducted. As shown in Figure 1A, animals on a standard rodent breeder diet (LabDiet) or the control diet (Harlan) exhibited normal levels of serum retinol (≥20 μg/dL [12;13]), whereas animals on the VAD diet (Harlan) had undetectable serum retinol levels, confirming their VAD status.
Figure 1. The VAD mouse model exhibits an impaired mucosal IgA AFC response toward FluMist.
Panel A: Prior to FluMist vaccinations, mice on a control or VAD diet (Harlan Laboratories; see Materials and Methods) were compared to mice on a standard diet (Rodent Breeder Diet #5013 produced by LabDiet) for serum retinol levels. Each bar represents results from an individual mouse. Panel B: One month following FluMist vaccinations, mice on control (n=3) and VAD (n=3) diets were tested for virus-specific IgA-producing AFCs in the d-NALT with an ELISPOT assay.
Adult VAD and control animals were administered FluMist vaccine and sacrificed one month later for the analysis of virus-specific IgA-producing AFCs in the URT. As shown by a representative experiment in Figure 1B, virus-specific IgA-producing AFC were significantly reduced in the d-NALT of VAD animals, precisely as had been observed in SeV experiments. Also similar to previous experiments, serum virus-specific IgG levels were not significantly impacted by VAD (data not shown)[10;14].
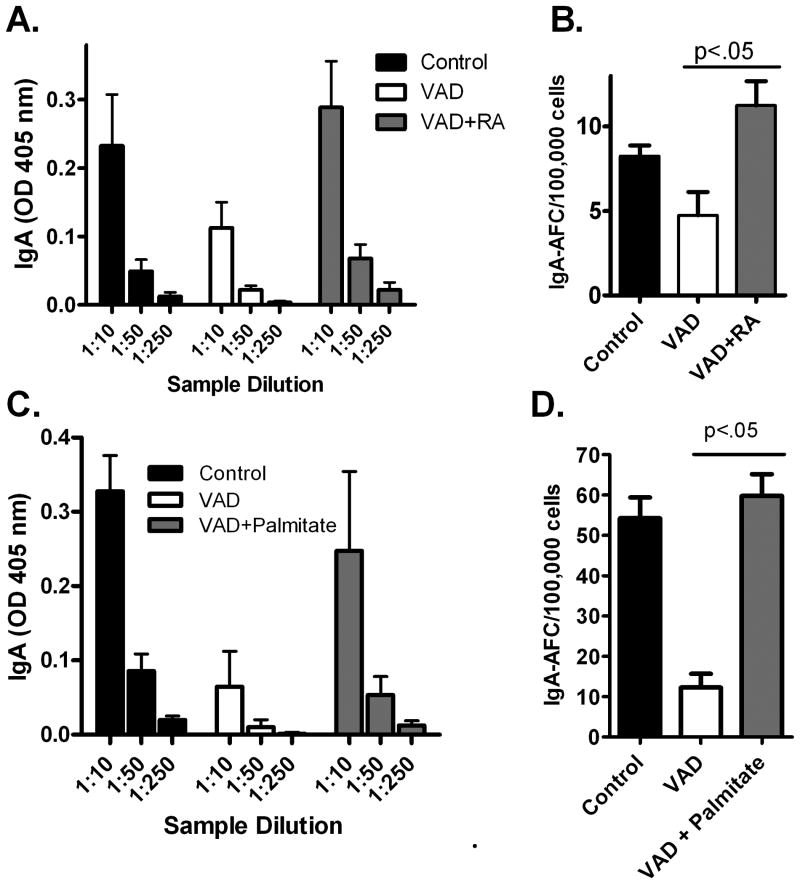
We next tested the hypothesis that an oral dosing of vitamin A at the time of vaccination might reverse the loss of vaccine-induced IgA-producing AFC in the URT mucosa. To this end, we first administered vitamin A in the form of retinoic acid (RA) by gavage on days 0,3, and 7 relative to vaccination with FluMist. As demonstrated in Figure 2A, the IgA responses in the VAD nasal washes were low, but IgA in the nasal wash was higher in VAD mice treated with RA (Figure 2A). Furthermore, there was a statistically significant increase in the number of IgA-producing AFC in the d-NALT of vitamin-supplemented VAD animals (Figure 2B).
Figure 2. Retinoic acid and retinyl palmitate rescues impaired mucosal IgA AFC responses toward FluMist in the VAD mouse.
Panel A: Control mice (n=3), VAD mice (n=2), and RA-supplemented VAD mice (n=3) were tested for mucosal IgA by ELISA. Panel B: Control mice (n=5), VAD mice (n=3), and RA-supplemented VAD mice (n=5) were tested for d-NALT IgA-producing AFCs with an ELISPOT assay. Panel C: Control mice (n=5), VAD mice (n=2), and retinyl palmitate -supplemented VAD mice (n=5) were tested for mucosal IgA. Panel D: Control mice (n=5), VAD mice (n=5), and retinyl palmitate -supplemented VAD mice (n=5) were tested for d-NALT IgA-producing AFCs. Some mortality of VAD mice during experiments yielded the variable group sizes described above.
Given that RA is not a preferred form of oral supplement in the clinical arena, we conducted additional experiments in which RA was replaced by retinyl palmitate. As shown in Figure 2C, IgA in the nasal wash was again improved in treated VAD mice. Most importantly, the URT d-NALT IgA-producing AFCs, responsible for IgA production at the mucosal surface, were significantly and repeatedly improved, exhibiting levels typical of control, healthy animals (Figure 2D).
A final question was whether the FluMist vaccine could prevent an influenza virus challenge in mice and whether VAD mice exhibited impaired protection compared to control animals. An initial test demonstrated that FluMist was not protective against a mouse adapted PR8 virus challenge in either VAD or control animals. This was not surprising as FluMist is a human vaccine that has not been adapted for amplification in rodents. As an alternative strategy for comparing vaccine efficacy in VAD and control animals, we immunized mice with a mouse-adapted, cold-adapted derivative of A/Puerto Rico/8/34 (PR8) virus, which was kindly provided to us by Drs. Huber and McCullers[15;16]. In a preliminary experiment, we demonstrated that IgA AFC responses were significantly impaired in VAD mice compared to control mice that received the cold-adapted vaccine. Control and VAD mice were also immunized with a dose of approximately 2.5×107 EID50 cold-adapted vaccine and challenged one month later with wildtype PR8 (approximately 1×104 EID50 per mouse). In one of two experiments, there was a significant difference between challenge virus amplification in VAD mice compared to controls (p<.05, Fisher's exact test). All control mice (10/10) were fully protected from infection with the challenge virus whereas only a fraction of VAD mice (3/6) were fully protected, Experiments are in progress to study the effects of VAD and vitamin supplementation on the protection conferred by additional respiratory virus vaccines in mice.
IgA and protective immunity
Results from this study demonstrated the effects of VAD on the induction of mucosal IgA responses following i.n. influenza virus vaccination. Previous studies have revealed the importance of IgA as a correlate of protection against respiratory virus infections [6-9], although a comprehensive study of IgA functions has not been conducted. While IgA is best known for its binding and neutralizing capacities, this antibody isotype is responsible for numerous additional functions that include modulation of cellular and cytokine immune effectors [17-21]. Future experiments are warranted to analyze the full impact of IgA and other isotypes on respiratory virus control, before and after [22] the infection of mammalian cells.
Vitamin A supplementation; efficacy and toxicity considerations
Decades ago, it was established that vitamin A supplements could protect VAD children from the morbidity and mortality caused by measles virus [23], and the World Health Organization established recommendations that supplements be administered orally to children at the time of routine measles vaccinations. The recommended dose of vitamin A for this purpose is 100,000 IU in infants and 200,000 IU in children 12 months or older [24]. Vitamin A palmitate is used as a preferred metabolite based on its safety and stability profile compared to retinoic acid. However, some toxicities are associated with high doses of vitamin A even when vitamin is administered in palmitate form. The precise human dose required to ensure efficacy without toxicity remains a point of debate. Studies have shown that doses between 100,000- 400,000 IU vitamin A associate with significant protection from morbidity and mortality in children with measles [23;25]. Lower doses (<50,000 IU) have been recommended to reduce the risks of acute side effects, but the benefit of lower doses is not always evident [26-29]. Clinical results differ depending on the regimen of supplementation and the degree of VAD in the target population. In our study, we tested 600 IU/∼20g VAD mouse, a relatively high dose (comparable to 300,000 IU in a 12 month old child of 10 kg). We are currently conducting additional murine experiments to define the lowest retinyl palmitate dose, preferably delivered only once during vaccination, sufficient to correct the d-NALT IgA-producing AFC response.
While mouse models provide proof-of-concept information, clinical studies of FluMist vaccine efficacy in individuals of known retinol status would be most informative to determine if VAD associates with impaired responses in humans. If an association is realized, vitamin A supplementation studies might then determine if weak immune responses in VAD hosts can be corrected. Programs directed toward a logistically-feasible improvement in URT responsiveness toward respiratory virus vaccines may enhance an important correlate of protection and thereby reduce the morbidity and mortality caused by respiratory virus infections worldwide.
Supplementary Material
Acknowledgments
This study was supported in part by NIH NIAID R01 AI088729, NIH NCI P30 CA21765 and the American Lebanese Syrian Associated Charities (ALSAC). We thank Drs. Victor Huber and Jon McCullers for the cold-adapted influenza virus vaccine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr. 1984;40:1090–5. doi: 10.1093/ajcn/40.5.1090. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Tarwotjo I, Djunaedi E, West KP, Jr, Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169–73. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- 3.Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, Surman S, Portner A, Slobod KS. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–93. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. Int Immunol. 2012 doi: 10.1093/intimm/dxs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine. 2012;30:6794–801. doi: 10.1016/j.vaccine.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy BR, Graham BS, Prince GA, Walsh EE, Chanock RM, Karzon DT, Wright PF. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986;23:1009–14. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills J, Van Kirk JE, Wright PF, Chanock RM. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971;107:123–30. [PubMed] [Google Scholar]
- 10.Surman SL, Rudraraju R, Sealy R, Jones B, Hurwitz JL. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 2012;25:341–4. doi: 10.1089/vim.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410:429–36. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Episkopou V, Maeda S, Nishiguchi S, Shimada K, Gaitanaris GA, Gottesman ME, Robertson EJ. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci U S A. 1993;90:2375–9. doi: 10.1073/pnas.90.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bennekum AM, Wei S, Gamble MV, Vogel S, Piantedosi R, Gottesman M, Episkopou V, Blaner WS. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276:1107–13. doi: 10.1074/jbc.M008091200. [DOI] [PubMed] [Google Scholar]
- 14.Rudraraju R, Surman SL, Jones BG, Sealy R, Woodland DL, Hurwitz JL. Reduced frequencies and heightened CD103 expression among virus-induced CD8(+) T cells in the respiratory tract airways of vitamin A-deficient mice. Clin Vaccine Immunol. 2012;19:757–65. doi: 10.1128/CVI.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber VC, Thomas PG, McCullers JA. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine. 2009;27:1192–200. doi: 10.1016/j.vaccine.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H, Zhou H, Lu B, Kemble G. Imparting temperature sensitivity and attenuation in ferrets to A/Puerto Rico/8/34 influenza virus by transferring the genetic signature for temperature sensitivity from cold-adapted A/Ann Arbor/6/60. J Virol. 2004;78:995–8. doi: 10.1128/JVI.78.2.995-998.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf HM, Hauber I, Gulle H, Samstag A, Fischer MB, Ahmad RU, Eibl MM. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol. 1996;105:537–43. doi: 10.1046/j.1365-2249.1996.d01-793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83:1278–88. [PubMed] [Google Scholar]
- 20.Mota G, Manciulea M, Cosma E, Popescu I, Hirt M, Jensen-Jarolim E, Calugaru A, Galatiuc C, Regalia T, Tamandl D, Spittler A, Boltz-Nitulescu G. Human NK cells express Fc receptors for IgA which mediate signal transduction and target cell killing. Eur J Immunol. 2003;33:2197–205. doi: 10.1002/eji.200323534. [DOI] [PubMed] [Google Scholar]
- 21.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J Immunol. 2005;174:2926–33. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- 22.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013;14:327–36. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussey GD, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med. 1990;323:160–4. doi: 10.1056/NEJM199007193230304. [DOI] [PubMed] [Google Scholar]
- 24.2013 http://www.who.int/vaccines/en/vitamina.shtml.
- 25.Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005:CD001479. doi: 10.1002/14651858.CD001479.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132:2902S–6S. doi: 10.1093/jn/132.9.2902S. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP, Jr, Sommer A. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr. 1996;128:489–96. doi: 10.1016/s0022-3476(96)70359-1. [DOI] [PubMed] [Google Scholar]
- 28.Rahman MM, Mahalanabis D, Wahed MA, Islam MA, Habte D. Administration of 25,000 IU vitamin A doses at routine immunisation in young infants. Eur J Clin Nutr. 1995;49:439–45. [PubMed] [Google Scholar]
- 29.Randomised trial to assess benefits and safety of vitamin A supplementation linked to immunisation in early infancy. WHO/CHD Immunisation-Linked Vitamin A Supplementation Study Group. Lancet. 1998;352:1257–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.