Abstract
Objective
The aim of this study was to examine the effect of acute tobacco abstinence on cortisol levels in regular smokers, and whether abstinence-induced changes in cortisol levels are correlated with various signs and symptoms of the tobacco withdrawal syndrome.
Methods
Smokers (N = 77, ≥15 cigarettes/day) attended two counterbalanced sessions (avg = 1 h), one following 12–20 h of abstinence and the other following ad lib smoking. At both sessions, salivary cortisol levels were measured at three time points. Additionally, a battery of self-report questionnaires, physiological assessments, and cognitive performance tasks were administered to measure signs and symptoms of tobacco withdrawal.
Results
Salivary cortisol levels were significantly lower during the abstinent session versus the non-abstinent session. No significant associations were found between abstinence-induced changes in cortisol and other tobacco withdrawal measures, although there was suggestive evidence that abstinence-induced changes in cortisol levels and hunger were inversely associated to a modest degree.
Conclusion
Acute tobacco abstinence was associated with decreased cortisol levels. Cortisol dampening during acute tobacco abstinence may reflect nicotine-mediated modulation of hypothalamic–pituitary–adrenal axis activity, which may be relevant to the maintenance of tobacco dependence. Tobacco-withdrawal cortisol changes do not appear to be a cause or consequence of many manifestations of acute tobacco withdrawal with the possible exception of hunger.
Keywords: acute tobacco abstinence, smoking deprivation, cortisol levels, withdrawal symptoms, HPA axis
INTRODUCTION
The hypothalamic-pituitary-adrenal (HPA) axis is a key biological pathway implicated in both stress response and nicotine addiction (Koob and Le Moal, 2001; Goeders, 2002; Koob and Kreek, 2007). Hence, understanding the interrelations between HPA axis activity, nicotine, and smoking behavior may yield important insights into the mechanisms underlying nicotine addiction and stress-related smoking behavior. Several studies have documented the stimulating effects of acute doses of nicotine delivered by tobacco smoking on the HPA axis (Wilkins et al., 1982; Pomerleau et al., 1983; Kirschbaum et al., 1992; Mendelson et al., 2005), with evidence of a nicotine dose-related increase in cortisol—a key hormone involved in the HPA axis—acutely following nicotine administration (Cryer et al., 1976, Winternitz and Quillen, 1977; Seyler et al., 1984; Mendelson et al., 2005).
Nicotine may trigger cortisol production through various mechanisms. For instance, after a nicotine administration, dose-dependent increases in brain activity have been noted in regions involved in emotion regulation and HPA responses to stress (Stein et al., 1998). Thus, nicotine may modulate the neural substrates of emotional processing, which could alter subjective stress and ultimately affect HPA axis-related cortisol production. More directly, nicotine stimulates cholinergic receptors in the hypothalamus, directly causing the release of corticotropic-releasing factors, which starts the HPA cascade that leads to the production of cortisol (Seyler et al., 1984; Fuxe et al., 1989; Pomerleau and Pomerleau, 1991; Dallman, 1993).
Although considerable research has documented nicotine’s stimulating effects on cortisol levels, the effects of tobacco abstinence on cortisol in regular smokers are not entirely clear. Understanding the effects of tobacco abstinence in its acute stages (i.e., the first 12–20 h) is particularly important because the biological changes that emerge following brief periods of abstinence that commonly occur in regular smokers may possibly maintain addictive smoking behavior among daily smokers attempting to quit (Chandra et al., 2007). Furthermore, such changes might underlie risk of smoking relapse following a quit attempt. Indeed, one study found that decreases in cortisol levels during the first day of abstinence predicted subsequent increased risk of early relapse, suggesting that endocrinological effects from acute tobacco abstinence may interfere with successful smoking cessation (al’Absi et al., 2004).
Acute tobacco abstinence could influence cortisol levels in several ways. Acute tobacco abstinence could result in a net reduction in cortisol levels among regular smokers, which could simply reflect the dissipation of nicotine’s stimulatory effects on cortisol production when transitioning from non-abstinent to abstinent states. Alternatively, acute tobacco abstinence could cause a reduction in cortisol, because chronic nicotine exposure is known to dysregulate the HPA axis and could perhaps result in a chronic dampening of the HPA axis when nicotine exposure is no longer present, resulting in a static stress system that is no longer adaptively responsive to stress or other factors that might activate the HPA axis (Koob and Le Moal, 2001). On the other hand, acute tobacco abstinence might increase cortisol levels. Indeed, the neural pathways involved in emotional processing become dysregulated during nicotine withdrawal (Froeliger et al., 2011). Therefore, acute tobacco abstinence could increase emotional vulnerability to stress in regular smokers. Because regular smokers come to rely on tobacco as a means of relaxation and stress reduction (Gough et al., 2009), acute abstinence from tobacco may be a direct psychological or physiologic stressor, which could activate the HPA axis and increase the cortisol levels. Some of the acute effects of abstinence (e.g., anxiety and irritability) resemble those that occur in response to acute stress (Hughes 1992).
The question of whether acute (i.e., 12–18 h) tobacco abstinence impacts cortisol levels in the absence of experimental stressors amongst regular smokers has received some attention in the empirical literature, and the findings are mixed. Several studies have shown no significant effects of short-term tobacco abstinence on cortisol levels (al’Absi et al., 2002; Teneggi et al., 2002; Wardle et al., 2011). However, each of these studies utilized sample sizes powered to detect only large-sized abstinence effects (n ≤ 30 per abstinent condition). Another study using a larger sample (n = 72) indicated that declines in cortisol concentrations after 24 h of smoking abstinence during an unaided quit attempt was greater in the group that ultimately relapsed in comparison with those who maintained abstinence (al’Absi et al., 2004) and that cortisol reduced from pre-quit to 24 h of abstinence. However, the main effects of abstinence in the overall sample in this study must be interpreted with the caveat of potential confounding of test order, as abstinent and non-abstinent sessions were not counterbalanced. Finally, one study found that while circadian variation was associated with plasma cortisol (plasma cortisol levels are greatest in the early morning and decrease to their lowest level in the late evening), tobacco abstinence did not impact the circadian variation of cortisol levels (Teneggi et al., 2002).
In addition, to examine the direct effects of acute tobacco abstinence on cortisol levels, another means of clarifying the role of HPA axis activity during tobacco abstinence is to examine a possible association between signs and symptoms of the tobacco withdrawal syndrome and abstinence-induced cortisol level changes. The tobacco withdrawal syndrome includes features such as anxiety, depression, restlessness, irritability, physical symptoms, and cognitive performance decrements (American Psychiatric Association, 2000; Hughes, 1992; Stitzer and Gross, 1988; Snyder et al., 1989). These symptoms begin within 4–24 h after smoking cessation and may contribute to risk for early relapse (Stitzer and Gross, 1988; Gritz et al., 1991; Shiffman, 1982; Carey et al., 1993). Importantly, there are marked individual differences in the severity of withdrawal signs and symptoms among acutely abstinent smokers (Leventhal et al., 2010). More extreme manifestations of withdrawal symptoms during acute tobacco abstinence may reflect more severe neuroadaptations caused by chronic nicotine exposure and higher levels of nicotine dependence (Shiffman et al., 2006; Leventhal et al., 2007; Panday et al., 2007; Bailey et al., 2009). Therefore, alterations of the HPA axis, such as changes in cortisol levels, during acute tobacco abstinence may couple with abstinence-induced changes in the severity of withdrawal symptoms. Yet, existing research of cortisol-withdrawal associations have utilized relatively restricted assessment of the various signs and symptoms of tobacco withdrawal syndrome and have focused on longer durations of tobacco abstinence (Pickworth et al., 1996; al’Absi et al., 2004). Whether these findings are evident during short-term abstinence (i.e., 12–20 h) and extend across various subjective, cognitive performance, and physiological manifestations of the tobacco withdrawal syndrome is unclear.
The current study examined the effects of experimentally-manipulated acute tobacco abstinence on salivary cortisol levels among regular smokers utilizing a sample adequately powered to detect medium-sized effects. As a secondary aim, we examined the association between abstinence-induced changes in cortisol levels and a variety of tobacco withdrawal signs and symptoms. Given some evidence that women may exhibit greater abstinence-induced reductions in cortisol than men (al’Absi et al., 2004), we also explored sex as a moderator of abstinence effects on cortisol levels.
METHODS
Participants
This report is a secondary paper from a previously published study that focused on quantifying the effects of acute tobacco abstinence on withdrawal signs and symptoms in 203 smokers that did not report cortisol data (Leventhal et al., 2010). The participants in this sample represent a subgroup of these individuals (n = 77) who underwent cortisol assessment procedures, which were introduced midstream into study recruitment. Participants included 17 male and 60 female adult smokers (average age: 38.2 year; range: 20–60 year) recruited from community announcements. Thirty-six participants were African American and 41 were Caucasian. On average, participants smoked 21.7 cigarettes/day (range: 15–40 cigarettes/day) and scores on the Fagerström Test of Nicotine Dependence (Heatherton et al., 1991) were 6.7 (range: 3–10).
The study inclusion criteria for participants were as follows: (i) 18 years or older; (ii) smokes at least 15 cigarettes/day; (iii) smoked for at least 2 years; (iv) regularly smokes a brand of cigarettes that delivers at least 11.0 mg tar and 0.7 mg nicotine as rated by the Federal Trade Commission method; and (v) A Fagerström Test of Nicotine Dependence score ≥ 3. The exclusion criteria were as follows: (i) recent history of certain diseases, including myocardial infarction, angina, heart failure, hypertension, stroke, and diabetes; (ii) use of nicotine replacement or smoking cessation treatments in the past 6 months; (iii) use of antidepressants in the past year; (iv) their estimated IQ on the Shipley Institute on Living Scale was less than 78; and (v) currently pregnant or nursing. Each participant underwent a medical examination to verify health status and provided informed consent. This study protocol was approved by the National Institute on Drug Abuse Intramural Research Program Internal Review Board and conducted at NIDA-IRP in Baltimore, MD.
Procedure
At an orientation session, participants practiced cognitive performance tasks to be completed in the experimental sessions (see in the succeeding text) and completed questionnaires assessing demographics and smoking history. Participants then attended two counterbalanced 2-h experimental sessions (abstinent and non-abstinent) between 1:00 pm and 3:00 pm on separate days. For the non-abstinent sessions, participants were instructed to smoke normally before the session and a cigarette within 20 min of the study session. During the abstinent sessions, participants were instructed to abstain from smoking overnight for at least 12 h (average of 16.9 h; range: 14–20 h) before the study session. Exhaled carbon monoxide (CO) was measured upon arrival at the laboratory on both abstinent (average 6.1 ppm; range: 2–11 ppm) and non-abstinent (average 29.7 ppm; range: 13–69 ppm) study sessions. A CO level of ≤11 ppm was required for the abstinent session. If participants arrived to the abstinent session with CO ≥ 12 ppm, the session was rescheduled.
The procedures were identical at both experimental sessions. Heart rate and blood pressure were measured while at rest during the beginning and end of the session. Subsequently, participants completed a battery of self-report, physiological, and computer-based cognitive performance tasks tapping the various manifestations of the tobacco withdrawal syndrome. Salivary cortisol samples were collected at three time points during each experimental session in order to capture the background variability of the circadian variations of cortisol levels: upon arrival right before completing the subjective measures (T1), after completing the cognitive performance tests (T2), and at the end of session (T3). The approximate timing of these three time points and the general outline of the session are indicated in Table 1. Certain measures administered during the study session (i.e., electroencephalography as well as questionnaires and computer tasks measuring attention bias toward smoking-related stimuli) were not analyzed in this paper because they were not expected to be related to HPA axis functioning or were not well-characterized manifestations of the tobacco withdrawal syndrome.
Table 1.
Approximate timeline of procedures for experimental sessions
| Procedure | Time (Minutes) |
|---|---|
| Exhaled CO | 2 |
| Heart rate/Blood pressure | 1 |
| Saliva sample for cortisol and cotinine analysis (T1) | 1 |
| Questionnairesa | 14 |
| Cognitive performance tasksb | 16 |
| Saliva sample for cortisol and cotinine analysis (T2) | 1 |
| Questionnairesc | 6 |
| Attentional bias tasks | 16 |
| Electroencephalogram | 13 |
| Heart rate/Blood pressure | 1 |
| Saliva sample for cortisol and cotinine analysis (T3) | 1 |
The following measures were administered at this time point: Positive and Negative Affect Scale; Hunger Questionnaire; Hughes Hatsukami Withdrawal Questionnaire; Wisconsin Smoking Withdrawal Scale; Subjective Attentional Bias Questionnaire; 12-item Tobacco Craving Questionnaire; and Brief Questionnaire of Smoking Urges.
The following cognitive measures were administered: Two-letter search task; serial math test; digital symbol substitution task; rapid information processing task.
The positive and negative affect scale and the Hughes Hatsukami Withdrawal Questionnaire were readministered at this time point.
Cortisol and cardiovascular measures
For cortisol assessment, participants provided 1–2 mL of saliva by placing a Salivette cotton collection device in their mouth for approximately 1 min. After the cotton was sufficiently saturated, it was deposited into a plastic tube (Salivette; Sarstedt Ltd.). All samples were stored at −20°C until analysis. Samples were assayed for salivary cortisol in duplicate using a highly sensitive enzyme immunoassay at SalimetricsLLLC (State College, PA). The test used 25 uL of saliva (for single determinations). Features of the test included a lower limit sensitivity of 0.003 ug/dL, range of sensitivity from 0.003 to 1.8 ug/dL, and average intra-assay and inter-assay coefficients of variation 4.8% and 8.8%, respectively. The accuracy of the method by spike recovery, and linearity, determined by serial dilution is 105% and 95%. Values from matched serum and saliva samples show the expected strong linear relationship, r (17) = >0.94, p < 0.0001. Systolic/diastolic blood pressures and heart rate were obtained using an automated vital sign monitor (IVAC, Corp., San Diego, CA).
Cognitive performance measures
Cognitive performance was assessed using four computerized tasks drawn from the Walter Reed performance assessment battery (Thorner et al., 1985) in the following order: Two-letter search test (ST), serial math test (MT), digit symbol substitution task, and the rapid information processing task (RIPT). For each test, the percentage of correct trials was recorded. The mean response time was recorded for the ST, MT, and RIPT tests.
Each test assessed for different cognitive capabilities. The ST test assessed visual scanning, recognition, and attention. The MT test assessed mathematical reasoning. The DSST assessed for psychomotor performance. The RIPT test assessed sustained attention. For more information on these cognitive performance measures, please refer to a previous publication discussing the original study (Leventhal et al., 2010).
Subjective assessments
Several questionnaires were administered to assess subjective effects of tobacco abstinence. The Hughes Hatsukami Tobacco Withdrawal Questionnaire (HHWQ; Hughes et al., 1986) assesses 11 signs and symptoms of tobacco withdrawal on 6-point Likert-type scales and produces a total score. A 23-item variant of the Wisconsin smoking withdrawal scale (WSWS; Welsch et al., 1999) which yields six subscales of tobacco withdrawal (anxiety, anger, hunger, concentration problems, craving, and sadness) and an overall severity scale. The Brief Questionnaire of Smoking Urges (QSU; Cox et al., 2001) examines desire for the positive effects of smoking, intention to smoke (Factor 1 scale), and desire for the relief of negative affect and urgent need to smoke (Factor 2 scale). It also produces a total score. The positive and negative affect schedule (PANAS; Watson et al., 1988) assesses positive affect (PA; 10 items, e.g., enthusiastic and strong) and negative affect (NA; 10 items, e.g., distressed and upset). Lastly, the hunger questionnaire (HQ; Hill and Blundell, 1982) includes eight items measuring feelings of hunger, fullness, and desire to eat on 10-point Likert scales (1 = not at all and 10 = extremely), which were averaged to create a total score. As demonstrated in prior analyses in the parent study (Leventhal et al., 2010), Cronbach’s alpha coefficients based on the intercorrelations of abstinence-induced changes for individual items within a scale illustrated that abstinence-induced change scores for each of the subjective measures described previously exhibit adequate internal consistency.
Data analysis
We modeled the effects of state (abstinent vs. non-abstinent), time (T1, T2, and T3), and the state × time interaction on cortisol levels using within-subject analysis of variance with a Greenhouse–Geiser Epsilon Adjusted p-values. We conducted additional models, including gender as an independent variable, because of hormonal sex differences possibly impacting the results. Results of these models demonstrated that the gender × state and gender × state × time interactions were nonsignificant. Therefore, results reported in final models do not include gender.
We then examined the effects of state on subjective, cognitive, and physiological measures of tobacco withdrawal using within-subject analysis of variance. For measures demonstrating significant differences by state, we created “abstinence-induced change scores” (i.e., score while abstinent and score while non-abstinent). Pearson’s correlation was examined between abstinence-induced change scores on these measures and abstinence-induced changes at each cortisol assessment. For the primary aim of testing the effects of abstinence on cortisol levels, the significance threshold was set to 0.05. For the secondary aim of testing correlations between abstinence-induced changes in cortisol and other withdrawal measures, we used a Bonferronicorrected significance threshold of 0.0007 (0.05/69 tests) and interpreted correlations with ps in between 0.0008 and 0.05 with caution. Sample sizes range from 74 to 77 in these analyses because of missing cortisol data.
Supplementary analysis was also conducted to permit the interpretation of the relative robustness of correlations between abstinence-induced cortisol changes and the subjective, cognitive, and physiological measures for comparative purposes: Pearson’s correlation was examined for abstinence-induced change scores for measures across subjective, cognitive, and physiological measures in which there were significant state effects.
RESULTS
Effects of abstinence on cortisol levels
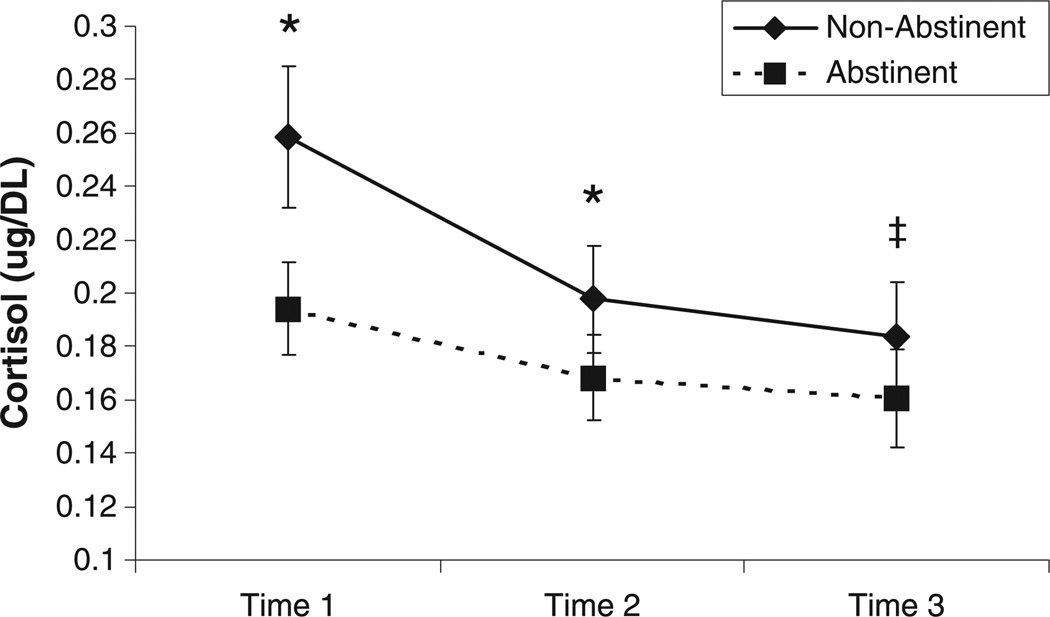
As illustrated in Figure 1, there was a significant main effect of state, F(1, 73) = 6.33, p = 0.01 on cortisol levels. Comparisons at each individual time point showed significantly lower cortisol levels in the abstinent (vs. non-abstinent) condition for T1 (F = 5.61, p < 0.05) and T2 (F = 4.09, p < 0.05) and a trend approaching significance for T3 (F = 3.43, p = 0.06).
Figure 1.
Effects of overnight abstinence on cortisol levels across three time points. Mean Cortisol levels (+ S.E.M.) are displayed. *Significant differences between abstinent and non-abstinent states (p > .05); ‡Differences between abstinent and non-abstinent states (p = .06).
There was also a significant main effect of time on cortisol levels, F(2, 146) = 15.76, p < 0.0001. Pairwise comparisons within the non-abstinent condition illustrated significant reductions in cortisol levels from T1 to T2 (F = 10.03, p = 0.002), T2 to T3 (F = 4.77, p = 0.03), and T1 to T3 (F = 10.91, p = 0.002). Within the abstinent condition, cortisol levels also significantly decreased from T1 to T2 (F = 5.81, p = 0.02) and T1 to T3 (F = 8.62, p = 0.004), but not from T2 to T3 (F = 1.78, p = 0.19). The state × time interaction was nonsignificant, F(2, 146) = 2.52, p = 0.11, demonstrating that the extent to which cortisol levels significantly declined over time did not differ as a function of abstinence.
Abstinence effects on tobacco withdrawal symptoms and signs
As illustrated in Table 2, there was a significant effect of overnight abstinence on all subjective measures: Time 1 and time 2 of HHWQ (both time points: p < 0.0001), WSWS–total, anger, anxiety, concentration, craving, hunger, and sadness subscales (all subscales: p < 0.0001), QSU–total, factor 1, and factor 2 subscales (total and both subscales: p < 0.0001), PANAS–both time points for PA (time 1: p < 0.003 and time 2: p < 0.0001) and NA (both time points: p < 0.0001), and HQ (p < 0.0001). There was a significant effect of overnight abstinence on some physiological measures in the expected direction: Time 1 and time 2 of heart rate (both time points: p < 0.0001) and Cotinine level (p < 0.0001). There were no significant effects of abstinence on both time points of systolic and diastolic blood pressure. Effects of abstinence on cognitive measures yielded few significant effects: Reaction time on the RIPT (p = 0.008), reaction time and error % on the ST (p < 0.0001 and p = 0.03, respectively), and reaction time on the MT (p = 0.004). There were no significant effects of abstinence on the following cognitive measures: RIPT (hit rate and false positives), MT (error percentage), and DSST (total correct and error percentage).
Table 2.
Effects of overnight abstinence on subjective, physiological, and cognitive measures
| Non-abstinent | Abstinent | State effect | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | |
| Subjective Measuresa | ||||||
| HHWQ | ||||||
| Time 1 | 0.70 | 0.62 | 1.96 | 0.94 | 154.67 | <0.0001 |
| Time 2 | 0.91 | 0.79 | 1.97 | 0.96 | 112.71 | <0.0001 |
| WSWS | ||||||
| Total | 1.28 | 0.64 | 2.15 | 0.70 | 115.64 | <0.0001 |
| Anger | 0.92 | 0.97 | 1.97 | 1.28 | 45.98 | <0.0001 |
| Anxiety | 1.25 | 0.84 | 2.11 | 0.78 | 77.27 | <0.0001 |
| Concentration | 0.98 | 0.76 | 1.82 | 0.94 | 52.33 | <0.0001 |
| Craving | 1.70 | 1.03 | 3.15 | 0.96 | 125.29 | <0.0001 |
| Hunger | 1.50 | 0.97 | 2.14 | 1.07 | 26.76 | <0.0001 |
| Sadness | 1.13 | 0.81 | 1.59 | 0.67 | 24.22 | <0.0001 |
| QSU | ||||||
| Total | 1.67 | 1.27 | 3.56 | 1.03 | 202.43 | <0.0001 |
| Factor 1 | 2.27 | 1.50 | 4.43 | 0.89 | 169.68 | <0.0001 |
| Factor 2 | 1.06 | 1.14 | 2.69 | 1.38 | 152.84 | <0.0001 |
| PANAS | ||||||
| PA–Time 1 | 3.14 | 0.97 | 2.91 | 0.93 | 9.39 | 0.003 |
| PA–Time 2 | 3.05 | 0.98 | 2.65 | 0.93 | 19.56 | <0.0001 |
| NA–Time 1 | 1.25 | 0.38 | 1.85 | 0.59 | 90.33 | <0.0001 |
| NA–Time 2 | 1.51 | 0.63 | 1.86 | 0.69 | 20.32 | <0.0001 |
| HQ | 2.89 | 1.82 | 3.97 | 2.20 | 17.23 | <0.0001 |
| Physiological measures | ||||||
| HR (bpm) | ||||||
| Time 1 | 82.50 | 11.80 | 64.20 | 9.20 | 62.26 | <0.0001 |
| Time 2 | 64.17 | 9.23 | 71.77 | 10.36 | 58.03 | <0.0001 |
| SBP (mmHg) | ||||||
| Time 1 | 120.90 | 13.50 | 120.80 | 13.90 | 0.00 | 0.99 |
| Time 2 | 117.22 | 14.74 | 118.34 | 14.61 | 0.75 | 0.39 |
| DBP (mmHg) | ||||||
| Time 1 | 74.30 | 9.60 | 74.30 | 9.60 | 0.87 | 0.35 |
| Time 2 | 72.24 | 9.99 | 72.96 | 8.90 | 0.71 | 0.40 |
| Cotinine (ng/mL)b | 327.70 | 159.90 | 190.30 | 128.80 | 70.27 | <0.0001 |
| Cognitive measures | ||||||
| RIPT | ||||||
| Hit rate (%) | 51.50 | 16.20 | 48.70 | 15.60 | 3.75 | 0.056 |
| RT (ms) | 539.60 | 90.20 | 576.50 | 118.40 | 7.52 | 0.008 |
| False positives/min | 4.04 | 5.25 | 4.24 | 5.53 | 0.45 | 0.51 |
| ST | ||||||
| RT (s) | 5.12 | 1.60 | 5.77 | 1.80 | 24.68 | <0.0001 |
| Errors (%) | 6.40 | 10.60 | 3.70 | 4.60 | 5.11 | 0.03 |
| MT | ||||||
| RT (s) | 2.01 | 0.80 | 2.19 | 0.91 | 9.09 | 0.004 |
| Errors (%) | 21.50 | 16.50 | 21.90 | 16.90 | 0.20 | 0.66 |
| DSST | ||||||
| Total correct | 23.60 | 11.30 | 22.60 | 11.40 | 1.03 | 0.31 |
| Errors (%) | 16.00 | 23.90 | 17.10 | 26.30 | 0.11 | 0.74 |
Mean cotinine score (from all three time points) reported. HHWQ, Hughes Hatsukami Withdrawal Questionnaire (scale: 0–5); WSWS, Wisconsin Smoking Withdrawal Scale (scale: 0–4); QSU, Brief Questionnaire of Smoking Urges (scale: 0–5); PANAS, Positive and Negative Affect Scale (scale: 1–5); HQ, Hunger Questionnaire (scale: 1–10); HR, Heart Rate; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; RIPT, Rapid Information Processing Task; ST, Two-letter search task; MT, serial addition and subtraction; DSST, Digit Symbol Substitution Task; RT, reaction time.
Mean scores per item displayed to enhance comparison across subscales.
Associations between abstinence-induced changes in cortisol levels and tobacco withdrawal symptoms and signs
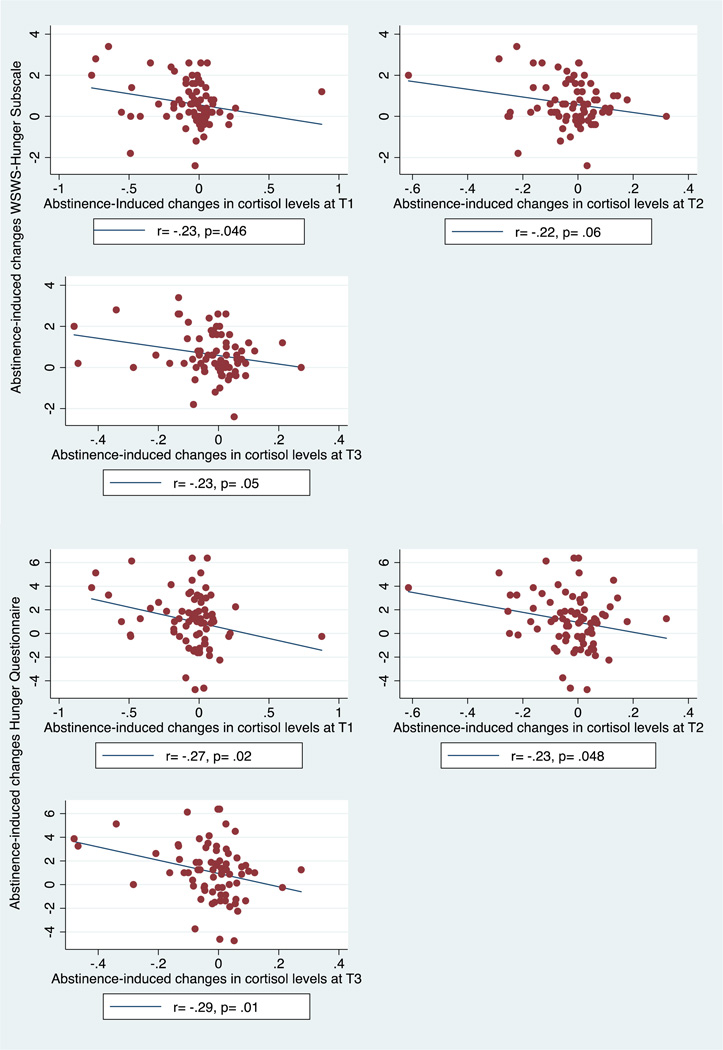
Among the tobacco withdrawal measures for which there were significant abstinence effects, there was suggestive evidence of inverse correlations between abstinence-induced changes in cortisol levels and corresponding abstinence-induced changes in hunger (Table 3 and Figure 2): T1 cortisol and WSWS-Hunger Subscale (r = −0.23; p = 0.046), T2 cortisol and WSWS-Hunger (r = −0.22; p = 0.06), T3 cortisol and WSWS-Hunger (r = −0.23; p = 0.05), T1 cortisol and Hunger Questionnaire (r = −0.27; p = 0.02), T2 cortisol and Hunger Questionnaire (r = −0.23; p = 0.048), and T3 cortisol and Hunger Questionnaire (r = −0.29; p = 0.01). Each of these associations did not surpass significance thresholds correcting for multiple tests (adjusted alpha = 0.0007) and therefore should be interpreted with caution. Associations between abstinence-related changes in cortisol and any of the other nicotine withdrawal signs and symptoms or abstinence-induced changes in cotinine levels at any time point were null (|r|: M = 0.09, SD = 0.06, and ps ≥ 0.11; Table 3), and sizably more modest in absolute magnitude than the corresponding results involving hunger.
Table 3.
Correlations between abstinence-induced changes in withdrawal symptoms to abstinence-induced changes in cortisol levels
| Cortisol T1 | Cortisol T2 | Cortisol T3 | ||||
|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | |
| Subjective measures | ||||||
| HHWQ | ||||||
| Time 1 | 0.11 | 0.33 | 0.05 | 0.67 | 0.09 | 0.47 |
| Time 2 | 0.08 | 0.49 | 0.10 | 0.41 | −0.02 | 0.88 |
| WSWS | ||||||
| Total | −0.07 | 0.54 | −0.04 | 0.76 | −0.01 | 0.92 |
| Anger | 0.08 | 0.49 | 0.07 | 0.53 | 0.13 | 0.27 |
| Anxiety | −0.07 | 0.54 | −0.07 | 0.53 | −0.03 | 0.78 |
| Concentration | 0.06 | 0.63 | 0.01 | 0.93 | 0.03 | 0.81 |
| Craving | −0.08 | 0.52 | <0.01 | 0.99 | <0.01 | 0.98 |
| Hunger | −0.23 | 0.05 | −0.22 | 0.06 | −0.23 | 0.05 |
| Sadness | 0.05 | 0.66 | 0.15 | 0.19 | 0.16 | 0.17 |
| QSU | ||||||
| Total | 0.10 | 0.42 | 0.07 | 0.53 | 0.05 | 0.67 |
| Factor 1 | −0.02 | 0.88 | 0.00 | 0.99 | −0.05 | 0.69 |
| Factor 2 | 0.22 | 0.06 | 0.15 | 0.20 | 0.16 | 0.17 |
| PANAS | ||||||
| PA–Time 1 | −0.12 | 0.32 | −0.09 | 0.42 | −0.06 | 0.58 |
| PA–Time 2 | −0.13 | 0.25 | −0.02 | 0.84 | −0.08 | 0.51 |
| NA–Time 1 | 0.07 | 0.53 | 0.06 | 0.61 | 0.13 | 0.28 |
| NA–Time 2 | 0.06 | 0.62 | 0.17 | 0.15 | 0.09 | 0.45 |
| HQ | −0.27 | 0.02 | −0.23 | 0.05 | −0.29 | 0.01 |
| Physiological measures | ||||||
| HR (bpm) | ||||||
| Time 1 | 0.19 | 0.11 | 0.17 | 0.14 | 0.18 | 0.13 |
| Time 2 | 0.18 | 0.12 | 0.14 | 0.22 | 0.16 | 0.16 |
| Cotinine (ng/mL) | 0.08 | 0.47 | 0.16 | 0.17 | 0.08 | 0.50 |
| Cognitive measures | ||||||
| RIPT | ||||||
| RT (ms) | −0.18 | 0.12 | −0.13 | 0.25 | −0.19 | 0.11 |
| ST | ||||||
| RT (s) | 0.07 | 0.58 | 0.03 | 0.80 | 0.19 | 0.11 |
| Errors (%) | 0.12 | 0.30 | 0.06 | 0.63 | 0.08 | 0.48 |
| MT | ||||||
| RT (s) | 0.13 | 0.28 | 0.12 | 0.31 | 0.13 | 0.27 |
Note. Pearson Correlation coefficient only analyzed for withdrawal symptoms with a significant state effect in Table 2. None of the associations exhibited a significance level that surpassed a type-I error correction for multiple tests (0.0007).
HHWQ, Hughes Hatsukami Withdrawal Questionnaire (scale: 0–5); WSWS, Wisconsin Smoking Withdrawal Scale (scale: 0–4); QSU, Brief Questionnaire of Smoking Urges (scale: 0–5); PANAS, Positive and Negative Affect Scale (scale: 1–5); HQ, Hunger Questionnaire (scale: 1–10); HR, Heart Rate; RIPT, Rapid Information Processing Task; ST, Two-letter search task; MT, serial addition and subtraction; RT, reaction time.
p-value ≤ 0.05.
Figure 2.
Scatter plots illustrating correlations between abstinence-induced changes in cortisol levels and hunger. Note. Pearson correlation coefficients calculated for relationship between abstinence induced changes in cortisol levels (at each timepoint) and abstinence-induced changes in hunger. Cortisol levels reported in µg/dL. WSWS, Wisconsin Smoking Withdrawal Scale (scale: 0–4); HQ, Hunger Questionnaire (scale: 1–10); T1, Time 1; T2, Time 2; T3, Time 3.
Associations of abstinence-induced changes in withdrawal phenomena across subjective, cognitive, and physiological domains
Supplementary tables 4 and 5 illustrate correlations in abstinence-induced changes across subjective, cognitive, and physiological domains. On average, the cross–domain correlation for each pair of measures was of small magnitude (|r|: M = 0.10 and SD = 0.07). Of the 131 relations tested, there were only a few correlations with ps < 0.05: abstinence-induced changes between PANAS-PA time 2 and HR time 1 (p = 0.04), HQ and HR time 1 (p = 0.05), HHWQ time 2 and ST % error (p = 0.01), WSWS-Hunger and RIPT reaction time (p = 0.04), PANAS-PA time 2 and RIPT reaction time (p = 0.04), HQ and RIPT reaction time (p = 0.05), and HQ and MT reaction time (p = 0.003).
DISCUSSION
In the current study, salivary cortisol levels were significantly lower among smokers following acute tobacco abstinence compared with a control non-abstinent state. Some previous studies using smaller sample sizes have not found significant effects of acute (12–24 h) tobacco abstinence on cortisol levels (al’Absi et al., 2002; Teneggi et al., 2002; Wardle et al., 2011). Though, a study employing a larger sample of active quitters found that cortisol reduced from prequit to 24 h of abstinence (al’Absi et al., 2004). With a sample sized powered to detect medium-sized effects and a within-participant counterbalanced design keying in on the first 12 to 20 h of abstinence, the present study presents a new finding suggesting HPA axis diminution associated with overnight tobacco abstinence.
We also found that cortisol levels declined over time at relatively similar rates in the abstinent and non-abstinent states, which is concordant with prior evidence that tobacco abstinence does not affect the natural circadian variations in plasma cortisol (Teneggi et al., 2002). Incidentally, cognitive performance tasks that were administered between T1 and T2 may suggest a possible disturbance of cortisol levels, as previous research has shown difficult cognitive tasks, such as public speaking and mental arithmetic stressors, may induce stress and increase cardiovascular and cortisol responses (Al’Absi et al., 1997). However, given the consistent within day cortisol reduction illustrated here, it appears that completing these cognitive tasks did not alter cortisol levels. It is likely that standard cognitive tasks administered here were not demanding enough to alter the stress response and confound typical circadian cortisol levels. Additionally, although extant research has shown that women may exhibit greater abstinence-induced reductions in cortisol than men (al’Absi et al., 2004), this study did not find significant gender differences in the effects of tobacco abstinence on salivary cortisol levels.
The abstinence-induced reduction in cortisol levels may reflect several underlying mechanisms. For instance, nicotine has been shown to stimulate cholinergic receptors that start the HPA cascade eventually leading to the production of cortisol; thus, the dissipation of nicotine levels after acute tobacco abstinence may explain the drop in cortisol levels (Seyler et al., 1984; Fuxe et al., 1989; Pomerleau and Pomerleau, 1991; Dallman, 1993). However, there was no significant association between abstinence-induced changes in cortisol and cotinine—a metabolite of nicotine—suggesting other mechanisms may account for this finding. Alternatively, the abstinence-induced reduction in cortisol levels may perhaps reflect the expression of biological adaptations associated with dampened HPA axis activity caused by nicotine dependence and withdrawal featured in stress-reward dysregulation models of addiction (Koob and Le Moal, 2001).
Prior research examining cortisol-withdrawal relations has utilized measures that amalgamate multiple nicotine withdrawal symptoms into a composite index (Pickworth et al., 1996; al’Absi et al., 2004), which may obscure associations that potentially exist between a particular symptom of tobacco withdrawal and HPA axis activity. In this study, abstinence-induced changes in most tobacco withdrawal signs and symptoms were not associated with tobacco abstinence-induced changes in salivary cortisol levels, which concords with a prior study utilizing composite-based indices (Pickworth et al., 1996). However, by measuring each withdrawal symptom separately, this study detected an inverse association between abstinence-induced changes in self-reported hunger and cortisol that may have been previously obscured in prior work. Specifically, there was suggestive evidence for this relation that was consistent across two indices of hunger and cortisol assessments at all three time points when applying uncorrected significance thresholds; no other measure appeared to follow this pattern. However, none of these relations surpassed the significance threshold corrected for multiple tests and should be interpreted with caution.1 It is worthy of noting that a recent study found that administration of oral hydrocortisone—a synthetic cortisol that increases cortisol production—versus placebo was marginally associated with lower self-reported hunger in recently abstinent smokers (Ussher et al., 2011). By and large, however, the overall pattern of results suggests that the effects of acute tobacco abstinence on cortisol and other subjective, cognitive, and physiological measures do not tend to couple together. Additionally, supplementary analyses illustrated that relations in abstinence effects across subjective, cognitive, and physiological domains were also minimal. Taken together, it is unlikely that the tobacco withdrawal syndrome is a homogenous phenotype across multiple domains of manifestation. Rather, different domains of withdrawal manifestations, including cortisol (or the endocrine domain), appear to reflect different phenotypic expressions of withdrawal that do not couple together across people.
There were certain limitations to this study. First, while the current design allowed us to focus on a precise period of abstinence (12–20 h) and apply a rigorous counterbalanced within-participant design in non-treatment-seeking smokers, we cannot extrapolate these results to longer periods of abstinence and naturalistic conditions of smoking cessation. Past research studying long term abstinence-induced changes in cortisol have shown an increase in cortisol after abstinence, suggesting that abstinence-induced cortisol level changes may be time-dependent (Pickworth et al., 1996; Pickworth and Fant, 1998). Second, this study only explored the role of one HPA axis hormone, cortisol. In order to better understand the effects of tobacco abstinence on the HPA axis, multiple measures of several biological processes that reflect HPA axis activity should be employed. Third, due the nature of the abstinence manipulation, we cannot disentangle the relative influence of pharmacologic factors (e.g., reduction in nicotine levels), loss of the sensory motor stimulation associated with the smoking ritual, and the activation of beliefs about the effects of tobacco abstinence on our findings. Fourth, as previously mentioned, evidence suggests that women may exhibit greater abstinence-induced reductions in cortisol than men (al’Absi et al., 2004). Because this study included a small number of men (n = 17), findings regarding gender differences should be interpreted with caution due to the high potential for: (i) insufficient statistical power and possible type-II error and (ii) limited generalizability to the overall population of men. Finally, the associations between abstinence-induced changes in hunger and cortisol are qualified by the large number of tests performed.
Overall, the current study advances endocrinology-related tobacco research, shedding light on cortisol reductions as a potential target for research on the determinants of early smoking relapse and factors that may maintain addictive smoking behavior. This study also indicates that cortisol may reflect a unique phenotypic manifestation of tobacco withdrawal that is separate from potentially several distinct phenotypic expressions of withdrawal across domains. Thus, continued investigation of the interrelation between nicotine, HPA axis activity, and tobacco withdrawal will be an important means for advancing psychopharmacology theory and smoking cessation practices.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by the National Institute on Drug Abuse, Intramural Research Program and National Institute on Drug Abuse grant DA025041.
Footnotes
Past work illustrates a systematic increase in salivary cortisol shortly after ingestion of a meal (Gibson et al., 1999). Thus, to consider the role of food ingestion in the current findings, we conducted a post-hoc analysis on the HHWQ item “increased eating”. Abstinence significantly increased the level of endorsement of this item (p < 0.001). Furthermore, abstinence-induced changes in cortisol were not significantly associated with abstinence-induced changes on the HHWQ-eating item (T1: r = −0.07, p = 0.57; T2: r = −0.15, p = 0.21; T3: r = −0.10, p = 0.38). Hence, it is unlikely that food ingestion impacted the observed decrease in salivary cortisol induced by tobacco abstinence or the inverse correlations between abstinence-induced changes in cortisol and hunger.
CONFLICT OF INTEREST
The authors have declared no competing interests.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web site.
REFERENCES
- Al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacol Biochem Behav. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Bailey SR, Harrison CT, Jeffery CJ, et al. Withdrawal symptoms over time among adolescents in a smoking cessation intervention: do symptoms vary by level of nicotine dependence? Addict Behav. 2009;34:1017–1022. doi: 10.1016/j.addbeh.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Kalra DL, Carey KB, Halperin S, Richards CS. Stress and unaided smoking cessation: a prospective investigation. J Consult Clin Psychol. 1993;61:831–838. doi: 10.1037//0022-006x.61.5.831. [DOI] [PubMed] [Google Scholar]
- Chandra S, Shiffman S, Scharf DM, Dang Q, Shadel WG. Daily smoking patterns, their determinants, and implications for quitting. Exp Clin Psychopharmacol. 2007;15:67–80. doi: 10.1037/1064-1297.15.1.67. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, Mcclernon FJ. Smoking abstinence and depressive symptoms modulate the executive control system during emotional information processing. Addict Biol. 2011;17:668–679. doi: 10.1111/j.1369-1600.2011.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gough B, Fry G, Grogan S, Conner M. Why do young adult smokers continue to smoke despite the health risks? A focus group study. Psychol Health. 2009;24:203–220. doi: 10.1080/08870440701670570. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Marcus AC. The tobacco withdrawal syndrome in unaided quitters. Br J Addict. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Blundell JE. Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res. 1982;17:203–212. doi: 10.1016/0022-3956(82)90023-1. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Skoog KP. Physical dependence on nicotine in gum. A placebo substitution trial. JAMA. 1986;255:3277–3279. [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrinol Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, et al. Associations between Cloninger’s temperament dimensions and acute tobacco withdrawal. Addict Behav. 2007;32:2976–2989. doi: 10.1016/j.addbeh.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35:1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday S, Reddy SP, Ruiter RA, Bergstrom E, De Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health. 2007;40:144–150. doi: 10.1016/j.jadohealth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Baumann MH, Fant RV, Rothman RB, Henningfield JE. Endocrine responses during acute nicotine withdrawal. Pharmacol Biochem Behav. 1996;55:433–437. doi: 10.1016/s0091-3057(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV. Endocrine effects of nicotine administration, tobacco and other drug withdrawal in humans. Psychoneuroendocrinology. 1998;23:131–141. doi: 10.1016/s0306-4530(97)00075-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig JB, Seyler LE, Jaffe J. Neuroendocrine reactivity to nicotine in smokers. Psychopharmacology (Berl) 1983;81:61–67. doi: 10.1007/BF00439275. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. Br J Addict. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Seyler LE, Jr, Fertig J, Pomerleau O, Hunt D, Parker K. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34:57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology (Berl) 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Gross J. Nicotine replacement effects on post-cessation withdrawal symptoms and weight gain. NIDA Res Monogr. 1988;81:53–58. [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: effect of nicotine patches on craving and withdrawal. Psychopharmacology (Berl) 2002;164:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Thorner MO, Evans WS, Vance M, et al. Human pancreatic tumor GH-releasing factor. Acta Neurochir (Wien) 1985;75:72–80. doi: 10.1007/BF01406325. [DOI] [PubMed] [Google Scholar]
- Ussher M, Aveyard P, Reid F, et al. A randomised placebo-controlled trial of oral hydrocortisone for treating tobacco withdrawal symptoms. Psychopharmacology (Berl) 2011;216:43–51. doi: 10.1007/s00213-011-2191-x. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Munafo MR, De Wit H. Effect of social stress during acute nicotine abstinence. Psychopharmacology (Berl) 2011;218:39–48. doi: 10.1007/s00213-010-2150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin smoking withdrawal scale. Exp Clin Psychopharmacol. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 1982;78:305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- Winternitz WW, Quillen D. Acute hormonal response to cigarette smoking. J Clin Pharmacol. 1977;17:389–397. doi: 10.1002/j.1552-4604.1977.tb04621.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.