Abstract
Objective
To assess functional connectivity in cortical networks in patients with nonbothersome tinnitus compared with a normal healthy nontinnitus control group by measuring low-frequency (<0.1 Hz) spontaneous blood oxygenation level–dependent (BOLD) signals at rest.
Design
Case-control.
Setting
Academic medical center.
Participants
Nonbothersome, idiopathic subjective tinnitus for at least 6 months (n = 18) and a normal healthy nontinnitus control group (n = 23).
Main Outcome Measure
Functional connectivity differences in 58 a priori selected seed regions of interest encompassing cortical loci in the default mode, attention, auditory, visual, somatosensory, and cognitive networks.
Results
The median age of the 18 subjects was 54 years (interquartile range [IQR], 52–57), 66% were male, 90% were white, median Tinnitus Handicap Inventory (THI) score was 8 (IQR, 4–14), and a median Beck Depression Index score was 1 (IQR, 0–5). The median age for the control group was 46 years (IQR, 39–54), and 52% were male. Of the 58 seeds analyzed, no regions had significantly different functional connectivity among the nonbothersome tinnitus group when compared with the control group.
Conclusion
Among nonbothersome tinnitus patients, the tinnitus percept does not appear to alter the functional connectivity of the auditory cortex or other key cortical regions.
Keywords: tinnitus, functional, imaging, rest, connectivity, fc-MRI, resting fMRI, human
Tinnitus is more than ringing in the ear; it is associated with a variety of nonauditory symptoms that include difficulty concentrating, inability to relax, frustration, and depression.1–4 However, this may represent only a small fraction of the actual tinnitus population. According to the American Tinnitus Association, tinnitus affects nearly 50 million Americans, yet only one-third will seek medical therapy because they are bothered. Although some investigators hypothesize tinnitus patients become “bothered” either by a lack of habituation5 or abnormal limbic activity,6–8 no study has focused on patients with nonbothersome tinnitus. These patients lack the cognitive and emotional sequelae commonly seen with bothersome tinnitus and can serve as a better cohort to understand the underlying neurobiology of tinnitus.
Neuroimaging provides a noninvasive approach to studying tinnitus. Early studies captured blood flow changes in frontal, parietal, and temporal areas via positron emission tomography (PET) with lidocaine injection,9–12 behavioral task,13 and tinnitus modulating behaviors.6,14 Using radioactive glucose in PET allowed visualization of asymmetrical increased metabolic activity of the auditory cortex.15–18 However, a major shortcoming of PET is the poor spatial resolution and radiation exposure. Even though magnetic resolution imaging (MRI) is associated with significant scanner noise, MRI has gained increasing popularity in studying tinnitus as it is cheaper, quicker, and provides better resolution without harmful radiation when compared with PET. Functional MRI (fMRI) measures cerebral blood flow based on the blood oxygen level–dependent (BOLD) signal.19 The BOLD signal acts as an in vivo contrast agent; brain regions with increased neural activity use the faster anaerobic glycolysis,20 resulting in proportionally increased amounts of oxyhemoglobin (ie, the BOLD signal). In tinnitus studies, an auditory stimulus is sent to the participant through headphones; the activity before and after the stimulus is then compared. A major limitation in task-based fMRI is that participants need to be able to perform task(s) or, in the case of tinnitus, hear the stimulus. A relatively underused technique in studying tinnitus using MRI involves measuring the spontaneous BOLD signal while the participant is at rest. Small fluctuations in the spontaneous BOLD activity below 0.1 Hz, originally considered to be “noise,” are significantly correlated across engaged brain networks. In 1995, Biswal et al21 found significant resting state temporal correlations within the somatomotor network. Subsequent functional connectivity analyses of spontaneous activity (fcMRI) revealed dorsal and ventral attention,22 cognitive control,23 auditory,24 visual,24,25 somatomotor,22 and default mode networks.21,24,26,27
In our previous work, we used fcMRI to study resting state activity within a cohort of participants with bothersome tinnitus compared with a cohort of controls without tinnitus. We found alterations in sensory and cognitive control networks.28 In this study of nonbothersome tinnitus, we hypothesize that there will be no differences in functional connectivity when compared with the control group used in our bothersome tinnitus study. This “sister study” duplicates the methodology of our previous study with regard to controls used, analytical methods, and seeds of interest analyzed.
Materials and Methods
Design and Setting
This was a single-institution (Washington University), case-control study to examine differences in functional connectivity in a nonbothersome tinnitus (NBT) group compared with a normal-hearing, healthy control group without tinnitus. The institutional review board provided approval prior to recruitment.
Participants
Adult participants were enrolled from October 2010 to April 2011 and were recruited from (Washington University) audiology or otolaryngology clinics. Of the 20 recruited participants, 2 were excluded from analysis due to excessive head motion. The remaining 18 participants had nonpulsatile subjective tinnitus, unilateral (n = 6) or bilateral (n = 12), for at least 6 months. Exclusion criteria included anyone with (1) an active diagnosis of any acute or chronic brain-related neurological conditions; (2) history of head trauma, seizure, or stroke; (3) a retrocochlear lesion or anatomic/structural lesion of the brain, skull base, temporal bone, or ear; or (4) active depression or anxiety disorder or who had recently began taking medications to treat depression or anxiety. Participants completed the following American Tinnitus Association data collection forms: (1) tinnitus description and history, (2) medical and health information, and (3) hearing history and occupation exposure. Participants also completed the Beck Depression Inventory-II (BDI-II)29 to evaluate level of depression. All tinnitus participants had a recent (<12-month) audiogram and underwent a focused ear, nose, and throat physical examination.
Resting State Functional Connectivity MRI
The resting state functional connectivity MRI (rs-fcMRI) data were obtained and processed as previously described.28
Image acquisition
All images were collected on a Siemens 3 T Trio scanner (Erlangen, Germany) while participants wore noise-reducing headphones. Three 164-frame echo-planar sequence (EPI) runs recorded spontaneous brain activity while participants were awake, performed no task, and kept their eyes closed in a darkened room. Structural images included both a T1-weighted magnetization-prepared rapid gradient echo sequence acquired across 176 sagittal slices and a T2-weighted structural image obtained across 36 axial slices.
Image preprocessing and computing functional connectivity maps
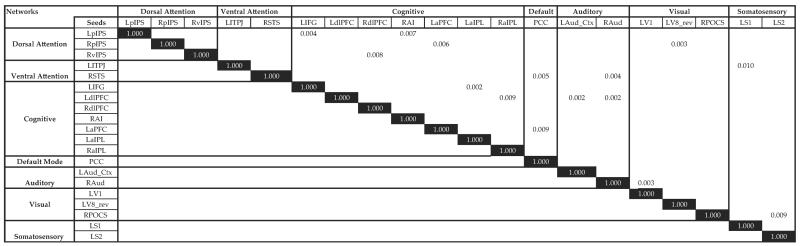
Preprocessing involved compensation for head motion, asynchronous slice acquisition, band-pass filtering to remove nuisance variables, and whole-brain signal normalization to mode 1000. Slices were resampled to 2-mm3 volumes (voxels) and registered to an atlas template by computing 12 parameter affine transforms between an average from the first frames of each EPI run and the atlas template.30 Using MP-RAGE images, an atlas template was created and conformed to Talairach atlas space.31 Fifty-eight spherical seed regions were chosen to represent established networks.21–27 An initial screening of possible group differences relied on computed temporal correlations between every pair of seed regions in each participant. The time series included 17.5 minutes of spontaneous activity and was averaged across all voxels. In the first stage of analysis, all correlations were transformed into z scores using Fisher's transformation; a t test, not corrected for multiple comparisons, evaluated group differences. In a second-stage analysis, we computed functional connectivity maps in each participant for seed regions whose paired temporal correlations had group differences with probabilities <0.01, as shown in Figure 1. The computed correlations were between the time series averaged across all voxels in a seed region and the time series in each 2-mm cubic volume in the brain.27 These voxel-based correlation values were then registered to participant-specific cortical surfaces using FreeSurfer (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts) and then deformed to the PALS-B12 atlas.32 Next, we computed a t statistic at each surface node to assess the null hypothesis that the Fisher's transform z scores for a seed region were comparable between the control and NBT groups. The t statistic was computed as the mean difference (control group z transform score minus NBT group z transform score) divided by the SEM difference. Significant clusters appear if they reach probability thresholds of 0.05 to 0.001 (t = ±1.7 and 3.3 for 39 df). We assessed the significance of clusters observed in the group contrast t statistic maps with a threshold-free cluster enhancement (TFCE) method. Clusters in the original t statistic map were judged significant at P = .05.
Figure 1.
Significant t test probabilities for the differences in connectivity within different seed regions between tinnitus and nontinnitus control groups. Black rectangles represent same-seed comparisons. Abbreviations for significant regions: LaIPL, left anterior inferior parietal lobule; LaPFC, left anterior prefrontal cortex; LAud_Ctx, left auditory cortex; LdlPFC, left dorsolateral prefrontal cortex; LIFG, left inferior frontal gyrus; LpIPS, left posterior intraparietal sulcus; LS1, left postcentral gyrus; LS2, left parietal operculum; LTPJ, left temporoparietal junction; LV1, left calcarine sulcal cortex; LV8, left fusiform gyrus; PCC, posterior cingulate cortex; RAI, right anterior insula; RaIPL, right anterior inferior parietal lobule; RAud, right auditory cortex; RdlPFC, right dorsolateral prefrontal cortex; RpIPS, right posterior intraparietal sulcus; RPOCS, right parieto-occipital sulcus; RSTS, right superior temporal sulcus; RvIPS, right ventral intraparietal sulcus.
Results
Participants
The median age of the NBT group (n = 18) was 54 years (interquartile range [IQR], 52–57), 66% were male, median Tinnitus Handicap Inventory (THI) score was 8 (IQR, 4–14), and a median BDI-II score was 1 (IQR, 0–5). A THI <16 characterizes the least severe tinnitus grade,33 and a BDI-II <14 is considered minimally depressed.34 Table 1 presents audiogram findings for each tinnitus participant. Overall, the group had a wide range of hearing loss from mild to severe. The median duration of tinnitus was 9 years and perceived loudness of 5 on a scale from 1 (low) to 10 (high). Study subjects reported a wide variety of sounds (Table 2).
Table 1.
Audiogram Findings for 18 Participants with Nonbothersome Tinnitus
| 500 Hz |
1 kHz |
2 kHz |
4 kHz |
8 kHz |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age, y | R | L | R | L | R | L | R | L | R | L | SRT in Worst Ear (Location) |
| M | 47 | 15 | 10 | 15 | 15 | 35 | 35 | 50 | 55 | 55 | 75 | 20 Equal |
| M | 56 | 10 | 5 | 10 | 5 | 15 | 10 | 35 | 35 | 60 | 60 | 10 Right |
| F | 54 | 70 | 25 | 65 | 25 | 50 | 15 | 30 | 25 | 45 | 25 | 50 Right |
| M | 58 | 20 | 20 | 20 | 20 | 20 | 0 | 35 | 45 | 50 | 60 | 15 Equal |
| M | 60 | 0 | 0 | 0 | 0 | 10 | 10 | 25 | 15 | 50 | 50 | 10 Equal |
| F | 53 | 5 | 10 | 10 | 5 | 15 | 15 | 30 | 45 | 35 | 45 | 10 Equal |
| F | 55 | 30 | 25 | 15 | 10 | 20 | 15 | 35 | 15 | 40 | 20 | NA |
| F | 55 | 0 | 10 | 5 | 5 | 5 | 5 | 35 | 25 | 65 | 50 | 10 Left |
| M | 57 | 25 | 5 | 40 | 25 | 35 | 15 | 45 | 45 | 55 | 60 | 35 Right |
| M | 60 | 0 | 5 | 5 | 15 | 20 | 35 | 55 | 70 | 75 | 65 | 20 Left |
| M | 57 | 10 | 5 | 10 | 5 | 5 | 5 | 35 | 45 | 45 | 70 | 10 Equal |
| M | 57 | 15 | 35 | 15 | 35 | 20 | 70 | 75 | 95 | 75 | 85 | 35 Left |
| M | 54 | 0 | 0 | 0 | 5 | 0 | 10 | 45 | 35 | 55 | 20 | 10 Left |
| F | 48 | 0 | 0 | 0 | 0 | 10 | 0 | 15 | 10 | 15 | 25 | 5 Equal |
| M | 52 | 5 | 5 | 10 | 0 | 0 | 0 | 15 | 15 | 60 | 50 | 10 Left |
| M | 50 | 5 | 10 | 10 | 0 | 10 | 60 | 30 | 60 | 20 | 60 | 10 Equal |
| F | 53 | 15 | 5 | 10 | 10 | 10 | 10 | 5 | 5 | 25 | 15 | 15 Right |
| M | 52 | 20 | 25 | 25 | 30 | 25 | 40 | 30 | 35 | 20 | 25 | 35 Left |
Abbreviations: F, female; M, male; NA, not available; SRT, speech reception threshold.
Table 2.
Tinnitus Characteristics of the 18 Participants with Nonbothersome Tinnitus
| THI | Duration, y | Perceived Tone | Location | Loudness (0–10) | |
|---|---|---|---|---|---|
| 4 | 6 | Ringing; hum | Both | 6 | |
| 12 | 8 | Ringing; hissing | Both | 4 | |
| 20 | 35 | Ringing; high-tension wire | Right | 5 | |
| 8 | 2 | Hissing; buzzing | Both | 3 | |
| 0 | 10 | Ringing | Both | 5 | |
| 8 | 10 | Ringing; buzzing | Both | 6 | |
| 8 | 5 | Hum; high-tension wire | Right | 3 | |
| 14 | 10 | Ringing; high-tension wire | Both | 4 | |
| 6 | 3 | Ringing; whistle | Both | 6 | |
| 4 | 15 | Ringing | Both | 5 | |
| 4 | 10 | Clear tone | Left | 2 | |
| 12 | 3 | Ringing; cicadas | Both | 4 | |
| 20 | 30 | Ringing; hissing; transformer noise | Right | 5 | |
| 4 | 1 | Ringing | Both | 7 | |
| 6 | 3 | Ringing; clicking | Both | 5 | |
| 14 | 12 | Ringing | Left | 5 | |
| 24 | 30 | Ringing; crickets; high-tension wire | Both | 7 | |
| 6 | 3.5 | Clear tone | Left | 2 | |
| Median | 8 | 9 | 5 |
Abbreviation: THI, Tinnitus Handicap Inventory.
The control group was composed of 23 individuals who served as healthy controls in our previous bothersome tinnitus study. The median age for the control group was 46 years (IQR, 39–54), and 52% were male. The control group had pure-tone average thresholds of <25 dB.
With respect to the control group, the NBT group was significantly older (Δ 8 years; 95% confidence interval [CI], 3.1–12.9, P = .002) but did not significantly differ in gender (Δ 14%, χ2 = 0.874, df = 1, P = .35).
Temporal Correlation Matrix and Functional Connectivity
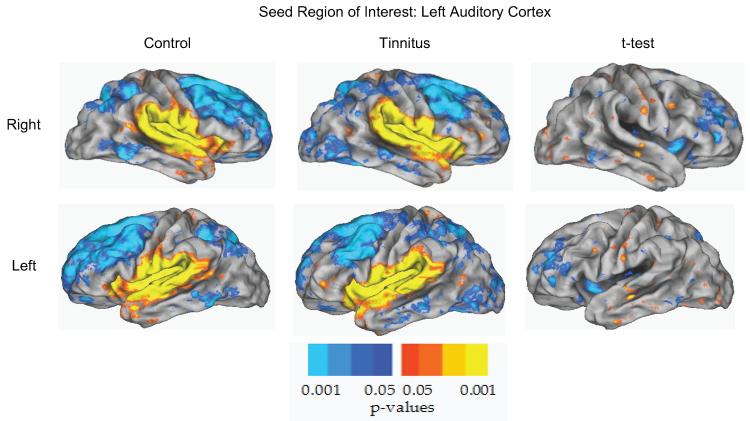
An initial screening of possible group differences relied on computed temporal correlations between every pair of seed regions in each participant. In the first stage of analysis, all correlations were transformed into z scores using Fisher's transformation and a t test evaluated group differences. Fifteen significant t tests (P < .01) shown in Figure 1 reflect the first stage of analysis. Only these significant seeds continued on to the permutation step, which included family-wide error corrections.35 None of the 15 significant seed regions from the temporal correlation were significant after the threshold-free cluster enhancement permutation analysis. Figure 2 depicts the control and NBT groups' functional connectivity maps of the left auditory cortex seed and a t test illustrating the non-significant difference between the 2 groups.
Figure 2.
Functional connectivity map for the left auditory cortex (LAud_Ctx) for the control and tinnitus groups. Functional connectivity for controls (column 1), tinnitus (column 2), and the difference between controls and tinnitus (column 3) displayed on a PALS-B12 atlas surface. The right hemisphere view (row 1), left hemisphere view (row 2), and auditory cortex seed region (black circle) are shown. The distribution of significant positive and negative correlations between time courses in the seed vs other brain regions is shown in shades of blue to yellow. The Difference column represents the difference in activity between controls and tinnitus based on Fisher z transforms of correlations. No significant clusters were identified using the left auditory cortex seed region.
Discussion
The current study examined functional connectivity in patients who often do not seek medical care because they are not bothered by their tinnitus. Using a seed-based approach, we examined 58 regions representative of established networks.21–27 When compared with a control group, there were no differences in connectivity. In contrast to our findings in a cohort with bothersome tinnitus,28 this negative finding indicates that less severe, nonbothersome tinnitus is not associated with abnormal cortical activity.
Prior imaging6,9,17,36,37 and behavioral38–41 studies commonly capture tinnitus severity but do not fully appreciate the wide variation of tinnitus severity. For example, the study population in Rossiter et al41 had a mean (SD) Tinnitus Reaction Questionnaire3 (TRQ) score of 36 (22), indicating moderate tinnitus severity, but a TRQ range of 0 to 74. This range spanned from those with bothersome tinnitus (TRQ of 74) to those with nonbothersome tinnitus (TRQ of 0). Consequently, it is unclear how to interpret the results of this study that found slower reaction times and poorer accuracy in a dual-task setting when compared with controls. In light of our recent findings, we propose that the findings by Rossiter et al would be even more robust in a more homogeneous cohort (ie, severe tinnitus). Inconsistent tinnitus findings in neuroimaging and behavioral studies may reflect the incorporation of highly variable tinnitus severities into these studies.
Origin of the “bother” in tinnitus
Andersson and McKenna42 proposed that tinnitus becomes annoying when it interferes with thinking and is more likely to become a problem in those who have an overall anxious or pessimistic outlook on life. This model highlights the heterogeneity of clinical complaints that range from difficulty in concentration to insomnia. Supportive of this model is our identification of significant functional cortical network connectivity differences among bothered (ie, annoyed) tinnitus patients28 that were not replicated in the nonbothersome tinnitus cohort.
Finding cortical network disruptions among bothered tinnitus patients may indicate a failed attempt of adaption or habituation toward the auditory percept. In the bothered tinnitus cohort,28 we found altered activity within the ventral attention network (VAN), which is important for involuntarily reorienting to salient stimuli.43,44 Disruptions of the VAN may explain the difficulty these patients have with responding or reorienting to unexpected stimuli, such as missing a phone call while in deep concentration (ie, a dual-task scenario as in Rossiter et al41). The dorsal attention network (DAN) is important for voluntarily shifting attention.43,44 Components of the DAN were not affected in our bothersome tinnitus study.28 An intact DAN explains why people with tinnitus can briefly “control” their tinnitus long enough to perform a specific task but often do poorly in dual-task situations in which they are required to maintain or shift attention.38,41 Difficulty in shifting attention in the bothered tinnitus group is supported by the disruption of the salience network (ie, frontoinsular network). The salience network helps maintain and adjust attention and has been proposed as a key component in tinnitus reaching consciousness by other researchers.45 Together, these functional connectivity findings support the model of annoyance proposed by Andersson and McKenna.42
Limitations
There are several limitations to our work. First, we only analyzed cortical activity between the 2 groups and did not explore deep brain or brainstem activity. In animal models, changes in activity of the dorsal46 or ventral47 cochlear nucleus have been proposed to play a role in tinnitus. There is also evidence from fMRI studies that tinnitus may originate in the inferior colliculus36,37,48 and cerebellum49 or arise from hyperactivity within the dorsal and ventral cochlear nucli.50,51 In addition, our analysis, based on a threshold-free cluster enhancement permutation technique,35 is very powerful but prone to type II (false-negative) errors. Consequently, this permutation analysis may be unable to detect small functional connectivity cluster differences between 2 groups. However, we published an fcMRI study of patients with bothersome tinnitus28 using similar methodology and found several dissociations not seen in the current study. The fact that differences were found in the bothersome cohort suggests our methodology is robust.
Conclusions and future directions
This analysis of resting state fcMRI found that patients with nonbothersome tinnitus do not exhibit a global cortical disorganization as seen in our previous study of bothered tinnitus patients. These complementary neuroimaging findings support a model of tinnitus wherein the level of annoyance relates to neurobiological changes as proposed by Andersson and McKenna.42 The current negative finding in patients with nonbothersome tinnitus and our previous findings in patients with bothersome tinnitus highlight the heterogeneity of this condition. Together these studies emphasize the need to incorporate techniques to identify unique subgroups of tinnitus patients based on the severity of the cognitive and emotional impairments, which we believe reflect the underlying neurobiology of this condition. Imaging, neurocognitive, or intervention studies in humans that recognize the heterogeneity of functional, cognitive, and emotional complaints in tinnitus are more likely to be informative than studies that ignore heterogeneity.
Acknowledgments
Sponsorships: AAO-HNS, NIH-NIDCD (R01 DC009095).
Funding source: 2010 Percy Memorial Research Award, Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32) titled “Development of Clinician/Researchers in Academic ENT” (5T32DC000022-22).
Trial Registration. ClinicalTrials.gov Identifier: NCT01049828
Footnotes
Author Contributions Andre M. Wineland, substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of the version to be published; Harold Burton, contributed to interpretation of data, critical manuscript revising, and final approval of the version to be published; Jay Piccirillo, substantial contributions to conception and design, critical manuscript revising, and final approval of the version to be published.
Disclosures Competing interests: None.
References
- 1.Gatehouse S. The role of non-auditory factors in measured and self-reported disability. Acta Otolaryngol Suppl. 1990;476:249–256. [PubMed] [Google Scholar]
- 2.Jacobson GP, Calder JA, Newman CW, Peterson EL, Wharton JA, Ahmad BK. Electrophysiological indices of selective auditory attention in subjects with and without tinnitus. Hear Res. 1996;97:66–74. [PubMed] [Google Scholar]
- 3.Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34:197–201. [PubMed] [Google Scholar]
- 4.Tyler RS, Baker LJ. Difficulties experienced by tinnitus sufferers. J Speech Hear Disord. 1983;48:150–154. doi: 10.1044/jshd.4802.150. [DOI] [PubMed] [Google Scholar]
- 5.Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy: a different view on tinnitus [Review] ORL J Otorhinolaryngol Relat Spec. 2006;68:23–29. doi: 10.1159/000090487. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 7.Haab L, Wallhausser-Franke E, Trenado C, Strauss DJ. Modeling limbic influences on habituation deficits in chronic tinnitus aurium. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4234–4237. doi: 10.1109/IEMBS.2009.5332696. [DOI] [PubMed] [Google Scholar]
- 8.Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirz F, Pedersen B, Ishizu K, et al. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–144. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 10.Andersson G, Lyttkens L, Hirvela C, Furmark T, Tillfors M, Fredrikson M. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol. 2000;120:967–972. doi: 10.1080/00016480050218717. [DOI] [PubMed] [Google Scholar]
- 11.Reyes SA, Salvi RJ, Burkard RF, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171:43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 12.Plewnia C, Reimold M, Najib A, et al. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2007;28:238–246. doi: 10.1002/hbm.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson G, Juris L, Classon E, Fredrikson M, Furmark T. Consequences of suppressing thoughts about tinnitus and the effects of cognitive distraction on brain activity in tinnitus patients. Audiol Neurootol. 2006;11:301–309. doi: 10.1159/000094460. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood AH, Wack DS, Burkard RF, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- 15.Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–199. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Tian J, Yin D. Positron emission tomography of tinnitus-related brain areas [in Chinese] Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35:420–424. [PubMed] [Google Scholar]
- 17.Langguth B, Eichhammer P, Kreutzer A, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus—first results from a PET study. Acta Otolaryngol Suppl. 2006;(556):84–88. doi: 10.1080/03655230600895317. [DOI] [PubMed] [Google Scholar]
- 18.Smith JA, Mennemeier M, Bartel T, et al. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope. 2007;117:529–534. doi: 10.1097/MLG.0b013e31802f4154. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 21.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 22.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 26.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton H, Dixit S, Litkowski P, Wingert JR. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res. 2009;26:90–104. doi: 10.3109/08990220903335742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012;13:3. doi: 10.1186/1471-2202-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steer RA, Ball R, Ranieri WF, Beck AT. Further evidence for the construct validity of the Beck Depression Inventory-II with psychiatric outpatients. Psychol Rep. 1997;80:443–446. doi: 10.2466/pr0.1997.80.2.443. [DOI] [PubMed] [Google Scholar]
- 30.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 31.Talairach J, Tousignant P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York: 1988. [Google Scholar]
- 32.Hill J, Dierker D, Neil J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCombe A, Baguley D, Coles R, et al. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin Otolaryngol Allied Sci. 2001;26:388–393. doi: 10.1046/j.1365-2273.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Smits M, Kovacs S, DeRidder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus [see comment] Neuroradiology. 2007;49:669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 37.Lanting CP, DeK leine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008;128:415–421. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- 38.Hallam RS, McKenna L, Shurlock L. Tinnitus impairs cognitive efficiency. Int J Audiol. 2004;43:218–226. doi: 10.1080/14992020400050030. [DOI] [PubMed] [Google Scholar]
- 39.Stevens C, Walker G, Boyer M, Gallagher M. Severe tinnitus and its effect on selective and divided attention. Int J Audiol. 2007;46:208–216. doi: 10.1080/14992020601102329. [DOI] [PubMed] [Google Scholar]
- 40.Cuny C, Norena A, El Massioui F, Chéry-Croze S. Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurootol. 2004;9:294–302. doi: 10.1159/000080267. [DOI] [PubMed] [Google Scholar]
- 41.Rossiter S, Stevens C, Walker G. Tinnitus and its effect on working memory and attention. J Speech Lang Hear Res. 2006;49:150–160. doi: 10.1044/1092-4388(2006/012). [DOI] [PubMed] [Google Scholar]
- 42.Andersson G, McKenna L. The role of cognition in tinnitus. Acta Otolaryngol Suppl. 2006;(556):39–43. doi: 10.1080/03655230600895226. [DOI] [PubMed] [Google Scholar]
- 43.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 45.De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psycho-physical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Baek JH, Smith PF, Darlington CL. Cannabinoid receptor down-regulation in the ventral cochlear nucleus in a salicylate model of tinnitus. Hear Res. 2007;228:105–111. doi: 10.1016/j.heares.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Melcher JR, Sigalovsky IS, Guinan JJ Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- 49.Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl. 2006;(556):20–26. doi: 10.1080/03655230600895309. [DOI] [PubMed] [Google Scholar]
- 51.Bledsoe SC, JrM, Koehler S, Tucci DL, Zhou J, Le PC, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102:886–900. doi: 10.1152/jn.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]