Abstract
Fine structural details of glycans attached to the conserved N-glycosylation site significantly not only affect function of individual immunoglobulin G (IgG) molecules but also mediate inflammation at the systemic level. By analyzing IgG glycosylation in 5,117 individuals from four European populations, we have revealed very complex patterns of changes in IgG glycosylation with age. Several IgG glycans (including FA2B, FA2G2, and FA2BG2) changed considerably with age and the combination of these three glycans can explain up to 58% of variance in chronological age, significantly more than other markers of biological age like telomere lengths. The remaining variance in these glycans strongly correlated with physiological parameters associated with biological age. Thus, IgG glycosylation appears to be closely linked with both chronological and biological ages. Considering the important role of IgG glycans in inflammation, and because the observed changes with age promote inflammation, changes in IgG glycosylation also seem to represent a factor contributing to aging.
Significance Statement
Glycosylation is the key posttranslational mechanism that regulates function of immunoglobulins, with multiple systemic repercussions to the immune system. Our study of IgG glycosylation in 5,117 individuals from four European populations has revealed very extensive and complex changes in IgG glycosylation with age. The combined index composed of only three glycans explained up to 58% of variance in age, considerably more than other biomarkers of age like telomere lengths. The remaining variance in these glycans strongly correlated with physiological parameters associated with biological age; thus, IgG glycosylation appears to be closely linked with both chronological and biological ages. The ability to measure human biological aging using molecular profiling has practical applications for diverse fields such as disease prevention and treatment, or forensics.
Key Words: Aging, Glycome, Glycosylation, Immunoglobulin G, Inflammation.
Aging is a complex process of accumulation of molecular, cellular, and organ damage, leading to loss of function and increased vulnerability to disease and finally to death (1). It is well known that lifestyle choices such as smoking and physical activity can hasten or delay the aging process (2). Such observations have led to the search for molecular markers of age that can be used to predict, monitor, and provide insight into age-associated physiological decline and disease.
Protein structure is defined by the sequence of nucleotides in the corresponding genes, thus the polypeptide sequence of a protein cannot change with age. However, an important structural and functional element of the majority of proteins are the glycans that participate in virtually all physiological processes (3). Glycans are product of a complex pathway that involves hundreds of different proteins and are encoded in a complex dynamic network of hundreds of genes (4). Epigenetic regulation of gene expression is expected to affect protein glycosylation and several publications recently reported this effect (5–8). Changes in glycosylation with age have been shown over 20 years ago (9) and have also replicated in recent large population studies (10–13).
Immunoglobulin G (IgG) is an excellent model glycoprotein because its glycosylation has been well defined (Figure 1), and many important functional effects of alternative IgG glycosylation have been described (14). For example, glycosylation acts as a switch between pro- and anti-inflammatory IgG functionality. Most of the IgG molecules are not sialylated and are proinflammatory. Terminal α2,6-sialylation of IgG glycans decreases the ability of IgG to bind to activating FcγRs and promotes recognition by DC-SIGN, which increases expression of inhibitory FcγRIIB and is anti-inflammatory (15). Another fascinating example is the role of core fucose in the modulation of antibody-dependent cellular cytotoxicity: IgG-containing glycans that lack core fucose have 100-fold increased affinity for FcγRIIIA and are therefore much more efficient in activating antibody-dependent cellular cytotoxicity than fucosylated glycoforms of the same molecule (16). On average, 95% of the IgG population is core fucosylated (12); thus, most of the immunoglobulins have a “safety switch,” which prevents them from activating antibody-dependent cellular cytotoxicity. Malfunction of this system appears to be associated with autoimmune diseases as indicated by both pleiotropic effects of genes that associate with IgG glycosylation on different inflammatory and autoimmune diseases, and the observed alterations in IgG glycosylation in systemic lupus erythematous (17) and many inflammatory diseases (18).
Figure 1.
UPLC analysis of immunoglobulin G (IgG) glycosylation. Each IgG contains one conserved N-glycosylation site on Asn197 of its heavy chain. Different glycans can be attached to this site and the process seems to be highly regulated. UPLC analysis can reveal composition of the glycome attached to a population of IgG molecules by separating total IgG N-glycome into 24 chromatographic glycan peaks (GP1–GP24), mostly corresponding to individual glycan structures.
Interindividual variability of IgG glycosylation in a population is large (12) and it appears to be affected by both variation in DNA sequence (19) and environmental factors (11). Most of the studies that investigated glycosylation changes with age were either of limited size or were performed on the total plasma glycome; thus, in addition to changes in glycosylation, the observed differences reflected changes in the concentration of individual plasma proteins. In this study, we focused on glycosylation of IgG and analyzed more than 5,000 individuals from four different European populations to provide definitive data about changes in IgG glycosylation through the lifetime.
Results
Changes in IgG Glycosylation With Age in Four Large Human Populations
IgG was isolated from plasma of 906 individuals from Croatian island Vis, 915 individuals from Croatian island Korcula, 2,035 individuals from Orkney Islands in Scotland, and 1,261 twins (560 monozygotic and 698 dizygotic) from the TwinsUK cohort. N-linked glycans were released, labeled with 2-aminobenzamide (2-AB), and analyzed by ultra performance liquid chromatography (UPLC) as described previously (12). A representative chromatogram with structures of all identified glycans is shown in Figure 1. More detailed structural explanation is presented in Supplementary Table S1. After correction for multiple testing, statistically significant associations with age were observed for 21 out of 24 directly measured glycan structures (Table 1, Figure 2, Supplementary Fig ure S1). These associations replicated very well between the four populations, though some population-specific differences existed. The strongest association with age was observed in the level of galactosylation. Nongalactosylated glycans (A2 and FA2) steadily increased with age, whereas digalactosylated glycans (A2G2, FA2G2, A2BG2, and FA2BG2) decreased with age. However, with monogalactosylated structures (A2G1 FA2G1, A2BG1, and FA2BG1), the situation was more complex, with some glycans increasing and some decreasing depending on the position of galactose and the presence of bisecting GlcNAc (Supplementary Figure S1). The observed changes were quite different in men and women, with more dynamic changes in women (Figure 2). The most pronounced age associations in IgG glycosylation in women occurred between the ages of 45 and 55 when the majority of women enter menopause. All other elements of IgG glycosylation (fucosylation, sialylation, and bisecting GlcNAc) also strongly associated with age, sometimes in a quite complex manner (Supplementary Fig ure S1).
Table 1.
Associations of Glycans With Age
| Glycan | Korcula | Orkney | TwinsUK | Vis | ||||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | |
| GP1 | .128 | 1.00E-04 | .372 | 1.55E-57 | .264 | 6.65E-18 | .024 | 4.70E-01 |
| GP2 | .332 | 7.31E-26 | .492 | 3.13E-114 | .438 | 1.59E-55 | .344 | 7.64E-27 |
| GP4 | .555 | 1.77E-85 | .645 | 7.04E-266 | .622 | 4.25E-152 | .574 | 5.02E-87 |
| GP5 | .003 | 1.73E-01 | .414 | 1.07E-73 | .335 | 5.69E-30 | .003 | 9.29E-01 |
| GP6 | .553 | 4.47E-81 | .713 | <1E-300 | .657 | 6.84E-185 | .549 | 5.43E-76 |
| GP7 | –.030 | 2.35E-03 | .181 | 3.68E-18 | .174 | 1.09E-07 | .019 | 4.37E-02 |
| GP8 | –.193 | 6.49E-08 | –.171 | 1.92E-12 | –.028 | 6.86E-03 | –.231 | 2.71E-11 |
| GP9 | .010 | 3.91E-01 | .024 | 3.89E-08 | .008 | 2.30E-03 | –.061 | 9.06E-02 |
| GP10 | .141 | 2.33E-07 | .335 | 4.69E-46 | .183 | 2.79E-10 | .043 | 1.69E-01 |
| GP11 | .264 | 1.05E-17 | .481 | 4.31E-113 | .314 | 3.77E-27 | .247 | 5.36E-14 |
| GP12 | –.308 | 2.44E-24 | –.227 | 8.74E-36 | –.260 | 2.21E-17 | –.241 | 1.13E-14 |
| GP13 | –.151 | 1.55E-07 | –.406 | 2.60E-82 | –.277 | 5.00E-21 | –.081 | 1.59E-02 |
| GP14 | –.599 | 9.20E-104 | –.716 | <1E-300 | –.656 | 9.56E-182 | –.624 | 6.75E-108 |
| GP15 | –.306 | 1.55E-22 | –.354 | 5.47E-64 | –.424 | 6.67E-51 | –.432 | 9.99E-42 |
| GP16 | .094 | 5.82E-03 | –.005 | 5.68E-06 | –.145 | 1.37E-05 | .031 | 1.55E-01 |
| GP17 | .031 | 8.81E-02 | .005 | 2.26E-09 | –.159 | 3.75E-06 | .043 | 4.55E-01 |
| GP18 | –.564 | 2.86E-87 | –.665 | 1.56E-295 | –.648 | 6.40E-177 | –.607 | 9.62E-100 |
| GP19 | .022 | 5.07E-01 | .020 | 4.05E-01 | .132 | 1.49E-04 | .057 | 9.11E-02 |
| GP20 | .005 | 1.38E-01 | –.212 | 6.35E-20 | –.121 | 3.03E-03 | –.047 | 2.27E-01 |
| GP21 | –.016 | 1.14E-02 | –.067 | 1.68E-02 | –.170 | 3.49E-05 | .052 | 1.25E-01 |
| GP22 | .033 | 4.17E-02 | .033 | 1.24E-02 | –.038 | 2.05E-01 | –.002 | 7.93E-02 |
| GP23 | –.313 | 4.39E-21 | –.355 | 3.83E-55 | –.293 | 5.03E-21 | –.276 | 4.99E-17 |
| GP24 | .032 | 3.28E-01 | .114 | 7.67E-06 | .058 | 4.17E-02 | 0029 | 3.88E-01 |
Note: GP1-24 = glycan peak 1-24; R = correlation coefficient; p = p-value.
Figure 2.
Relationship between age and selected glycan structures. Plots indicate associations between the individual contributions of four selected glycan structures to the total immunoglobulin G glycome and chronological age in four different human populations. Blue and red curves are fitted local regression models describing gender-specific relationship between age and glycan structure. The shaded region is a pointwise 95% confidence interval on the fitted values (there is 95% confidence that the true regression curve lies within the shaded region).
To verify that longitudinal changes in IgG glycosylation within an individual follow trends observed at the population level, we have analyzed IgG glycosylation in 26 individuals first sampled in year 2003 and then again in year 2013. Two time points of relative levels of selected glycans in the same individuals are presented in Figure 3 and Supplementary Fig ure S2. In the majority of individuals, the change was very well aligned with changes observed at the population level, with women displaying more intensive changes than men.
Figure 3.
Individual changes in immunoglobulin G (IgG) glycans with age. Two time points of relative levels of glycans (percentages of individual glycan structures within the total IgG glycome), which associated strongly with age within an individual, are presented on the background showing levels of the same glycans for different individuals from the same population.
Prediction of Chronological Age From IgG Glycosylation
Because most of the analyzed glycans strongly associated with age, we attempted to build a predictive model of aging on the ORCADES cohort (2,035 individuals) using a multivariate analysis of covariance. The maximal model included 96 gender-specific glycomic parameters. The backward regression was performed and the best minimal model was determined according to the Akaike’s information criterion (20). The minimal model was trained and validated by repeated random subsampling, using two thirds (1,357) of samples as the “training set” and one third (678) of samples as the “validation set.” In 1,000 replicas, regression coefficients were estimated on the training set and goodness of fit was obtained by applying the model estimated on the training set to the validation set. Final model estimates were calculated as median values of 1,000 iterations of random subsampling. The resulting GlycanAge index is composed of three glycan variables:
 |
 |
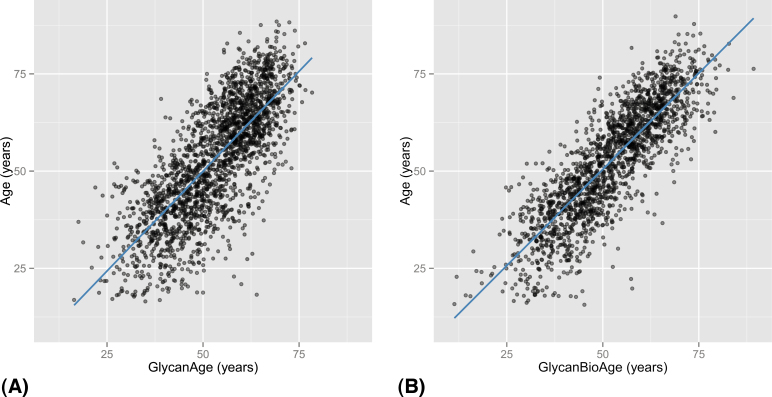
All three glycan structures selected by the optimal model were shown to be strongly associated with age, but each of the three glycan structures revealed different patterns between genders. The model explained 58% of variation in chronological age (with 95% of replicas giving R 2 between 54% and 61%), with correlation between age and predicted age of .76 (.73–.78) (Table 3). When applied to the full Orkney data set, the standard deviation (SD) of residuals was 9.7 years (Figure 4A). The predictive power of our GlycanAge index was better for women (64% of variance explained) than for men (49% of variance explained).
Table 3.
Goodness-of-Fit and Spearman’s Correlations of Chronological Age and Age Predicted by Various Models
| Training | Test | Population | Female | Male | |||
|---|---|---|---|---|---|---|---|
| R 2 | Correlation | R 2 | Correlation | R 2 | Correlation | ||
| GlycanAge Index | |||||||
| Orkney | Orkney | 57.8% [54.1–61.3%] | .76 [.73 to .78] | 64.0% [60.0–68.1%] | .80 [.77 to 0.82] | 49.4% [41.9–56.7%] | .70 [.64 to .75] |
| Orkney | Korcula | 41.3% | .64 | 50.6% | .71 | 25.2% | .50 |
| Orkney | Vis | 41.5% | .64 | 49.1% | .70 | 31.4% | .56 |
| Orkney | TwinsUK | 48.0% | .69 | 48.0% | .69 | NA | NA |
| Korcula | Korcula | 42.9% [34.2–49.7%] | .65 [.58 to .70] | 51.1% [40.5–58.9%] | .71 [.63 to 0.76] | 26.8% [16.1–36.3%] | .51 [.40 to .60] |
| Vis | Vis | 43.0% [36.6–51.0%] | .65 [.60 to .71] | 49.8% [41.3–57.8%] | .70 [.64 to .76] | 34.6% [24.6–46.2%] | .58 [.49 to .67] |
| TwinsUK | TwinsUK | 49.5% [42.9–55.3%] | .70 [.65 to 0.74] | 49.5% [42.9–55.3%] | .70 [.65 to .74] | NA | NA |
| Combined Glycan Age Index | |||||||
| Orkney | Orkney | 71.3% [68.2–73.9%] | .84 [.83 to 0.86] | 75.7% [72.7–78.4%] | .87 [.85 to .89] | 63.7% [57.1–69.2%] | .80 [.76 to .83] |
| Orkney | Korcula | 64.5% | .80 | 69.5% | .83 | 56.3% | .75 |
| Korcula | Korcula | 65.2% [59.7–70.0%] | .81 [.77 to 0.84] | 69.6% [63.8–74.8%] | .83 [.80 to .86] | 57.4% [46.5–67.0%] | .76 [.68 to .82] |
Note: Numbers in brackets are 95% range of 1,000 replicas of random subsampling validation.
Figure 4.
Prediction of chronological age from measured immunoglobulin G glycans. Using the simplified GlycanAge model, predicted age was calculated for 2,035 individuals from the Orkney cohort and plotted against real chronological age of the same individual (A). The prediction of age was further improved by inclusion of forced expiratory volume and systolic blood pressure (B).
The same model was tested on the remaining three populations (Vis = 906, Korcula = 915, and TwinsUK = 1,261). Samples from these populations were processed in the same way as samples from Orkney and used to predict age with a model that was trained on the Orkney cohort. The new predictions based on the three independent populations were somewhat lower, with correlation between age and predicted age of .64, .64, and .69 for Vis, Korcula, and TwinsUK, respectively. To see whether underperformance of prediction in these three populations was due to overspecificity of the trained model, starting from the same minimal model as in ORCADES and repeating the same procedure, we built additional models for each of the cohorts. Three models, trained separately on Vis, Korcula, and TwinsUK cohort, showed only marginally better prediction performance on their training cohorts (R = .65, .65, and .70) compared with the model trained on the Orkney cohort. This indicates two things. First, different populations vary slightly in their associations between age and glycans. Glycans are more strongly associated with age in the Orkney population than in other three populations, and so accuracy of the age prediction in Orkney population is the highest. Secondly, although each population shows a different strength of association between age and glycans, glycan profiles change in similar way through lifetime across all four populations. As a consequence, a model trained on one population is capable of explaining almost all of existing association between age and glycans in another population. In our case, a model trained on Orkney cohort explained 42%, 41%, and 48% of variation of age in Vis, Korcula, and TwinsUK cohorts compared with 43%, 43%, and 50% of variation explained by models trained specifically on these three populations.
The same model was applied to a subpopulation of individuals from Vis who were sampled again in 2013, 10 years after the original sampling in 2003. Although chronological difference between two samplings was 10 years for all individuals, the median value of age difference predicted from the GlycanAge index was 9.6 years for women and 0.6 years for men (Supplementary Table S2).
IgG Glycosylation and Biological Age
To identify factors that may be responsible for the remaining variability in the GlycanAge index, we performed an association analysis with all available biochemical and physiological traits in our databases. Associations with statistical significance after correction for multiple testing are shown in Table 2. Virtually all traits with strong association with GlycanAge in one or more studied populations (insulin, HbA1c, BMI, triglycerides, etc.) are known to be associated with unhealthy lifestyles.
Table 2.
Association of GlycanAge Index With Biochemical and Physiological Traits After Correcting for Chronological Age and Sex
| Orkney | Vis and Korcula | |||
|---|---|---|---|---|
| Beta | p | Beta | p | |
| Insulin | 0.0755 | 9.22E-08 | 0.0402 | 3.50E-01 |
| Fibrinogen | 0.0157 | 1.98E-06 | 0.0167 | 8.83E-05 |
| HbA1c | 0.1106 | 2.63E-06 | 0.0084 | 3.16E-03 |
| BMI | 0.0585 | 1.67E-04 | 0.0344 | 1.04E-02 |
| Triglycerides | 0.0092 | 1.75E-04 | 0.0140 | 1.20E-04 |
| Glucose | 0.0113 | 2.09E-04 | 0.0091 | 4.77E-02 |
| Waist circumference | 0.1468 | 2.08E-04 | ||
| Calcium | 0.0010 | 2.35E-04 | 0.0002 | 7.04E-01 |
| D-dimer | 2.9670 | 8.24E-04 | ||
| Cholesterol | 0.0036 | 3.07E-01 | 0.0201 | 5.51E-08 |
| LDL | 0.0031 | 3.26E-01 | 0.0146 | 6.08E-06 |
| Uric acid | 1.0773 | 4.02E-02 | 0.7620 | 9.68E-04 |
Note: HbA1c = glycosylated hemoglobin; BMI = body mass index; LDL = low-density lipoprotein; p = p value; beta = regression coefficient.
Both glycans and chronological age correlated significantly with a number of these parameters (Supplementary Table S3); therefore, we attempted to build a model that would combine biological information in glycans and other biological parameters. The inclusion of forced expiratory volume in the first second (FEV1) and systolic blood pressure into the model significantly improved the prediction of chronological age. The extended model was trained and validated in the same way as the GlycanAge index and explained 71% (68%–74%) of variation in chronological age of the Orkney cohort, with correlation between age and predicted age of .84 (.83–.86). Just as for glycan age, the predictive power of this model was better for women (76% of variance explained) than for men (64% of variance explained). Using a minimal model constructed of two glycans and two biological parameters was
 |
 |
The model was tested on the Korcula cohort and the correlation between age and age predicted with the model trained on the Orkney cohort was .80. The model trained on the Korcula cohort explained 65% of variation in age in that cohort (70% in women and 57% in men), with a correlation of chronological and predicted age of .81 (.83 for women and .76 for men). An overview of all results, together with corresponding 95% range of replicas, can be seen in Table 3.
Discussion
Fine details of glycan structures attached to IgG Fc promote binding of IgG to different receptors and in this way modulate action of the immune system (14,21). Individual variability in IgG glycosylation is large (12) and this variability in glycosylation contributes to individual differences in function of the immune system (22,23). As it was clearly demonstrated for intravenous immunoglobulin therapy even slight changes in IgG glycosylation can direct pro- and anti-inflammatory actions of immunoglobulins (24). IgG Fc glycosylation modulates inflammation (25,26) and through promotion or suppression of inflammation, it may significantly contribute to the process of biological aging (27–29). This hypothesis is further supported by the observed decrease in galactosylation in some premature ageing syndromes (13,30).
Up to 50% of plasma glycome variability is estimated to be heritable (12,31). The remaining variability is apparently caused by environmental factors, but the observed long-term stability of the glycome (32) argues in favor of long-term epigenetic regulation of the glycan biosynthesis pathways (33). As we have shown in this study, a large part of this nongenetic variability can be explained by age and physiological variables related to age. In the total plasma glycome, as opposed to the IgG glycome, age was reported to have only minor effects on most of the glycans (31). Nearly all IgG glycans (21 out of 24 directly measured), however, were significantly affected by age (Table 1). The heavily influenced feature of IgG glycosylation was galactosylation, which decreased to less than 50% of its maximal value through lifetime. The decrease in IgG galactosylation with age was initially reported more than 25 years ago (9) and replicated in a number of subsequent studies (34). Recently we reported that in childhood and adolescence, the galactosylation of IgG glycans actually increases with age (35). Indeed, from the population studies, it appears that total galactosylation is increasing until early adulthood (mid to late 20s) and decreases afterward. However, the situation is apparently more complex because the pattern of changes in men and women differ considerably (Figure 2). Also, galactosylated glycans with (GP15) and without bisecting GlcNAc (GP14) have different patterns of change.
Galactosylation strongly decreases proinflammatory function of IgG (15); thus, both age-related and individual variation in the decrease of IgG galactosylation with age is affecting inflammation and can actively contribute to the deterioration of an aging organism through the inflammation-based process named inflammaging (27,36). The decrease in expression of the corresponding galactosyltransferase (β4-GalT1) is clearly involved (37), but the reason and molecular mechanisms underlying such dramatic age changes in IgG galactosylation are not known. Our recent genome-wide association study of IgG glycosylation identified polymorphisms in IL6ST, SMARCD3, HLA-DQA2/B2, and BACH2 that affect IgG galactosylation (17). None of these genes was previously reported to be involved in IgG glycosylation; thus, their functional role in this process is unknown. Recent study of genome-wide methylation profiles identified a number of genes whose expression changed significantly with aging (38) and BACH2 was one of them. The combined observations that (i) BACH2 affects IgG galactosylation and (ii) that epigenetic regulation of BACH2 expression is changing with age suggest a new and intriguing hypothesis that changes in IgG galactosylation may actually represent a by-product of some other age-driven processes. In our data set, array methylation data for BACH2 and glycans were available for only 310 individuals, but even on this small data set and despite the “methylation noise” due to genetic and lifestyle factors, and DNA from other types of leukocytes, several significant associations between glycans and BACH2 promoter methylation were observed (Supplementary Table S4). With current knowledge, it is not possible to speculate whether associations of BACH2 promoter methylation changes with age and IgG glycosylation is causal or just a secondary by-product of BACH2 function. If changes in IgG glycosylation would be caused by changes in BACH2 promoter methylation status with aging, they would contribute to the deterioration of the organism with age by promoting inflammation, as proposed by Franceschi and colleagues (28,29).
The accurate prediction of chronological and biological ages from biochemical parameters is a priority in the aging field (39–41). Here, we introduce a novel GlycanAge index that combines one nongalactosylated glycan (GP6) and two digalactosylated glycans (GP14 and GP15). This index predicts chronological age with error of 9.7 years and importantly explains 58% of variation in chronological age and sex. The explanation of nearly 60% of variance of age is impressive compared with other so-called age biomarkers like telomere length where most studies show 15%–25% (42); thus, our new GlycanAge index appears to be more closely related to age than telomere lengths. After correcting for chronological age, the GlycanAge index correlated strongly with physiological parameters associated with measurements related to biological age (Table 2); thus, IgG glycosylation appears to be closely linked with both chronological and biological ages. We have further supported this by including forced expiratory volume in first second and systolic blood pressure in our prediction model, which significantly improved prediction and cumulatively explained 71% of variation in chronological age (Figure 4B).
The GlycanAge index, which includes only three glycans, seems to be a practical way to address both chronological and biological ages of an individual. The ability to measure human biological aging using molecular profiling has practical applications for diverse fields such as disease prevention and treatment, or forensics. The evaluation of aging immune system is here particularly interesting because glycosylation strongly affects function of immunoglobulins (14) and the immune system in general (43). Whether aging causes changes in glycosylation of IgG or do glycosylation changes in IgG contribute to aging by promoting inflammation is very difficult to distinguish without longitudinal studies. Even though both of these aspects are probably partly true, it is clear that changes that occur in IgG glycosylation with aging are closely regulated and integrated with other physiological processes in the body.
Materials and Methods
Human Samples
This study was based on plasma samples obtained from 906 individuals (377 men and 529 women) from Croatian island Vis, 915 individuals (320 men and 595 women) from Croatian island Korcula (44), 2,035 individuals (797 men and 1238 women) from the Orkney Islands in Scotland, and 1,261 female twins (560 monozygotic and 698 dizygotic) from the TwinsUK cohort (45). The participants from Vis cohort were men and women between 18 and 88 years of age (median age 56.0 and SD 15.2) and 18 and 93 years of age (median 56.5, SD 16.1), respectively. Male participants of Korcula cohort were aged between 20 and 90 (median age 57.8, SD 14.4) and female participants between 18 and 98 years of age (median 55.4, SD 14.0). In Orkney cohort, men were aged between 16 and 100 (median 53.7, SD 15.1) and women between 17 and 97 years of age (median 52.9 and SD 15.3). TwinsUK cohort consisted of women aged between 27 and 83 years, with mean age of 58.6 and SD of 9.5.
A subset of samples from the Croatian island Vis (n = 26) were sampled twice, first time in 2003 and second time in 2013. All human participants included in this study signed an informed consent and the study was approved by the appropriate Ethics Committee of the University of Split Medical School, Institute for Anthropological Research, Research Ethics Committees in Orkney and Aberdeen, and Kings College London. This study conformed to ethical guidelines of the 1975 Declaration of Helsinki.
Analysis of IgG glycans
Isolation of IgG from human plasma.—
The IgG was isolated using protein G monolithic plates (BIA Separations, Ajdovščina, Slovenia) as described previously (12). Briefly, 50–90 µL of plasma was diluted 10× with 1× phosphate-buffered saline, pH 7.4, applied to the protein G plate and instantly washed with 1× phosphate-buffered saline, pH 7.4, to remove unbound proteins. IgGs were eluted with 1mL of 0.1M formic acid (Merck, Darmstadt, Germany) and neutralized with 1M ammonium bicarbonate (Merck).
Glycan release and labeling.—
Glycan release and labeling of Korčula and Vis IgG samples was performed essentially as described by Royle and coworkers (46). Briefly, IgG was immobilized in a block of sodium dodecyl sulfate–polyacrylamide gel and N-glycans were released by digestion with PNGase F (ProZyme, Hayward, CA). Each step was done in a 96-well microtiter plate to achieve the best throughput of sample preparation. After deglycosylation, N-glycans were labeled with 2-AB fluorescent dye. Excess of label was removed by solid-phase extraction using Whatman 3MM chromatography paper. Finally, glycans were eluted with water and stored at −20°C until usage.
Orkney and TwinsUK IgG samples were first denatured with addition of 30 μL 1.33% sodium dodecyl sulfate (w/v) (Invitrogen, Carlsbad, CA) and by incubation at 65°C for 10min. Subsequently, 10 μL of 4% Igepal-CA630 (Sigma–Aldrich, St. Louis, MO) and 1.25 mU of PNGase F (ProZyme) in 10 μL 5× phosphate-buffered saline were added to the samples. The samples were incubated overnight at 37°C for N-glycan release. The released N-glycans were labeled with 2-AB. The labeling mixture was freshly prepared by dissolving 2-AB (Sigma–Aldrich) in dimethyl sulfoxide (Sigma–Aldrich) and glacial acetic acid (Merck) mixture (85:15, v/v) to a final concentration of 48mg/mL. A volume of 25 μL of labeling mixture was added to each N-glycan sample in the 96-well plate. Also, 25 μL of freshly prepared reducing agent solution (106.96mg/mL 2-picoline borane [Sigma–Aldrich] in dimethyl sulfoxide) was added and the plate was sealed using adhesive tape. Mixing was achieved by shaking for 10min, followed by 2-hour incubation at 65°C. Samples (in a volume of 100 μL) were brought to 80% acetonitrile (ACN) (v/v) by adding 400 μL of ACN (J.T. Baker, Phillipsburg, NJ). Free label and reducing agent were removed from the samples using hydrophilic interaction chromatography–solid-phase extraction. An amount of 200 μL of 0.1g/mL suspension of microcrystalline cellulose (Merck) in water was applied to each well of a 0.45 μm GHP filter plate (Pall Corporation, Ann Arbor, MI). Solvent was removed by application of vacuum using a vacuum manifold (Millipore Corporation, Billerica, MA). All wells were prewashed using 5×200 μL water, followed by equilibration using 3×200 μL acetonitrile/water (80:20, v/v). The samples were loaded to the wells. The wells were subsequently washed seven times using 200 μL acetonitrile/water (80:20, v/v). Glycans were eluted two times with 100 μL of water and combined eluates were stored at −20°C until usage.
Hydrophilic interaction chromatography-UPLC.—
Fluorescently labeled N-glycans were separated by hydrophilic interaction chromatography on a Waters Acquity UPLC instrument (Waters, Milford, MA) consisting of a quaternary solvent manager, sample manager, and a FLR fluorescence detector set with excitation and emission wavelengths of 330 and 420nm, respectively. The instrument was under the control of Empower 2 software, build 2145 (Waters). Labeled N-glycans were separated on a Waters ethylene bridged hybrid (BEH) Glycan chromatography column, 100×2.1mm i.d., 1.7 μm BEH particles, with 100mM ammonium formate, pH 4.4, as solvent A and acetonitrile as solvent B. Separation method used linear gradient of 75%–62% acetonitrile (v/v) at flow rate of 0.4mL/min in a 25-minute analytical run. Samples were maintained at 5°C before injection, and the separation temperature was 60°C. The system was calibrated using an external standard of hydrolyzed and 2-AB-labeled glucose oligomers from which the retention times for the individual glycans were converted to glucose units. Data processing was performed using an automatic processing method with a traditional integration algorithm after which each chromatogram was manually corrected to maintain the same intervals of integration for all the samples. The chromatograms were all separated in the same manner into 24 peaks and the amount of glycans in each peak was expressed as percentage of total integrated area. In addition to 24 directly measured glycan structures, 53 derived traits were calculated as described previously (12). These derived traits average particular glycosylation features (galactosylation, fucosylation, and sialylation) across different individual glycan structures. Consequently, they are more closely related to individual enzymatic activities and underlying genetic polymorphisms (47).
Statistical Analysis
To obtain normally distributed variables for 24 glycan structures, a log transformation was performed on glycan variables. Batch correction was performed for each glycan group separately using linear mixed model. In this model, dependent variable was log-transformed fraction of glycan group in total glycome. Age and gender were described as fixed effects, and technical sources of variation were described as random effects. To obtain corrected values for fractions of glycan groups, estimated batch effect (technical source of variation) was subtracted from each log-transformed glycan measurement.
The predictive model of age (GlycanAge model) was built using multivariate analysis of covariance approach implemented in “stats” package for R programming language (48). The maximal model included gender-specific linear and quadratic terms for each of 24 glycan structures—96 parameters in total. Feature selection was performed using backward elimination implemented in “step” function of “stats” package for R, with Akaike information criterion (20) as optimization criteria. Reported goodness of fit, correlations, and regression coefficients were estimated as median values of 1,000 iterations of repeated random subsampling validation, with two thirds (1,357 samples) of Orkney cohort as “training set” and one third (678 samples) as “validation set.”
To identify factors that may be responsible for the differences between chronological age and predicted age, we performed association analysis with all available biochemical and physiological traits in our databases. For each trait, we defined two models:
Likelihood ratio test was performed on those models to see if trait variable significantly improves model prediction. False discovery rate was controlled using Benjamini–Hochberg procedure.
For combined biological and glycan age model, biochemical and physical parameters that were present in at least two cohorts were selected from available databases. The list contained many biomarkers that were previously shown to be related to human aging (49) and their correlations with age may be seen in Supplementary Table S3. Not all biomarkers were collected in all samples from Orkney, so resulting data set consisted of 1,728 people. The most significant biomarkers for the analysis were absent in Croatian Vis cohort and TwinsUK, so these populations were excluded from the analysis.
The predictive model of combined biological and glycan biomarkers was built in the same way as GlycanAge model. A full model consisted of gender-specific polynomial of second degree for each of the 24 biological parameters included in the analysis, and three glycans included in GlycanAge, resulting in 108 parameters. Penalty for large number of parameters was selected based on 1,000 iterations of 10-fold cross-validation on Orkney’s training set, with robustness of goodness of fit (adjusted for the number of predictors) as selection criteria. The performance of the model was additionally tested on Korcula cohort. Training and validating on Korcula cohort were performed in the same manner.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The CROATIA-Vis study in the Croatian island of Vis was supported by grants from the Medical Research Council (UK), the Ministry of Science, Education and Sport of the Republic of Croatia (108-1080315-0302), and the European Union framework program 6 European Special Populations Research Network project (contract LSHG-CT-2006 -018947). ORCADES was supported by the Chief Scientist Office of the Scottish Government, the Royal Society, and the European Union Framework Programme 6 EUROSPAN project (contract LSHG-CT-2006 -018947). Glycome analysis was supported by the Ministry of Science, Education and Sport of the Republic of Croatia (309-0061194-2023) and the European Commission GlycoBioM (contract # 259869), HighGlycan (contract # 278535), MIMOmics (contract # 305280), IBD-BIOM (contract # 305479), and Integra-Life (contract # 315997) grants. The work of Y.A. was supported by Russian Foundation for Basic Research grant 12-04-33182. The collection of samples at island Vis was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (196-1962766-2751 to P.R.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
TwinsUK. The study was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
Conflict of Interest
G.L. declares that he is a founder and owner, and J.K., F.V., I.B., M.P.-B., L.K., M.N., and D.P. declare that they are employees of Genos Ltd., which offers commercial service of glycomic analysis and has several patents in this field.
Acknowledgments
J.K., T.K., I.B., M.P.-B., M.N., and K.T. performed experiments; M.M., P.R., N.N., J.S., S.M., I.K., O.P., and C.H. provided samples and resources; F.V., C.M., and L.K. analyzed data; I.R., H.C., Y.A., J.F.W., O.G., V.Z., T.S., and G.L. planned experiments; and A.V., T.S., V.Z., and G.L. wrote the manuscript. Tim Spector is holder of an ERC Advanced Principal Investigator award. SNP Genotyping was performed by The Wellcome Trust Sanger Institute and National Eye Institute via NIH/CIDR.
References
- 1. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401 [DOI] [PubMed] [Google Scholar]
- 3. Walt D, Aoki-Kinoshita KF, Bendiak B, et al. Transforming Glycoscience: A Roadmap for the Future. Washington, DC: National Academies Press; 2012 [PubMed] [Google Scholar]
- 4. Lauc G, Rudan I, Campbell H, Rudd PM. Complex genetic regulation of protein glycosylation. Mol Biosyst. 2010;6:329–335. 10.1039/b910377e [DOI] [PubMed] [Google Scholar]
- 5. Horvat T, Mužinić A, Barišić D, Bosnar MH, Zoldoš V. Epigenetic modulation of the HeLa cell membrane N-glycome. Biochim Biophys Acta. 2012;1820:1412–1419. 10.1016/j.bbagen.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 6. Zoldoš V, Horvat T, Novokmet M, et al. Epigenetic silencing of HNF1A associates with changes in the composition of the human plasma N-glycome. Epigenetics. 2012;7:164–172. 10.4161/epi.7.2.18918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saldova R, Dempsey E, Pérez-Garay M, et al. 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 2011;6:1362–1372. 10.4161/epi.6.11.17977 [DOI] [PubMed] [Google Scholar]
- 8. Lauc G, Vojta A, Zoldoš V. Epigenetic regulation of glycosylation is the quantum mechanics of biology. Biochim Biophys Acta. 2013;1840:65–70. 10.1016/j.bbagen.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 9. Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988;167:1731–1736. 10.1084/jem.167.5.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruhaak LR, Uh HW, Beekman M, et al. Plasma protein N-glycan profiles are associated with calendar age, familial longevity and health. J Proteome Res. 2011;10:1667–1674. 10.1021/pr1009959 [DOI] [PubMed] [Google Scholar]
- 11. Knezevic A, Gornik O, Polasek O, et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology. 2010;20:959–969. 10.1093/glycob/cwq051 [DOI] [PubMed] [Google Scholar]
- 12. Pucić M, Knezević A, Vidic J, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 2011;10M111.010090. 10.1074/mcp.M111.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanhooren V, Dewaele S, Libert C, et al. Serum N-glycan profile shift during human ageing. Exp Gerontol. 2010;45:738–743. 10.1016/j.exger.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 14. Gornik O, Pavić T, Lauc G. Alternative glycosylation modulates function of IgG and other proteins—implications on evolution and disease. Biochim Biophys Acta. 2012;1820:1318–1326. 10.1016/j.bbagen.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 15. Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med. 2012;18:1401–1406. 10.1038/nm.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuda K, Kubota T, Kaneko E, et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44:3122–3131. 10.1016/j.molimm.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 17. Lauc G, Huffman JE, Pučić M, et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013;9:e1003225. 10.1371/journal.pgen.1003225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Dis Markers. 2008;25:267–278. http://dx.doi.org/10.1155/2008/493289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lauc G, Huffman J, Pučić M, et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013;9e1003225. 10.1371/journal.pgen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. 10.1109/TAC.1974.1100705 [Google Scholar]
- 21. Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcgammaRs in vivo. Curr Opin Organ Transplant. 2011;16:7–14. 10.1097/MOT.0b013e328342538f [DOI] [PubMed] [Google Scholar]
- 22. Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170–180. 10.1007/s10875-010-9405-6 [DOI] [PubMed] [Google Scholar]
- 23. Johnson JL, Jones MB, Ryan SO, Cobb BA. The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 2013;34:290–298. 10.1016/j.it.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. 10.1146/annurev.immunol.26.021607.090232 [DOI] [PubMed] [Google Scholar]
- 25. Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A. 2008;105:15005–15009. 10.1073/pnas.0808248105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lux A, Aschermann S, Biburger M, Nimmerjahn F. The pro and anti-inflammatory activities of immunoglobulin G. Ann Rheum Dis. 2010;69(suppl 1):i92–i96. 10.1136/ard.2009.117101 [DOI] [PubMed] [Google Scholar]
- 27. Dall’olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C. N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing Res Rev. 2013;12:685–698. 10.1016/j.arr.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 28. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. 10.1016/j.febslet.2005.02.055 [DOI] [PubMed] [Google Scholar]
- 29. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 30. Vanhooren V, Desmyter L, Liu XE, et al. N-glycomic changes in serum proteins during human aging. Rejuvenation Res. 2007;10:521–531a. 10.1089/rej.2007.0556 [DOI] [PubMed] [Google Scholar]
- 31. Knezević A, Polasek O, Gornik O, et al. Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res. 2009;8:694–701. 10.1021/pr800737u [DOI] [PubMed] [Google Scholar]
- 32. Gornik O, Wagner J, Pucić M, Knezević A, Redzic I, Lauc G. Stability of N-glycan profiles in human plasma. Glycobiology. 2009;19:1547–1553. 10.1093/glycob/cwp134 [DOI] [PubMed] [Google Scholar]
- 33. Zoldoš V, Novokmet M, Bečeheli I, Lauc G. Genomics and epigenomics of the human glycome. Glycoconj J. 2013;30:41–50. 10.1007/s10719-012-9397-y [DOI] [PubMed] [Google Scholar]
- 34. Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj J. 1997;14:401–405. 10.1023/A: 1018582930906 [DOI] [PubMed] [Google Scholar]
- 35. Pucic M, Muzinic A, Novokmet M, et al. Changes in plasma and IgG N-glycome during childhood and adolescence. Glycobiology. 2012;22:975–982. 10.1093/glycob/cws062 [DOI] [PubMed] [Google Scholar]
- 36. O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. 10.1038/nature11862 [DOI] [PubMed] [Google Scholar]
- 37. Keusch J, Lydyard PM, Delves PJ. The effect on IgG glycosylation of altering beta1, 4-galactosyltransferase-1 activity in B cells. Glycobiology. 1998;8:1215–1220. http://dx.doi.org/10.1093/glycob/8.12.1215 [DOI] [PubMed] [Google Scholar]
- 38. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitnitski A, Rockwood K. Biological age revisited. J Gerontol A Biol Sci Med Sci. in press. 10.1093/gerona/glt137 [DOI] [PubMed] [Google Scholar]
- 40. Sanders JL, Minster RL, Barmada MM, et al. Heritability of and mortality prediction with a longevity phenotype: The Healthy Aging Index. J Gerontol A Biol Sci Med Sci. in press. 10.1093/gerona/glt117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A Biol Sci Med Sci. 2007;62:1096–1105 [DOI] [PubMed] [Google Scholar]
- 42. Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12:509–519. http://dx.doi.org/10.1016/j.arr.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 43. Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. 10.1126/science.291.5512.2370 [DOI] [PubMed] [Google Scholar]
- 44. Rudan I, Marusić A, Janković S, et al. “10001 Dalmatians:” Croatia launches its national biobank. Croat Med J. 2009;50:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and healthy ageing twin study. Int J Epidemiol. 2013;42:76–85. 10.1093/ije/dyr207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Royle L, Campbell MP, Radcliffe CM, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 2008;376:1–12. 10.1016/j.ab.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 47. Lauc G, Essafi A, Huffman JE, et al. Genomics meets glycomics: The first GWAS study of human N-glycome identifies HNF1alpha as a master regulator of plasma protein fucosylation. PLoS Genet. 2010;6:e1001256. 10.1371/journal.pgen.1001256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/ Accessed May 2013.
- 49. Crimmins E, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Adv Clin Chem. 2008;46:161–216. 10.1016/S0065-2423(08)00405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]