Abstract
Our knowledge of the mechanisms and regulation of intestinal absorption of water-soluble vitamins under normal physiological conditions, and of the factors/conditions that affect and interfere with theses processes has been significantly expanded in recent years as a result of the availability of a host of valuable molecular/cellular tools. Although structurally and functionally unrelated, the water-soluble vitamins share the feature of being essential for normal cellular functions, growth and development, and that their deficiency leads to a variety of clinical abnormalities that range from anaemia to growth retardation and neurological disorders. Humans cannot synthesize water-soluble vitamins (with the exception of some endogenous synthesis of niacin) and must obtain these micronutrients from exogenous sources. Thus body homoeostasis of these micronutrients depends on their normal absorption in the intestine. Interference with absorption, which occurs in a variety of conditions (e.g. congenital defects in the digestive or absorptive system, intestinal disease/resection, drug interaction and chronic alcohol use), leads to the development of deficiency (and sub-optimal status) and results in clinical abnormalities. It is well established now that intestinal absorption of the water-soluble vitamins ascorbate, biotin, folate, niacin, pantothenic acid, pyridoxine, riboflavin and thiamin is via specific carrier-mediated processes. These processes are regulated by a variety of factors and conditions, and the regulation involves transcriptional and/or post-transcriptional mechanisms. Also well recognized now is the fact that the large intestine possesses specific and efficient uptake systems to absorb a number of water-soluble vitamins that are synthesized by the normal microflora. This source may contribute to total body vitamin nutrition, and especially towards the cellular nutrition and health of the local colonocytes. The present review aims to outline our current understanding of the mechanisms involved in intestinal absorption of water-soluble vitamins, their regulation, the cell biology of the carriers involved and the factors that negatively affect these absorptive events.
Keywords: ascorbate, biotin, folate, intestinal transport, riboflavin, thiamin
INTRODUCTION
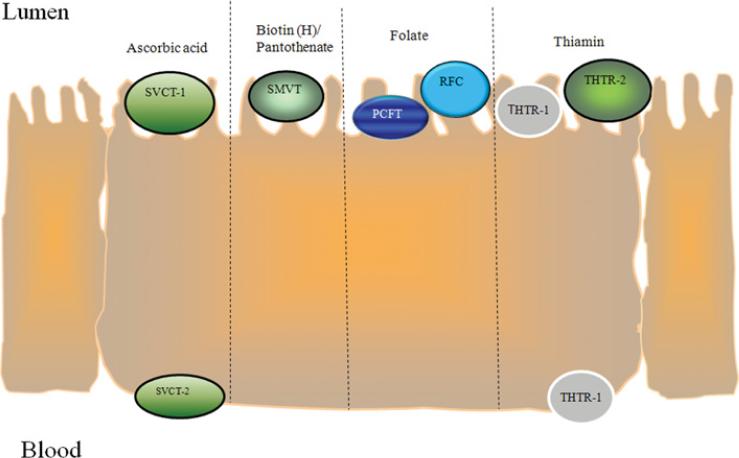
The water-soluble vitamins are a structurally dissimilar group of organic compounds that share the common features of being essential for normal cellular functions, growth and development. Although they exist in minute quantities in the diet, they play major roles in maintaining normal metabolic, energy, differentiation and growth status of cells. Thus it is not surprising that their deficiency (or sub-optimal levels) negatively affect human health, whereas optimizing their body levels brings positive benefits to human health (e.g. prevention of neural tube defects via optimization of the folate level). Humans have lost the ability to synthesize water-soluble vitamins (with the exception of some synthesis of niacin); rather, they obtain these compounds from exogenous sources via intestinal absorption. Because of that and since a variety of conditions and factors (both inherited as well as secondary causes) interfere with their normal intestinal absorption, detailed understanding of the mechanisms and regulation of their intestinal uptake are of high physiological/pathophysiological and nutritional significance. The aim of the present review is to describe our current understanding of the cellular and molecular mechanisms involved in intestinal absorption of these vitamins, their regulation, the cell biology of the carriers involved and their membrane expression (Figure 1), and the factors/conditions that interfere with these events. The focus will be on those water-soluble vitamins that are transported by carrier-mediated mechanisms, and thus no coverage of intestinal absorption of cobalamin (vitamin B12) will be presented; an excellent review of the latter subject has been published recently [1]. It is my hope that such a review will stimulate further investigations in these areas under physiological and pathophysiological conditions.
Figure 1.
Schematic depiction of the membrane expression of well-characterized water-soluble vitamin transporters in polarized intestinal epithelial cells
ASCORBATE
Dietary ascorbate (vitamin C) exists in the reduced [(i.e. AA (ascorbic acid)] and oxidized [DHAA (dehydro-L-ascorbic acid)] forms. The vitamin acts as a cofactor in a variety of critical metabolic reactions that include the synthesis of collagen, carnitine and catecholamine as well as in peptide amidation and tyrosine metabolism; it is also involved in maintaining metal ions (like iron and copper) in their reduced forms and serves as a scavenger for free radicals. A role for AA in the regulation of CFTR (cystic fibrosis transmembrane conductance regulator)-mediated chloride secretion in epithelial cells has also been suggested [2]. Deficiency of this vitamin leads to a variety of clinical abnormalities that include scurvy, poor wound healing, vasomotor instability and connective tissue disorders. With regard to DHAA, this compound is structurally different from AA; rather, it is similar to glucose. DHAA is converted into AA in intestinal epithelial cells via the action of DHAA reductase. Converting DHAA into AA helps maintain a low (non-toxic) level of the compound [3–5].
Physiological and molecular aspects of the intestinal ascorbate absorption process
Most mammals can generate AA from D-glucose endogenously. However, humans (and other primates as well as the guinea pig) cannot do so as they lack the enzyme L-gulonolactone oxidase; rather, they obtain the vitamin from dietary sources via intestinal absorption. Unlike a number of other water-soluble vitamins, which are also produced by the normal microflora of the large intestine, there appears to be no net production of ascorbate by these bacteria [6]. Studies on the mechanism of intestinal absorption of AA in the small intestine have shown the involvement of a concentrative, Na+ -dependent, carriermediated mechanism (reviewed in [7–9]). Absorption of dietary DHAA, however, occurs via a Na+ -independent carrier-mediated mechanism that is competitively inhibited by hexoses [7–9]. The molecular identity of the intestinal AA uptake systems has been delineated in recent years [10,11]. Both SVCT-1 [sodium-dependent vitamin C transporter-1, the product of the SLC23A1 (solute carrier family 23 member 1) gene] and SVCT-2 (the product of the SLC23A2 gene) are expressed in the intestine, with expression of the former being higher than that of the latter [10,11]. The SVCT-1 (a 598 amino acid protein) and SVCT-2 (a 650 amino acid protein) systems share considerable similarity with one another, and both proteins have 12 predicted TMDs (transmembrane domains). In addition, both polypeptides are predicted to have multiple potential protein kinase phosphorylation motifs and N-glycosylation sites (and indeed both proteins appear to be glycosylated [12]). At the functional level, SVCT-1 and -2 have a higher selectivity for L-ascorbic acid than for D-isoascorbic acid, and neither transports DHAA. With regard to the molecular identity of the system(s) involved in intestinal absorption of DHAA, GLUT1 (glucose transporter 1), GLUT3 and GLUT4 [but not GLUT2 and GLUT5 or SGLT-1 (sodium/glucose cotransporter-1)] have been reported to mediate the transport of this compound (reviewed in [13]).
With the determination of molecular identity of the intestinal AA transporters, it became possible to study certain structure– activity features of these systems. Thus an essential role of the histidine residue at position 51 of the SVCT-1 polypeptide and of the histidine residue at position 109 of the SVCT-2 polypeptide for the function of these transporters has been reported [14]. In addition, the N-glycosylation sites of the hSVCT-1 (human SVCT-1) polypeptide (located at positions 138 and 144) and those of the hSVCT-2 polypeptide (located at positions 188 and 196) are important for functionality and are glycosylated [12].
Cell biology of the intestinal AA absorption process: membrane targeting and intracellular trafficking of hSVCT- 1 and hSVCT-2
Aspects of the cell biology of hSVCT-1 and -2 such as membrane targeting and intracellular trafficking in intestinal epithelial cells have been studied in recent years using a live-cell confocal imaging approach. Using human intestinal epithelial Caco-2 cells expressing hSVCT-1 fused to YFP (yellow fluorescent protein), i.e. hSVCT1–YFP, it has been shown that the protein is exclusively expressed at the apical membrane domain of these cells [15] (see Figure 1 for a diagrammatic depiction of the membrane domains at which well-characterized vitamin transporters, including those of ascorbate, are expressed in intestinal epithelial cells). Some of the protein was also observed to be inside a heterogeneous population of intracellular structures (can be viewed at http://www.jbc.org/cgi/content/full/M400876200/DC1) [15]. The mobility of these structures was influenced by temperature and was dependent on an intact microtubule network. The molecular signal that dictates the targeting of hSVCT-1 to the apical membrane domain was shown be embedded in the cytoplasmic C-terminal sequence PICPVFKGFS (i.e. amino acids 563–572) [15]. As to the cell biology of the SVCT-2 system in intestinal epithelial cells, there is little known on the subject besides the finding that this transporter appears to be expressed at the basolateral domain of these cells [16] (Figure 1).
Regulatory aspects of the intestinal AA absorption process
Intestinal AA absorption is regulated by extracellular and intracellular factors. Knowledge about basal transcriptional activity of the SLC23A1 and SLC23A2 genes was gained from a study involving cloning and characterization of the 5′ -regulatory regions (promoters) of these genes with the use of the luciferase reporter-gene approach [17]. The characterization, however, was performed in human liver cells and identified a 135-bp sequence upstream of the transcriptional start site as the minimal promoter region required for basal activity of the SLC23A1 promoter. A role for HNF-1 (hepatocyte nuclear factor-1), a cis-regulatory element, in regulating the activity of the SLC23A1 gene was also reported [17]. As to the SLC23A2 gene, a role for the cis-element KLF (Krüppel-like factor)/Sp1 (stimulating protein-1) in regulating this gene has been described [18]. Although these studies provide important information about the transcriptional activity of the SLC23A1 and SLC23A2 genes in human liver cells, it is unclear if they also apply to human intestinal epithelial cells. Further studies are needed to address this issue.
Intestinal AA absorption appears to be adaptively regulated by the dietary level of the vitamin. Supplementing guinea pigs with AA led to a down-regulation in intestinal AA absorption [19,20]; similar observations were reported in a study using human intestinal epithelial Caco-2 cells [21]. In the latter study, the decrease in AA uptake upon supplementation was associated with a decrease in the level of expression of hSVCT-1. In contrast, an induction in the level of expression of SVCT-1 in the intestine of SMP30/GNI-knockout mice (animals that have lost the ability to synthesize AA endogenously) was observed upon feeding a vitamin C-deficient diet [22].
The intestinal AA uptake process also undergoes differentiation-dependent regulation. A study with Caco-2 cells (which differentiate spontaneously in culture upon reaching confluence to become mature enterocyte-like cells [23]), showed that the intestinal AA uptake process is regulated during differentiation [24]. This regulation was associated with changes in the level of expression of hSVCT-1, but not hSVCT-2 [24].
BIOTIN
In mammals, biotin (vitamin H) serves as a cofactor for five carboxylases that are involved in a variety of metabolic reactions including fatty acid biosynthesis, gluconeogenesis, and catabolism of certain amino acids and fatty acids. The vitamin also plays a role in regulating the expression of oncogenes and the cellular level of the second messenger cGMP [25–28]. Deficiency of biotin leads to growth retardation, dermatological abnormalities and neurological disorders. Animal studies have also shown that biotin deficiency during pregnancy can lead to congenital malformation and death [29–32]. Biotin deficiency and sub-optimal levels occur in subjects with inborn errors of biotin metabolism [33], those on long-term therapy with anticonvulsant drugs [34–37], those on long-term parenteral nutrition [38], in chronic alcoholics [39], during pregnancy [40] and in subjects with inflammatory bowel disease [41].
Physiological and molecular aspects of the intestinal biotin absorption process
Humans obtain biotin from dietary and bacterial sources. The latter source is provided by the normal microflora of the large intestine [6]. The contribution of the bacterial source towards total body biotin nutrition is not clear. However, considering that the large intestine is capable of absorbing luminal biotin [42–44], that colonocytes possess an efficient carrier-mediated mechanism for biotin uptake [45] and that luminal content remains relatively longer in the large intestine than in the small intestine, it is reasonable to suggest that this source of vitamin is of nutritional value to the host and especially to the local colonocytes.
In the diet, biotin exists in the free and protein-bound forms. Protein-bound biotin is digested by gastrointestinal proteases and peptidases to biocytin (biotinyl-L-lysine) and biotin–short peptide conjugates, which are converted into free biotin prior to absorption by the action of the enzyme biotinidase [46]. Mutation in this enzyme leads to ‘biotinidase deficiency’, a condition in which biocytin (and other biotin–short peptides) cannot be converted (recycled) into free biotin, resulting in impairment in cellular uptake including absorption in the gut [46,47].
The mechanism of absorption of biotin in the small and large intestine has been studied using a variety of intestinal preparations from a number of species (reviewed in [8,9]). These studies have shown the involvement of an Na+ -dependent carrier-mediated uptake mechanism in both regions of the gut. This carrier was also found to transport two other functionally unrelated nutrients, pantothenic acid (a water-soluble vitamin involved in the synthesis of coenzyme A and acyl carrier proteins in mammalian cells) and lipoate (a potent intracellular and extracellular antioxidant) [48,49]. It is for this reason that the biotin uptake system is now referred to as the ‘SMVT’ (sodium-dependent multivitamin transporter).
Studies using purified intestinal BBMVs [BBM (brush-border membrane) vesicles] have shown that the intestinal Na+ -dependent carrier-mediated mechanism of biotin uptake is functional only at the apical BBM domain of the polarized intestinal epithelial cells [50–54]. Subsequent immunological studies using specific polyclonal anti-SMVT antibodies and confocal imaging investigations confirmed this conclusion (Figure 1) [55,56]. As to how the negatively charged biotin leaves the intestinal absorptive epithelial cells across the BLM (basolateral membrane), this issue was also addressed using purified intestinal BLMVs (basolateral membrane vesicles) and involves a specialized Na+ -independent carrier-mediated mechanism [53,54].
The molecular identity of the human SMVT protein (the product of the SLC19A6 gene) and that of a number of other species have been delineated in recent years ([57–59] and GenBank® accession number AY572835). The hSMVT (human SMVT) protein is predicted to have 12 TMDs and a number of potential post-translational modification sites that include four N-glycosylation motifs and two protein kinase phosphorylation sites. Recent studies from my laboratory have shown that the hSMVT protein is indeed glycosylated and that this glycosylation is important for the function and stability of the carrier protein (A. Ghosal, V.S. Subramanian and H.M. Said, unpublished work). The relative contribution of the hSMVT system towards total carrier-mediated biotin uptake in the intestine has also been determined using gene-specific siRNA (small interfering RNA) [60]. Knocking down the hSMVT system in Caco-2 cells leads to a severe inhibition of carrier-mediated biotin uptake, suggesting that the hSMVT system is the major (if not the only) biotin uptake mechanism in these cells [60]. Further studies involving SMVT-knockout mouse models are needed to confirm this conclusion in native intestinal tissue in vivo.
With the delineation of the molecular identity of the SMVT system, knowledge about the structure–function relationship of the system has also been forthcoming. Thus, in a recent study using site-directed mutagenesis, an important role for His115and His254 of the hSMVT polypeptide in the function of the transporter has been reported [61]. Specifically, mutating these sites was found to lead to a significant inhibition of carrier-mediated biotin uptake via a significant reduction in the Vmax, but not the apparent Km, of the uptake process. This effect was not related to the charge of the histidine residues or to changes in transcriptional or translational efficiency of the mutated hSMVT [61]. Rather, the inhibition appeared to be due to a significant reduction in the level of expression of the mutated hSMVT at the cell surface [61].
Cell biology of the intestinal biotin absorption process and identification of an accessory protein
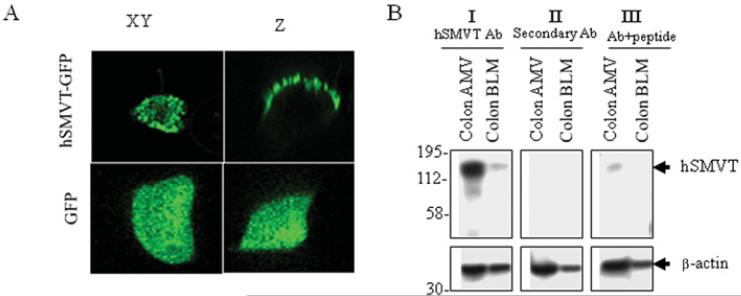
Membrane targeting and intracellular trafficking of the hSMVT system in intestinal epithelial cells has been investigated recently [55]. A live-cell confocal imaging study has shown that the hSMVT protein [fused to GFP (green fluorescent protein)] is expressed exclusively at the apical membrane domain of epithelial cells, and its C-terminal tail is important for membrane targeting and function (Figure 2) [55]. The intracellular movement of hSMVT involves distinct trafficking vesicles, and the mobility of these vesicles depends on the existence of an intact microtubule network and is impaired by dynamitin (p50), brefeldin and monensin [55].
Figure 2. Distribution of hSMVT fused to GFP in human intestinal epithelial Caco-2 cells grown on filters.
(A) XY and Z confocal images showing a Caco-2 cell expressing hSMVT–EGFP (enhanced GFP) and DsRed (a cytoplasmic dye) imaged 48 h after transient transfection. The lower panels show distribution of EGFP alone. (B) Western blot showing the expression of hSMVT at human colonic apical (but not basolateral) membrane. Ab, antibody; AMV, apical membrane vesicles. Adapted from The American Journal of Physiology: Cell Physiology, vol. 296 (2010), Subramanian, V.S., Marchant, J.S., Boulware, M.J., Ma, T.Y. and Said, H.M., Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells, pp. C663–C671, used with permission from The American Physiological Society.
Another recent study, using yeast two-hybrid screening of a human intestinal cDNA library, has identified PDZD11 (PDZ domain-containing protein 11), a PDZ-containing protein, as an interacting partner with hSMVT [62]. The interaction between hSMVT with PDZD11 was confirmed further in vitro [by a GST (glutathione transferase) pull-down assay] and in vivo (by two-hybrid luciferase and co-immunoprecipitation assays), as well as by confocal imaging of living cells. Furthermore, the interaction had a functional consequence in that co-expression of hSMVT with PDZD11 led to an increase in biotin uptake, whereas knocking down PDZD11 (with the use of gene-specific siRNA) led to an inhibition of uptake. Moreover, the PDZ-binding domain of the hSMVT polypeptide that interacts with PDZD11 was localized to the C-terminal tail of the transporter [62].
Regulatory aspects of the intestinal biotin absorption process
The process of biotin absorption in the intestine is regulated by extracellular and intracellular factors/conditions via transcriptional and post-transcriptional mechanisms. Information about basal transcriptional activity of the SLC5A6 gene has been forthcoming as a result of cloning of the 5′-regulatory region of this gene, and by characterization of its activity in vitro and in vivo [63,64]. The 5′-regulatory region of the human SLC5A6 gene harboured two distinct promoters (P1 and P2) [64]. Both P1 and P2 were TATA-less, CAAT-less and had multiple putative cis-regulatory elements. The minimal promoter region required for basal activity of P1 was shown to be in a sequence between– 4830 and – 4603, and for P2 in a sequence between –4417 and – 4303 [64]. A role for the cis-regulatory elements [KLF-4 and AP-2 (activator protein 2)] in the function of these promoters has also been demonstrated [65]. Functionality of the cloned human SLC5A6 5′-regulatory region was confirmed in vivo in transgenic mice [65].
Intestinal biotin uptake is adaptively regulated by the substrate level in the diet [66,67]. A significant and specific up-regulation in intestinal uptake was observed in rats fed on a biotin-deficient diet compared with pair-fed controls. On the other hand, a significant down-regulation in biotin uptake was observed in rats over-supplemented with biotin. This adaptive regulation was mediated via changes in the Vmax (but not the apparent Km) of the biotin uptake process, suggesting that the effect is by changes in the number (and/or activity) of the biotin transporters, but not in their affinity. Similar adaptive regulation in biotin uptake was observed in a study with human intestinal epithelial Caco-2 cells [67]. In the latter study, the induction in biotin uptake in biotin deficiency was further shown to be associated with an induction in the level of expression of hSMVT via what appears to be a transcriptionally mediated mechanism. A biotin deficiency-responsive region was also identified and was mapped to a 103-bp sequence in the SLC5A6 promoter [67]. Furthermore, a GKLF (gut-enriched KLF) cis-regulatory element was involved in this adaptive response of the SLC5A6 promoter [67].
Intestinal biotin absorption is developmentally regulated during the early stages of life [56,68]. An increase in biotin uptake in the proximal small intestine was observed with maturation, and was mediated via an increase in the Vmax and the apparent Km of the biotin uptake process; it was also associated with an increase in the level of expression of SMVT and in the transcription rate of the Slc5a6 gene [56]. These findings suggest that developmental regulation of the intestinal biotin absorption process, at least in part, involves a transcriptional mechanism(s).
Finally, intestinal biotin uptake was found to be under the regulation of an intracellular PKC (protein kinase C)-mediated pathway [69,70]. Activation of this pathway leads to a significant decrease in biotin uptake, whereas its inhibition leads to a slight (but significant) increase in the vitamin uptake by human intestinal epithelial cells [69,70]. This PKC-mediated regulation of intestinal biotin uptake was mediated via a decrease in the Vmax (not the apparent Km), suggesting that the effect is via changes in the activity (and/or number), but not affinity, of the carrier system. More recent studies in our laboratory using site-directed mutagenesis have shown that both of the two potential PKC phosphorylation sites of the hSMVT polypeptide (Thr286 and Ser283) are involved in mediating the PKC effect on biotin uptake (A. Ghosal, V.S. Subramanian and H.M. Said, unpublished work). A role for an intracellular Ca2+ /CaM (calmodulin)-mediated pathway in the regulation of the intestinal biotin uptake process has also been suggested [70].
Factors that negatively affect the intestinal biotin absorption process
Chronic alcohol use in humans is associated with a significant reduction in plasma biotin levels [39,71]. A rat model of chronic alcohol feeding showed that chronic alcohol feeding leads to a significant inhibition of intestinal biotin absorption [72]. This inhibition affected the biotin transport process across the BBM and BLM domains of the polarized enterocytes; it was also associated with a significant reduction in the level of expression of the SMVT protein, mRNA and hnRNA (heterogeneous nuclear RNA) [72]. Chronic alcohol feeding also inhibited carrier-mediated biotin uptake in the colon [72]. When the effect of chronic alcohol feeding on intestinal biotin uptake was examined in transgenic mice carrying the human SLC5A6 5′-regulatory region, a significant inhibition of the activity of the SLC5A6 5′-regulatory region in the intestine of these animals was also observed [72]. Finally, chronic exposure of Caco-2 cells to alcohol, which leads to a significant inhibition of biotin uptake, also causes a significant inhibition in activity of the human SLC5A6 promoters [72]. Collectively, these findings showed that chronic alcohol feeding exerts profound negative effects on intestinal biotin absorption and that the effect is, at least in part, exerted at the transcriptional level. It is of relevance to mention here that chronic alcohol use also inhibits the renal biotin uptake process via a similar mechanism(s) [73].
Intestinal biotin uptake also appears to be sensitive to the effect of the anticonvulsant drugs carbamazepine and primidone [74], and both biotin deficiency and sub-optimal levels have been reported in patients on long-term use of these agents [34,36].
FOLATE
The term folate (vitamin B9) refers to folic acid and its derivatives, compounds that act as coenzymes for cellular one-carbon metabolism and consequently for the synthesis of thymidine and purine as well as in the metabolism of several amino acids, e.g. homocysteine. Folate deficiency (which is a highly prevalent vitamin deficiency worldwide) leads to clinical abnormalities that range from megaloblastic anaemia to growth retardation and congenital (neural tube) defects. On the other hand, optimizing folate body homoeostasis leads to a significant reduction in the incidence of neural tube defects. A variety of conditions and factors affect and interfere with the normal intestinal folate absorption process. This includes congenital defects in the uptake system {as in the case of mutations in the PCFT (proton-coupled folate transporter), which occur in patients with HFMS (hereditary folate malabsorption syndrome) [75,76] , intestinal diseases (e.g. coeliac disease and tropical sprue), drug interaction (e.g. sulfasalazin, trimethoprim, pyrimethamine and diphenylhydantoin) and chronic alcohol use.
Physiological and molecular aspects of the intestinal folate absorption process
The human gut is exposed to two sources of folate, a dietary source (which is processed and absorbed mainly in the small intestine) and a bacterial source (where the vitamin is produced by the normal microflora of the large intestine and absorbed in that region of the gut [6]). Although the relative contribution of the bacterial source to total body nutrition of folate is not clear, the existence of an efficient carrier-mediated mechanism for folate uptake by human colonocytes [77,78], when combined with the considerable time luminal contents stay in the large intestine, suggests that this source of folate contributes to host nutrition, especially towards the cellular nutrition of the local colonocytes.
In the diet, folate exists in the form of mono- and polyglutamates. Folate polyglutamates are hydrolysed to folate monoglutamates prior to absorption. This digestion process occurs mainly in the proximal part of the small intestine and involves the enzyme folylpoly-γ -glutamate carboxypeptidase. With regard to the bacterial-provided folate in the large intestine, a substantial portion of this folate exists in the folate monoglutamate form and thus is available for absorption [79].
The mechanism of uptake of folate monoglutamates in the small and large intestine has been studied using a variety of human and animal small intestinal and colonic preparations (reviewed in [8,9,80,81]). In both regions of the gut, a specific pH-dependent, Na+ -independent carrier-mediated mechanism has been found. The uptake process was also found to be sensitive to the inhibitory effect of the anion transport inhibitors DIDS (4,4′-di-isothiocyanostilbene-2,2′-disulfonate), and SITS (4-acetimido-4′-isothiocyanostilbene-2,2′-disulfonate). Other studies used purified intestinal BBMV and BLMV preparations to demonstrate the involvement of a carrier at each of the membrane domains of the polarized enterocytes (see [8,9,81] and references therein).
The molecular identity of the systems involved in intestinal folate absorption has been delineated, with both the RFC (reduced folate carrier, the product of the SLC19A1 gene) [82–84] and the PCFT (the product of the SLC46A1 gene) [75,85] involved (see detailed reviews in [8,9,80,81]). hRFC (human RFC) (a 591 amino acid protein) is predicted to have 12 TMDs together with a number of putative protein kinase phosphorylation sites, and one N-glycosylation motif (indeed, the latter site appears to be glycosylated). hRFC shares a high degree of homology with that of other mammals [85]. The RFC protein is expressed at the apical membrane domain of intestinal epithelial cells and functions at neutral pH [85–87]. hPCFT (human PCFT, a 459 amino acid protein) is predicted to have 12 TMD and two potential N-glycosylation sites (both of which appear to be glycosylated [88]). The protein was originally cloned as a haem transporter [89], but a subsequent study demonstrated that the system is an efficient folate transporter [75]. Expression of PCFT is restricted to the apical membrane domain of the polarized intestinal epithelial cells [90]; in addition, the protein is expressed mainly in the proximal part of the human small intestine, with low expression in the distal small intestine and the large intestine [75,85,89]. Transport of the negatively charged folate by the PCFT system is acidic-pH-dependent (proton coupled) and electrogenic, i.e. it occurs via a folate− –H+ symport, and uses the energy generated by the downhill movement of protons (see [75,80] and references therein) (an inwardly directed proton gradient exists across the apical membrane domain of the proximal small intestine due to the existence of the intestinal surface acid microclimate [91]).
Since both the PCFT and RFC proteins are expressed at the apical membrane domain of the polarized intestinal epithelial cells (Figure 1), but have different pH optima, it is possible to speculate that the contribution of each system towards total folate absorption in vivo depends on their level of expression and on the prevailing pH at the site of absorption. Thus it is reasonable to suggest that the PCFT system predominates in the proximal half of the small intestine where the surface pH is acidic [91], whereas the hRFC system operates in the distal small intestine and in the colon where surface pH is neutral [91]. An important role for hPCFT in intestinal folate absorption has been supported by the identification of subjects with HFMS where loss-of-function mutations in this transporter have been identified [75,80].
Little is known about the molecular identity of the folate transport system across the BLM domain of intestinal epithelial cells. However, a role for a member(s) of the MDR (multidrug resistance) proteins (see [80] and references therein) has been proposed.
Knowledge about the structure–function activity relationship of the PCFT and RFC systems has been forthcoming from both clinical findings and experimental investigations. With regard to RFC, experimental evidence suggests a role for the amino acid residues at position 45, 46, 104, 105, 127, 130, 297 and 309 in the function of the transporter (reviewed in [85]). Other studies have reported a role for the intracellular loop between TMDs 6 and 7 of the RFC protein [92], whereas no functional role was found for the N- and C-termini [93].
With regard to hPCFT, clinical mutations identified in patients with HFMS at positions 65, 66, 113, 147, 318, 376 and 425 appear to be important for functionality (see [80] and references therein). These mutations lead to a spectrum of changes, including early truncation and frameshift, that result in impairment in intracellular trafficking, membrane targeting and/or changes in protein stability (see [80] and references therein). A role for the conserved and positively charged histidine residues at positions 247 and 281 in the functionality of hPCFT in transporting the negatively charged folate has been reported [94].
Cell biology of the intestinal folate absorption process: membrane targeting and intracellular trafficking of hRFC and hPCFT
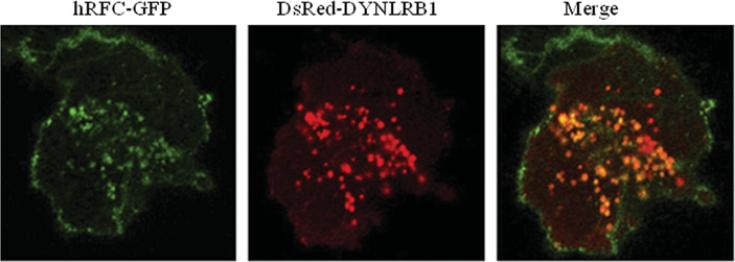
The mechanisms involved in membrane targeting and intracellular trafficking of the folate transporters hRFC and hPCFT in epithelial cells have been investigated in recent years using live-cell confocal imaging. Molecular determinants that dictate the targeting of hRFC to the cell surface reside within the hydrophobic backbone of the polypeptide, with no role for the N- or C-termini [93,95,96]. In addition, integrity of the hRFC backbone was critical for trafficking of the protein from the endoplasmic reticulum to the cell surface. Intracellular movement of hRFC involves trafficking vesicles, whose mobility depends on an intact microtubule network [96] (trafficking vesicles can be seen at http://www.jbc.org/cgi/content/full/277/36/33325/DC1). Another study used a bacterial two-hybrid system to show that the DYNLRB1 (dynein, light chain, roadblock-type 1) protein is an interacting partner with hRFC in human intestinal epithelial cells [97]. The interaction between hRFC and DYNLRB1 was further confirmed by in vitro (pull-down assay) and in vivo (mammalian two-hybrid luciferase assay and co-immunoprecipitation) assays [97], as well as by confocal imaging of live intestinal epithelial cells (Figure 3). Co-expression of DYNLRB1 with hRFC led to an increase in folate uptake, whereas its knockdown (by means of gene-specific siRNA) produced a decrease in folate uptake [97].
Figure 3. Co-localization of hRFC with DYNLRB1 in human intestinal epithelial cells.
Human intestinal epithelial HuTu-80 cells were grown on glass-bottomed Petri dishes and cotransfected with hRFC–GFP (left-hand panel) and DsRed–DYNLRB1 (middle panel); the right-hand panel is a merged image showing co-localization of hRFC and DYNLRB1 (yellow). Adapted from The American Journal of Physiology: Gastrointestinal and Liver Physiology, vol. 297 (2009), Ashokkumar, B., Nabokina, S.M., Ma, T.Y. and Said, H.M., Identification of dynein light chain road block-1 as a novel interaction partner with the human reduced folate carrier, pp. G480–G487, used with permission from The American Physiological Society.
As to hPCFT, a live-cell confocal imaging study showed the protein to be exclusively expressed at the apical membrane domain of epithelial cells (as depicted in Figure 1) and that a β-turn sequence separating the predicted TMD2 and TMD3 of the hPCFT protein was essential for its membrane targeting [90]. In addition, delivery of the hPCFT polypeptide to the cell surface depends on an intact microtubule network and was inhibited by overexpression of the dynamitin (p50) subunit of the dynactin complex [90].
Regulatory aspects of the intestinal folate absorption process
The process of folate absorption in the intestine is under the regulation of a variety of extracellular and intracellular conditions that act via transcriptional and/or post-transcriptional mechanisms. Basal transcriptional activity of the SLC19A1 gene (which encodes hRFC) involves at least six alternative promoters. The activity of these promoters leads to the generation of 15 distinct 5′ -untranslated regions (variants) that share a common hRFC open reading frame [85]. These promoters appear to be regulated by ubiquitous [SP and USF (upstream stimulatory factor)] and tissue-specific [e.g. AP-1 and C/EBP (CCAAT/enhancer-binding protein)] nuclear regulatory factors, and by methylation [85]. In the intestine, variant I appears to be the predominant hRFC variant and is driven by promoter B of the SLC19A1 gene [81,84]. The basal transcriptional activity of the SLC46A1 gene (which encodes hPCFT) has been studied, with the minimal region required for basal activity mapped to a sequence of 157 bp upstream of the ATG codon and containing putative GC-box sites as well as enhancer elements [YY1 (Yin and Yang 1) and AP-1] [98].
Another study showed that the intestinal folate digestion and absorption processes are both adaptively regulated by the substrate level in the diet. The activity of folylpoly-γ -glutamate carboxypeptidase increased in folate deficiency [99]. Similarly, a specific and significant induction in the jejunal carrier-mediated folate uptake process occured in folate deficiency [99–101]. The induction in uptake was mediated via an increase in the Vmax (no change in apparent Km) and was associated with an increase in the level of expression of PCFT and RFC [99–102]. The induction in expression of RFC in folate deficiency was observed in the small and large intestines [99]. Similar findings were observed in studies with human intestinal epithelial Caco-2 cells maintained under a folate-deficient condition [101,102]. In the latter studies, folate deficiency also led to an induction in the activity of SLC19A1 promoter B [101]. The folate deficiency-responsive region of the SLC19A1 promoter was mapped to a sequence between – 2016 and – 1431 [101]. There is little known at present about the molecular mechanism(s) involved in the induction of PCFT expression in folate deficiency.
Both the intestinal folate digestion and absorption processes are developmentally regulated during early stages of life [103–105]. With regard to folate uptake, a decrease in this parameter was seen with maturation and was mediated via a decrease in the Vmax (not the apparent Km) of the folate uptake process; it was also associated with a decrease in the expression of RFC and in the transcription rate of the Slc19a1 gene, suggesting the involvement of transcriptional mechanism(s) [105]. Little is known about PCFT expression during early development and the molecular mechanism(s) involved in any possible regulation.
Intestinal folate uptake was further shown to undergo differentiation-dependent regulation. Enterocytes gain functional maturity (differentiation status) as they move from their place of birth in the crypts to the villus region [106]. Studies using human intestinal epithelial Caco-2 cells (which differentiate spontaneously in culture upon reaching confluence) have shown a significant increase in carrier-mediated folate uptake with differentiation [106]. This increase was associated with an increase in the expression of hRFC and hPCFT and the activity of their respective promoters. The latter suggests that the differentiation-dependent regulation in intestinal folate uptake may involve a transcriptional mechanism(s) [106]. These findings on differentiation-dependent regulation of the intestinal folate uptake process were further confirmed in a study with native mouse intestine [106].
Finally, intestinal folate uptake was found to be under the regulation of an intracellular protein-tyrosine-kinase-mediated pathway [78,107]. This regulatory pathway appears to affect the folate uptake process by changes in Vmax, not its apparent Km, suggesting that the effect is mediated via changes in the activity (and/or number), but not affinity, of the folate uptake carriers. A role for an intracellular cAMP-mediated pathway that is independent of PKA (protein kinase A) has also been reported [78,107].
Factors that negatively affect the intestinal folate absorption process
Folate deficiency is common in alcoholics. Although a variety of factors contribute to the development of this deficiency, an inhibition in intestinal processing of the vitamin has been recognized as being a major contributing factor [108,109]. Indeed chronic alcohol use inhibits both the digestion process of dietary folate polyglutamates and the uptake phase of liberated folate monoglutamates [108,109]. The inhibition in the intestinal folate uptake process by chronic alcohol use was associated with a significant reduction in the level of expression of RFC (the level of PCFT was not examined in these studies) [109,110]. It may be of relevance to mention that a recent study reported that chronic alcohol use inhibits pancreatic folate uptake, and the inhibition is associated with a significant reduction in the level of expression of both the RFC and PCFT systems [111].
Other studies have reported an impairment in the activity of the folate-hydrolysing enzyme folylpoly-γ -glutamate carboxypeptidase in disease conditions such as coeliac disease and tropical sprue [112,113] and as a result of prolonged use of sulfasalazine [114,115]. Sulfasalazine also appears to inhibit the intestinal folate absorption process [114] via an effect on the transport systems involved [116–118].
NIACIN (NICOTINIC ACID)
Niacin (vitamin B3) is a precursor for the coenzymes NAD and NADP, both of which are involved in metabolic reactions that maintain the redox state of the cell, including glycolysis and the pentose phosphate shunt. The vitamin also has a lipid-lowering effect. Niacin deficiency leads to pellagra, a disease that is characterized by inflammation of mucous membranes, skin lesions and diarrhoea. Deficiency and sub-optimal levels occur in alcoholics and in patients with Hartnup disease; individuals with the latter disease have mutations in the membrane transporter of the amino acid tryptophan (the precursor of endogenous niacin synthesis). Humans obtain their requirements for niacin from endogenous and exogenous sources. The former source is provided via the metabolic conversion of tryptophan into niacin, whereas the latter source is the diet (there appears to be little contribution by the normal microflora of the large intestine as they tend to retain their niacin intracellularly [6]).
Early studies on the mechanism of intestinal niacin uptake produced conflicting reports with some reporting the process as being via simple diffusion, whereas others reported the process as being carrier-mediated with an apparent Km in the milimolar range [119–122]. The high apparent Km reported in these studies has raised significant doubt regarding the physiological relevance of such a system since niacin exists in the micromolar range in the intestinal lumen [123]. To clarify these issues, we used human intestinal epithelial Caco-2 cells and purified human intestinal BBMVs, and physiological conditions, to show the involvement of a specific high-affinity (apparent Km of 0.53± 0.08 μM), acidic-pH-dependent, Na+ -independent carrier-mediated + mechanism for niacin uptake [124]. A similar high-affinity carrier-mediated mechanism (apparent Km of 0.73 ±0.16 μM) for niacin uptake was subsequently identified in human liver cells [125]. With regard to the molecular identity of the uptake system involved in intestinal absorption of physiological concentrations of niacin, this issue still needs further studies, although a role for hOAT-10 (human organic anion transporter-10) in the uptake process has been proposed [126]. Other studies [127,128] have suggested a role for the sodium-coupled monocarboxylate transporter SLC5A8 in intestinal uptake of niacin, but the reported apparent Km for this system of 0.23–0.3 mM and its apparent non-specificity raises some concern regarding its role in the absorption of physiological concentrations of niacin (which as mentioned above exist in the micromolar range). It is possible that the SLC5A8 system is involved in the absorption of high pharmacological doses of niacin that are used clinically as a lipid-lowering agent. Nothing is known about the mechanism involved in niacin exit out of the enterocytes across the BLM.
With regard to regulation of the intestinal niacin absorption process, a study involving human Caco-2 cells suggested a role for an intracellular protein-tyrosine-kinase-mediated pathway in regulating vitamin uptake [124].
PANTOTHENIC ACID
Pantothenic acid (vitamin B5) is needed for the synthesis of coenzyme A and acyl carrier protein, which are involved in carbohydrate, fat and protein metabolism. Owing to the ubiquitous distribution of pantothenic acid, no known cases of deficiency have been reported in humans.
Humans obtain pantothenic acid from a dietary source and a bacterial source; the latter is provided by the normal microflora of the large intestine and is absorbed in that region of the gut [6]. Both sources appear to contribute to the body's need for the vitamin, although the exact level of contribution is not defined. In the diet, pantothenic acid exists mainly in the form of coenzyme A, which is hydrolysed to free pantothenic acid prior to intestinal absorption [129]. The mechanism of uptake of pantothenic acid in the small and large intestines is the same and occurs via SMVT [48,49,58,69]. As discussed above in the ‘Biotin’ section, SMVT also transports biotin and lipoate. There is no information available as to how pantothenic acid leaves the intestinal absorptive cells across the BLM.
PYRIDOXINE (AND ITS DERIVATIVES)
Pyridoxine, together with pyridoxal and pyridoxamine, are collectively referred to as vitamin B6. The vitamin acts as a cofactor in a number of metabolic reactions involving carbohydrate, protein and lipid metabolism. Pyridoxal 5′ phosphate is the most biologically active form of the vitamin. Deficiency of vitamin B6 (leading to a variety of clinical abnormalities, including neurological disorders and anaemia) occurs in chronic alcoholism, in patients with diabetes mellitus and those with coeliac disease; it also occurs in patients on long-term use of the therapeutic agents isoniazid and penicillamine. Sub-optimal levels of vitamin B6 have also been reported in patients with vitamin-B6-dependent seizure, an autosomal-recessive disorder believed to be due to an impairment in pyridoxine transport into cells [130].
Physiological aspects of the intestinal pyridoxine absorption process
Humans obtain vitamin B6 from dietary and bacterial sources (the latter is produced by the normal microflora of the large intestine [6]). Although the relative contribution of the bacterial source to total body nutrition of vitamin B6 is well defined, evidence exists to suggest that this source is bioavailable [131] and that the large intestine possesses an efficient mechanism for vitamin B6 uptake (see below). In the diet, vitamin B6 compounds exist in the free and phosphorylated forms; the latter form is hydrolysed to the free form prior to absorption [132–134]. Earlier studies on the mechanism of intestinal vitamin B6 absorption concluded that the process is non-saturable [132,135]. However, more recent studies from our laboratory using the enterocyte-like human intestinal epithelial Caco-2 cells, mouse-derived colonic epithelial (YAMC) cells and purified human colonic apical membrane vesicle preparations, isolated from the colon of organ donors, as models and proper physiological conditions have shown the existence of a specific acidic pH (but not Na+ )-dependent carrier-mediated mechanism for pyridoxine uptake [136,137]. Studies have also shown the vitamin B6 uptake process to be sensitive to the effect of the diuretic amiloride [136]. Nothing is currently known about the molecular identity of the intestinal pyridoxine carrier of any mammalian species.
Regulatory aspects of the intestinal vitamin B6 absorption process
The process of pyridoxine absorption in the intestine appears to be under the regulation of extracellular and intracellular factors. Adaptive up-regulation of intestinal pyridoxine uptake has been reported in intestinal epithelial cells maintained in the presence of a low vitamin level [137]. This up-regulation appears, at least in part, to be transcriptionally mediated [137]. Pyridoxine uptake by intestinal epithelial cells also appears to be under the regulation of an intracellular PKA-mediated pathway [136]. An increase in intracellular cAMP level was found to lead to a significant inhibition in pyridoxine uptake, an effect that is mediated via a significant reduction in the Vmax, but not the apparent Km, of the uptake process. These findings suggest a decrease in the activity (and/or the number), but not affinity, of pyridoxine uptake [136].
RF (RIBOFLAVIN)
RF (vitamin B2) in its coenzyme forms, FMN and FAD, plays key metabolic roles in a variety of reactions involving carbohydrates, amino acids and lipids, and the conversion of folic acid and vitamin B6 into their active coenzyme forms. Deficiency and suboptimal levels of RF (which occur in patients with inflammatory bowel disease, chronic alcoholism and Brown–Vialetto–Van Laere syndrome) leads to a variety of clinical abnormalities that include degenerative changes in the nervous system, endocrine dysfunction, skin disorders and anaemia. In contrast, optimization of RF status reduces the risk of oesophageal squamous cell carcinoma (see [138] and references therein).
Physiological and molecular aspects of the intestinal RF absorption process
As in the case of a number of other water-soluble vitamins, the human intestine is exposed to two sources of RF, one being dietary (which is processed and absorbed in the small intestine) and the other bacterial (where the vitamin is generated by the normal microflora of the large intestine and is absorbed in that region of the intestinal tract [6]). Again, although the contribution of the latter source to total body RF homoeostasis is not clear, what is clear is that the large intestine is capable of absorbing luminal RF [43,139] and that colonocytes possess an efficient uptake mechanism for RF (see below).
In the diet, RF exists in the free and FMN and FAD forms. The latter two forms are hydrolysed to free RF prior to absorption by intestinal phosphatases [140]. As to the bacterially produced RF in the large intestine, a considerable amount of this RF exists in the free (absorbable) form [141,142]. The mechanism of RF absorption in the small and large intestines has been studied using a variety of preparations from a number of species (reviewed in [8,9]). These studies have shown the involvement of an efficient and specific Na+ -independent carrier-mediated mechanism for RF uptake located at the apical membrane domain. Other studies have characterized the exit process of RF out of the polarized enterocyte (transport across the BLM) and showed the event to also be via a specific carrier-mediated mechanism (reviewed in [8,9]). Studies have delineated the molecular identity of the intestinal RF uptake systems. Both RFT-1 (RF transporter-1) and RFT-2 (GenBank® accession numbers NM_017986 and NM_033409 respectively; [143,144]) are expressed in the small intestine, with expression of the latter being significantly higher than that of the former [143,144]. Furthermore, hRFT-2 (human RFT-2) appears to be a more efficient RF transporter than hRFT-1 [144]; in addition, the apparent Km of RF uptake by RFT-2 is similar to the apparent Km of the RF uptake process in intestinal epithelial cells (both being in the sub-micromolar range) [145,146]. A third RFT (RFT-3) has been described recently, but it appears to be brain-specific [147]. hRFT-1 (a 448 amino acid protein) is predicted to have ten TMDs [143], whereas hRFT-2 (a 468 amino acid protein) is predicted to have 11 TMDs; in addition, the two proteins share 43% identity with one another [144].
Knowledge about the structure–activity relationship of the RF transporters has also begun to emerge from both clinical findings and laboratory investigations. Many mutations in hRFT-2 have been identified recently in patients with Brown–Vialetto–Van Laere syndrome, a rare neurological disorder caused by mutation(s) in this transporter, and are associated with RF deficiency and sub-optimal levels [148–150]. The affected amino acids were reported to be at positions 28, 36, 71, 132, 213, 224, 413 and 457 of the hRFT-2 polypeptide. These findings suggest a role for these sites in the function and/or cell biology of hRFT-2, although no detailed characterizations of their function and cell biology have been performed so far. Another study used thiol-group-specific reagents to indicate the possible involvement of such groups in the function of the intestinal RF uptake process [145].
Regulatory aspects of the intestinal RF absorption process
The process of RF absorption in the intestine is regulated by extracellular and intracellular factors/conditions. Specifically, intestinal RF uptake was adaptively regulated by extracellular substrate levels [145,146,151]. Studies using cultured intestinal epithelial cells and rats have shown that RF deficiency leads to a significant up-regulation in RF uptake, whereas over-supplementation with RF leads to a significant down-regulation [145,146,151]. These adaptive changes in the intestinal RF uptake by substrate levels were mediated via changes in the Vmax (but not the apparent Km) of the uptake process, suggesting a change in the number (and/or activity), but not the affinity, of the RF carriers [145]. Furthermore, the up-regulation in RF uptake in deficiency was suppressed by the transcription inhibitor actinomycin D, suggesting the possible involvement of a transcriptional regulatory mechanism(s).
Intestinal RF uptake also appears to be developmentally regulated during early stages of life, with a decrease in RF uptake occurring with maturation [152]. This decrease appears to be mediated via a decrease in the Vmax and an increase in the apparent Km of the RF uptake process, suggesting a decrease in the number (and/or activity) and affinity of the RF uptake carriers with maturation.
Finally, intestinal RF uptake is under the regulation of specific intracellular regulatory pathways. A role for a PKA-mediated signalling pathway was reported where activation of the pathway leads to a significant inhibition in uptake [153]. The PKA-mediated inhibition in intestinal RF uptake was reversible and was mediated via a reduction in the Vmax (but not the apparent Km) of the RF uptake process. A role for an intracellular Ca2+ /CaM-mediated pathway in the regulation of intestinal RF uptake has also been reported [153].
Factors that negatively affect the intestinal RF absorption process
Laboratory investigations show that the intestinal RF uptake process is sensitive to the inhibitory effect of the Na+ /H+ exchanger inhibitor amiloride [145]. In addition, the tricyclic phenothiazine drug chlorpromazine (a compound that shares structural similarity with RF) inhibits intestinal RF uptake [154]. Whether these pharmacological agents also affect RF absorption in human intestine in vivo is not clear and requires further investigation.
THIAMIN
Thiamin (vitamin B1) is the first member of the family of water-soluble vitamins to be described, appearing in Chinese medical literature some 4000 years ago. The vitamin acts as a coenzyme in a variety of critical metabolic reactions related to energy metabolism; it is also involved in the generation of chemical-reducing power in cells. Deficiency of this vitamin leads to a variety of clinical abnormalities that include neurological and cardiovascular disorders (reviewed in [155]). The incidence of thiamin deficiency (and sub-optimal levels) is common in chronic alcoholics and diabetic patients [156–159]. It has also been reported in patients with coeliac disease [160] and those on long-term use of the diuretic furosemide [161]. Tissue-specific (i.e. localized) deficiency of thiamin also occurs in humans, as in the case of patients with TRMA (thiamin-responsive megaloblastic anaemia) and those with thiamin-responsive Wernicke's-like encephalopathy. The autosomal-recessive disorder TRMA is caused by mutations in hTHTR-1 [human THTR-1 (thiamin transporter-1)] (the product of the SLC19A2 gene) [162–164], which is highly expressed in the affected tissues. TRMA is characterized by megaloblastic anaemia, sensorineural deafness and Type 2 diabetes mellitus [165,166]. The thiamin-responsive Wernicke's-like encephalopathy is believed to be caused by mutations in the other major thiamin transporter, hTHTR-2 (the product of the SLC19A3 gene) [167].
Physiological and molecular aspects of the intestinal thiamin absorption process
Humans obtain thiamin from dietary and bacterial sources; the latter is provided by the normal microflora of the large intestine [6] and is absorbed in that region of the gut [42,139]. Again, the relative contribution of the bacterial source towards the total body requirement of thiamin is not clear. However, the recent identification of an efficient and specific uptake mechanism for luminal thiamin by human colonocytes (see below) when considered in combination with the time luminal content stays in the large intestine raises the possibility that this source of thiamin is of nutritional value to the host, especially for the cellular nutrition of the local colonocytes.
In the diet, thiamin exists mainly in the phosphorylated form, which is converted by phosphatases into free thiamin prior to absorption in the proximal part of the small intestine [168]. As to the bacterially produced thiamin in the large intestine, up to 50% of this thiamin exists in the free absorbable form [169–171]. The mechanism of absorption of free thiamin in the small and large intestine has been studied using a variety of intestinal and colonic preparations from a number of species. These studies indicate the involvement of a similar specialized carrier-mediated mechanism for thiamin uptake in both regions of the gut (reviewed in [8,9]). Others studies have shown that thiamin transport across the individual membrane domain of the polarized enterocyte is via a pH (but not Na+ )-dependent, electroneutral carrier-mediated mechanism [172–174]. The molecular identity of the systems involved in intestinal thiamin uptake has been delineated in recent years following the cloning of THTR-1 and THTR-2 from different tissues of a number of species [162–164,175,176]. Both hTHTR-1 (a 497 amino acid protein) and hTHTR-2 (a 496 amino acid protein) are expressed in the small and large intestines with expression of the former being significantly higher than that of the latter. These transport proteins are predicted to have 12 TMDs and multiple potential post-translational modification sites. The transporters also share considerable similarity with one another and with hRFC. However, neither of these thiamin transporters handles folate nor does RFC handle free thiamin. hTHTR-1 functions at the micromolar range, whereas hTHTR-2 functions at the nanomolar range. In addition, although the hTHTR-1 protein is expressed at both the BBM and BLM domains of the polarized enterocyte, expression of the hTHTR-2 protein is restricted to the BBM domain of these cells [177,178].
The relative contribution of THTR-1 and THTR-2 towards total carrier-mediated thiamin uptake in the intestine has also been determined using gene-specific siRNAs to selectively knock down the individual transporter [178]. That study showed that the transporters are involved in intestinal thiamin uptake and account for total carrier-mediated thiamin uptake [178]. A more recent study used THTR-1- and THTR-2-knockout mouse models to show a significant and specific inhibition in thiamin uptake in the intestine of THTR-2-knockout mice (Figure 4), whereas normal uptake was observed in the intestine of THTR-1-knockout mice [179]. The latter observation is most probably due to the significant induction of THTR-2 observed in the intestine (and kidney) of the THTR-1-knockout mice compared with their wild-type littermates. Collectively, these findings establish an important role for THTR-2 in the process of intestinal thiamin absorption. They may explain the normal plasma level of thiamin observed in patients with TRMA despite dysfunctional THTR-1 [180]. The latter could be due to an induction in hTHTR-2 expression in the intestine (and kidney) of the affected subjects, which may translate into a higher intestinal absorption (and renal re-uptake) of thiamin and, hence, a thiamin plasma level within the normal range [179].
Figure 4. Effect of the loss of THTR-2 on intestinal thiamin uptake.
Initial rate of carrier-mediated thiamin uptake in vitro in freshly isolated intestinal epithelial cells (A) and in vivo by intact intestinal loops (B) from THTR-2-knockout mice (THTR-2−/− ) and their sex-matched littermates [P < 0.01 for (A and B)]. (C) Initial rate of carrier-mediated biotin uptake in vivo by intact intestinal loops from THTR-2+/+ and THTR-2−/− mice. Reprinted from Gastroenterology, vol. 138, Reidling, J.C., Lambrecht, N., Kassir, M. and Said, H.M., Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice, pp. 1802–1809, © 2010, with permission from Elsevier.
Knowledge about the structure–function relationship of hTHTR-1 and -2 has been emerging from both clinical findings and experimental investigations. Thus 16 missense and nonsense mutations have been found in hTHTR-1 in patients with TRMA [180,181]. These mutations lead to a spectrum of physiological and biological phenotypes (including changes in protein stability, membrane targeting and/or transport activity) that result in impaired functionality [182,183]. Another study reported an important role for the amino acid at position 138 of the hTHTR-1 polypeptide (the only conserved anionic amino acid residue in the TMD of the polypeptide) in the transport of positively charged thiamin [183]. No role, however, was found for the putative N-glycosylation sites (predicted to be at positions 63 and 314 of the polypeptide) in the function or membrane targeting of hTHTR-1 [183]. With regard to the structure–function relationship of the hTHTR-2 polypeptide, a number of clinical mutations have also been identified recently. Two such mutations (K44E and E320Q) were identified in patients with thiamin-responsive Wernicke's-like encephalopathy, and both were shown to lead to a significant impairment in function [167]. In addition, although the E320Q mutant trafficked to the cell membrane, the K44E mutant failed to do so [167]. Few other additional mutations in the hTHTR2 polypeptide have been reported in patients with biotin-responsive basal ganglia disease [184], but it is unclear how mutations in a specific thiamin transporter contribute to the pathology of a condition that responds to biotin, since hTHTR-2 does not transport biotin [185]. A role for the three conserved negatively charged glutamic acid residues in the TMDs of both the hTHTR-1 and hTHTR-2 polypeptides (located at positions 120, 320 and 346) in the function of hTHTR-2 in transporting the positively charged vitamin has also been reported [185]. On the other hand, the two putative N-glycosylation sites (located at positions 45 and 166) of the hTHTR-2 polypeptide are not important for function or targeting of the protein to the cell membrane [185].
Cell biology of the intestinal thiamin absorption process: membrane targeting and intracellular trafficking of hTHTR-1 and hTHTR-2
Significant progress in our understanding of the cell biology of hTHTR-1 and -2 with regard to membrane targeting and intracellular trafficking to the cell surface has been made [177,186]. Live-cell confocal imaging of human intestinal epithelial cells expressing full-length and truncated hTHTR-1 constructs fused to GFP show that the full-length construct is expressed at both the apical and the BLM domains of these cells, with expression slightly higher at the latter than the former domain (Figure 5) [177]. These studies show an essential role for the N-terminal and backbone of the hTHTR-1 polypeptide (but not its C-terminal tail) in membrane targeting. Furthermore, truncation within a region of the hTHTR-1 polypeptide where several TRMA mutations are clustered leads to an intracellular retention of the mutant protein [177]. With regard to intracellular trafficking of hTHTR-1, this protein was found inside trafficking vesicles, whose mobility requires an intact microtubule network and is temperature-dependent ([177]; a movie of this can be seen at http://www.jcb.org/cgi/content/full/278/6/3976/DC1). The cell biology of hTHTR-2 in epithelial cells has been studied using live-cell confocal imaging, with results showing the exclusive expression of the protein at the apical membrane domain of these cells (Figure 5) [186]. Again, an essential role for the transmembrane backbone of the hTHTR-2 polypeptide, but not its cytoplasmic C-terminus, was found [186]. With regard to intracellular trafficking of the hTHTR-2 protein, this protein was again found to be inside numerous trafficking vesicles, whose mobility depends on an intact microtubule network and is affected by overexpression of dynamitin (p50) (a subunit of dynactin, which is involved in trafficking of vesicles via the minus-end-directed motor protein dynein) [186].
Figure 5. Expression of hTHTR-1 (A and B) and hTHTR-2 (C and D) fused to GFP in polarized living human intestinal epithelial Caco-2 cells.
AP, apical membrane; BL, basolateral membrane. Adapted from The American Journal of Physiology: Gastrointestinal and Liver Physiology, vol. 286 (2003), Said, H.M., Balamurugan, K., Subramanian, V.S. and Marchant, J.S., Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine, G491–G498, used with permission from The American Physiological Society.
Regulatory aspects of the intestinal thiamin absorption process
The process of thiamin absorption in the intestine is regulated by extracellular and intracellular factors/conditions via transcriptional and post-transcriptional mechanisms. Our understanding of the basal transcriptional regulation of hTHTR-1 and hTHTR-2 arises from studies in which the 5′-regulatory region (promoter) of their respective genes (SLC19A2 and SLC19A3 respectively) were cloned and characterized. The minimal promoter region required for basal activity of the SLC19A2 promoter in intestinal epithelial cells was found in a sequence between –356 and – 36, and included multiple putative cis-regulatory elements [187,188]. A number of these cis-elements [GKLF, NF-1 (nuclear factor-1) and Sp1] were shown experimentally (by mutational analysis, oligonucleotide competition assays, and electrophoretic mobility shift and supershift assays) to be important for promoter activity [187,188]. Functionality of the cloned human SLC19A2 promoter was also confirmed in vivo in transgenic mice [188]. With regard to the minimal promoter region required for basal activity of the SLC19A3 promoter, this region was found in a sequence between − 77 and + 59, and contains a number of putative cis-regulatory elements. An important role for the Sp1/GC-box-binding site (located at position − 48/− 45 bp) in promoter activity was established; in addition, this site appears to interact with both Sp1 and Sp3 [189]. Functionality of the human SLC19A3 promoter was also confirmed in vivo in transgenic mice [189].
The intestinal thiamin uptake process is adaptively regulated by the substrate level in the diet. Thiamin deficiency leads to a specific and significant induction in intestinal thiamin uptake [190,191]. This effect was associated with a significant induction in the level of expression of THTR-2 (but not THTR-1). When transgenic mice carrying the human SLC19A2 and SLC19A3 promoter–luciferase constructs were fed on a thiamin-deficient diet, a significant induction in the activity of the SLC19A3 (but not the SLC19A2) promoter in the intestine was observed compared with pair-fed transgenic controls [191]. These findings suggest that the up-regulation in THTR-2 is, at least in part, mediated via transcriptional mechanism(s).
The intestinal thiamin uptake process is developmentally regulated during early stages of life, with a decrease in thiamin uptake occurring with maturation [192]. This decrease was associated with a reduction in the expression of endogenous mouse THTR-1 and THTR-2 [192]. Furthermore, it was associated with a decrease in the activity of both the human SLC19A2 and SLC19A3 promoters in the small intestine of transgenic mice carrying these constructs [192]. These observations suggest that the regulation of intestinal thiamin uptake during development is, at least partially, mediated via a transcriptional mechanism(s). A similar pattern of developmental regulation was observed in thiamin uptake in the kidney [192].
The intestinal thiamin uptake process was further shown to undergo a differentiation-dependent regulation [193]. Studies using Caco-2 cells, which differentiate spontaneously in culture upon reaching confluence to become enterocyte-like cells [23], show a significant induction in carrier-mediated thiamin uptake with differentiation [193]. This induction was associated with a significant increase in the expression of hTHTR-1 and hTHTR-2, and in the activity of their respective promoters. The differentiation-responsive region is located between −356 and − 275 bp for the SLC19A2 promoter, and between− 77 −13 bp for the SLC19A3 promoter [193]. An important role for an NF-1-binding site (located between −348 and −345 bp of the SLC19A2 promoter) and for a Sp1/GC-box-binding− − site (located between − 48 and − 45 bp of the SLC19A3 promoter) in the differentiation-dependent response was also demonstrated. The differentiation-dependent regulation of intestinal thiamin uptake was also observed in studies with wild-type and transgenic mice carrying the human SLC19A2 and SLC19A3 promoters. In these studies, a significantly higher thiamin uptake, level of expression of the endogenous THTR-1 and THTR-2, and activity of SLC19A2 and SLC19A3 promoters were observed in intestinal villus compared with crypt epithelial cells [193].
Finally, the intestinal thiamin uptake process is under the regulation of specific intracellular regulatory pathways. A role for an intracellular Ca2+ /CaM-mediated pathway in the regulation of intestinal thiamin uptake has been reported [194,195]. This pathway functions by a change in the Vmax, but not the apparent Km, of the uptake process (i.e. via an effect on the activity and/or number, but not affinity, of the uptake process) [194,195]. The same intracellular pathway appears to regulate thiamin uptake in other non-intestinal cellular systems [196–198].
Factors that negatively affect the intestinal thiamin absorption process
It has been well recognized that chronic alcohol use leads to thiamin deficiency and that inhibition in intestinal thiamin absorption plays a role in causing this deficiency [199,200]. A A recent study in rats investigated the cellular and molecular parameters of the intestinal thiamin absorption process that were affected by chronic alcohol use [201]. Chronic alcohol feeding inhibited carrier-mediated thiamin transport across the BBM and BLM domains of the polarized enterocyte, and the inhibition was apparent as early as 2 weeks after the start of chronic alcohol feeding. This inhibition was associated with a marked decrease in the level of expression of THTR-1 (but not THTR-2) at the protein, mRNA and hnRNA levels, suggesting that the effect is being exerted, at least in part, at the level of transcription [201]. Similarly, thiamin uptake in rat colon was inhibited by chronic alcohol feeding, suggesting that this ingestion negatively affects the ability of the colon to absorb the bacterially synthesized thiamin [201]. Similar inhibition in thiamin uptake by chronic alcohol feeding was observed in the kidney [202]. These findings demonstrate that chronic alcohol use leads to inhibition in the entry of thiamin into the body (via the intestine) and its salvaging (reabsorption) by the kidneys, thus compounding its negative impact on normal thiamin homoeostasis in the body.
The intestinal thiamin uptake process is also negatively affected by infection with Gram-negative EPEC (enteropathogenic Escherichia coli) [203]. EPEC, a food-borne pathogen, causes a significant inhibition in thiamin uptake by Caco-2 cells [203]. This inhibition was not generalized to all members of the B family of vitamins as uptake of riboflavin, folate and biotin was not affected (A. Ghosal, B. Ashokkumar and H.M. Said, unpublished work). EPEC inhibition of thiamin uptake was mediated via a suppression in the function and expression of both hTHTR-1 and hTHTR-2; it was also associated with a decrease in the level of expression of hTHTR-1 and THTR-2 proteins at the cell surface [203]. EPEC also affected the transcription rate of SLC19A2 and SLC19A3. Thus EPEC infection of intestinal epithelial cells appears to have a rapid effect on thiamin uptake (mediated by inhibition in the function and membrane expression of the thiamin transporters) and a prolonged effect (exerted at the transcriptional level). The structural components of EPEC that are needed to cause the inhibition in thiamin uptake were also investigated, with results showing a role for the espF and espH genes (both of which encode effector proteins) [203].
Finally, expression of THTR-1 and -2 in the small intestine was shown to be significantly reduced in an animal model of chronic kidney disease [204]. Whether the same occurs in humans with kidney disease and what functional consequence this inhibition may have on intestinal thiamin absorption require further investigations.
Acknowledgments
FUNDING
My work is supported by the National Institutes of Health [grant numbers DK 56061 and DK58057] and the Department of Veterans Affairs.
Abbreviations used
- AA
ascorbic acid
- AP
activator protein
- BBM
brush-border membrane
- BBMV
brush-border membrane vesicle
- BLM
basolateral membrane
- BLMV
basolateral membrane vesicle
- CaM
calmodulin
- DHAA
dehydro-L-ascorbic acid
- DYNLRB1
dynein, light chain, roadblock-type 1
- EPEC
enteropathogenic Escherichia coli
- GFP
green fluorescent protein
- GLUT
glucose transporter
- HFMS
hereditary folate malabsorption syndrome
- HNF
hepatocyte nuclear factor
- hnRNA
heterogeneous nuclear RNA
- KLF
Krüppel-like factor
- GKLF
gut-enriched KLF
- NF
nuclear factor
- PCFT
proton-coupled folate transporter
- hPCFT
human PCFT
- PDZD11
PDZ domain-containing protein 11
- PKA
protein kinase A
- PKC
protein kinase C
- RF
riboflavin
- RFC
reduced folate carrier
- hRFC
human RFC
- RFT
RF transporter-1
- hRFT
human RFT
- siRNA
small interfering RNA
- SLC
solute carrier
- SMVT
sodium-dependent multivitamin transporter
- hSMVT
human SMVT
- Sp1
stimulating protein-1
- SVCT
sodium-dependent vitamin C transporter
- hSVCT
human SVCT
- THTR
thiamin transporter
- hTHTR
human THTR
- TMD
transmembrane domain
- TRMA
thiamin-responsive megaloblastic anaemia
- YFP
yellow fluorescent protein
REFERENCES
- 1.Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br. J. Haematol. 2010;148:195–204. doi: 10.1111/j.1365-2141.2009.07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc. Nat. Acad. Sci. U.S.A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi J, Wilson FA, Rose RC. Dehydroascorbic acid and ascorbic acid transport in the guinea pig ileum. Am. J. Physiol. 1986;250:G461–G468. doi: 10.1152/ajpgi.1986.250.4.G461. [DOI] [PubMed] [Google Scholar]
- 4.Choi JL, Rose RC. Regeneration of ascorbic acid by rat colon. Proc. Soc. Exp. Biol. Med. 1989;190:369–374. doi: 10.3181/00379727-190-42874. [DOI] [PubMed] [Google Scholar]
- 5.Schell DA, Bode AM. Measurement of ascorbic acid and dehydroascorbic acid in mammalian tissue utilizing HPLC and electrochemical detection. Biomed. Chromatogr. 1993;7:267–272. doi: 10.1002/bmc.1130070506. [DOI] [PubMed] [Google Scholar]
- 6.Wrong OM, Edmonds CJ, Chadwick VS. The Large Intestine: its Role in Mammalian Nutrition and Homeostasis. Wiley and Sons; New York: 1981. [Google Scholar]
- 7.Rose RC. In: Intestinal absorption of water-soluble vitamins. Physiology of the Gastrointestinal Tract. Johnson LR, editor. Raven Press; New York: 1987. pp. 1581–1596. [DOI] [PubMed] [Google Scholar]
- 8.Said HM. Recent advances in carrier-mediated absorption of water-soluble vitamins. Annu. Rev. Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 9.Said HM, Seetharam B. Intestinal absorption of vitamins. In: Johnson LR, Barrett KE, Ghishan FK, Merchand JM, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4th edn Vol. 2. Academic Press; New York: 2006. pp. 1791–1826. [Google Scholar]
- 10.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (l-ascorbic acid) transporter SVCT1. Biochim. Biophys. Res. Commun. 2000;267:488–494. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian VS, Marchant JS, Reidling JC, Said HM. N-Glycosylation is required for Na+-dependent vitamin C transporter functionality. Biochem. Biophys. Res. Commun. 2008;374:123–127. doi: 10.1016/j.bbrc.2008.06.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 14.Varma S, Campbell CE, Kuo S. Functional role of conserved transmembrane segment 1 residues in human sodium-dependent vitamin C transporters. Biochemistry. 2008;47:2952–2960. doi: 10.1021/bi701666q. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J. Biol. Chem. 2004;279:27719–27728. doi: 10.1074/jbc.M400876200. [DOI] [PubMed] [Google Scholar]
- 16.Boyer JC, Campbell CE, Sigurdson WJ, Kuo S. Polarized localization of vitamin C transports, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys. Res. Commun. 2005;334:150–156. doi: 10.1016/j.bbrc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 17.Michels AJ, Hagen TM. Hepatocyte nuclear factor 1 is essential for transcription of sodium-dependent vitamin C transporter protein 1. Am. J. Physiol. Cell Physiol. 2009;297:C1220–C1227. doi: 10.1152/ajpcell.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reidling JC, Rubin SA. Promoter analysis of the human ascorbic acid transporters SVCT1 and SVCT2: mechanism of adaptive regulation in liver epithelial cells. J. Nutr. Biochem. 2011;22:344–350. doi: 10.1016/j.jnutbio.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karasov WH, Darken BW, Bottum MC. Dietary regulation of intestinal ascorbate uptake in guinea pigs. Am. J. Physiol. 1991;260:G108–G118. doi: 10.1152/ajpgi.1991.260.1.G108. [DOI] [PubMed] [Google Scholar]
- 20.Rose RC, Nahrwold DL. Intestinal ascorbic acid transport following diets of high or low ascorbic acid content. Int. J. Vitam. Nutr. Res. 1978;48:382–386. [PubMed] [Google Scholar]
- 21.MacDonald L, Thumser AE, Sharp P. Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br. J. Nutr. 2002;87:97–100. doi: 10.1079/BJN2001492. [DOI] [PubMed] [Google Scholar]
- 22.Amano A, Aigaki T, Maruyama N, Ishigami A. Ascorbic acid depletion enhaces expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch. Biochem. Biophys. 2010;496:38–44. doi: 10.1016/j.abb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Pinto M, Leon SR, Tappay M, Kedinger M, Triadou N, Dussayix M, Lacvrix B, Simon-Asswan P, Haffer N, Fiugh J, Zwiebaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell. 1988;47:323–340. [Google Scholar]
- 24.Maulén NP, Henriquez EA, Kempe S, Cárcamo JG, Schmid-Kotsas A, Bachem M, Grünert A, Bustamante ME, Nualart F, Vera JC. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated Caco-2 cells. J. Biol. Chem. 2003;278:9035–9041. doi: 10.1074/jbc.M205119200. [DOI] [PubMed] [Google Scholar]
- 25.Scheerger SB, Zempleni J. Expression of oncogenes depends on biotin in human small cell lung cancer cells NCI-H69. Int. J. Vit. Nutr. Res. 2003;73:461–467. doi: 10.1024/0300-9831.73.6.461. [DOI] [PubMed] [Google Scholar]
- 26.Collins JC, Paietta E, Green R, Morell AG, Stockert RJ. Biotin-dependent expression of the asialoglycoprotein receptor in HepG2 cells. J. Biol. Chem. 1988;263:11280–11283. [PubMed] [Google Scholar]
- 27.Vesely DL. Biotin enhances guanylate cyclase activity. Science. 1982;216:1329–1330. doi: 10.1126/science.6123152. [DOI] [PubMed] [Google Scholar]
- 28.Spence JT, Koudelka AP. Effect of biotin upon the intracellular level of cGMP and the activity of glucokinase in cultured rat hepatocytes. J. Biol. Chem. 1984;259:6393–6396. [PubMed] [Google Scholar]
- 29.Watanabe T. Teratogenic effect of biotin deficiency in mice. J. Nutr. 1983;113:574–581. doi: 10.1093/jn/113.3.574. [DOI] [PubMed] [Google Scholar]
- 30.Cooper WA, Brown SO. Tissue abnormalities in newborn rats from biotin-deficient mothers. Texas J. Sci. 1958;10:60–68. [Google Scholar]
- 31.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J. Nutr. 2003;133:2519–2525. doi: 10.1093/jn/133.8.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc. Soc. Exp. Biol. Med. 2000;223:14–21. doi: 10.1046/j.1525-1373.2000.22303.x. [DOI] [PubMed] [Google Scholar]
- 33.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu. Rev. Nutr. 1986;6:314–343. doi: 10.1146/annurev.nu.06.070186.001533. [DOI] [PubMed] [Google Scholar]
- 34.Krause KH, Berlit P, Bonjour JP. Impaired biotin status in anticonvulsant therapy. Ann. Neurol. 1982;12:485–486. doi: 10.1002/ana.410120513. [DOI] [PubMed] [Google Scholar]
- 35.Lampen J, Hahler G, Peterson W. The occurrence of free and bound biotin. J. Nutr. 1942;23:11–21. [Google Scholar]
- 36.Krause KH, Bonjour J, Berlit P, Kochen W. Biotin status of epileptics. Ann. N. Y. Acad. Sci. 1985;447:297–313. doi: 10.1111/j.1749-6632.1985.tb18447.x. [DOI] [PubMed] [Google Scholar]
- 37.Forbes GM, Forbes A. Micronutrient status in patients receiving home parenteral nutrition. Nutrition. 1997;13:941–944. doi: 10.1016/s0899-9007(97)00334-1. [DOI] [PubMed] [Google Scholar]
- 38.Mock DM, deLorimer AA, Liebman WM, Sweetman L, Baker H. Biotin deficiency: an unusual complication of parenteral alimentation. N. Engl. J. Med. 1981;304:820–823. doi: 10.1056/NEJM198104023041405. [DOI] [PubMed] [Google Scholar]
- 39.Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int. J. Vitam. Nutr. Res. 1980;50:425–440. [PubMed] [Google Scholar]
- 40.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J. Nutr. 1997;127:710–716. doi: 10.1093/jn/127.5.710. [DOI] [PubMed] [Google Scholar]
- 41.Banares FF, Lacruz AA, Gine JJ, Esteve M, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1989;84:744–748. [PubMed] [Google Scholar]
- 42.Sorrell MF, Frank O, Thomson AD, Aquino A, Baker H. Absorption of vitamins from the large intestine. Nutr. Res. Int. 1971;3:143–148. [Google Scholar]
- 43.Barth CA, Frigg M, Homogemeister H. Biotin absorption from the hindgut of the pig. J. Anim. Physiol. Anim. Nutr. 1986;55:128–134. [Google Scholar]
- 44.Brown BB, Rosenberg JH. Biotin absorption by distal rat intestine. J. Nutr. 1987;117:2121–2126. doi: 10.1093/jn/117.12.2121. [DOI] [PubMed] [Google Scholar]
- 45.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am. J. Physiol. 1998;275:C1365–C1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 46.Wolf B, Heard GS, Secor-McVoy JR, Raetz HM. Biotinidase deficiency: the possible role of biotinidase in the processing of dietary protein-bound biotin. J. Inherit. Metab. Dis. 1984;7:121–122. doi: 10.1007/978-94-009-5612-4_35. [DOI] [PubMed] [Google Scholar]
- 47.Blanton SH, Pandya A, Landa BL, Javaheri R, Xia XJ, Nance WE, Pomponio RJ, Norrgard KJ, Swango KL, Demirkol M, et al. Fine mapping of the human biotinidase gene and haplotype analysis of five common mutations. Hum. Hered. 2000;50:102–111. doi: 10.1159/000022897. [DOI] [PubMed] [Google Scholar]
- 48.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by the human colonic epithelial cells NCM460: a carrier-mediated process shared with pantothenic acid. Am. J. Physiol. 1998;44:C1365–C1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 49.Said HM. Cellular uptake of biotin: mechanisms and regulation. J. Nutr. 1999;129:490S–493S. doi: 10.1093/jn/129.2.490S. [DOI] [PubMed] [Google Scholar]
- 50.Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport system for biotin in human intestinal brush border membrane vesicles. Am. J. Physiol. 1987;253:G631–G636. doi: 10.1152/ajpgi.1987.253.5.G631. [DOI] [PubMed] [Google Scholar]
- 51.Said HM, Redha R. Biotin transport in brush border membrane vesicles of rat small intestine. Biochim. Biophys. Acta. 1988;945:195–201. doi: 10.1016/0005-2736(88)90482-8. [DOI] [PubMed] [Google Scholar]
- 52.Said HM, Nylander W, Redha R. Biotin transport in human intestine: site of maximum transport and effect of pH. Gastroenterology. 1988;95:1312–1317. doi: 10.1016/0016-5085(88)90366-6. [DOI] [PubMed] [Google Scholar]
- 53.Said HM, Redha R. Biotin transport in basolateral membrane vesicles of human intestine. Gastroenterology. 1988;94:1157–1163. doi: 10.1016/0016-5085(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 54.Said HM. Movement of biotin across the rat intestinal basolateral membrane: studies with membrane vesicles. Biochem. J. 1991;279:671–674. doi: 10.1042/bj2790671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am. J. Physiol. Cell Physiol. 2009;296:C663–C671. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabokina SM, Subramanian VS, Said HM. Comparative analysis of ontogenic changes in renal and intestinal biotin transport in the rat. Am. J. Physiol. Renal Physiol. 2003;284:F737–F742. doi: 10.1152/ajprenal.00364.2002. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Huang W, Fei Y-J, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Cloning, functional expression, gene structure, and chromosomal localization. J. Biol. Chem. 1999;274:14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- 58.Prasad PD, Wang H, Huang W, Fei Y, Leibach FH, Devoe LD, Ganapathy V. Cloning and functional characterization of the intestinal Na+ -dependent multivitamin transporter. Arch. Biochem. Biophys. 1999;366:95–106. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- 59.Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am. J. Physiol. 1999;277:C605–C613. doi: 10.1152/ajpcell.1999.277.4.C605. [DOI] [PubMed] [Google Scholar]
- 60.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G73–G77. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- 61.Ghosal A, Said HM. Structure–function activity of the human sodium-dependent multivitamin transporter: role of His115 and His254. Am. J. Physiol. Cell Physiol. 2011;300:C97–C104. doi: 10.1152/ajpcell.00398.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nabokina SM, Subramanian VS, Said HM. Association of PDZ-containing protein PDZD11 with the human sodium-dependent multivitamin transporter. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;300:G561–G567. doi: 10.1152/ajpgi.00530.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatterjee NS, Rubin SA, Said HM. Molecular characterization of the 5′ regulatory region of rat sodium-dependent multivitamin transporter gene. Am. J. Physiol. Cell Physiol. 2001;280:C548–C555. doi: 10.1152/ajpcell.2001.280.3.C548. [DOI] [PubMed] [Google Scholar]
- 64.Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim. Biophys. Acta. 2002;1574:187–192. doi: 10.1016/s0167-4781(02)00226-9. [DOI] [PubMed] [Google Scholar]
- 65.Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am. J. Physiol. Cell Physiol. 2007;292:C1305–C1312. doi: 10.1152/ajpcell.00360.2006. [DOI] [PubMed] [Google Scholar]
- 66.Said HM, Mock DM, Collins J. Regulation of intestinal biotin transport in the rat: effect of biotin deficiency and supplementation. Am. J. Physiol. 1989;256:G306–G311. doi: 10.1152/ajpgi.1989.256.2.G306. [DOI] [PubMed] [Google Scholar]
- 67.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: a study of the hSMVT system. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G275–G281. doi: 10.1152/ajpgi.00327.2006. [DOI] [PubMed] [Google Scholar]
- 68.Said HM, Redha R. Ontogenesis of the intestinal transport of biotin in the rat. Gastroenterology. 1988;94:68–72. doi: 10.1016/0016-5085(88)90611-7. [DOI] [PubMed] [Google Scholar]
- 69.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin SA. Biotin uptake by the human colonic epithelial cells NCM460: A carrier-mediated process shared with pantothenic acid. Am. J. Physiol. 1998;275:C1365–C1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 70.Said HM. Cellular uptake of biotin: mechanisms and regulation. J. Nutr. 1999;129:490S–493S. doi: 10.1093/jn/129.2.490S. [DOI] [PubMed] [Google Scholar]
- 71.Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic: I, Aetiological role of aneurin and other B-complex vitamins. Br. Med. J. 1964;2:1290–1292. doi: 10.1136/bmj.2.5420.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subramanya SB, Subramanian VS, Kumar JS, Hoiness R, Said HM. Inhibition of intestinal biotin absorption by chronic alcohol feeding: cellular and molecular mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G494–G501. doi: 10.1152/ajpgi.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramanian VS, Subramanya SB, Said HM. Chronic alcohol exposure negatively impacts the physiological and molecular parameters of the renal biotin reabsorption process. Am. J. Physiol. Renal Physiol. 2011;300:F611–F617. doi: 10.1152/ajprenal.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Said HM, Redha R, Nylander W. Biotin transport in the human intestine: inhibition by anticonvulsant drugs. Am. J. Clin. Nutr. 1987;49:127–131. doi: 10.1093/ajcn/49.1.127. [DOI] [PubMed] [Google Scholar]
- 75.Qiu A, Jansen M, Sakaris A, Min SH, Chattopahyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 76.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine. 2002;81:51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Dudeja PK, Torania SA, Said HM. Evidence for the existence of an electroneutral, pH-dependent, DIDS-sensitive carrier-mediated folate uptake mechanism in the human colonic luminal membrane vesicles. Am. J. Physiol. 1997;272:G1408–G1415. doi: 10.1152/ajpgi.1997.272.6.G1408. [DOI] [PubMed] [Google Scholar]
- 78.Kumar CK, Moyer MP, Dudeja PK, Said HM. A protein-tyrosine kinase regulated, pH-dependent carrier-mediated uptake system for folate by human normal colonic epithelial cell line NCM 460. J. Biol. Chem. 1997;272:6226–6231. doi: 10.1074/jbc.272.10.6226. [DOI] [PubMed] [Google Scholar]
- 79.Rong NI, Selhub J, Goldin BR, Rosenberg I. Bacterially synthesized folate in rat large intestine is incorporated into host tissue folylpolyglutamates. J. Nutr. 1991;121:1955–1959. doi: 10.1093/jn/121.12.1955. [DOI] [PubMed] [Google Scholar]
- 80.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009;11:1–27. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu. Rev. Nutr. 1999;19:91–122. doi: 10.1146/annurev.nutr.19.1.91. [DOI] [PubMed] [Google Scholar]
- 82.Dixon KH, Lampher BC, Chiu J, Kelly K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotraxate sensitivity to transport deficient cells. J. Biol. Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- 83.Williams FMR, Murray RC, Underhill TM, Flintoff WF. Isolation of hamster cDNA clone coding for a function involved in methotrexate uptake. J. Biol. Chem. 1994;269:5810–5816. [PubMed] [Google Scholar]
- 84.Nguyen TT, Dyer DL, Dunning DD, Rubin SA, Said HM. Human intestinal folate transport: cloning, expression and distribution of complementary RNA. Gastroenterology. 1997;112:783–791. doi: 10.1053/gast.1997.v112.pm9041240. [DOI] [PubMed] [Google Scholar]
- 85.Matherly LH, Hou Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 2008;79:145–184. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Zhao R, Russel RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim. Biophys. Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 87.Dudeja PK, Kode A, Alnounou M, Tyagi S, Torania S, Subramanian VS, Said HM. Mechanism of folate transport across the human colonic basolateral membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G54–G60. doi: 10.1152/ajpgi.2001.281.1.G54. [DOI] [PubMed] [Google Scholar]
- 88.Unal ES, Zhao R, Qiu A, Coldman D. N-linked glycosylation and its impact on the electrophoreticmobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim. Biophys. Acta. 2008;1778:1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 90.Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled folate transporter in polarized epithelia. Am. J. Physiol. Cell Physiol. 2008;294:C233–C240. doi: 10.1152/ajpcell.00468.2007. [DOI] [PubMed] [Google Scholar]
- 91.Said HM, Blair JA, Lucas ML, Hilburn ME. Intestinal surface acid microclimate in vitro and in vivo in the rat. J. Lab. Clin. Med. 1986;107:420–424. [PubMed] [Google Scholar]
- 92.Liu XY, Witt TL, Matherly LH. Restoration of high-level transport activity by human reduced folate carrier/ThTr1 thiamine transporter chimaeras: role of the transmembrane domain 6/7 linker region in reduced folate carrier function. Biochem. J. 2003;369:31–37. doi: 10.1042/BJ20020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadlish H, Williams F, Flintoff W. Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function. Biochem. J. 2002;364:777–786. doi: 10.1042/BJ20011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J. Biol. Chem. 2009;284:17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subramanian VS, Marchant JS, Parker I, Said HM. Intracellular trafficking/membrane targeting of human reduced folate carrier expressed in Xenopus oocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1477–G1486. doi: 10.1152/ajpgi.2001.281.6.G1477. [DOI] [PubMed] [Google Scholar]
- 96.Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in mammalian epithelial cells. J. Biol. Chem. 2002;277:33325–33333. doi: 10.1074/jbc.M205955200. [DOI] [PubMed] [Google Scholar]
- 97.Ashokkumar B, Nabokina SM, Ma TY, Said HM. Identification of dynein light chain road block-1 as a novel interaction partner with the human reduced folate carrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G480–G487. doi: 10.1152/ajpgi.00154.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stark M, Gonen N, Assaraf YG. Functional elements in the minimal promoter of the human proton-coupled folate transporter. Biochem. Biophys. Res. Commun. 2009;388:79–85. doi: 10.1016/j.bbrc.2009.07.116. [DOI] [PubMed] [Google Scholar]
- 99.Said HM, Chatterjee H, Haq RU, Subramanian VS, Ortiz A, Matherly LH, Sirotnak FM, Halsted C, Rubin SA. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am. J. Physiol. Cell Physiol. 2000;279:C1889–C1895. doi: 10.1152/ajpcell.2000.279.6.C1889. [DOI] [PubMed] [Google Scholar]
- 100.Qiu A, Min SH, Jansen M, Usha M, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure functional properties and response to dietary folate restriction. Am. J. Physiol. Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 101.Subramanian VS, Chatterjee N, Said HM. Folate uptake in the human intestine: promoter activity and effect of folate deficiency. J. Cell. Physiol. 2003;196:403–408. doi: 10.1002/jcp.10324. [DOI] [PubMed] [Google Scholar]
- 102.Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am. J. Clin. Nutr. 2007;86:159–166. doi: 10.1093/ajcn/86.1.159. [DOI] [PubMed] [Google Scholar]
- 103.Shafizadeh TB, Halsted CH. Postnatal ontogeny of intestinal GCPII and the RFC in pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G476–G481. doi: 10.1152/ajpgi.00446.2007. [DOI] [PubMed] [Google Scholar]
- 104.Said HM, Ghishan FK, Murrell JE. Ontogenesis of intestinal transport of 5-methyltetrahydrofolate in the rat. Am. J. Physiol. 1985;249:G567–G571. doi: 10.1152/ajpgi.1985.249.5.G567. [DOI] [PubMed] [Google Scholar]
- 105.Balamurugan K, Said HM. Ontogenic regulation of folate transportacross rat jejunal brush-border membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G1068–G1073. doi: 10.1152/ajpgi.00188.2003. [DOI] [PubMed] [Google Scholar]
- 106.Subramanian VS, Reidling JC, Said HM. Differentiation-dependent regulation of the intestinal folate uptake process: studies with Caco-2 cells and native mouse intestine. Am. J. Physiol. Cell Physiol. 2008;295:C828–C835. doi: 10.1152/ajpcell.00249.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Said HM, Ma TY, Ortiz A, Tapia A, Valerio CK. Intracellular regulation of intestinal folate uptake: studies with cultured IEC-6 epithelial cells. Am. J. Physiol. 1997;272:C729–C736. doi: 10.1152/ajpcell.1997.272.2.C729. [DOI] [PubMed] [Google Scholar]
- 108.Halsted CH, Robles EA, Mezey E. Decreased jejunal uptake of labeled folic acid (3 H-PGA) in alcoholic patients: roles of alcohol and nutrition. N. Engl. J. Med. 1971;285:701–706. doi: 10.1056/NEJM197109232851301. [DOI] [PubMed] [Google Scholar]
- 109.Villanueva JA, Devlin AM, Halsted CH. Reduced folate carrier: tissue distribution and effects of chronic ethanol intake in the micropigAlcohol. Clin. Exp. Res. 2001;25:415–420. [PubMed] [Google Scholar]
- 110.Hamid A, Wani NA, Rana S, Vaiphei K, Mahmood A, Kaur J. Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism. FEBS J. 2007;274:6317–6328. doi: 10.1111/j.1742-4658.2007.06150.x. [DOI] [PubMed] [Google Scholar]
- 111.Said HM, Mee L, Sekar VT, Ashokkumar B, Pandol SJ. Mechanism and regulation of folate uptake by pancreatic acinar cells: effect of chronic alcohol consumption. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G985–G993. doi: 10.1152/ajpgi.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Halsted CH, Reisenauer AM, Romero JJ, Cantor DS, Ruebner B. Jejunal perfusion of simple and conjugated folates in celiac sprue. J. Clin. Invest. 1977;59:933–940. doi: 10.1172/JCI108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Halsted CH, Reisenauer AM, Shane B, Tamura T. Availability of monoglutamyl and polglutamyl folates in normal subjects and in patients with celiac sprue. Gut. 1978;19:886–891. doi: 10.1136/gut.19.10.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franklin JL, Rosenberg IH. Impaired folic acid absorption in inflammatory bowel disease: effects of salicylazosulfapyridine (azulfidine). Gastroenterology. 1973;64:517–525. [PubMed] [Google Scholar]
- 115.Halsted CH, Gandhi G, Tamura T. Folate levels inflammatory bowel disease. N. Engl. J. Med. 1982;306:1488. doi: 10.1056/NEJM198206173062416. [DOI] [PubMed] [Google Scholar]
- 116.Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G248–G254. doi: 10.1152/ajpgi.00224.2009. [DOI] [PubMed] [Google Scholar]
- 117.Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, Hatakeyama M, Hayashi Y, Otagiri M, Yuasa H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G660–G668. doi: 10.1152/ajpgi.00309.2007. [DOI] [PubMed] [Google Scholar]
- 118.Jansen G, Van der Heijden J, Oerlemans R, Lems WF, Ifergan I, Scheper RJ, Assaraf YG, Dijkmans BA. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum. 2004;50:2130–2139. doi: 10.1002/art.20375. [DOI] [PubMed] [Google Scholar]
- 119.Fox KR, Adrian C, Hogben M. Nicotinic acidactive transport by in vitro bullfrog small intestine. Biochim. Biophys. Acta. 1974;332:336–340. [Google Scholar]
- 120.Sadoogh-Abasian F, Evered DF. Absorption of nicotinic acid and nicotinamide from rat small intestine in vitro. Biochim. Biophys. Acta. 1980;598:385–391. doi: 10.1016/0005-2736(80)90016-4. [DOI] [PubMed] [Google Scholar]
- 121.Simanjuntak MT, Tamai I, Terasaki T, Tsuji A. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. J. Pharmacobiodyn. 1990;13:301–309. doi: 10.1248/bpb1978.13.301. [DOI] [PubMed] [Google Scholar]
- 122.Takanaga H, Maeda H, Yabuuchi H, Tamai I, Higashida H, Tsuji A. Nicotinic acid transport mediated by pH-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. J. Pharm. Pharmacol. 1996;48:1073–1077. doi: 10.1111/j.2042-7158.1996.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 123.Guilarte TR, Pravlik K. Radiometric-microbiologic assay of niacin using Kloeckera brevis: analysis of human blood and food. J. Nutr. 1983;113:2587–2594. doi: 10.1093/jn/113.12.2587. [DOI] [PubMed] [Google Scholar]
- 124.Nabokina SM, Kashyap ML, Said HM. Mechanism and regulation of human intestinal niacin uptake. Am. J. Physiol. Cell Physiol. 2005;289:C97–C103. doi: 10.1152/ajpcell.00009.2005. [DOI] [PubMed] [Google Scholar]
- 125.Said HM, Nabokina SM, Balamurugan K, Hohammed ZM, Urbina C, Kashyap ML. Mechanism of nicotinic acid transport in human liver cells: experiments with HepG2 cells and primary hepatocytes. Am. J. Physiol. Cell Physiol. 2007;293:C1773–C1778. doi: 10.1152/ajpcell.00409.2007. [DOI] [PubMed] [Google Scholar]
- 126.Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J. Biol. Chem. 2008;283:16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- 127.Gopal E, Fei YJ, Miyauchi S, Zhuang L, Prasad PD, Ganapathy V. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem. J. 2005;388:309–316. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, Smith SB, Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1(SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm. Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 129.Shibata K, Gross CJ, Henderson LM. Hydrolysis and absorption of pantothenate and its coenzymes in the rat small intestine. J. Nutr. 1983;113:2107–2115. doi: 10.1093/jn/113.10.2107. [DOI] [PubMed] [Google Scholar]
- 130.Gospe SM. Pyridoxine-dependent seizures: finding from recent studies pose new questions. Pediatr. Neurol. 2002;26:181–185. doi: 10.1016/s0887-8994(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 131.Linkswiler H, Baumann CA, Snell EE. Effect of aureomycin on the response of rats to various forms of vitamin B6. J. Nutr. 1951;43:565–573. doi: 10.1093/jn/43.4.565. [DOI] [PubMed] [Google Scholar]
- 132.Hamm MW, Hehansho H, Henderson LM. Transport and metabolism of pyridoxamine and pyridoxamine phosphate in the small intestine. J. Nutr. 1979;109:1552–1559. doi: 10.1093/jn/109.9.1552. [DOI] [PubMed] [Google Scholar]
- 133.Middleton HM. Intestinal absorption of pyridoxal-5′ phosphate disappearance from perfused segments of rat jejunum in vivo. J. Nutr. 1979;109:975–981. doi: 10.1093/jn/109.6.975. [DOI] [PubMed] [Google Scholar]
- 134.Middleton HM. Uptake of pyridoxine by in vivo perfused segments of rat small intestine: a possible role for intracellular vitamin metabolism. J. Nutr. 1985;115:1079–1088. doi: 10.1093/jn/115.8.1079. [DOI] [PubMed] [Google Scholar]
- 135.Yoshida S, Hayashi K, Kawasaki T. Pyridoxine transport in brush border membrane vesicles of guinea pig jejunum. J. Nutr. Sci. Vitaminol. 1981;27:311–317. doi: 10.3177/jnsv.27.311. [DOI] [PubMed] [Google Scholar]
- 136.Said HM, Ortiz A, Ma TY. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am. J. Physiol. Cell Physiol. 2003;285:C1219–C1225. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 137.Said ZM, Subramanian VS, Vaziri ND, Said HM. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am. J. Physiol. Cell Physiol. 2008;294:C1192–C1197. doi: 10.1152/ajpcell.00015.2008. [DOI] [PubMed] [Google Scholar]
- 138.Wang L-D, Zhou F-Y, Li X-M, Sun L-D, Song X, Jin Y, Li J-M, Kong G-Q, Qi H, Cui J, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat. Genet. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 139.Kasper H. Vitamin absorption in the colon. Am. J. Proctol. 1970;21:341–345. [PubMed] [Google Scholar]
- 140.Daniel H, Binninger E, Rehner G. Hydrolysis of FMN and FAD by alkaline phosphatase of the intestinal brush border membrane. Int. J. Vitam. Nutr. Res. 1983;53:109–114. [PubMed] [Google Scholar]
- 141.Iinuma S. Synthesis of riboflavin by intestinal bacteria. J. Vitam. 1995;2:6–13. doi: 10.5925/jnsv1954.1.2_6. [DOI] [PubMed] [Google Scholar]
- 142.Ocese O, Pearson PB, Schwiegert BS. The synthesis of certain B vitamins by the rabbit. J. Nutr. 1948;35:577–590. doi: 10.1093/jn/35.5.577. [DOI] [PubMed] [Google Scholar]
- 143.Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am. J. Physiol. Cell Physiol. 2008;295:C632–C641. doi: 10.1152/ajpcell.00019.2008. [DOI] [PubMed] [Google Scholar]
- 144.Yamamoto S, Inoue K, Ohta K, Fukatsu R, Maeda J, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J. Biochem. 2009;145:437–443. doi: 10.1093/jb/mvn181. [DOI] [PubMed] [Google Scholar]
- 145.Said HM, Ma TY. Mechanism of riboflavin uptake by Caco-2 human intestinal epithelial cells. Am. J. Physiol. 1994;266:G15–G21. doi: 10.1152/ajpgi.1994.266.1.G15. [DOI] [PubMed] [Google Scholar]
- 146.Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am. J. Physiol. Cell Physiol. 2000;278:C270–C276. doi: 10.1152/ajpcell.2000.278.2.C270. [DOI] [PubMed] [Google Scholar]
- 147.Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K-I. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J. Nutr. 2010;140:1220–1226. doi: 10.3945/jn.110.122911. [DOI] [PubMed] [Google Scholar]
- 148.Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin J-P, Raymon FL, Childs A-M, Sheridan E, Edwards S, Josifova DJ. Brown–Vialetto–Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in C20orf54. Am. J. Hum. Genet. 2010;86:485–489. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnson JO, Gibbs JR, Maldergem LV, Houlden H, Singleton AB. Exome sequencing in Brown–Vialetto–Van Laere syndrome. Am. J. Hum. Genet. 2010;87:567–570. doi: 10.1016/j.ajhg.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bosch AM, Abeling NGGM, IJ1st L, Knoester H, Van der Pol WL, Stroomer AEM, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown–Vialetto–Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J. Inherit. Metab. Dis. 2011;34:159–164. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Said HM, Khani R. Uptake of riboflavin across the brush border membrane of rat intestine: regulation by dietary vitamin levels. Gastroenterology. 1993;105:1294–1298. doi: 10.1016/0016-5085(93)90131-u. [DOI] [PubMed] [Google Scholar]
- 152.Said HM, Ghishan FK, Greene HL, Hollander D. Developmental maturation of riboflavin intestinal transport in the rat. Pediatr. Res. 1985;19:1175–1178. doi: 10.1203/00006450-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 153.Said HM, Ma TY, Grant K. Regulation of riboflavin intestinal uptake by protein kinase A: studies with Caco-2 cells. Am. J. Physiol. 1994;267:G955–G959. doi: 10.1152/ajpgi.1994.267.6.G955. [DOI] [PubMed] [Google Scholar]
- 154.Yanagawa N, Shih RN, Jo OD, Said HM. Riboflavin transport by isolated perfused rabbit renal proximal tubules. Am. J. Physiol. Cell Physiol. 2000;279:C1782–C1786. doi: 10.1152/ajpcell.2000.279.6.C1782. [DOI] [PubMed] [Google Scholar]
- 155.Said HM. In: Thiamin. In Encyclopedia of Dietary Supplements. Coats PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, editors. Informa HealthCare; New York: 2010. pp. 752–757. [Google Scholar]
- 156.Tallaksen CME, Bohmer T, Bell H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol. Clin. Exp. Res. 1992;16:320–325. doi: 10.1111/j.1530-0277.1992.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 157.Leevy CM, Baker H. Vitamins and alcoholism. Am. J. Clin. Nutr. 1968;21:1325–1328. doi: 10.1093/ajcn/21.11.1325. [DOI] [PubMed] [Google Scholar]
- 158.Tallaksen CM, Bell H, Bohmer T. Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Werincke encephalopathy. Alcohol. Clin. Exp. Res. 1993;17:712–716. doi: 10.1111/j.1530-0277.1993.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 159.Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamin levels in outpatients with diabetes mellitus. J. Nutr. Sci. Vitaminol. 1987;33:421–430. doi: 10.3177/jnsv.33.421. [DOI] [PubMed] [Google Scholar]
- 160.Thomson AD. The absorption of sulfur-labeled thiamin hydrochloride in control subjects and in patients with intestinal malabsorption. Clin. Sci. 1966;31:167–179. [PubMed] [Google Scholar]
- 161.Seligmann H, Halkin H, Rauchfleisch S, Kaufmann N, Motro M, Vered Z, Ezra D. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am. J. Med. 1991;91:151–155. doi: 10.1016/0002-9343(91)90007-k. [DOI] [PubMed] [Google Scholar]
- 162.Diaz GA, Banikazemai M, Oishi K, Desnick RJ, Gelb BD. Mutations in a new gene encoding a thiamin transporter cause thiamin-responsive megaloblastic anaemia syndrome. Nat. Genet. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- 163.Labay V, Raz T, Baron D, Mandel H, Williams H, Barrett T, Szargel R, McDonald L, Shalata A, Nosaka K, et al. Mutations in SLC19A2 cause thiamin-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nat. Genet. 1999;22:300–304. doi: 10.1038/10372. [DOI] [PubMed] [Google Scholar]
- 164.Fleming JC, Tartaglini E, Steinkamp MP, Schorderet DF, Cohen N, Neufeld EJ. The gene mutated in thiamine-response anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat. Genet. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- 165.Mandel H, Berant M, Hazani A, Naveh Y. Thiamine-dependent beriberi in the thiamine-responsive anemia syndrome. N. Engl. J. Med. 1984;311:836–838. doi: 10.1056/NEJM198409273111307. [DOI] [PubMed] [Google Scholar]
- 166.Rindi G, Patrini C, Laforenza U, Mandel H, Berant M, Viana MB, Poggi V, Zarra AN. Further studies on erythocyte thiamin transport and phosphorylation in seven patients with thiamin-responsive megaloblastic anaemia. J. Inherit. Metab. Dis. 1994;17:667–677. doi: 10.1007/BF00712009. [DOI] [PubMed] [Google Scholar]
- 167.Kono S, Miyajima H, Yoshida K, Togawa A, Shirakawa K, Suzuki H. Mutations in a thiamine-transporter gene and Wernicke's-like encephalopathy. N. Engl. J. Med. 2009;360:1792–1794. doi: 10.1056/NEJMc0809100. [DOI] [PubMed] [Google Scholar]
- 168.Sklan D, Trostler N. Site and extend of thiamin absorption in the rat. J. Nutr. 1977;107:353–356. doi: 10.1093/jn/107.3.353. [DOI] [PubMed] [Google Scholar]
- 169.Guerrant NB, Dutcher RA. The assay of vitamins B and G as influenced by coprophagy. J. Biol. Chem. 1932;98:225–235. [Google Scholar]
- 170.Guerrant NB, Dutcher RA, Brown RA. Further studies concerning formation of B vitamins in digestive tract of rat. J. Nutr. 1937;13:305–315. [Google Scholar]
- 171.Najjar VA, Holt LE. The biosynthesis of thiamin in man and its implication in human nutrition. JAMA, J. Am. Med. Assoc. 1943;123:683–684. [Google Scholar]
- 172.Rindi G, Laforenza U. Thiamin intestinal transport and related issues: recent aspects. Proc. Soc. Exp. Biol. Med. 2000;224:246–255. doi: 10.1046/j.1525-1373.2000.22428.x. [DOI] [PubMed] [Google Scholar]
- 173.Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamin uptake by human jejunal brush-border membrane vesicles. Am. J. Physiol. Cell Physiol. 2001;281:C786–C792. doi: 10.1152/ajpcell.2001.281.3.C786. [DOI] [PubMed] [Google Scholar]
- 174.Dudeja PK, Tyagi S, Gill R, Said HM. Evidence for carrier-mediated mechanism for thiamin transport to human jejunal basolateral membrane vesicles. Digest. Dis. Sci. 2003;48:109–115. doi: 10.1023/a:1021794600864. [DOI] [PubMed] [Google Scholar]
- 175.Rajgopal A, Edmondson A, Goldman D, Zhao R. SLC19A3 encodes a second thiamin transporter ThTr2. Biochim. Biophys. Acta. 2001;1537:175–178. doi: 10.1016/s0925-4439(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 176.Eudy JD, Spiegelstein O, Baber RC, Wlodarczyk BJ, Talbot J, Finnell RH. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol. Gen. Metabol. 2000;71:581–590. doi: 10.1006/mgme.2000.3112. [DOI] [PubMed] [Google Scholar]
- 177.Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamin transporter-1 (hTHTR1): intracellular trafficking and membrane targeting mechanisms. J. Biol. Chem. 2003;278:3976–3984. doi: 10.1074/jbc.M210717200. [DOI] [PubMed] [Google Scholar]
- 178.Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;286:G491–G498. doi: 10.1152/ajpgi.00361.2003. [DOI] [PubMed] [Google Scholar]
- 179.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology. 2010;138:1802–1809. doi: 10.1053/j.gastro.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Neufeld EJ, Fleming JC, Tyartaglini E, Steinkamp MP. Thiamine-responsive megaloblastic anemia syndrome: a disorder of high-affinity thiamine transport. Blood Cells Mol. Dis. 2001;27:135–138. doi: 10.1006/bcmd.2000.0356. [DOI] [PubMed] [Google Scholar]
- 181.Lagarde WH, Underwood LE, Moats-Staats BM, Calikoglu AS. Novel mutation in the SLC19A2 gene in an African-American female with thiamine-responsive megaloblastic anemia syndrome. Am. J. Med. Genet. A. 2004;125A:299–305. doi: 10.1002/ajmg.a.20506. [DOI] [PubMed] [Google Scholar]
- 182.Subramanian VS, Marchant JS, Said HM. Targeting and intracellular trafficking of clinically relevant hTHTR1 mutations in human cell lines. Clin. Sci. 2007;113:93–102. doi: 10.1042/CS20060331. [DOI] [PubMed] [Google Scholar]
- 183.Balamurugan K, Said HM. Functional role of specific amino acid residues in human thiamin transporter SLC19A2: mutational analysis. Am. J. Physiol. Gastrointest Liver Physiol. 2002;283:G37–G43. doi: 10.1152/ajpgi.00547.2001. [DOI] [PubMed] [Google Scholar]
- 184.Zeng WQ, Al-Yamani E, Acierno JS, Jr., Slaugenhaupt S, Gillis T, MacDonald ME, Ozand PT, Gusella JF. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am. J. Hum. Genet. 2005;77:16–26. doi: 10.1086/431216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am. J. Physiol. Cell Physiol. 2006;291:C851–C859. doi: 10.1152/ajpcell.00105.2006. [DOI] [PubMed] [Google Scholar]
- 186.Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J. Biol. Chem. 2006;281:5233–5245. doi: 10.1074/jbc.M512765200. [DOI] [PubMed] [Google Scholar]
- 187.Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim. Biophys. Acta. 2002;1561:180–187. doi: 10.1016/s0005-2736(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 188.Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am. J. Physiol. Cell Physiol. 2003;285:C633–C641. doi: 10.1152/ajpcell.00076.2003. [DOI] [PubMed] [Google Scholar]
- 189.Nabokina S, Said HM. Characterization of the 5′ -regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G822–G829. doi: 10.1152/ajpgi.00234.2004. [DOI] [PubMed] [Google Scholar]
- 190.Laforenza U, Patrini C, Alvisi C, Faelli A, Licandro A, Rindi G. Thiamin uptake in human intestinal biopsy specimens, including observations from a patient with acute thiamin deficiency. Am. J. Clin. Nutr. 1997;66:320–326. doi: 10.1093/ajcn/66.2.320. [DOI] [PubMed] [Google Scholar]
- 191.Reidling JC, Said HM. Adaptive regulation of intestinal thiamin uptake: molecular mechanism using wild-type and transgenic mice carrying hTHTR-1 and -2 promoters. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1127–G1134. doi: 10.1152/ajpgi.00539.2004. [DOI] [PubMed] [Google Scholar]
- 192.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J. Cell. Physiol. 2006;206:371–377. doi: 10.1002/jcp.20492. [DOI] [PubMed] [Google Scholar]
- 193.Nabokina SM, Reidling JC, Said HM. Differentiation-dependent up-regulation of intestinal thiamin uptake: cellular and molecular mechanisms. J. Biol. Chem. 2005;280:32676–32682. doi: 10.1074/jbc.M505243200. [DOI] [PubMed] [Google Scholar]
- 194.Said HM, Ortiz A, Kumar CK, Chatterjee N, Dudeja PK, Rubin SA. Transport of thiamin in the human intestine: mechanism and regulation in intestinal epithelial cell model Caco-2. Am. J. Physiol. 1999;277:C645–C651. doi: 10.1152/ajpcell.1999.277.4.C645. [DOI] [PubMed] [Google Scholar]
- 195.Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamin uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G144–G150. doi: 10.1152/ajpgi.2001.281.1.G144. [DOI] [PubMed] [Google Scholar]
- 196.Ashokkumar B, Vaziri ND, Said HM. Thiamin uptake by the human-derived renal epithelial (HEK-293) cells: cellular and molecular mechanisms. Am. J. Physiol. Renal Physiol. 2006;291:F796–F805. doi: 10.1152/ajprenal.00078.2006. [DOI] [PubMed] [Google Scholar]
- 197.Subramanian VS, Mohammed ZM, Molina A, Marchant JS, Vaziri ND, Said HM. Vitamin B1 (thiamine) uptake by human retinal pigment epithelial (ARPE-19) cells: mechanism and regulation. J. Physiol. 2007;582:73–85. doi: 10.1113/jphysiol.2007.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM. Pancreatic beta cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G197–G206. doi: 10.1152/ajpgi.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hoyumpa AM., Jr Mechanisms of thiamin deficiency in chronic alcoholism. Am. J. Clin. Nutr. 1980;33:2750–2761. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- 200.Gastaldi G, Casirola D, Ferrari G, Rindi G. Effect of chronic ethanol administration on thiamin transport in microvillous vesicles of rat small intestine. Alcohol Alcohol. 1989;24:83–89. doi: 10.1093/oxfordjournals.alcalc.a044888. [DOI] [PubMed] [Google Scholar]
- 201.Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal absorption: effects on physiological and molecular parameters of the uptake process. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G23–G31. doi: 10.1152/ajpgi.00132.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am. J. Physiol. Renal Physiol. 2010;299:F28–F34. doi: 10.1152/ajprenal.00140.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ashokkumar B, Kumar JS, Hecht GA, Said HM. Enteropathogenic Escherichia coli inhibits intestinal vitamin B1 (thiamin) uptake: studies with human-derived intestinal epithelial Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G825–G833. doi: 10.1152/ajpgi.00250.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bukhari FJ, Moradi H, Gollapudi P, Kim HJ, Vaziri ND, Said HM. Effect of chronic kidney disease on the expression of thiamin and folic acid transporters. Nephrol. Dial. Transplant. 2010;26:2137–2144. doi: 10.1093/ndt/gfq675. [DOI] [PMC free article] [PubMed] [Google Scholar]