Abstract
Background
Approximately 50 to 70% of childbearing-aged women consume alcohol and up to 23% of pregnancies have some level of prenatal alcohol exposure.
Methods
Using data from the Pregnancy Risk Assessment Monitoring System from 2004 to 2008, 111,644 women who completed questions relating to periconceptional alcohol use and multivitamin supplement use were included in the study. This study explored associations between periconceptional alcohol use and multivitamin supplementation use. Weighted multivariable logistic regression was used to explore associations, adjusting for maternal education, maternal ethnicity, maternal age, household income, and parity.
Results
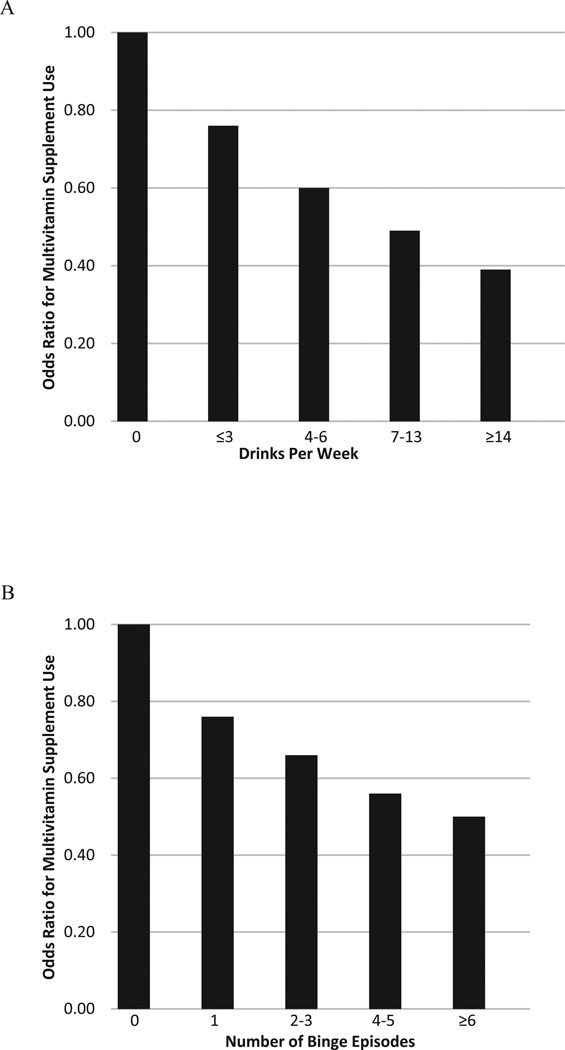
During the periconceptional period, a dose-dependent association was found where women who consumed alcohol (≤ 3 drinks/wk, odds ratio [OR] = 0.76; 4 to 6 drinks/wk, OR = 0.60; 7 to 13 drinks/wk, OR = 0.49; ≥ 14 drinks/wk, OR = 0.39) and binged on alcohol (1 time, OR = 0.76; 2 to 3 times, OR = 0.66; 4 to 5 times, OR = 0.56; ≥ 6 times, OR = 0.50) were significantly less likely to take a multivitamin supplement compared with those that did not consume alcohol.
Conclusions
These findings emphasize the importance of periconceptional multivitamin supplement use, especially among alcohol-consuming women of childbearing age.
Keywords: Supplements, Alcohol, Pregnancy, Prenatal Alcohol Exposure
Surveys in the United States show a high prevalence of alcohol consumption among women of childbearing age that has been found to range from roughly 53% (Center for Disease Control, 2009) to approximately 60 to 70% (Caetano et al., 2006), with 12 to 14% reporting “binge” or heavy episodic drinking (Centers for Disease Control and Prevention [CDC], 2009; Naimi et al., 2003; Paintner et al., 2012). Alcohol consumption among this group of women is of public health concern since more than 50% of pregnancies are unplanned (Henshaw, 1998), and it is likely that a woman may unintentionally drink during the early stages of pregnancy (Paintner et al., 2012).
It has been estimated that approximately 12 to 23% of pregnancies have some level of prenatal alcohol exposure (Center for Disease Control, 2009; Meschke et al., 2013). Maternal alcohol consumption, especially binge drinking, is the cause of the specific pattern of adverse birth outcomes known as fetal alcohol spectrum disorders (FASD). The range of adverse outcomes associated with prenatal alcohol exposure includes increased risks of structural malformations, pre- and postnatal growth deficiency, preterm delivery, stillbirth, spontaneous abortion, and cognitive and behavioral deficits (Feldman et al., 2012). FASD is estimated to affect approximately 1 in 100 births, rivaling autism spectrum disorders as one of the most common developmental disabilities (May and Gossage, 2001; Sampson et al., 1997).
Although it is well-known that alcohol intake during pregnancy may lead to FASD, not every pregnant woman who consumes alcohol, even at high levels, will have a child affected with the disorder (Abel, 2001). A variety of factors are thought to modify the risk of FASD among women who consume alcohol. These include previous history of having an affected child (Li et al., 2001), older maternal age (Chiodo et al., 2010), and alcohol metabolizing genotype (Jacobson et al., 2006; Li et al., 2001; Warren and Li, 2005). There is ample animal data, and some human evidence to suggest that maternal nutrition may also play an important role (Paintner et al., 2012; Riley et al., 2011).
Alcohol alters the metabolism of nutrients, resulting in reduced absorption and utilization, as well as increased absorption and can also interfere with the nutritional supply from the placenta to the developing fetus (Dreosti, 1993). In animal models, maternal diets that are nutritionally inadequate (especially in antioxidant nutrients, choline, iron, and folic acid) have been shown to exacerbate the detrimental effects of alcohol consumption on the fetus and demonstrate that nutrient supplementation may attenuate these alcohol-induced outcomes (Dreosti, 1993; Rufer et al., 2012; Thomas et al., 2009). Pregnancy increases nutritional requirements for several vitamins and minerals that are vital for the growing fetus (Pick et al., 2005). Due to the increasing demand for nutrients during pregnancy, many women do not meet the recommended intake through diet alone (Pick et al., 2005). Prenatal multivitamin supplementation use before and during pregnancy can improve maternal nutrition and help to meet the vitamin and mineral requirements which can more than double for many nutrients during pregnancy (Menard, 1997).
The importance of periconceptual multivitamin supplement intake has been clearly demonstrated for the folic acid content of these supplements, which affords protection against neural tube defects and certain other congenital anomalies (Wilson et al., 2007). As embryonic development begins at conception, before most women are aware of their pregnancy, the benefits of multivitamin supplementation can best be obtained by supplementation that is initiated in the period prior to pregnancy (CDC, 2004; Wolff et al., 2009). However, surveys show that less than half of childbearing-aged women (Ammon Avalos et al., 2009; Kim et al., 2011; Sullivan et al., 2009) and less than one-third of women in the month prior to pregnancy (CDC, 2004; Yu et al., 1996) use multivitamin supplements.
Although it is highly recommended that women of childbearing age who consume alcohol take a daily multivitamin supplement (Institute of Medicine, 1992; Menard, 1997), very little research has investigated multivitamin supplement use among alcohol consumers in the periconceptional period. Two studies have shown that multivitamin supplement use during early pregnancy may modify the risks of small-for-gestational-age, preterm birth (Avalos et al., 2011), and miscarriage (Ammon Avalos et al., 2009) associated with low-to-moderate alcohol intake; however, no studies to our knowledge have investigated dose–response associations between alcohol consumption and multivitamin supplement use before pregnancy. Therefore, we explored associations between periconceptional alcohol use (drinking and “binge” or heavy episodic drinking) and multivitamin supplementation use in participants from Pregnancy Risk Assessment and Monitoring System (PRAMS).
MATERIALS AND METHODS
Sample and Procedures
We investigated the association between periconceptional alcohol intake and multivitamin supplement use using data from PRAMS from 2004 to 2008, which is a surveillance project of the CDC and state health departments that collects information on maternal demographics, lifestyle factors and behaviors, and experiences before, during, and after delivery of a live infant (CDC, 2012). The PRAMS sample of women who have had a recent live birth is drawn from the state’s birth certificate file. Each participating state samples between 1,300 and 3,400 women per year. Selected women are first contacted by mail which commences 2 to 4 months after delivery. If there is no response to repeated mailings, women are contacted 7 to 14 days after mailing the last questionnaire and interviewed by telephone. Data collection procedures and instruments are standardized to allow comparisons between states (detailed PRAMS methodology can be found at http://www.cdc.gov/prams/Methodology.htm).
From Phase 5 (2004 to 2008) of PRAMS core questionnaire, participants were asked about alcohol and vitamin supplement use in the period prior to pregnancy. The following questions were selected for this analysis: (1) During the 3 months before you got pregnant, how many alcoholic drinks did you have in an average week? The following were the potential responses: (a) 14 drinks or more a week; (b) 7 to 13 drinks a week; (c) 4 to 6 drinks a week; (d) 1 to 3 drinks a week; (e) <1 drink a week; (f) I didn’t drink then. These responses were recategorized into (a) ≤ 3 drinks/wk; (b) 4 to 6 drinks/wk; (c) 7 to 13 drinks/wk; (d) ≥ 14 drinks/wk; (2) During the 3 months before you got pregnant, how many times did you drink 5 alcoholic drinks or more in 1 sitting? (defined in this analysis as “binge” or heavy episodic drinking). The following were the potential responses: (a) 6 or more times, (b) 4 to 5 times, (c) 2 to 3 times, (d) 1 time, (e) I didn’t have 5 drinks or more in 1 sitting, (f) I didn’t drink then. These answers were recategorized into: (a) never drink or binge, (b) 1 time, (c) 2 to 3 times, (d) 4 to 5 times, (e) ≥ 6 times; (3) During the month before you got pregnant with your new baby, how many times a week did you take a multivitamin or a prenatal vitamin? These are pills that contain many different vitamins and minerals. The following were the potential responses: (a) I did not take a multivitamin or a prenatal vitamin at all; (b) 1 to 3 times a week; (c) 4 to 6 times a week; (d) every day of the week. These responses were recategorized into yes and no.
Women who completed all the questions relating to periconceptional alcohol use and multivitamin supplement use were included in the analyses (n = 111,644). A subset of women answered the question relating to periconceptional binge drinking (n = 111,210), and they were included in the analysis of binge drinking.
Statistical Analyses
PRAMS data are statistically weighted to adjust for the complex survey design. Weighted multivariable logistic regression was used to explore associations between periconceptional alcohol use and multivitamin supplement use, adjusting for maternal education, maternal ethnicity, maternal age, household income, and parity. The covariates chosen were found to be associated with both alcohol consumption and multivitamin supplement use in the current study. SPSS version 19 (IBM Corp., Armonk, NY) was used for data analyses. Because of the nature of the public use data set, the study was considered exempt by the institutional review board at the University of California San Diego.
RESULTS
Demographic characteristics of the participants showed that majority of the women were aged 20 to 29, were white or Hispanic, and educated. More than half of the women reported not using a multivitamin supplement in the month prior to pregnancy (53.7%) and approximately 82% of women reported consuming alcohol (≤ 3 drinks/wk, 67.3%; 4 to 6 drinks/wk, 9.2%; 7 to 13 drinks/wk, 3.8%; ≥ 14 drinks/wk, 1.7%). Approximately 30% reported at least 1 episode of binge drinking defined as 5 or more drinks per sitting (1 time, 10.9%; 2 to 3 times, 12.3%; 4 to 5 times, 3.4%; ≥ 6 times, 3.3%) in the 3 months before pregnancy (Table 1).
Table 1.
Selected Demographic Characteristics of 111,644 Women Who Reported Using or not Using Alcohol Before Pregnancy, PRAMS, 2004 to 2008
| Characteristica | Number | Percent |
|---|---|---|
| Maternal age (years) | ||
| <19 | 8,536 | 6.4 |
| 20 to 29 | 59,256 | 52.7 |
| ≥ 30 | 43,852 | 40.9 |
| Maternal education | ||
| High school or less | 45,471 | 37.6 |
| Some college or more | 66,173 | 62.4 |
| Maternal race/ethnicity | ||
| Black | 17,421 | 12.7 |
| Asian | 6,146 | 2.7 |
| Other (includes white, Hispanic) | 95,445 | 84.6 |
| Household income (dollars/y) | ||
| <35,000 | 55,772 | 44.9 |
| 35,000 to 49,999 | 12,265 | 10.9 |
| ≥ 50,000 | 43,607 | 44.2 |
| Parity | ||
| 0 | 50,576 | 44.4 |
| 1 to 2 | 51,574 | 48.0 |
| ≥ 3 | 9,494 | 7.6 |
| Use multivitamin 1 month before pregnancy | ||
| No | 61,594 | 53.7 |
| Yes | 50,050 | 46.3 |
| Drink 3 months before pregnancyb | ||
| Never | 22,171 | 17.9 |
| ≤ 3 drinks/wk | 73,261 | 67.3 |
| 4 to 6 drinks/wk | 9,845 | 9.2 |
| 7 to 13 drinks/wk | 4,186 | 3.8 |
| ≥ 14 drinks/wk | 2,181 | 1.7 |
| Binge drink (5 or more drinks/sitting) 3 months before pregnancyc | ||
| Never drink or binge | 78,677 | 70.1 |
| 1 time | 11,617 | 10.9 |
| 2 to 3 times | 13,373 | 12.3 |
| 4 to 5 times | 3,770 | 3.4 |
| ≥ 6 times | 3,773 | 3.3 |
PRAMS, Pregnancy Risk Assessment and Monitoring System.
Numbers are unweighted, percents are weighted.
n = 111,644.
n = 111,210.
Table 2 shows results of multivariable logistic regression analysis of alcohol consumption in the 3 months before pregnancy on odds of multivitamin use in the month prior to pregnancy, adjusting for maternal education, maternal ethnicity, household income, maternal age, and parity. A dose–response association was found between both drinking and binge drinking and use of multivitamin supplements. Women who consumed alcohol (≤ 3 drinks/wk, odds ratio [OR] = 0.76; 4 to 6 drinks/wk, OR = 0.60; 7 to 13 drinks/ wk, OR = 0.49; ≥ 14 drinks/wk, OR = 0.39) and binged on alcohol (1 time, OR = 0.76; 2 to 3 times, OR = 0.66; 4 to 5 times, OR = 0.56; ≥ 6 times, OR = 0.50) were significantly less likely to take a multivitamin supplement compared with those that did not consume alcohol.
Table 2.
Multivariable Logistic Regression of Alcohol Use 3 months Before Pregnancy on Odds of Using a Multivitamin Supplement 1 month Before Pregnancy, PRAMS, 2004 to 2008
| Multivitamin use | ||
|---|---|---|
| Exposure variable | OR | 95% CI |
| Drink 3 months before pregnancya | ||
| Never | 1.00 | – |
| ≤ 3 drinks/wk | 0.76 | 0.72, 0.80 |
| 4 to 6 drinks/wk | 0.60 | 0.56, 0.66 |
| 7 to 13 drinks/wk | 0.49 | 0.44, 0.55 |
| ≥ 14 drinks/wk | 0.39 | 0.32, 0.47 |
| p-for-trend | <0.001 | |
| Binge drink (5 or more drinks/sitting) 3 months before pregnancyb | ||
| Never drink or binge | 1.00 | – |
| 1 time | 0.76 | 0.71, 0.81 |
| 2 to 3 times | 0.66 | 0.62, 0.70 |
| 4 to 5 times | 0.56 | 0.50, 0.64 |
| ≥ 6 times | 0.50 | 0.44, 0.56 |
| p-for-trend | <0.001 | |
PRAMS, Pregnancy Risk Assessment and Monitoring System.
Adjusted for maternal education, maternal ethnicity, household income, maternal age, and parity.
n = 111,644.
n = 111,210.
Figure 1 illustrates the dose–response association between periconceptual alcohol consumption and odds of multivitamin supplement use (drinks per week [A], binge drinking [B]).
Fig. 1.
Dose–response association between drinks per week (A) and episodes of binge drinking (B) on odds of multivitamin use. Pregnancy Risk Assessment and Monitoring System, 2004 to 2008.
DISCUSSION
We found significant dose–response associations between periconceptional alcohol use and multivitamin supplement use. Women who were more likely to drink alcohol were less likely to take multivitamin supplements in the periconceptional period. Two studies have reported that multivitamin supplementation in early pregnancy reduced the risk of preterm and small-for-gestational-age births (Avalos et al., 2011) and miscarriage (Ammon Avalos et al., 2009) among alcohol users. However, this study is the first of which we are aware that has explored associations between multivitamin use and alcohol consumption in women during the periconceptual period.
Optimal nutrition during pregnancy is vital for the normal growth and development of the fetus. The process of pregnancy increases the requirements for vitamins, minerals, and other nutrients that are critical for the growing fetus (Pick et al., 2005) and the diets of many pregnant women have been reported to be deficient in these vital nutrients (Shah and Ohlsson, 2009). In addition, nutrient deficiencies can arise as a result of alcohol consumption due to the altered metabolism of nutrients, resulting in reduced absorption and utilization of many nutrients (e.g., choline, folate, zinc), as well as increased absorption of others (e.g., iron, manganese) (Dreosti, 1993; Zidenberg-Cherr et al., 1988). It has been well established that alcohol also interferes with nutritional supply to the fetal–placental unit (Goodlett and Horn, 2001). Maternal diets that are deficient in essential nutrients may exacerbate the effects of alcohol on the fetus (Shankar et al., 2006), and it has been reported that women who consume alcohol during pregnancy are often also malnourished (Shankar et al., 2006). It has been suggested that risk of FASD may be amplified by nutritional deficiencies brought on by alcohol consumption before and during pregnancy.
While there is little published data in humans, animal studies have shown that several defects associated with FASD can be attenuated and/or prevented with vitamin or mineral supplementation. One study found that supplementation with folic acid prevented alcohol-related cardiac defects in mice (Serrano et al., 2010), while another reported that antioxidant supplementation (vitamin E) diminished alcohol-induced congenital malformations (Wentzel et al., 2006) and neurotoxicity in rats (Heaton et al., 2000). Another study in rats found that alcohol reduced maternal zinc levels during pregnancy and lactation (Yeh and Cerklewski, 1984) and another reported that zinc supplementation during pregnancy protected against fetal dysmorphology and improved postnatal survival after prenatal alcohol exposure in mice (Summers et al., 2009). Prenatal choline supplementation was found to mitigate the adverse effects of alcohol exposure on development in rats (Thomas et al., 2009) and most recently, a new study found that poor maternal iron status exacerbated FASD outcomes in rats (Rufer et al., 2012).
Currently, a human intervention trial with prenatal multivitamin supplementation initiated in alcohol-consuming pregnant women during the late first or early second trimester is being completed in Ukraine. This study, as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) funded by the NIH-NIAAA, to our knowledge, will be the first to compare neurobehavioral outcomes in moderate to heavy prenatal alcohol exposed children to those without prenatal alcohol exposure with or without multivitamin supplementation (Keen et al., 2010).
The exact mechanism whereby multivitamin supplementation during pregnancy may reduce or lessen the effects of alcohol on the fetus is unknown but several theories have been suggested. It has been hypothesized that multivitamin supplementation may improve immune function/immune defense system, which is important for the health of the growing fetus (Keen et al., 2003). Alcohol-related defects may in part be due to alcohol-related oxidative damage to the fetus and sufficient amounts of antioxidant nutrients may protect the fetus from this damage (alcohol-induced micronutrient deficiencies) (Dreosti, 1993). More recently, it has been suggested that epigenetic changes resulting from a diet poor in methyl donors may play a role in the etiology of FASD (Dominguez-Salas et al., 2012; Wang et al., 2009; Zeisel, 2011). While research suggests the benefits of a favorable nutritional status in women consuming alcohol during pregnancy, the exact “optimal” levels of nutrients that would be protective has yet to be elucidated.
The current study has several strengths. The PRAMS surveillance system employs a standardized sample collection that allows for multistate analysis of a large sample size. In addition, the results are generalizable due to the large population- based sample. Our study also has some limitations. Self-reported alcohol consumption (reporting bias) may be subject to misclassification due to potential stigma of drinking alcohol during pregnancy. In addition, recall of alcohol use and/or vitamin use more than 12 months earlier than the postnatal interview may be subject to error. Furthermore, the data set is restricted to pregnancies ending in live births. Thus, pregnancies ending in spontaneous abortion or stillbirth, outcomes that occur with increased frequency in alcohol- consuming mothers, were not included. We were unable to assess associations between alcohol use and multivitamin supplement use later in pregnancy because the question relating to supplement use in the last 3 months of pregnancy was only asked by a few states. Finally, findings from this study may not be generalizable to all women of childbearing age because the participants included in this study recently had a pregnancy that ended in a live birth.
The current study found that periconceptional alcohol consumption was associated with lack of multivitamin supplement use in a dose-dependent relationship. These findings highlight the importance of supplement use periconceptionally, especially among women of reproductive age who are consuming alcohol. Although the explanation for the association between the lack of periconceptional supplement use and alcohol consumption may relate to unplanned pregnancies, these findings clearly reinforce the need for targeted intervention in alcohol consumers of childbearing age.
ACKNOWLEDGMENTS
Pregnancy Risk Assessment and Monitoring System Working Group: Alabama—Izza Afgan, MPH, Alaska—Kathy Perham-Hester, MS, MPH, Arkansas—Mary McGehee, PhD, Colorado—Alyson Shupe, PhD, Delaware— George Yocher, MS, Florida—Avalon Adams-Thames, MPH, CHES, Georgia—Chinelo Ogbuanu, MD, MPH, PhD, Hawaii—Emily Roberson, MPH, Illinois—Theresa Sandidge, MA, Iowa—Sarah Mauch, MPH, Louisiana— Amy Zapata, MPH, Maine—Tom Patenaude, MPH, Maryland— Diana Cheng, MD, Massachusetts—Emily Lu, MPH, Michigan—Cristin Larder, MS, Minnesota—Judy Punyko, PhD, MPH, Mississippi—Brenda Hughes, MPPA. Missouri —Venkata Garikapaty, MSc, MS, PhD, MPH, Montana— JoAnn Dotson, Nebraska—Brenda Coufal, New Hampshire —David J. Laflamme, PhD, MPH, New Jersey—Lakota Kruse, MD, New Mexico—Eirian Coronado, MPH, New York State—Anne Radigan-Garcia, New York City—Candace Mulready-Ward, MPH, North Carolina—Kathleen Jones-Vessey, MS, North Dakota—Sandra Anseth, Ohio—Connie Geidenberger, PhD, Oklahoma—Alicia Lincoln, MSW, MSPH, Oregon—Kenneth Rosenberg, MD, MPH, Pennsylvania—Tony Norwood, Rhode Island—Sam Viner-Brown, PhD, South Carolina—Mike Smith, MSPH, Texas—Rochelle Kingsley, MPH, Tennessee—David Law, PhD, Utah—Lynsey Gammon, MPH, Vermont—Peggy Brozicevic, Virginia—Marilyn Wenner, Washington—Linda Lohdefinck, West Virginia—Melissa Baker, MA, Wisconsin —Katherine Kvale, PhD, Wyoming—Amy Spieker, MPH, CDC PRAMS Team, Applied Sciences Branch, Division of Reproductive Health.
REFERENCES
- Abel EL. Fetal alcohol syndrome: when the end does not justify the means. J Pediatr. 2001;138:295–296. doi: 10.1067/mpd.2001.107612. [DOI] [PubMed] [Google Scholar]
- Ammon Avalos L, Kaskutas LA, Block G, Li DK. Do multivitamin supplements modify the relationship between prenatal alcohol intake and miscarriage? Am J Obstet Gynecol. 2009;201:563 e1–563.e9. doi: 10.1016/j.ajog.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos LA, Kaskutas L, Block G, Abrams B, Li DK. Does lack of multivitamin supplementation during early pregnancy increase vulnerability to alcohol-related preterm or small-for-gestational-age births? Matern Child Health J. 2011;15:1324–1332. doi: 10.1007/s10995-010-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30:1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Spina bifida and anencephaly before and after folic acid mandate—United States 1995–1196 and 1999–2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Alcohol use among pregnant and nonpregnant women of childbearing age—United States, 1991–2005. MMWR Morb Mortal Wkly Rep. 2009;58:529–532. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Pregnancy Risk Assessment Monitoring System (PRAMS) [Accessed September 1, 2012];2012 Available at: http://www.cdc.gov/prams.
- Chiodo LM, da Costa DE, Hannigan JH, Covington CY, Sokol RJ, Janisse J, Greenwald M, Ager J, Delaney-Black V. The impact of maternal age on the effects of prenatal alcohol exposure on attention. Alcohol Clin Exp Res. 2010;34:1813–1821. doi: 10.1111/j.1530-0277.2010.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Salas P, Cox SE, Prentice AM, Hennig BJ, Moore SE. Maternal nutritional status, C(1) metabolism and offspring DNA methylation: a review of current evidence in human subjects. Proc Nutr Soc. 2012;71:154–165. doi: 10.1017/S0029665111003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti IE. Nutritional factors underlying the expression of the fetal alcohol syndrome. Ann N Y Acad Sci. 1993;678:193–204. doi: 10.1111/j.1749-6632.1993.tb26122.x. [DOI] [PubMed] [Google Scholar]
- Feldman HS, Jones KL, Lindsay S, Slymen D, Klonoff-Cohen H, Kao K, Rao S, Chambers C. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36:670–676. doi: 10.1111/j.1530-0277.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000;24:512–518. [PubMed] [Google Scholar]
- Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24–29. 46. [PubMed] [Google Scholar]
- Institute of Medicine (IOM), Subcommittee for a Clinical Applications Guide. Nutrition During Pregnancy and Lactation: An Implementation Guide. Washington, DC: The National Academies Press; 1992. [Google Scholar]
- Jacobson SW, Carr LG, Croxford J, Sokol RJ, Li TK, Jacobson JL. Protective effects of the alcohol dehydrogenase-ADH1B allele in children exposed to alcohol during pregnancy. J Pediatr. 2006;148:30–37. doi: 10.1016/j.jpeds.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, Oteiza P, Uriu-Adams JY. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J Nutr. 2003;133:1597S–1605S. doi: 10.1093/jn/133.5.1597S. [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. BioFactors. 2010;36:125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cain R, Viner-Brown S, Yang MF. Multivitamin use prior to pregnancy in Rhode Island. Med Health R I. 2011;94:276–278. [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O’Connor S, Ramchandani VA. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res. 2001;25:136–144. [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- Menard MK. Vitamin and mineral supplement prior to and during pregnancy. Obstet Gynecol Clin North Am. 1997;24:479–498. doi: 10.1016/s0889-8545(05)70318-1. [DOI] [PubMed] [Google Scholar]
- Meschke LL, Holl J, Messelt S. Older not wiser: risk of prenatal alcohol use by maternal age. Matern Child Health J. 2013;17:147–155. doi: 10.1007/s10995-012-0953-7. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC. Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics. 2003;111:1136–1141. [PubMed] [Google Scholar]
- Paintner A, Williams AD, Burd L. Fetal alcohol spectrum disorders—implications for child neurology, part 2: diagnosis and management. J Child Neurol. 2012;27:355–362. doi: 10.1177/0883073811428377. [DOI] [PubMed] [Google Scholar]
- Pick ME, Edwards M, Moreau D, Ryan EA. Assessment of diet quality in pregnant women using the Healthy Eating Index. J Am Diet Assoc. 2005;105:240–246. doi: 10.1016/j.jada.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer ES, Tran TD, Attridge MM, Andrzejewski ME, Flentke GR, Smith SM. Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders. PLoS ONE. 2012;7:e47499. doi: 10.1371/journal.pone.0047499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Serrano M, Han M, Brinez P, Linask KK. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am J Obstet Gynecol. 2010;203:75.e7–75.e15. doi: 10.1016/j.ajog.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Shah PS, Ohlsson A. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ. 2009;180:E99–E108. doi: 10.1503/cmaj.081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FA, Badger TM, Ronis MJ. Physiologic and genomic analyses of nutrition- ethanol interactions during gestation: implications for fetal ethanol toxicity. Exp BiolMed (Maywood) 2006;231:1379–1397. doi: 10.1177/153537020623100812. [DOI] [PubMed] [Google Scholar]
- Sullivan KM, Ford ES, Azrak MF, Mokdad AH. Multivitamin use in pregnant and nonpregnant women: results from the Behavioral Risk Factor Surveillance System. Public Health Rep. 2009;124:384–390. doi: 10.1177/003335490912400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BL, Rofe AM, Coyle P. Dietary zinc supplementation throughout pregnancy protects against fetal dysmorphology and improves postnatal survival after prenatal ethanol exposure in mice. Alcohol Clin Exp Res. 2009;33:591–600. doi: 10.1111/j.1530-0277.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Warren KR, Li TK. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. 2005;73:195–203. doi: 10.1002/bdra.20125. [DOI] [PubMed] [Google Scholar]
- Wentzel P, Rydberg U, Eriksson UJ. Antioxidative treatment diminishes ethanol-induced congenital malformations in the rat. Alcohol Clin Exp Res. 2006;30:1752–1760. doi: 10.1111/j.1530-0277.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- Wilson RD, Johnson JA, Wyatt P, Allen V, Gagnon A, Langlois S, Blight C, Audibert F, Désilets V, Brock JA, Koren G, Goh YI, Nguyen P, Kapur B Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada and The Motherrisk Program. Preconceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2007;29:1003–1026. doi: 10.1016/S1701-2163(16)32685-8. [DOI] [PubMed] [Google Scholar]
- Wolff T, Witkop CT, Miller T, Syed SB. Folic acid supplementation for the prevention of neural tube defects: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:632–639. doi: 10.7326/0003-4819-150-9-200905050-00010. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Cerklewski FL. Interaction between ethanol and low dietary zinc during gestation and lactation in the rat. J Nutr. 1984;114:2027–2033. doi: 10.1093/jn/114.11.2027. [DOI] [PubMed] [Google Scholar]
- Yu SM, Keppel KG, Singh GK, Kessel W. Preconceptional and prenatal multivitamin-mineral supplement use in the 1988 National Maternal and Infant Health Survey. Am J Public Health. 1996;86:240–242. doi: 10.2105/ajph.86.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol. 2011;44:185–191. doi: 10.1007/s12035-011-8165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidenberg-Cherr S, Benak PA, Hurley LS, Keen CL. Altered mineral metabolism: a mechanism underlying the fetal alcohol syndrome in rats. Drug Nutr Interact. 1988;5:257–274. [PubMed] [Google Scholar]