Abstract
Infections induce severe respiratory muscle weakness. Currently there are no treatments for this important clinical problem. We tested the hypothesis that β-hydroxy-β-methylbutyrate (HMB) would prevent sepsis-induced diaphragm weakness. Four groups of adult male mice were studied: controls (saline-injected), sepsis (intraperitoneal lipopolysaccharide), sepsis+HMB (injected intravenously), and HMB. Diaphragm force generation and indices of caspase 3, calpain, 20S proteasomal subunit, and double-stranded RNA-dependent protein kinase (PKR) activation were assessed after 24 hours. Sepsis elicited large reductions in diaphragm specific force generation at all stimulation frequencies. Endotoxin also activated caspase 3, calpain, the 20S proteasomal subunit and PKR in the diaphragm. HMB blocked sepsis-induced caspase 3, 20S proteasomal and PKR activation, but did not prevent calpain activation. Most importantly, HMB administration significantly attenuated sepsis-induced diaphragm weakness, preserving muscle force generation at all stimulation frequencies (p<0.01). We speculate that HMB may prove to be an important therapy in infected patients, with the potential to increase diaphragm strength, to reduce the duration of mechanical ventilation and to decrease mortality in this patient population
Keywords: Diaphragm, sepsis, caspase 3, PKR, β-hydroxy-β-methylbutyrate (HMB), proteolysis
1. Introduction
Critically ill, mechanically ventilated patients are extremely weak, with studies demonstrating that this patient population has diaphragm strength levels that, on average, are only 20% of that observed in normal healthy individuals (Demoule et al., 2013; Hermans et al., 2010; Laghi et al., 2003; Supinski and Callahan, 2013; Watson et al., 2001). While several factors appear to contribute to the development of muscle weakness in critically ill patients, two recent papers indicate that the presence of systemic infection is a major cause of diaphragm weakness in these patients (Demoule et al., 2013; Supinski and Callahan, 2013). These two studies suggest, moreover, that infection-induced diaphragm weakness may be responsible for poor outcomes in MICU patients, making it difficult or impossible to successfully wean patients from mechanical ventilation. As a result, infection-induced diaphragm weakness is associated with a higher mortality (Demoule et al., 2013; Supinski and Callahan, 2013) and in patients that survive their ICU stays, an extremely prolonged requirement for mechanical ventilation (Supinski and Callahan, 2013).
In view of these considerations, development of safe, practical treatments to prevent or reverse infection-induced diaphragm weakness should, theoretically, provide a means to significantly improve the outcomes of mechanically ventilated MICU patients. Recent reports indicate that HMB is a safe and effective agent that can improve skeletal muscle function in several patient populations, including the elderly and patients with cancer (Flakoll et al., 2004; May et al., 2002). In addition, HMB has been shown to improve leg muscle function in an animal model of hindlimb unloading (Hao et al., 2011), and in cell based studies, to inhibit activation of several of the proteolytic pathways (caspase, the proteasome) that are activated in skeletal muscle with sepsis (Eley et al., 2008; Smith et al., 2005) Based on these considerations, the purpose of the present study was to test the hypothesis that administration of HMB would prevent the development of diaphragm weakness in an animal model of endotoxin-induced sepsis. Animals were sacrificed at 24 hours after endotoxin administration and diaphragm specific force generation as well as diaphragm mass, caspase 3 activity, calpain activity, 20S proteasomal subunit activity, and PKR phosphorylation levels were determined.
2. Materials and Methods
2.1 Experimental Protocol
Studies were approved by the University of Kentucky Institutional Animal Care and Use Committee. Four groups of adult male mice were studied (n=4–5/group), including: (a) saline injected controls, (b) LPS (lipopolysaccharide) injected animals (60,000,000 endotoxin units/kg, intraperitoneally), (c) animals receiving both LPS and HMB (150 μmol/kg, intravenously), and (d) animals given both saline and HMB. To induce sepsis, we used LPS from Escherichia coli 055:B5 (Sigma-Aldrich Corp., St. Louis, MO, USA). At 24 hours after injections, animals were sacrificed and the diaphragm removed en bloc. Diaphragm strips dissected from the left costal diaphragm were used for determination of the diaphragm force-frequency relationship. Total costal diaphragm mass was also assessed, and the protein content of diaphragm samples were measured using the Bradford technique (Biorad Protein Assay, Biorad, Hercules, CA). The remaining costal diaphragm was frozen, stored at −80°C and subsequently used for activity assays as well as for determination of levels of selected proteins using Western blot techniques.
2.2 Diaphragm Force–Frequency Curves and Muscle Mass
To assess diaphragm specific force generation, diaphragm strips were excised from the costal diaphragm and mounted in vertical water jacketed organ baths (25°C) as previously described (Supinski et al., 2009). The rib end of each diaphragm strip was secured to a hook in the base of the bath and the central tendon end of each strip was tied to a force transducer above the bath (Scientific Instruments, Heidelberg, Germany). Baths contained Krebs Henselheit solution that was continuously bubbled with a 95% oxygen-5% carbon dioxide gas mixture. Platinum field electrodes, connected to a constant current amplifier driven by a Grass 48 stimulator (Grass, West Warwick, RI, USA) were placed around the strip in the organ bath and strips were allowed to equilibrate for a 15 minute period. Muscle strips were then adjusted to optimal length, defined as the length for which twitch tension was maximal, and current was adjusted to supramaximal levels. After an additional 5 minute rest period, strips were sequentially stimulated to contract in response to trains of 1, 10, 20, 50, 100, and 150 Hz (Hertz) impulses (train duration 800 msec, with 30 second rest periods between adjacent trains). Muscle strip optimal length was measured with a micrometer, and strips were blotted to remove excess moisture and weighed to determine “wet” muscle mass. Muscle strip cross sectional area (CSA) was calculated according to the formula of Close, i.e. CSA= muscle weight times density (1.06) divided by optimal length (Close, 1972). Specific muscle force for each stimulation frequency tested was subsequently calculated as raw force divided by cross sectional area (Close, 1972). Muscle strips were then placed in a dessicator for 48 hours; strips were then weighed to determine “dry weight” and the wet/dry weight ratio for each strip was calculated.
2.3 Caspase 3 Activity Assay
To assess caspase 3 activity, diaphragm muscle homogenates (100 μg of protein) were added to assay buffer and a caspase-3-specific fluorogenic substrate (30 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin, Ac-DEVD-AMC) as previously described in detail (Supinski and Callahan, 2006). Duplicate determinations were made with muscle homogenate, assay buffer, Ac-DEVD-AMC, and a specific caspase-3 inhibitor (DEVD-CHO, 20 nM). Immediately after substrate was added, a baseline fluorescent measurement of AMC was performed using a SpectraMax® M2 spectrofluorophotometer (Molecular Devices Corporation, Sunnyvale, CA, USA), at an excitation frequency of 360 nm and an emission frequency of 460 nm. This measurement was then repeated after 0.5 h of incubation at 30°C. The increase in fluorescence from the initial reading to the final reading was calculated to obtain the raw increase in fluorescence; the increase in reading for the DEVD-CHO duplicate was subtracted from the raw reading to determine the caspase 3 specific increase in fluorescence for a given sample.
2.4 20S Proteasome Subunit Activity Assay
Proteasome activity of diaphragm homogenates was measured using the Calbiochem 20S Proteasome Subunit Activity kit assay (Calbiochem, San Diego, CA) according to the manufacturer’s protocol. AMC standards were used to calibrate fluorescent measurements of proteasomal activity.
2.5 Determination of Calpain Specific Spectrin Degradation and Phospho-PKR
Western blot techniques were employed to measure diaphragm levels of a 136 kDa calpain-specific spectrin degradation product and phospho-PKR protein levels. For determination of the 136 kDa calpain specific spectrin degradation product (Higuchi et al., 2005; Takano et al., 2005), diaphragm muscle homogenates were diluted with an equal volume of loading buffer (126 mM Tris·HCl, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromphenol blue, pH 6.8) and loaded onto Tris glycine polyacrylamide gels. Protein mixtures were then separated by electrophoresis (Novex Minicell II, Carlsbad, CA) and proteins transferred to polyvinylidene fluoride membranes and incubated over night at 4°C with primary antibodies to the 136 kDa calpain specific spectrin degradation product (Higuchi et al., 2005; Takano et al., 2005) (kindly supplied by Dr. Takaomi C. Saido, RIKEN Brain Science Institute, Saitama, Japan). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies and antibody binding detected on film using enhanced chemiluminescence (Western Lightning, Perkin Elmer, Boston, MA, USA). Densitometry was performed using a Microtek scanner (Carson, CA, USA) and UN-SCAN-IT software (Silk Scientific, Orem, UT, USA). Membranes were then reprobed with antibodies to GAPDH (Santa Cruz Biotechnology, CA, USA) to verify equal lane loading since sepsis does not alter GAPDH in skeletal muscle.
Because phospho-PKR levels are difficult to detect in skeletal muscle, we employed immunoprecipitation techniques. Diaphragm homogenates containing 250 μg of protein from each sample were mixed with one microliter of anti-PKR (Biosource, Lincoln, NE, USA) overnight at 4°C. We then added 20 microliters of protein A agarose beads and incubated the mixture at 4°C for 3 hours. This mixture was then centrifuged at 13,000 G, and the pellet washed 4 times with lysis buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 5 mM DTT, 0.1 mM EDTA, 500 microliters/sample). The pellet was resuspended in 50 microliter of buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA), 25 microliters of 3X SDS Sample buffer was added, and the mixture heated to 95°C for 5 minutes. Aliquots of the resulting mixture (30 microliters) were then loaded onto Tris glycine polyacrylamide gels, proteins separated by electrophoresis, and proteins transferred to polyvinylidene fluoride membranes. Membranes were incubated over night at 4°C with primary antibodies to phospho-PKR, followed by washing and incubation in horseradish peroxidase-conjugated secondary antibodies. Antibody binding was then detected using enhanced chemiluminescence as described in detail in the preceding paragraph. Note that this procedure detects only two proteins, i.e. phospho-PKR and the excess primary antibody. Levels of phospho-PKR were quantitated using a Microtek scanner (Carson, CA, USA) and UN-SCAN-IT software (Silk Scientific, Orem, UT, USA).
2.6 Statistical Analysis
ANOVA was be used to compare variables (e.g. force) across groups of animals treated with different agents, with post-hoc testing (Tukeys) to determine differences between groups. A p value of less than .05 was taken as indicating statistical significance for all experiments. Data are reported as the mean ± 1 standard error of the mean.
3. Results
3.1 Effect of LPS and HMB on Diaphragm Specific Force and Mass
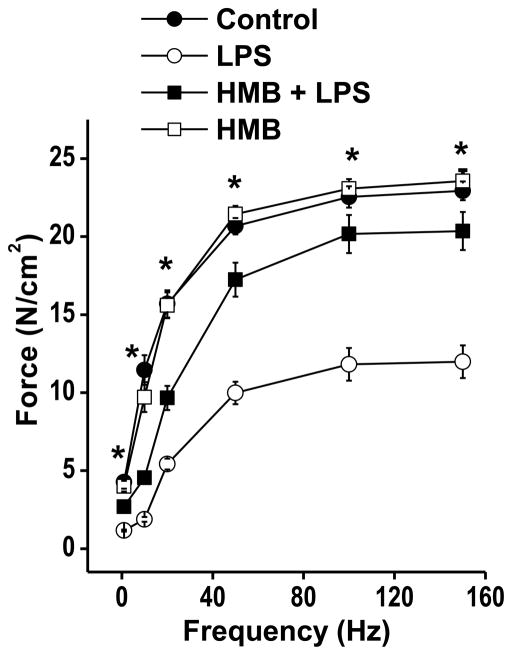
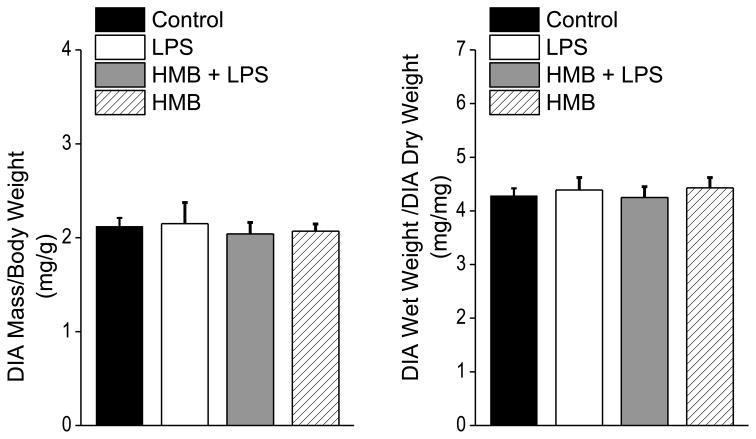
Administration of LPS elicited a substantial reduction in the muscle specific force generating capacity of the diaphragm, as shown in Figure 1. Specifically, the entire force-frequency curve of the diaphragm was downshifted at 24 hours after LPS administration, as compared to saline treated control animals, with significant reductions in specific force at all stimulation frequencies tested (i.e. 1 Hz-150 Hz.). Concomitant administration of HMB almost completely prevented sepsis-induced decrements in diaphragm force generation, with force at all stimulation frequencies significantly higher for the HMB + LPS group compared to the animals given LPS alone. Force-frequency curves for animals given HMB alone were similar to curves for saline treated control animals. Specific force in response to 150 Hz stimulation averaged 22.9 ± 0.6 N/cm2 for saline treated controls, 12.0 ± 1.0 N/cm2 for LPS treated animals, 20.4 ± 1.2 N/cm2 for animals given both HMB and LPS, and 23.6 ± 0.7 N/cm2 for the animals given HMB alone (p< 0.001 for comparison of the LPS treated group to the other three groups). Muscle specific force measurements provide an index of muscle quality, i.e. the ability of a given muscle cross sectional area to generate force. Total force production by muscle, however, is determined both by muscle quality (force/unit cross sectional area or force/unit mass) and muscle quantity (i.e. total cross sectional area or mass). To assess the effects of LPS and/or HMB on diaphragm quantity, we measured diaphragm mass, corrected for initial animal weight, as shown in Figure 2A. To exclude the possibility that LPS and/or HMB could artifactually alter diaphragm mass by producing tissue edema, we also determined diaphragm wet weight to dry weight ratios as shown in Figure 2B. We found that all experimental groups had similar levels of diaphragm mass and diaphragm wet-to-dry weight ratios, arguing that over the first 24 hours after LPS and/or HMB administration, neither intervention significantly alters diaphragm quantity.
Figure 1.
This figure displays diaphragm specific force-frequency relationships for the four experimental groups of animals. Symbols represent mean forces and error bars indicate 1 standard error of the mean. Diaphragm specific force in the saline treated control group (solid circles) was significantly higher than force generated by the LPS treated animals (open circles) at all stimulation frequencies tested (* indicates a significant difference between the LPS group and the other three groups). Diaphragm specific force generation in animals given both HMB and LPS was similar to saline treated controls and significantly higher than that of the LPS treated group. Diaphragms from animals given only HMB generated specific forces similar to controls.
Figure 2.
The figure displays diaphragm weight/animal weight ratios on the left and wet diaphragm weight/dry diaphragm weight ratios on the right for control, LPS, HMB+LPS and HMB treated groups.. Values for both parameters were similar for all groups.
3.2 Effect of LPS and HMB on Indices of Diaphragm Caspase, Calpain, and Proteasomal Proteolytic Pathway Activation
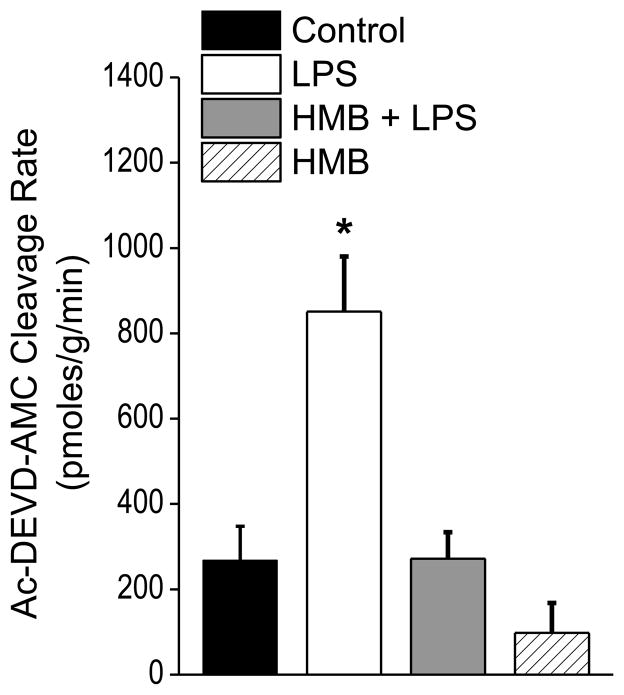
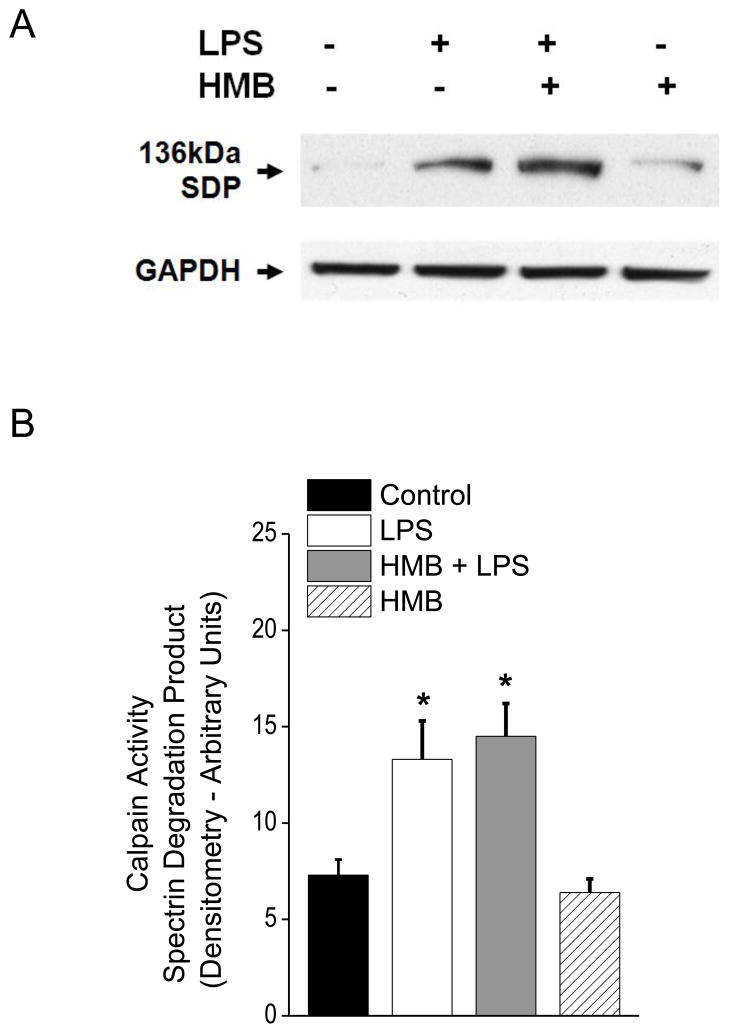
Several proteolytic pathways are known to be activated in the diaphragm in animal models of sepsis and are thought to contribute to sepsis induced reductions in diaphragm function. These pathways include the caspase, calpain and proteasomal proteolytic systems (Klaude et al., 2012; Supinski and Callahan, 2006, 2010). To determine if HMB improved diaphragm function in LPS treated animals by inhibiting one or more of these pathways, we assessed indices of activation of each of these three proteolytic systems. As shown in Figure 3, sepsis elicited a large increase in diaphragm caspase 3 activity (p<0.001) and concomitant administration of HMB completely blocked sepsis-induced caspase 3 activation. We also found that LPS administration increased a marker of diaphragm calpain activation (p<0.05), i.e. formation of the calpain dependent spectrin degradation product (Figure 4). HMB administration, however, failed to decrease this LPS-induced calpain dependent spectrin degradation, suggesting that HMB does not completely block sepsis-induced calpain activation in the diaphragm. LPS administration also increased diaphragm 20S proteasomal subunit activity (p<0.001), as shown in Figure 5. Administration of HMB prevented this latter LPS induced process, with 20S proteasomal activity for diaphragm homogenates from animals given both HMB and LPS similar to levels observed for diaphragms from saline treated controls.
Figure 3.
This figure displays caspase 3 activity levels, expressed as AMC substrate cleavage rates, for diaphragm homogenates from the four experimental groups. LPS administration elicited a significant increase in diaphragm caspase 3 activity compared to controls (* indicates statistical significance). HMB blocked this increase in diaphragm caspase 3 activity, with HMB+LPS or HMB alone groups demonstrating caspase 3 activity levels similar to saline treated controls.
Figure 4.
This figure presents diaphragm protein levels of the 136 kDa calpain specific spectrin degradation product (SDP) for the four experimental groups, with a representative blot shown in Panel A and group mean densitometry data presented in Panel B. GAPDH was used as a loading control. LPS administration elicited a large increase in this calpain specific SDP (* indicates a significant difference compared to controls). HMB administration did not block this increase, with diaphragm SDP levels for the HMB+LPS group similar to the LPS alone group and significantly higher than controls. Animals given HMB alone had SDP levels similar to saline treated controls.
Figure 5.
This figure displays 20S Proteasomal activity for diaphragm homogenates from the four experimental groups. LPS elicited a large increase in diaphragm proteasomal activity, while animals given both HMB and LPS had diaphragm proteasomal activity levels similar to controls (* indicates a significant difference from control). Diaphragm proteasomal activity levels for animals given HMB alone were also similar to controls.
3.3 Effect of LPS and HMB on Diaphragm Levels of Phospho-PKR
PKR is activated in the diaphragm in animal models of sepsis (Supinski and Callahan, 2011). Moreover, our previous work indicates that administration of inhibitors of PKR blocks sepsis-induced diaphragm dysfunction (Supinski and Callahan, 2011). To determine if HMB may have improved diaphragm function after LPS administration by inhibiting PKR activation, we assessed diaphragm levels of activated, phosphorylated PKR as shown in Figure 6. LPS administration elicited a large increase in diaphragm levels of phosphorylated PKR, when compared to levels for saline treated control animals (p<0.05). Concomitant administration of HMB with LPS completely blocked LPS induced PKR phosphorylation, with phospho-PKR levels for the HMB + LPS group similar to controls (NS) and significantly lower than phospho-PKR levels for LPS treated animals (p<0.05). Recent studies indicate that PKR activation is linked to downstream activation of caspase and proteasomal pathways in skeletal muscle (Russell et al., 2010; Supinski and Callahan, 2011). As a result, the effect of HMB to inhibit activation of PKR in the present study may be responsible for its action to also block both caspase 3 and 20S proteasomal subunit activation in the diaphragm following LPS administration.
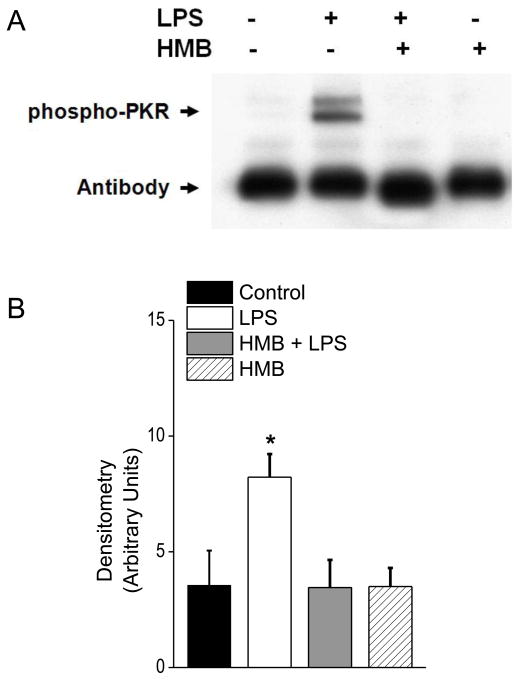
Figure 6.
This figure presents diaphragm phospho-PKR protein levels for the four experimental groups, with a representative blot shown in Panel A and group mean data in Panel B. LPS administration elicited a large increase in diaphragm phospho-PKR levels while animals given both HMB and LPS had phospho-PKR levels similar to controls (* indicates a statistical difference from control values). Phospho-PKR levels for animals given HMB alone were also similar to controls.
4. Discussion
In the present study we found that endotoxin induced sepsis induces severe diaphragm muscle weakness in mice. This finding has important implications for critically ill patients, many of whom have significant respiratory muscle weakness (Demoule et al., 2013; Hermans et al., 2010; Laghi et al., 2003; Supinski and Callahan, 2013; Watson et al., 2001). It is reasonable to believe that development of severe infection induced diaphragm weakness in patients with either preexisting lung dysfunction or acute lung disease can easily precipitate respiratory failure. This would account for the observation that most mechanically ventilated MICU patients are both infected and weak. Specifically, two recent reports indicate that systemic infections are a major cause of severe diaphragm weakness in mechanically ventilated MICU patients (Demoule et al., 2013; Supinski and Callahan, 2013). These two studies also found that mechanically ventilated patients with the most severe diaphragm weakness have poor outcomes, with the weakest patients having much higher mortality rates (Demoule et al., 2013; Supinski and Callahan, 2013). Additionally, in the weakest patients who survive, weaning from mechanical ventilation is prolonged (Supinski and Callahan, 2013). This work also suggests that weakness may affect mortality by making it extremely difficult to wean patients from mechanical ventilation, potentially influencing decisions to terminate this mode of life support (Supinski and Callahan, 2013).
Since infections are a major factor in producing severe diaphragm weakness in mechanically ventilated MICU patients and since weakness is associated with death and the need for protracted mechanical ventilation, implementation of treatments to prevent or reverse infection induced diaphragm weakness should, theoretically, improve MICU patient outcomes. While a number of previous animal studies have shown that it is possible to prevent diaphragm weakness in animal models of infection using chemical inhibitors of various cellular pathways, none of these previous studies utilized pharmaceutical agents that can be safely given to human patient populations (Supinski and Callahan, 2006, 2010; Supinski et al., 2010). The present study demonstrates that it is possible to prevent the development of diaphragm weakness in an animal model of infection by administration of HMB a compound that has been used in numerous human clinical trials (Flakoll et al., 2004; May et al., 2002). Specifically, we found that diaphragm force generation fell to 50% of its normal value by 24 hours after administration of LPS and that concomitant administration of HMB completely prevents this LPS-induced diaphragm weakness. We should note that the dosage of HMB utilized in the present study (150 μmol/kg or 41 mg/kg) is comparable to the dosages that have been used safely in previous human clinical trials (2000–3000 mg per day for an adult or 29–43 mg/kg for a 70 kg adult) (Wilson et al., 2013). All previous clinical studies indicate, moreover, that this dosage of HMB is extremely safe in human patients (Gallagher et al. 2005; Nissen et al. 2005; Rathmacher et al 2004). In addition, an animal study found that a far larger dosage of HMB (3.5 and 4.2 gm/kg given to male and female rats, respectively) for three months had no adverse effects on clinical observations, hematology, clinical chemistry or organ weights (Baxter et al., 2005). As a result, there are no known side effects to HMB that have been reported at either the dosage used in the present study or in response to higher doses of this biopharmaceutical.
Previous use of HMB in humans includes a number of studies in which this agent has been shown to increase indices of muscle function in a variety of patient groups and normal subjects (May et al., 2002) (Flakoll et al., 2004). Prior animal studies have also explored the potential mechanisms by which HMB may improve muscle mass and strength. Several papers have suggested that HMB administration may alter factors that regulate one or more steps required for protein synthesis (Kornasio et al., 2009. Smith et al., 2005 Katta et al., 2012). HMB also appears to influence the function of key proteolytic pathways, including the proteasome and caspase proteolytic pathways (Supinski and Callahan, 2006, Eley et al. 2006, Smith et al. 2005). The present work is consistent with these previous reports that HMB inhibits key proteolytic pathways. We found that LPS administration to animals elicited large increases in diaphragm caspase 3 activity and also increased the activity of the 20S proteasomal subunit. HMB administration completely prevented LPS induced increases in both caspase 3 and 20S proteasomal activity, paralleling the effect of this agent to also attenuate LPS induced diaphragm weakness. The other important proteolytic pathway known to be activated in the diaphragm during sepsis is calpain. In the present study, however, a marker of calpain mediated protein degradation, the 136 kDa calpain specific spectrin degradation product, was not attenuated by HMB administration to LPS treated animals. This finding suggests HMB did not completely block sepsis induced skeletal muscle calpain activation in our model.
We also found that HMB prevented phosphorylation of PKR in the diaphragm of septic animals. We have previously shown that PKR becomes phosphorylated and activated in the diaphragm in animal models of sepsis and that PKR phosphorylation/activation is associated with loss of diaphragm specific force generating capacity (Supinski and Callahan, 2011). We also previously found that diaphragm caspase activation was downstream of PKR phosphorylation following LPS administration, with 2-aminopurine (a PKR inhibitor) blocking caspase 8 and caspase 3 activation in the diaphragm in parallel with its effects to inhibit PKR and prevent diaphragm weakness (Supinski and Callahan, 2011). As a result, our present and previous findings are consistent with the possibility that the effect of HMB to prevent diaphragm caspase activation following LPS administration may well be a consequence of HMB mediated inhibition of PKR phosphorylation. Our finding that HMB inhibits PKR activation in skeletal muscle is also consistent with previous reports by investigators who found that PKR inhibition prevented LPS induced protein degradation by the proteasomal system in isolated murine myotubes (Eley et al., 2008; Russell and Tisdale, 2009; Smith et al., 2005). Other studies have shown that activated (i.e. phosphorylated) PKR activates the mTOR phosphatase, thereby reducing mTOR activation, and also phosphorylates eIF2α, inhibiting mRNA-ribosomal interactions (Morel et al., 2009). As a result, an effect of HMB to inactivate PKR could account for the known effects of HMB to enhance protein synthesis and to reduce proteolysis. All of the findings of the current study (HMB attenuation of PKR, caspase and proteasomal activation) are consistent with this possibility.
One limitation of the present report is that we only demonstrated that HMB prevents sepsis induced skeletal muscle weakness. This report, by itself, does not provide data as to whether or not HMB can restore strength when it is given later in the course of sepsis. Previous human studies suggest, however, that is likely that HMB can increase the function of previously damaged muscle. Specifically, results of human studies in which HMB is given to cachectic patients with cancer and to elderly patients with sarcopenia indicate that HMB can increase muscle mass and function even when given after muscle weakness is present (Flakoll et al., 2004; May et al., 2002). We believe that this experimental question may be best studied in infected ICU patients, and we are currently testing the ability of HMB to improve the strength of this patient population.
5. Conclusions
In summary, we found that administration of HMB blocked the development of diaphragm weakness in an animal model of infection. The dosage of HMB required to achieve this effect is comparable to that employed in previous clinical trials using this agent to improve muscle function in exercising humans. Moreover, this dosage of HMB is considered safe, with no known side effects. We also found that sepsis increased PKR, caspase and proteasomal activation in the diaphragm and HMB blocked each of these effects. These findings may have important clinical implications, because infections are a major cause of diaphragm weakness in critically ill MICU patients and diaphragm weakness contributes to poor outcomes in this patient population. Additional studies are needed to determine if HMB administration can improve diaphragm function in critically ill MICU patients, and if so, can decrease the time required to wean these patients from mechanical ventilators and reduce ICU mortality.
Highlights.
β-hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm weakness
HMB blocks sepsis-induced diaphragm caspase 3 and 20S proteasomal activation but not calpain activation
HMB prevents phosphorylation of PKR in the diaphragm of septic animals
HMB may improve infection-induced diaphragm weakness in critically ill patients
Acknowledgments
We would like to thank Dr. Lin Wang for her technical expertise in performing these studies.
We would also like to thank Dr. Saido for his generous contribution of the 136 kDa calpain specific spectrin degradation antibody.
This work was supported by the following grants from the National Institutes of Health: HL080429, HL081525, and HL112085.
Footnotes
Authorship
G.S.S. and L.A.C. conception and design of research; G.S.S. and L.A.C. performed experiments; G.S.S. and L.A.C analyzed data, interpreted results of experiments, prepared figures; G.S.S. drafted manuscript; G.S.S. and L.A.C. edited and revised manuscript; and G.S.S. and L.A.C approved final version of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter JH, Carlos JL, Thurmond J, Rehani RN, Bultman J, Frost D. Dietary toxicity of calcium β-hydroxy-beta-methyl butyrate (CaHMB) Food Chem Toxicol. 2005;43:1731–1741. doi: 10.1016/j.fct.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S. Diaphragm Dysfunction on Admission to ICU: Prevalence, Risk Factors and Prognostic Impact - a Prospective Study. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1417–1426. doi: 10.1152/ajpendo.90567.2008. [DOI] [PubMed] [Google Scholar]
- Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20:445–451. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc. 2000;32:2116–2119. doi: 10.1097/00005768-200012000-00023. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. beta-Hydroxy-beta-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R701–715. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Tomioka M, Takano J, Shirotani K, Iwata N, Masumoto H, Maki M, Itohara S, Saido TC. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J Biol Chem. 2005;280:15229–15237. doi: 10.1074/jbc.M500939200. [DOI] [PubMed] [Google Scholar]
- Katta A, Kakarla SK, Manne ND, Wu M, Kundla S, Kolli MB, Nalabotu SK, Blough ER. Diminished muscle growth in the obese Zucker rat following overload is associated with hyperphosphorylation of AMPK and dsRNA-dependent protein kinase. J Appl Physiol (1985) 2012;113:377–384. doi: 10.1152/japplphysiol.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaude M, Mori M, Tjader I, Gustafsson T, Wernerman J, Rooyackers O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci (Lond) 2012;122:133–142. doi: 10.1042/CS20110233. [DOI] [PubMed] [Google Scholar]
- Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2009;1793:755–763. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kovarik M, Muthny T, Sispera L, Holecek M. Effects of beta-hydroxy-beta-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. Journal of physiology and biochemistry. 2010;66:311–319. doi: 10.1007/s13105-010-0037-3. [DOI] [PubMed] [Google Scholar]
- Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- Luo YM, Hart N, Mustfa N, Man WD, Rafferty GF, Polkey MI, Moxham J. Reproducibility of twitch and sniff transdiaphragmatic pressures. Respir Physiol Neurobiol. 2002;132:301–306. doi: 10.1016/s1569-9048(02)00115-5. [DOI] [PubMed] [Google Scholar]
- May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Morel M, Couturier J, Pontcharraud R, Gil R, Fauconneau B, Paccalin M, Page G. Evidence of molecular links between PKR and mTOR signalling pathways in Abeta neurotoxicity: role of p53, Redd1 and TSC2. Neurobiol Dis. 2009;36:151–161. doi: 10.1016/j.nbd.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC., Jr beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130:1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Duguet A, Luo Y, Hughes PD, Hart N, Hamnegard CH, Green M, Similowski T, Moxham J. Anterior magnetic phrenic nerve stimulation: laboratory and clinical evaluation. Intensive Care Med. 2000;26:1065–1075. doi: 10.1007/s001340051319. [DOI] [PubMed] [Google Scholar]
- Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, D’Olimpio J, Abumrad NN. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr. 2004;28:65–75. doi: 10.1177/014860710402800265. [DOI] [PubMed] [Google Scholar]
- Russell ST, Siren PM, Siren MJ, Tisdale MJ. Mechanism of attenuation of protein loss in murine C2C12 myotubes by D-myo-inositol 1,2,6-triphosphate. Exp Cell Res. 2010;316:286–295. doi: 10.1016/j.yexcr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem. 2009;330:171–179. doi: 10.1007/s11010-009-0130-5. [DOI] [PubMed] [Google Scholar]
- Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65:277–283. [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol. 2006;100:1770–1777. doi: 10.1152/japplphysiol.01288.2005. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2010;42:80–87. doi: 10.1165/rcmb.2008-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Double-stranded RNA-dependent protein kinase activation modulates endotoxin-induced diaphragm weakness. J Appl Physiol. 2011;110:199–205. doi: 10.1152/japplphysiol.01203.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17:R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R825–834. doi: 10.1152/ajpregu.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Ji XY, Callahan LA. p38 Mitogen-activated protein kinase modulates endotoxin-induced diaphragm caspase activation. Am J Respir Cell Mol Biol. 2010;43:121–127. doi: 10.1165/rcmb.2008-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tomioka M, Tsubuki S, Higuchi M, Iwata N, Itohara S, Maki M, Saido TC. Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. J Biol Chem. 2005;280:16175–16184. doi: 10.1074/jbc.M414552200. [DOI] [PubMed] [Google Scholar]
- Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB) Journal of the International Society of Sports Nutrition. 2013;10:6. doi: 10.1186/1550-2783-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]