Abstract
The design, synthesis, properties, and cell imaging applications of a series of pyridine-disulfide based fluorescent probes (WSP1, WSP2, WSP3, WSP4 and WSP5) for hydrogen sulfide detection are reported. The strategy is based on the dual-nucleophilicity of hydrogen sulfide. A hydrogen sulfide mediated tandem nucleophilic substitution-cyclization reaction is used to release the fluorophores and turn on the fluorescence. The probes showed high sensitivity and selectivity for hydrogen sulfide over other reactive sulfur species including cysteine and glutathione.
Keywords: fluorescent probes, hydrogen sulfide, cell imaging, cyclization, fluorescence
Introduction
Hydrogen sulfide (H2S), although historically known as a toxic gas, has been recently classified as a critical cell signalling molecule like nitric oxide (NO).[1–6] Literature published in the past few years increasingly suggests that H2S is a mediator of many physiological and/or pathological processes, especially in cardiovascular systems.[1–6] The production of H2S in mammalian systems has been attributed to at least three endogenous enzymes:[5–10] cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfur-transferase. These enzymes use cysteine or cysteine derivatives as the substrates and convert them into H2S within different organs and tissues.
To date, H2S’s exact mechanisms of action are still under active investigation. As a highly reactive molecule, H2S is known to react with a number of biological targets and these reactions may be responsible for the biological functions of H2S. For example, H2S reacts readily with methemoglobin to form sulfhemoglobin, which might act as a metabolic sink for H2S. H2S is a powerful reducing agent likely to react with endogenous oxidants such as peroxynitrite, superoxide and hydrogen peroxide. H2S can also cause protein S-sulfhydration (i.e. converting cysteine residues –SH to persulfides –S-SH). Although the detailed mechanism of S-sulfhydration is still unclear this process has been considered as an important posttranslational modification. It provides a possible mechanism by which H2S alters the functions of a wide range of cellular proteins and enzymes.[11–16] It is possible that many more important reactions of H2S are to be discovered. Nevertheless, the production of endogenous H2S and the exogenous administration of H2S have been demonstrated to exert protective effects in many pathologies.[1–8] It is important, therefore, to understand the chemistry and properties of H2S and to develop effective and convenient methods for the detection of H2S in biological systems.
Fluorescence based assays can be very useful in this field due to the high sensitivity and convenience. Fluorescence methods are suitable for non-destructive detection of bio-targets in live cells or tissues with readily available instruments. In 2011 our laboratory and several other groups reported the first reaction-based fluorescent probes for H2S detection.[17–21] These works have inspired many researchers to develop new H2S fluorescent probes and a number of papers have been published in the past two years.[22,23] One of the main challenges for current fluorescent probes is the high selectivity for H2S versus other biothiols. The strategy used in the design of our first generation H2S probe was based on a unique H2S-mediated nucleophilic addition followed by an intramolecular cyclization to turn on the fluorescence signals.[18] This method is highly specific for H2S. We have expanded this strategy and a series of new probes have been prepared and evaluated. This article is to report the design and synthesis of these probes, the optimization of the detection conditions, as well as the applications of the probes in cell imaging.
Results and Discussion
Design and synthesis of probes
H2S can be considered as a reactive nucleophile in biological systems and it should undergo nucleophilic substitution or addition reactions easily. In order to selectively detect H2S from the biological thiol pool, the key is to differentiate H2S from other biothiols such as cysteine (Cys) and glutathione (GSH). H2S is a non-substituted thiol that can undergo nucleophilic reaction two times; while other thiols like Cys and GSH are mono-substituted thiols which can only pursue nucleophilic reaction one time. Based on this property, we believe that compounds containing dual-electrophilic enters are useful reagents for H2S detection. As shown in Scheme 1, H2S should react with the most electrophilic component of probe A to form a free SH containing intermediate B1. If another electrophilic reaction center is presented at a suitable position, like the ester group shown in B1, the SH group should undergo a spontaneous cyclization to release the fluorophore and form product P. This strategy not only can capture H2S as a stable and analyzable product P, but also allows visualizing H2S signals via convenient and sensitive fluorescence measurements. It should be noted that substrate A may also react with biothiols (RSH). However, product B2 should not undergo the cyclization to release the fluorophore. In addition, if appropriate electrophiles are employed, it is possible for the first reaction with biothiols to be reversible or non-productive so that the probes won’t be consumed by biothiols. Another advantage of this strategy is that we can easily modify the structures of all three subunits of the template (electrophile, linker, and fluorophore). Therefore a large library of probes can be prepared and tested. This has great potential to produce ideal fluorescent probes with optimized properties.
Scheme 1.
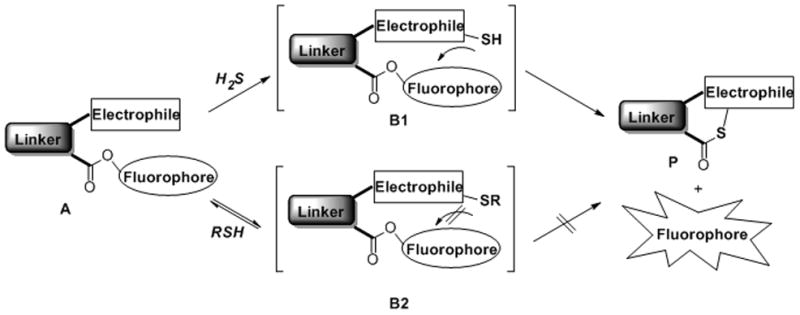
General design of the nucleophilic substitution-cyclization based fluorescent probes for H2S.
To test this idea, we first prepared a model compound 1 and tried the reaction with H2S (using NaHS as the equivalent in a buffer system, Scheme 2). In this model compound we envisioned S-pyridium disulfide was an effective electrophile for trapping H2S and benzene was an appropriate linker. The rigidity of benzene 1,2-substitutions should facilitate the proposed intramolecular cyclization. As expected, the reaction went well and the desired cyclization product 2 and phenol were obtained in good yield (83%).
Scheme 2.
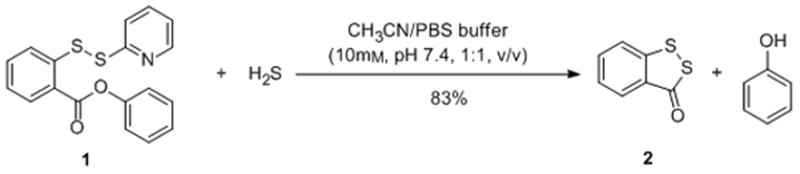
Model reaction between compound 1 and H2S.
Based on the structure of the model compound, we believed the introduction of pseudo- fluorophores through the ester linkage should result in selective fluorescent probes for H2S. Therefore a series of probes were synthesized (shown in Scheme 3). The common intermediate, 2-(2-pyridinyldithio)-benzoic acid 3, was readily prepared from a mix-disulfide formation between 2-mercaptobenzoic acid and 2, 2′-dipyridinyl disulfide. Compound 3 was then coupled with –OH containing fluorophores to give the probes WSP1-5 (Washington State Probes). We chose methoxy fluorescein, 7-hydroxycoumarin, resorufin, and 2-methyl TokyoGreen as the fluorophores because of their readily availability, excellent fluorescence properties, and easy fluorescence quenching via hydroxyl substitution.[24] For WSP5, two reaction centers were introduced to the core structure of fluorescein. Upon reaction with H2S, it should produce highly fluorescent species.
Scheme 3.
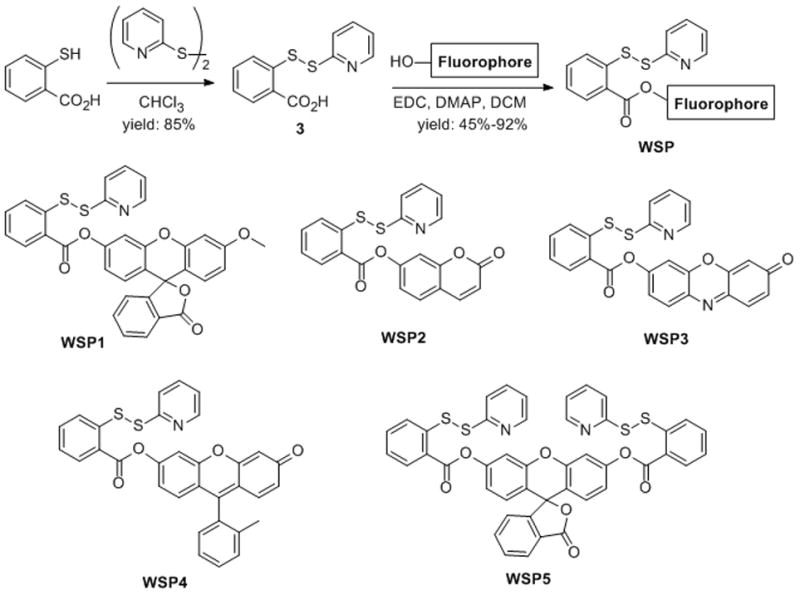
Preparation of fluorescent probes WSP1-5.
Fluorescence properties and responses of probes WSP1-5 to H2S
With these probes in hand, we tested their fluorescent properties. As expected, these probes exhibited very weak fluorescence with low quantum yields (Φf < 0.1, as shown in Table 1) due to the esterification of the hydroxyl group of fluorophores. This low background fluorescence is critical for highly sensitive detection of H2S.
Table 1.
Fluorescent properties of probes WSP1-5.
| Probes | λex (nm) | λem (nm)[a] | Φf[b] |
|---|---|---|---|
| WSP1 | 476 | 516 | 0.003 |
| WSP2 | 385 | 456 | 0.003 |
| WSP3 | 550 | 586 | 0.014 |
| WSP4 | 512 | 531 | 0.088 |
| WSP5 | 502 | 525 | 0.020 |
The maximal emission of the probes.
The fluorescence quantum yield.
We then tested their fluorescence responses to H2S and optimized the fluorescence measurement conditions. WSP1 was used as the representative in these studies. As shown in Figure 1, the fluorescence intensity of WSP1 increased dramatically when H2S was present in the solution. We also found that media plays an important role in this process. When a mixed CH3CN/PBS buffer (10 mM, pH 7.4, 1:1, v/v) solution was used (due to poor water solubility of the probe), the fluorescence turn-on rate was somewhat slow. The intensity could reach the maximum in about 30 min (36 fold increase). However, when a small amount of surfactant hexadecyltrimethylammonium bromide (CTAB) was added into detection system, the turn on rate was significantly increased and the fluorescence intensity was also significantly enhanced (110-fold). The effects of CTAB may be attributed to 1) CTAB can increase the solubility of the probe in aqueous buffers; and 2) CTAB is a cationic surfactant, which may absorb sulfide anion (HS−) and facilitate the reaction between sulfide anion and the probe. These effects were also noted in previous work on fluorescent probes.[25]
Figure 1.
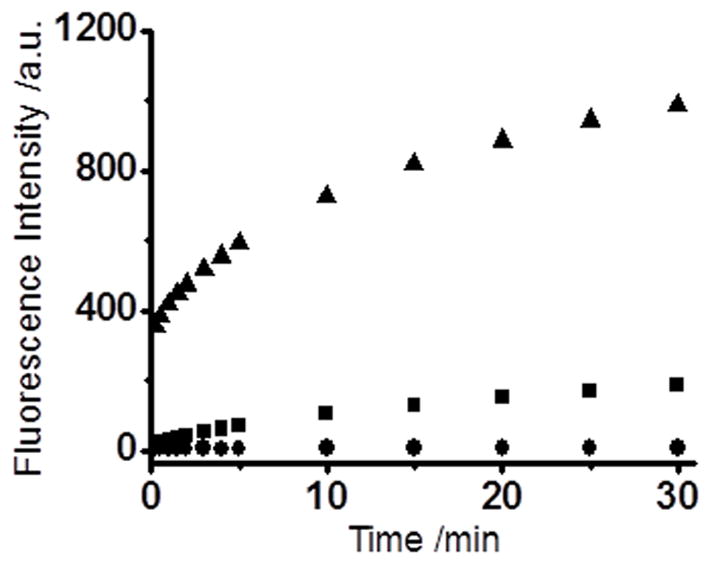
Time-dependent fluorescence changes of 10 μM WSP1 in the presence (■ and ▲) or absence (●) of 50 μM NaHS. (▲) and (●) data were obtained in 10 mM PBS buffer (pH 7.4) containing 1 mM CTAB. (■) data were obtained in CH3CN/PBS buffer (10 mM, pH 7.4, 1:1, v/v). Data were acquired at 516 nm with excitation at 476 nm.
We next applied this optimized condition to other probes. As shown in Figure 2 and 3, all of them (WSP2, WSP3, WSP4, and WSP5) exhibited fast fluorescence turn-on toward H2S and usually the fluorescence signals can reach a steady state in a few minutes, the intensities increased 275-, 68-, 20-, and 60-fold (for WSP2, WSP3, WSP4, and WSP5, respectively). This may be very favorable for fast detection of H2S. Upon gradual introduction of H2S, the fluorescence intensity at the maximal emission wavelength of the probes increased drastically. As can be seen in Figure S1, good linear relationships were obtained between the fluorescence intensity at the maximal emission wavelength of the probes and different hydrogen sulfide concentrations. The detection limits[26] were determined to be 60, 79, 47, 266 and 47 nM for WSP1, WSP2, WSP3, WSP4, and WSP5, respectively (Table 2).
Figure 2.
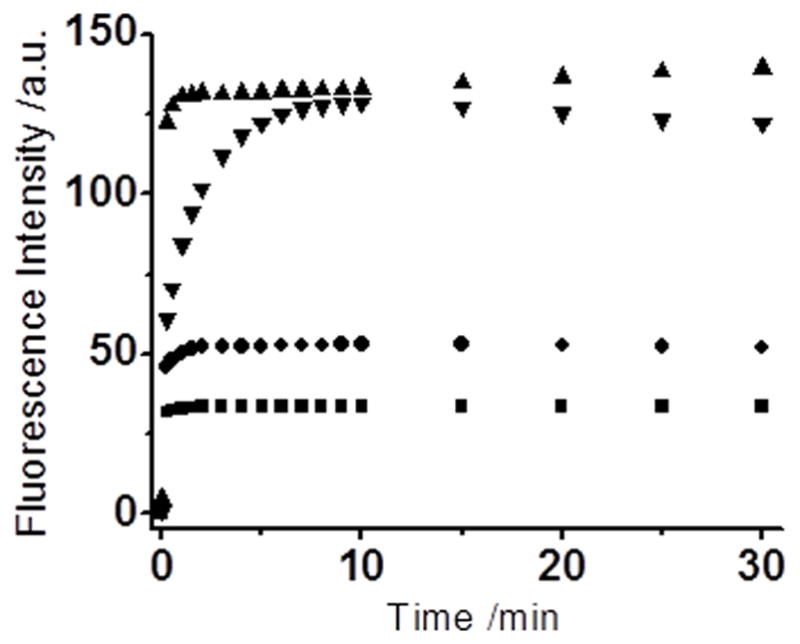
Time-dependent fluorescence changes of the probes (10 μM) in the presence of NaHS (50 μM), The reactions were carried out for 30 min at room temperature in PBS buffer (10 mM, pH 7.4) with 1 mM CTAB. Data were acquired at 456 nm with excitation at 385 nm for WSP2 (■); at 586 nm with excitation at 550 nm for WSP3 (●); at 531 nm with excitation at 512 nm for WSP4 (▲); at 525 nm with excitation at 502 nm for WSP5 (▼).
Figure 3.
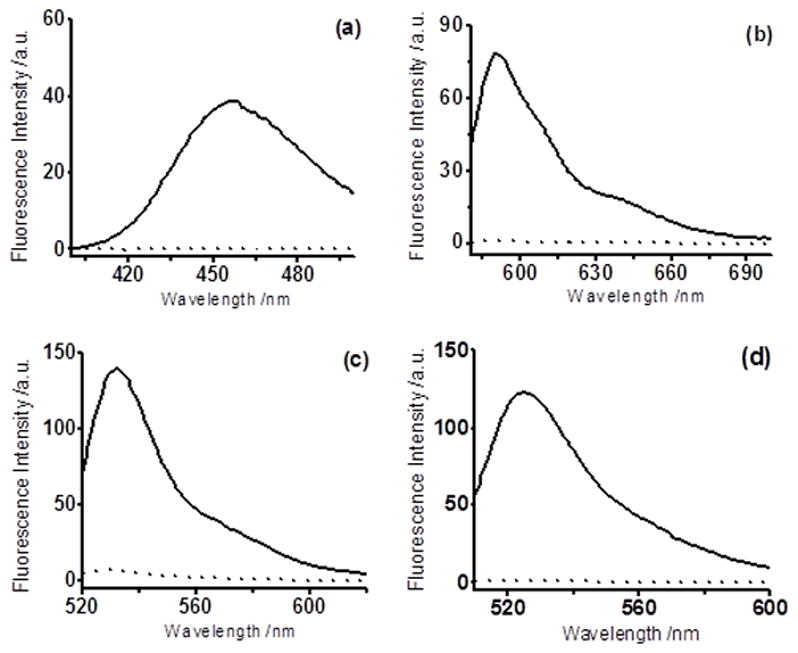
Fluorescence spectra changes of probes (10 μM) in the absence (dot line) or presence (solid line) of NaHS (50 μM), The reactions were carried out for 5 min at room temperature in PBS buffer (10 mM, pH 7.4) with 1 mM CTAB. Data were acquired with excitation at 385 nm for WSP2 (a); with excitation at 550 nm for WSP3 (b); with excitation at 512 nm for WSP4 (c); with excitation at 502 nm for WSP5 (d).
Table 2.
Turn-on fold changes and detection limits (DL) of probes WSP1-5.
| Probes | Turn-on folds | DL[a]/nM |
|---|---|---|
| WSP1 | 130 | 60 |
| WSP2 | 275 | 79 |
| WSP3 | 68 | 47 |
| WSP4 | 20 | 266 |
| WSP5 | 60 | 47 |
DL is the detection limit (3S/m, in which S is the standard deviation of blank measurements, n = 11, and m is the slope of the linear equation).[26]
To evaluate the potential applications of the probes in different biological environments, we studied pH effects for all of the five probes (Figure 4). In general these probes worked well under normal biological pH ranges (6 ~ 9). However, WSP3 and WSP4 showed increased background fluorescence under pH 10, which might be due to the hydrolysis of the ester linkage under this pH.
Figure 4.
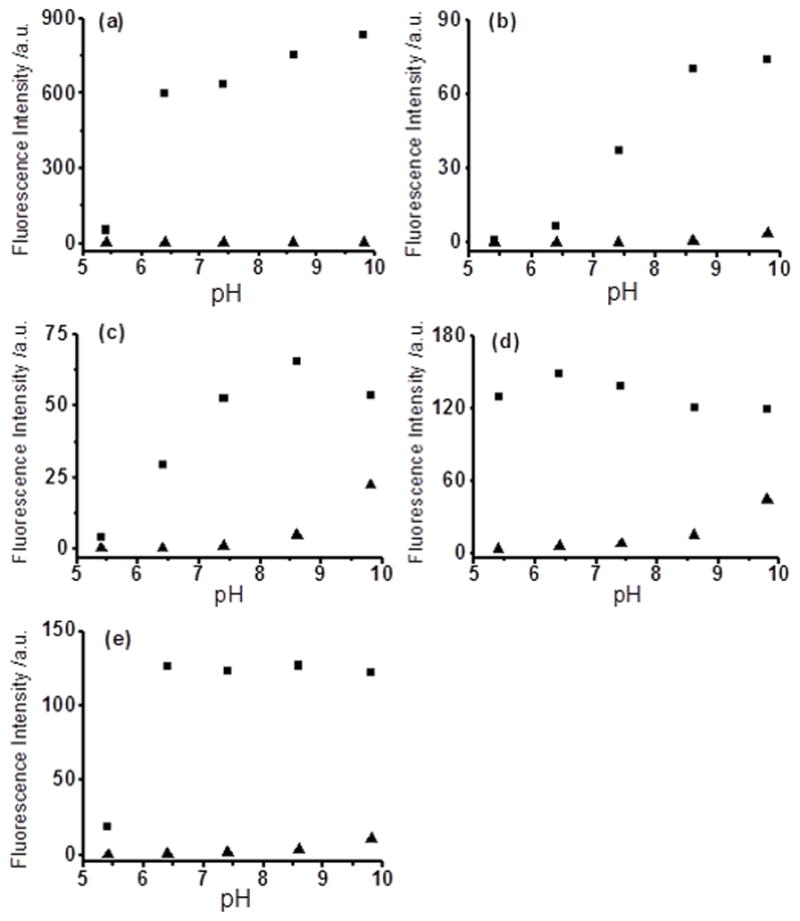
Fluorescence intensity changes of the probes (10 μM) at different pH values in the absence (▲) or presence (■) of NaHS (50 μM), The reactions were carried out for 5 min at room temperature in 10 mM PBS solution with 1 mM CTAB. WSP1 (a), WSP2 (b), WSP3 (c), WSP4 (d), and WSP5 (e).
We next investigated the probes’ selectivity for H2S, which is the most important property to justify the effectiveness of the probes. In these experiments, each probe (10 μM) was treated with different reactive sulfur species including Cys, GSH, Hcy, SO32-, S2O32- (all at 200 μM) under the optimized conditions and fluorescence signals were recorded after mixing for 5 min. As shown in Figure 5, these sulfur-containing species did not lead to any significant fluorescence responses while NaHS (50 μM) gave very strong signals for all probes. Therefore all the probes are proved to have good selectivity for H2S over other reactive sulfur species including Cys and GSH. As one can imagine, these disulfide-based probes may also react with biothiols in biological systems. Although such reactions won’t turn on fluorescence, it could consume the probes and higher loading of the probes may be needed. This is a potential problem for this type of probes. However we realized that the fluorescence turn-on rates of our probes under the optimized conditions were quite fast (within a few minutes). Therefore it is possible that the probes can give significant fluorescence signals even when H2S co-exists with biothiols. To prove this, a solution of NaHS (50 μM) and Cys or GSH (200 μM) was prepared and the probe (10 μM) was then loaded. Pleasantly we still observed significant fluorescence signals for each probe (Figure 5), although at decreased levels compared to NaHS only. These results suggested that the probes were effective for H2S detection in the presence of biothiols.
Figure 5.
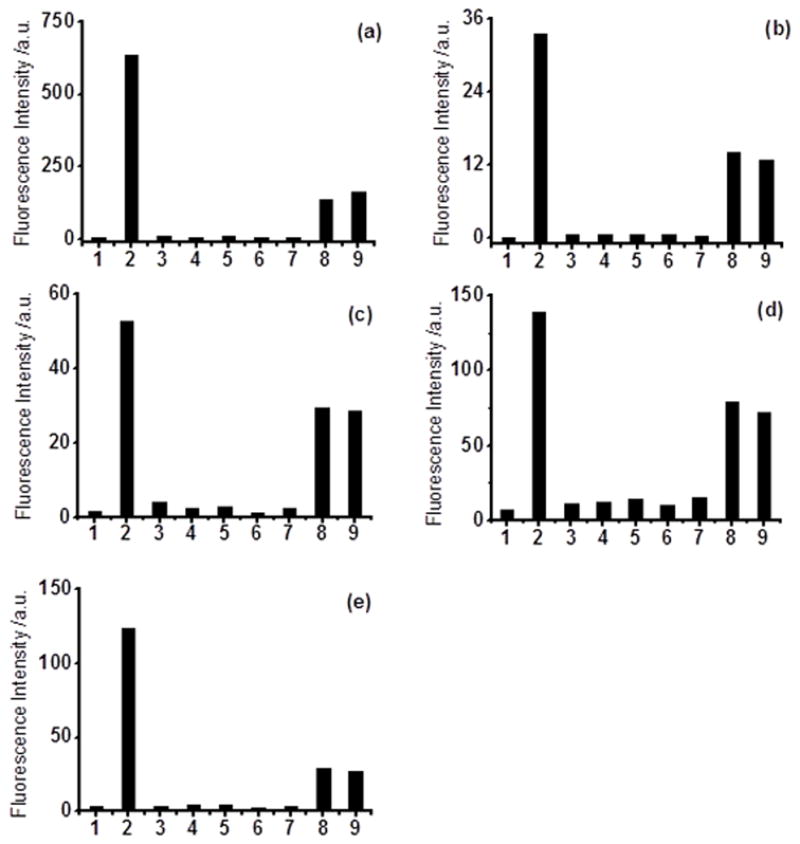
Fluorescence intensity of the probes (10 μM) in the presence of various reactive sulfur species: 1) control; 2) 50 μM NaHS; 3) 200 μM Cys; 4) 200 μM GSH; 5) 200 μM Hcy; 6) 200 μM Na2SO3; 7) 200 μM Na2S2O3; 8) 50 μM NaHS + 200μM Cys; 9) 50 μM NaHS + 200μM GSH. WSP1 (a), WSP2 (b), WSP3 (c), WSP4 (d), and WSP5 (e).
After proving the sensitivity and selectivity of this series of probes for H2S in aqueous buffers, we decided to explore their applications in imaging H2S in living cells. WSP4 and WSP5 were selected for this study because of their strong fluorescent intensity observed when treating with H2S. Before we conducted cell imagine experiments, we realized that our probes contain the fluorophores through an ester linkage. One concern is that such a linkage may be labile in the presence of cellular esterases. In order to address this question, we tested the stability of the probes (WSP1, WSP4 and WSP5) in the presence of esterase (esterase E-0887, from rabbit liver). As shown in Figure 6, the probes were incubated with the enzyme for 30 min and no significant fluorescence increase was observed. After that NaHS was added into the mixture and a strong fluorescence increase was observed. These results suggested that the probes were stable to esterases.
Figure 6.
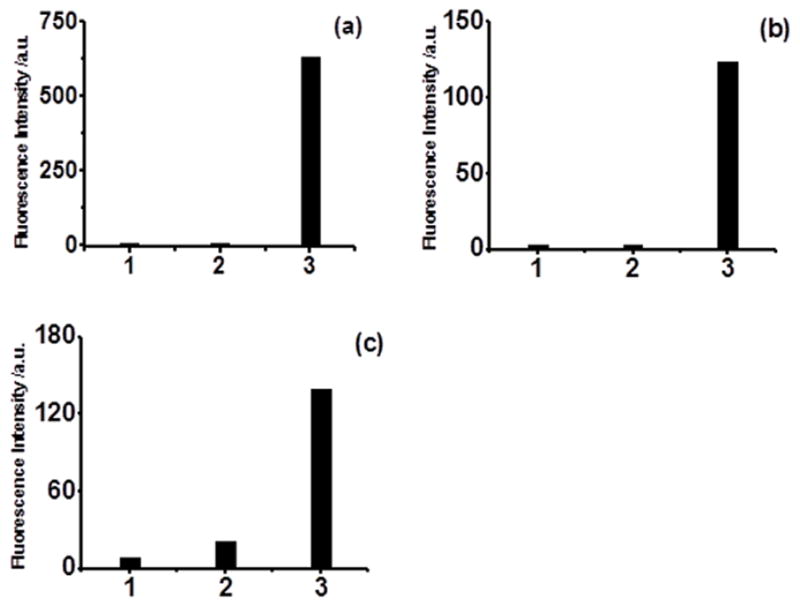
Fluorescence intensity of the probe (10 μM). 1) control, without the esterase; 2) incubate with esterase (0.06 U/mL) at room temperature for 30 min; 3) incubate with 50 μM NaHS for 5 min after 2). WSP1 (a), WSP4 (b), WSP5 (c).
Living cell imaging studies
Having demonstrated the stability of the probes, we then used them in monitoring H2S in live cells. In brief, freshly cultured Hela cells were incubated with probe WSP4 for 30 min. Then the cells were washed by medium buffer to remove excess probe and treated with different concentrations of NaHS. As shown in Figure 7, we did not observe significant fluorescent cells when NaHS was absent. However, strong fluorescence in the cells was observed after treating with NaHS for 30 min. Cells treated with 60 μM NaHS displayed obviously stronger fluorescence than cells treated with 30 μM NaHS. In addition, WSP5 was also tested by using the same protocol and similar results were observed. Thus we concluded that these probes can be used for the detection of H2S in living cells.
Figure 7.
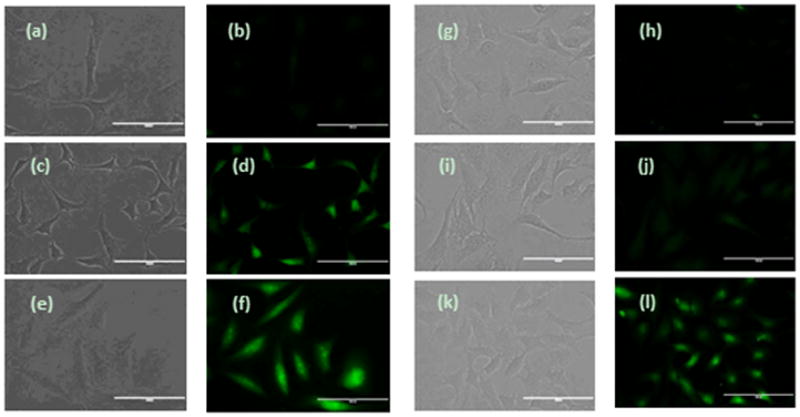
Fluorescence images of H2S in HeLa cells using WSP4 and WSP5. (a-f) Cells on 24-well plate were incubated with WSP4 (30 μM) for 30 min, then washed and subjected to different treatments. (a and b) control (no NaHS was added); (c and d) treated with 30 μM NaHS; (e and f) treated with 60 μM NaHS. (g-l) Cells on 24-well plate were incubated with WSP5 (50 μM) for 30 min, then washed and subjected to different treatments. (g and h) control (no NaHS was added); (i and j) treated with 50 μM NaHS; (k and l) treated with 100 μM NaHS. (Scale bar: 100 nm)
To further explore the applications of the probes in H2S study we used them in the evaluation of novel H2S donors. H2S donors are another type of important research tools in this field and our laboratory has recently developed several different controllable H2S donors.[27] Among these the perthiol-based donors are of particularly interest because they have exhibited promising activity against myocardial ischemia-reperfusion injury.[27a] To monitor H2S generation in cells from this type of donors, we applied our newly developed fluorescent probes. Briefly, Hela cells were first incubated with YZ-4-074, a perthiol-based donor, for 30min. After that, the exo-cellular donor was removed by washing with buffers. The cells were then incubated with WSP4. As shown in Figure 8, donor-treated cells showed much enhanced fluorescent signals compared to vehicle-treated cells. We also applied WSP5 in the same protocol and similar results were observed (Figure S2 in supporting information). These results demonstrated that these probes can be used to evaluate synthetic H2S donor in cells.
Figure 8.
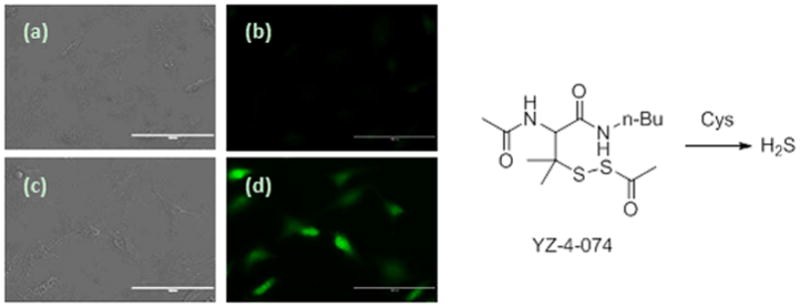
Fluorescence images of H2S production from a H2S donor YZ-4-074 in HeLa cells. Cells on 24-well plate were incubated with or without YZ-4-074 for 30 min, then washed and incubated with 30 μM WSP4 for 30 min. (a and b) controls (no donor was added); (c and d) treated with 100 μM YZ-4-074. (Scale bar: 100 nm)
Conclusion
In summary, we reported in this study the development of a series of nucleophilic substitution-cyclization based fluorescent probes for H2S. Five probes (WSP1-5) were prepared and evaluated. These probes proved to be selective for H2S over other sulfur-containing species including Cys and GSH. Moreover, fluorescence ‘turn-on’ are fast. The efficiencies of these probes were demonstrated in aqueous solution and in cell imaging. Further development of nucleophilic substitution-cyclization based probes and application of these probes in H2S studies are currently ongoing in our laboratory.
Experimental Section
Synthesis of the probes
Compound 3 was prepared using the protocol described previously[18]. General procedure for probes synthesis: to a mixture of compound 3 (262 mg, 1.0 mmol), OH-containing fluorophore (1.0 mmol), EDC (192 mg, 1.0 mmol), and DMAP (12.2 mg, 0.1 mmol) in a 50 mL round bottom flask was added dry CH2Cl2 (25 mL) at room temperature. The mixture was stirred for 12 h. Then solvent was removed under reduced pressure and resulted crude product was purified by fresh column chromatography to provide the desired product.
Compound 1 was obtained as a white solid (273 mg, 80.5% yield). M.p. 113 °C–115 °C; 1H NMR (300 MHz, [D1]CDCl3, 25 °C, TMS) δ= 7.06–7.11 (m, 1H, HAr), 7.24–7.36 (m, 4H, HAr), 7.41–7.47 (m, 2H, HAr), 7.50–7.57 (m, 3H, HAr), 7.97 (dd, J1=8.1 Hz, J2=2.4 Hz, 1H, HAr), 8.30 (dd, J= 7.8, 1.5 Hz, 1H, HAr), 8.45–8.47 (m, 1H, HAr); 13C NMR (75 MHz, CDCl3, 25 °C, TMS) δ=119.7, 121.0, 121.7, 125.8, 126.0, 126.2, 126.4, 129.8, 132.0, 133.8, 137.4, 141.4, 149.6, 150.5, 159.0, 164.9; IR (thin film, cm−1) 3999.0, 3072.0, 3041.0, 1707.0, 1562.5; MS (ESI) [M+Na]+ calcd for C18H13NO2S2Na, 362.0; found, 362.0; HRMS [M+H]+ calcd for C18H14NO2S2, 340.0466; found, 340.0468.
WSP1: Data was the same as previously reported.[18]
WSP2 was obtained as a white solid (374 mg, 92% yield). M.p. 165 °C–167 °C; 1H NMR (300 MHz, [D1]CDCl3, 25 °C, TMS) δ= 6.44 (d, J=9.3 Hz, 1H, HAr), 7.09–7.14 (m, 1H, HAr), 7.23 (d, J=2.1 Hz, 1H, HAr), 7.28–7.29 (m, 1H, HAr), 7.34–7.39 (m, 1H, HAr), 7.52–7.61 (m, 4H, HAr),7.74 (d, J=9.9 Hz, 1H, HAr), 7.99–8.01 (m, 1H, HAr), 8.31 (dd, J1=7.8 Hz, J2=1.2 Hz, 1H, HAr), 8.48 (dd, J=4.2, 0.9 Hz, 1H, HAr); 13C NMR (75 MHz, CDCl3, 25 °C, TMS) δ=110.9, 116.5, 117.2, 118.8, 120.0, 121.4, 125.8, 126.2, 126.5, 129.0, 132.4, 134.5, 137.6, 142.2, 143.1, 149.9, 153.3, 155.0, 158.9, 160.5, 164.4; IR (thin film, cm−1) 3098.6, 3064.4, 3034.0, 1733.6, 1707.0; MS (ESI) [2M+Na]+ calcd for C42H26N2O8S4Na, 837.0; found, 836.7; HRMS [M+H]+ calcd for C21H14NO4S2, 408.0364; found, 408.0362.
WSP3 was obtained as an orange solid (266 mg, 58% yield). M.p. 139 °C–141 °C; 1H NMR (300 MHz, [D1]CDCl3, 25 °C, TMS) δ=6.37 (d, J=1.8 Hz, 1H, HAr), 6.90 (dd, J1=9.6, J2=1.8 Hz, 1H, HAr), 7.11–7.15 (m, 1H, HAr), 7.31–7.41 (m, 3H, HAr), 7.48 (d, J=9.9 Hz, 1H, HAr), 7.54–7.63 (m, 3H, HAr), 7.89 (d, J=8.1 Hz, 1H, HAr), 8.02 (d, J=7.5 Hz, 1H, HAr), 8.32 (dd, J1=7.8 Hz, J2=0.9 Hz, 1H, HAr), 8.50 (d, J=3.9 Hz, 1H, HAr); 13C NMR (75 MHz, CDCl3, 25 °C, TMS) δ=107.2, 109.9, 119.4, 119.9, 121.2, 125.4, 126.0, 126.3, 131.2, 131.4, 132.1, 134.3, 134.8, 135.2, 137.5, 141.9, 144.4, 148.4, 149.2, 149.5, 153.2, 158.5, 163.9, 186.2; IR (thin film, cm−1) 3100.1, 3064.4, 3040.6, 1704.4, 1624.0; MS (ESI) [2M+Na]+ calcd for C48H28N4O8S4Na, 939.1; found, 938.8; HRMS [M+H]+ calcd for C24H15N2O4S2, 459.0473; found, 459.0487.
WSP4 was obtained as an orange solid (301mg, 55% yield). M.p. 114 °C–117 °C; 1H NMR (300 MHz, [D1]CDCl3, 25 °C, TMS) δ=2.12 (s, 3H; CH3), 6.47 (d, J=1.8 Hz, 1H, HAr), 6.60 (dd, J1=9.9 Hz, J2=1.8 Hz, 1H, HAr), 6.99 (d, J=9.9 Hz, 1H, HAr), 7.10–7.14 (m, 3H, HAr), 7.19–7.21 (m, 1H, HAr), 7.34–7.47 (m, 4H, HAr), 7.50 (d, J=8.4 Hz, 1H, HAr), 7.54–7.62 (m, 3H, HAr), 8.00 (d, J= 8.1 Hz, 1H, HAr), 8.30 (dd, J1=7.8 Hz, J2=1.8 Hz, 1H, HAr), 8.47–8.50 (m, 1H, HAr); 13C NMR (75 MHz, CDCl3, 25 °C, TMS) δ=19.6(s; CH3), 106.1, 110.4, 118.4, 118.6, 119.6, 120.4, 121.0, 125.3, 125.9, 126.1, 126.2, 129.0, 129.1, 129.6, 130.5, 130.6, 130.9, 131.9, 132.0, 134.2, 136.1, 137.3, 141.9, 148.0, 149.6, 153.0, 154.0, 158.4, 158.4, 163.8, 185.8; IR (thin film, cm−1) 3053.0, 2946.6, 2916.1, 1726.0, 1596.7; MS (ESI) [2M+Na]+ calcd for C64H42N2O8S4Na, 1117.2; found, 1116.9; HRMS [M+H]+ calcd for C32H22NO4S2, 548.0990; found, 548.0974.
WSP5 was obtained as a light yellow solid (370 mg, 45% yield). M.p. 125 °C–128 °C; 1H NMR (300 MHz, [D1]CDCl3, 25 °C, TMS) δ=6.92–6.95 (m, 2H, HAr), 7.00–7.03 (m, 2H, HAr), 7.10–7.12 (m, 2H, HAr), 7.23–7.38 (m, 5H, HAr), 7.52–7.61 (m, 6H, HAr), 7.64–7.75 (m, 2H, HAr), 7.99 (d, J=7.8 Hz, 2H, HAr), 8.07 (d, J=7.2 Hz, 1H, HAr), 8.30 (d, J=7.2 Hz, 2H, HAr), 8.47 (d, J=4.2 Hz, 2H, HAr); 13C NMR (75 MHz, CDCl3, 25 °C, TMS) δ 81.6, 110.7, 116.8, 118.0, 119.8, 121.1, 124.1, 125.3, 125.8, 126.0, 126.2, 129.2, 130.2, 132.1, 134.1, 135.4, 137.4, 141.8, 149.6, 151.6, 151.9, 153.0, 158.8, 164.3, 169.2; IR (thin film, cm−1) 3060.6, 2954.2, 2923.7, 1771.6, 1718.4; MS (ESI) [M+Na]+ calcd for C44H26N2O7S4Na, 845.1; found, 845.1; HRMS [M+H]+ calcd for C44H27N2O7S4, 823.0701; found, 823.0714.
General procedure for H2S determination
Unless otherwise noted, all the measurements were carried out at room temperature for 5 min in 10 mM PBS buffer (pH 7.4) containing 1 mM CTAB according to the following procedure: in a test tube, 3.5 mL of 10 mM PBS buffer (pH 7.4) and 40 μL of the stock solution of CTAB were mixed. To this mixture was then added 16 μL of the stock solution of the probe. The resulting solution was well-mixed, followed by the addition of the requisite volume of testing species solution. The final volume of the solution was adjusted to 4 mL with 10 mM PBS buffer (pH 7.4). After mixing and standing for 5 min at room temperature, 3 mL of the solution was transferred into a 1 cm quartz cell and fluorescence signal was recorded.
Cell imaging experiments with H2S
Hela cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM, Cellgro company) supplemented with 10% fetal bovine serum, 4 mM glutamine, 100 IU/mL penicillin, and 100 ug/mL streptomycin at 37 °C and with 5% CO2 for two days. One day before imaging, cells were transferred to 24-well plates. Before use, the adherent cells were washed one time with FBS-free DMEM. For intracellular H2S imaging, the cells were incubated with the probe in FBS-free DMEM at 37 °C for 30 min. After removal of excess probe and washed with PBS (pH 7.4), the cells were incubated with NaHS for 30 min in PBS buffer (pH 7.4, containing 100 μM CTAB). Cell imaging was carried out after washing the cells three times with PBS (pH 7.4). All microscopy images were taken on a fluorescence microscope with excitation at 490 nm (green channel).
Cell imaging experiments with H2S donor
Medium was removed from the cell plate. The cells were washed by FBS free medium once before they were incubated with or without the donor in FBS free medium. After 30 min, the medium was removed and the cells were washed by FBS free medium. Then the cells were incubated in FBS free medium with the probe (containing 100 μM CTAB) for 30 min. After washing by PBS twice, the cells were ready for imaging. Fluorescence imaging was performed with fluorescence microscope with GFP light cube for fluorescence channel and 40 × objectives.
Supplementary Material
Acknowledgments
This work is supported by an American Chemical Society-Teva USA Scholar Grant and NIH (R01GM088226). We thank Prof. Viveka Vadyvaloo for providing the fluorescence microscope for this work.
References
- 1.Li L, Rose P, Moore PK. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 2.Szabo C. Nat Rev Drug Disco. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 3.Wang R. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 4.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Chem Res Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Kimura H. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]; b) Kimura H, Shibuya N, Kimura Y. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson KR. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Qu K, Lee SW, Bian JS, Low CM, Wong PTH. Neurochem Int. 2008;52:155–165. doi: 10.1016/j.neuint.2007.05.016. [DOI] [PubMed] [Google Scholar]; b) Predmore BL, Lefer DJ, Gojon G. Antioxid Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteman M, Moore PK. J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stipanuk MH, Ueki I. J Inherit Metab Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob C, Anwar A, Burkholz T. Planta Med. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Antioxid Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan N, Fu C, Pappin DJ, Tonks NK. Sci Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan J, Carroll KS. ACS Chem Biol. 2013;8:1110–1116. doi: 10.1021/cb4001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francoleon NE, Carrington SJ, Fukuto JM. Arch Biochem Biophys. 2011;516:146–153. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Lippert AR, New EJ, Chang CJ. J Am Chem Soc. 2011;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Angew Chem. 2011;123:10511–10513. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. Angew Chem. 2011;123:9846–9849. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian Y, Karpus J, Kabil O, Zhang SY, Zhu HL, Banerjee R, Zhao J, He C. Nat Commun. 2011;2:495–501. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- 21.Sasakura T, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T. J Am Chem Soc. 2011;133:18003–18005. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 22.a) Lin VS, Chang CJ. Curr Opin Chem Biol. 2012;16:595–601. doi: 10.1016/j.cbpa.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xuan W, Sheng C, Cao Y, He W, Wang W. Angew Chem. 2012;124:2325–2330. doi: 10.1002/anie.201107025. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:2282–2284. doi: 10.1002/anie.201107025. [DOI] [PubMed] [Google Scholar]; c) Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973–984. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Peng H, Chen W, Cheng Y, Hakuna L, Strongin R, Wang B. Sensors. 2012;12:15907–15946. doi: 10.3390/s121115907. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Li X, Gao X, Shi W, Ma H. Chem Rev. 2013 doi: 10.1021/cr300508p. [DOI] [PubMed] [Google Scholar]
- 23.a) Lin VS, Lippert AR, Chang CJ. Proc Natl Acad Sci USA. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu J, Sun YQ, Zhang J, Yang T, Cao J, Zhang L, Guo W. Chem Eur J. 2013;19:4717–4722. doi: 10.1002/chem.201300455. [DOI] [PubMed] [Google Scholar]; c) Liu T, Xu Z, Spring DR, Cui J. Org Lett. 2013;15:2310–2313. doi: 10.1021/ol400973v. [DOI] [PubMed] [Google Scholar]; d) Wan Q, Song Y, Li Z, Gao X, Ma H. Chem Commun. 2013;49:502–504. doi: 10.1039/c2cc37725j. [DOI] [PubMed] [Google Scholar]; e) Liu C, Peng B, Li S, Park C, Whorton AR, Xian M. Org Lett. 2012;14:2184–2187. doi: 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Zheng H, Zhan X, Bian Q, Zhang X. Chem Commun. 2013;49:429–447. doi: 10.1039/c2cc35997a. [DOI] [PubMed] [Google Scholar]; b) Seo H, Jun ME, Egorova OA, Lee K, Kim KJ, Ahn KH. Org Lett. 2012;14:5062–5065. doi: 10.1021/ol302291c. [DOI] [PubMed] [Google Scholar]; c) Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. J Am Chem Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]; d) Kawaguchi M, Okabe T, Okudaira S, Hanaoka K, Fujikawa Y, Terai T, Komatsu T, Kojima H, Aoki J, Nagano T. J Am Chem Soc. 2011;133:12021–12030. doi: 10.1021/ja201028t. [DOI] [PubMed] [Google Scholar]; e) Li Z, Gao X, Shi W, Li X, Ma H. Chem Commun. 2013;49:5859–5861. doi: 10.1039/c3cc42610f. [DOI] [PubMed] [Google Scholar]; f) Chen W, Li Z, Shi W, Ma H. Chem Commun. 2012;48:2809–2811. doi: 10.1039/c2cc17768d. [DOI] [PubMed] [Google Scholar]; g) Zhang YY, Chen W, Feng D, Shi W, Li XH, Ma H. Analyst. 2012;137:716–721. doi: 10.1039/c2an15952j. [DOI] [PubMed] [Google Scholar]; h) Yuan L, Lin W, Xie Y, Chen B, Zhu S. J Am Chem Soc. 2012;134:1305–1315. doi: 10.1021/ja2100577. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Yang X, Hakuna L, Barve A, Escobedo JO, Lowry M, Strongin RM. Sensors. 2012;12:5940–5950. doi: 10.3390/s120505940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Li Z, Li X, Gao X, Zhang Y, Shi W, Ma H. Anal Chem. 2013;85:3926–3932. doi: 10.1021/ac400750r. [DOI] [PubMed] [Google Scholar]; b) Chen W, Liu C, Peng B, Zhao Y, Pacheco A, Xian M. Chem Sci. 2013;4:2892–2996. doi: 10.1039/C3SC50754H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Zhao Y, Bhushan S, Yang C, Otsuka H, Stein JD, Pacheco A, Peng B, Devarie-Baez NO, Aguilar HC, Lefer DJ, Xian M. ACS Chem Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao Y, Wang H, Xian M. J Am Chem Soc. 2011;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Devarie-Baez NO, Bagdon PE, Peng B, Zhao Y, Park C, Xian M. Org Lett. 2013;15:2786–2789. doi: 10.1021/ol401118k. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Park C, Zhao Y, Zhu Z, Pacheco A, Peng B, Devarie-Baez NO, Bagdon P, Zhang H, Xian M. Mol BioSyst. 2013;9:2430–2434. doi: 10.1039/c3mb70145j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


