Abstract
Background and Purpose
Migraine with aura is a risk factor for ischemic stroke. The goals of this study are to examine the association between migraine and subclinical cerebrovascular damage in a race/ethnically diverse older population-based cohort study.
Methods
In the Northern Manhattan Study, we quantified subclinical brain infarctions (SBI) and white matter hyperintensity volumes (WMHV) among participants with self-reported migraine, confirmed by the International Classification of Headache Disorders-2 criteria (ICHD-2).
Results
Of 546 study participants with imaging and migraine data (41% men, mean age at MRI=71±8 yrs, mostly Hispanic (65%)), those reporting migraine overall had double the odds of SBI (adjusted OR 2.1, 95% CI 1.0-4.2), compared to those reporting no migraine, after adjusting for sociodemographics and vascular risk factors. No association was observed between migraine with or without aura and WMHV.
Conclusion
Migraine may be a risk factor for SBI. Prospective studies are needed in race/ethnically diverse populations.
Keywords: Migraine, Epidemiology, Biomarkers, Ethnic Groups, Cerebral Infarction, Leukoaraiosis, Risk Factors
Introduction
Migraine with aura has been associated with a slightly elevated stroke risk1. However, the association between migraine and subclinical cerebrovascular disease is limited to a few predominantly Caucasian population studies2. In the Northern Manhattan Study (NOMAS), a racially/ethnically diverse population-based urban cohort, we hypothesized that migraine is associated with white matter hyperintensity volume (WMHV) and silent brain infarction (SBI).
Methods
Study Participants
NOMAS includes 3289 participants followed prospectively to determine stroke incidence, risk factors, and prognosis. The study is approved by the IRBs of Columbia University and the University of Miami and participants provided written informed consent. Details of the study have been published previously3-6. From the entire NOMAS cohort, we excluded 378 participants with history of meningitis, head trauma, or radiation to rule out secondary headache.
Baseline evaluation
Baseline data on demographics, socioeconomic factors, medical history and medication use, vascular risk factors, family history and other health-related information was collected. Participants recruited after 1998 were interviewed about their migraine history (some participants enrolled between 1996-1997 were re-interviewed) as previously described)7,8.
MRI sub-study
All participants age >55 remaining clinically stroke-free were screened for recruitment into the brain MRI sub-study (N=1091)9. Protocols to determine white matter hyperintensity volumes (WMHV) and SBI have been described10.
Statistical Analysis
Data on migraine was available for 1380 participants, of whom 546 had MRI data available. The unadjusted associations between migraine and WMHV and SBI were examined, using linear regression for WMHV and logistic regression for SBI. Multivariable-adjusted regression models were constructed including covariates that were associated with migraine in the full NOMAS cohort at p<0.10, those that were associated with WMHV or SBI in multivariable-adjusted regression models at p<0.10, as well as the time span from baseline interview to the time of MRI. Migraine was examined as a dichotomous variable. In secondary exploratory analyses, migraine with and without aura were analyzed separately vs. no migraine. We examined possible interactions of migraine with sex and race/ethnicity in relation to the outcomes, but we did not observe effect modification at p<0.10 so stratified analyses were not conducted.
Results
Table 1 shows the distribution of the demographic and vascular risk factors in the study population across migraine categories. Covariates included in the fully adjusted models were age, sex, race/ethnicity, insurance status, high school completion, smoking, mild-moderate alcohol use, diabetes mellitus, hypertension, and body mass index (BMI). Among those with MRI data, the frequency of migraine was 19% (N=546), 6% with aura, 13% without aura. The prevalence of self reported migraine was 17%.
Table 1.
Northern Manhattan Study: Sample Characteristics
| No Migraine N(%) | Migraine N(%) | P value | |
|---|---|---|---|
| Overall cohort | 442 (81) | 104 (19) | |
| Female | 246 (56) | 74 (71) | 0.0039 |
| Race/ethnicity | 0.7750 | ||
| Black | 59 (13) | 13 (13) | |
| White | 53 (12) | 11 (11) | |
| Hispanic | 314 (71) | 78 (75) | |
| Age at Baseline | 0.2962 | ||
| <60 | 134 (30) | 41 (39) | |
| 60-69 | 191 (43) | 41 (39) | |
| 70-79 | 101 (23) | 20 (19) | |
| 80+ | 16 (4) | 2 (2) | |
| Hypertension | 298 (67) | 87 (84) | 0.0011 |
| Diabetes | 90 (20) | 22 (21) | 0.8572 |
| Hypercholesterolemia | 264 (60) | 70 (67) | 0.1536 |
| BMI | 0.7905 | ||
| Normal (<25) | 100 (23) | 26 (25) | |
| Overweight (25-29.9) | 213 (48) | 50 (49) | |
| Obese (30+) | 128 (29) | 27 (26) | |
| Smoking | 0.2382 | ||
| Never | 208 (47) | 55 (53) | |
| Former | 169 (38) | 40 (38) | |
| Current | 65 (15) | 9 (9) | |
| Moderate alcohol use | 192 (43) | 35 (34) | 0.0685 |
| Moderate-heavy physical activity | 42 (10) | 6 (6) | 0.2312 |
| Depression | 64 (17) | 21 (23) | 0.3918 |
| Medicaid and no insurance | 218 (49) | 69 (66) | 0.0018 |
| High school completion | 197 (45) | 30 (29) | 0.0034 |
Years between baseline and MRI ranged from 2-11 (mean±SD=5.7±1.5, median=5.4). Fifty-six participants (10%) had SBI, of whom 15 also had migraines, and only 2 had aura (which prevented further analysis of the effects of migraine with aura separately). The mean (±SD) WMHV was 0.65 (±0.84; interquartile range=0.20-0.71% median=0.34 TCV). Table 2 shows the relationship between migraine overall and the two outcomes. Migraine overall was associated with more than a two-fold greater odds of SBI after adjusting for covariates. The association between migraine without aura and SBI was even stronger (model 2; OR=2.6, 95% CI 1.3-5.5). Figure 1 shows the percentage of participants with an SBI stratified by migraine status and age categories. Infarcts were found most commonly in the white matter (13%), cerebellum (10%), and frontal cortex (7%). There was no association between migraine status and WMHV in either unadjusted or fully adjusted models.
Table 2.
Northern Manhattan Study: Association of Migraine with WMHV and SBI
| Migraine vs. no migraine | ||
|---|---|---|
| Unadjusted | Adjusted | |
| White matter hyperintensity volume: Beta (p-value) | -0.12945 (0.21) | -0.16258 (0.09) |
| Silent brain infarcts: OR (95% CI) for | 1.65 (0.87-3.11) | 2.07 (1.03-4.17) |
Unadjusted model: univariate; adjusted model: controlling for age, sex, race/ethnicity, insurance status, high school completion, smoking, mild-moderate alcohol use, diabetes, hypertension, BMI, and the time from baseline to MRI.
Figure 1.
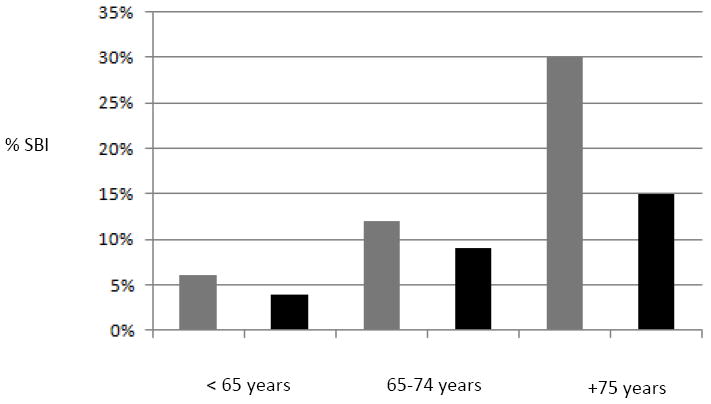
SBI Distribution by Age and Migraine Status. Legend: Proportion of participants with migraine (gray bars) and without migraine (black bars) in the Northern Manhattan Study sample denotes % of SBI found in participants with migraine vs. no migraine history respectively at the time of MRI.
Discussion
We found that participants with a self-reported history of migraine were more likely to have SBI, while there was no association between migraine and white matter lesion load. The association between migraine and SBI was independent of cardiovascular risk factors and was significant in the subgroup with migraine without aura. Migraine appears to be an important stroke risk factor for younger individuals11; however, we have shown that migraine may also be an important risk factor even for much older populations (Table 2). We did not find race/ethnic differences in the odds of SBI by migraine diagnosis. Unexpectedly, we found no associations between WMHV and migraine or its subgroups. Perhaps the group difference in WMH was not detected due to the high burden of other cardiovascular risk factors in our racially diverse older cohort.
The strengths of this study include the large stroke-free population-based race/ethnically diverse older cohort that includes a large proportion of Hispanic participants that are typically underrepresented in studies of migraine. However, due to the cross-sectional design, we are unable to infer a causal or temporal association between migraine and SBI. Migraine was identified by self-report, although the details of the history of headache allowed classification as migraine without selection bias. The questionnaire was not systematically validated; however, the questions were based on the ICHD-2 criteria8. Our definition of visual auras confined to ‘visual changes such as spots, stars, lines, flashing lights’ is a restrictive one. However, we do not believe this was a significant problem as the prevalence of migraine aura in our sample size was representative when historically compared,11,12 and non-visual migraine symptoms are much less common.
Although the risk of ischemic stroke in people with migraine is considered small, the potential for more aggressive measures of risk factor reduction in individuals with migraine found to have SBI and other vascular risk factors needs consideration. Larger prospective multiethnic studies are necessary to elucidate the potential for SBI accumulation as a biomarker for subclinical cerebrovascular disease in individuals with migraine.
Acknowledgments
Sources of Funding
Teshamae Monteith: NIH/NINDS Supplements to Promote Diversity in Health-Related Research; NIH/NINDS R37 NS 29993, PIs Ralph Sacco/Mitchell Elkind; Clinton Wright: (K02 NS 059729, R01 HL 108623) and American Heart Association SDG 0735387N; Tatjana Rundek: NINDS K24 NS 062737 grant.
Footnotes
Disclosures/COI:
None.
References
- 1.Kurth T, Slomke MA, Kase CS, Cook NR, Lee IM, Gaziano JM, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64:1020–1026. doi: 10.1212/01.WNL.0000154528.21485.3A. [DOI] [PubMed] [Google Scholar]
- 2.Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: A systematic review and meta-analysis. Neurology. 2013;81:1260–8. doi: 10.1212/WNL.0b013e3182a6cb32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. American journal of epidemiology. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA : the journal of the American Medical Association. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke; a journal of cerebral circulation. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 6.Kargman DE, Sacco RL, Boden-Albala B, Paik MC, Hauser WA, Shea S. Validity of telephone interview data for vascular disease risk factors in a racially mixed urban community: the Northern Manhattan Stroke Study. Neuroepidemiology. 1999;18:174–184. doi: 10.1159/000026209. [DOI] [PubMed] [Google Scholar]
- 7.Rundek T, Elkind MS, Di Tullio MR, Carrera E, Jin Z, Sacco RL, et al. Patent foramen ovale and migraine: a cross-sectional study from the Northern Manhattan Study (NOMAS) Circulation. 2008;118:1419–1424. doi: 10.1161/CIRCULATIONAHA.108.771303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The International Classification of Headache Disorders. 2. Suppl 1. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 9.Willey JZ, Moon YP, Paik MC, Yoshita M, DeCarli C, Sacco RL, et al. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology. 2011;76:2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke; a journal of cerebral circulation. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merikangas KR, Fenton BT, Cheng SH, Stolar MJ, Risch N. Association between migraine and stroke in a large-scale epidemiological study of the United States. Archives of neurology. 997;54:362–368. doi: 10.1001/archneur.1997.00550160012009. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WF, Linet MS, Celentano DD, Van Natta M, Ziegler D. Age- and sex-specific incidence rates of migraine with and without visual aura. American journal of epidemiology. 1991;134:1111–1120. doi: 10.1093/oxfordjournals.aje.a116014. [DOI] [PubMed] [Google Scholar]


