Abstract
Perfluorooctanesulphonicacid (PFOS) is an organic contaminant that is ubiquitous in the environment, wildlife, and humans. Few studies have assessed the effects of chronic PFOS exposure on central nervous system function in aquatic organisms. The present study defined the behavioral effects of varying life span chronic exposures to low dose PFOS in zebrafish. The zebrafish were treated with vehicle control or 0.5μM PFOS during 1–21, 21–120, or 1–120 day post fertilization (dpf). Chronic PFOS exposure impaired the adult zebrafish behavior mode under the tapping stimulus. The movement speed of 1–120 dpf exposed fish was significantly increased compared with control, while 1–21 and 21–120 dpf exposed groups were not severely affected. PFOS residues in F1 embryos derived from parental exposure during both the 1–120 and 21–120 dpf groups was significantly higher than control, and F1 embryos in these two groups showed obvious malformations, such as uninflated swim bladder (USB) and bent spine (BS). Larvae of the parental exposed to PFOS from 1–21 or 21–120 dpf elicited a higher swim rate than control in both the light and dark periods. Embryos derived from the 1–120 dpf group showed a statistically lower speed in the light period and a higher speed in the dark period as compared with control. Though there is little PFOS residue in 1–21 dpf group, the adverse behavioral effects on both adult and F1 larvae indicate that exposure during the first 21 dpf induce long-term neurobehavior toxicity. Our findings demonstrate that chronic exposure to low dose PFOS in different life stage adversely impacts adult behavior, subsequent offspring malformation, and larval behavior.
Keywords: zebrafish embryo, chronic exposure, perfluorooctanesulfonic acid, behavior
1. Introduction
Fluorinated organic compounds (FOCs) such as perfluorooctanesulfonic acid (PFOS) have been manufactured for over 50 years, and are used as components of fire retardants, lubricants, adhesives, paper coatings, pharmaceuticals, cosmetics, and insecticides (Key et al. 1997; Lein et al. 2008; Renner 2001). As a pollutant in the global ecosystem, PFOS values (median, range) are between 18.8, 8.1 150.7μng/L in the serum of non-occupational exposed humans (Wilhelm et al. 2009). In the aquatic environment, high concentrations of PFOS is detected in a variety of fish species. For example, PFOS concentrations in the liver of smallmouth bass (Micropterus dolomieu) and largemouth bass (Micropterus salmoides) from New York State are present from 9 to 315 ng/g wet weight, and the average concentrations of PFOS in the fish are 8,850 times greater than those found in the surface water (Sinclair et al. 2006).
The role of PFOS in developmental toxicity, reproductive toxicity, hepatotoxicity, immunotoxicity, and neurotoxicity effects have been widely studied in mammalian and aquatic fish model species (Hagenaars et al. 2008; Han and Fang 2010; Johansson et al. 2009; Peden-Adams et al. 2008). Several prior studies on the reproductive and developmental effects of PFSO have included assessments of the developing nervous system in rodents, and one recent study evaluated the effect of PFOS on the development of PC12 cells (Grasty et al. 2003; Slotkin et al. 2008). Nishikawa (Nishikawa 2004) found that PFOS caused backward swimming and prolonged the duration of high K+-induced backward swimming among paramecia. Huang (Huang et al. 2010) investigated behavioral changes in zebrafish larvae under acute PFOS exposure, and Wang (Wang et al. 2011) found that chronic PFOS exposure induced behavioral deficits and primary motor neuron and muscle developmental malformations in F1 embryos. However, the potential long term effects of chronic PFOS exposure during different life stages have yet to be explored.
We conducted this study using the zebrafish model. The zebrafish is the most appropriate model for this type of study because embryos develop externally, all of their organs form within 5 days, and the fish mature to adulthood in just 3 months (http://zfin.org/zf_info/zfbook/zfbk.html). Furthermore, due to the predictable swimming habits of zebrafish, both larval and adult behavioral tests using locomotor activity as the endpoint can be quickly and efficiently conducted (Truong et al. 2012). Our previous study demonstrated that chronic low dose PFOS exposures induced adverse effects on both the parental F0 generation and F1 offspring. Specifically, the 0.5μM PFOS chronic exposure group resulted in altered sex ratios and produced F1 larval developmental toxicity including reduced fertilization rate, induced malformations and mortality (Wang et al. 2011). The juvenile zebrafish have acquired most adult characteristics with the absence of sexual maturity (Parichy et al. 2009), and the zebrafish sex determination period beginning at 21 dpf (von Hofsten and Olsson 2005). Based on these studies, we elected to use 0.5μM PFOS exposure during three different life stages: 1–21 days post fertilization (dpf), 21–120 dpf, and 1–120 dpf. Our goal was to identify the which life span were most sensitive to PFOS exposure. Specifically, we wanted to detect the impacts of PFOS exposure on the behavior of adult zebrafish and F1 offspring.
Materials and methods
2.1. Fish husbandry and embryo collection
Adult zebrafish (Danio rerio) of the (US-AB strain) were raised at the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University under standard laboratory conditions of temperature 28 (± 0.5°C), pH 7.2 (± 0.2), and a 14:10 dark/light photoperiod according to standard zebrafish breeding protocols (Westerfield 1995). Water supplied to the system was filtered by reverse osmosis (pH 7.0–7.5) and Instant Ocean® salt was added to the water to raise the conductivity to 450–1000 μS/cm (system water). The adult fish were fed twice daily with live Artemia and dry flake food (Zeigler, Aquatic Habitats, Apopka, Florida).
Zebrafish embryos were obtained from spawning adults in tanks with a sex ratio of 1:1. Embryos were collected within 1 hour after spawning and rinsed in an embryo medium (EM) (Westerfield 1995). The fertilized embryos were inspected and staged using a stereomicroscope (Nikon, Japan) according to the description of (Kimmel et al. 1995).
2.2. PFOS stock solutions and chronic exposure protocol
Perfluorooctanesulphonicacid (PFOS; CAS # 1763-23-1, purity>96%) was purchased from Sigma–Aldrich Chemical (St. Louis, MO, USA) and stock solutions (0.5 mM PFOS in DMSO) were prepared by dissolving it in 100% dimethyl sulphoxide (DMSO). High quality 8-hour post fertilization (hpf) embryos were divided into four treatment groups:control vehicle control (0.01% v/v, in fish water), 0.5 μM PFOS exposed from 1–21, 21–120, and 1–120 dpf groups. 0.01% DMSO exposure from 1–120 dpf was used as a vehicle control for all three PFOS treatment groups, which was based the previous study that showed 0.01% DMSO chronic exposure have no adverse effects on zebrafish (Wang et al. 2011). Embryos were first exposed to PFOS in a petri dish (100 embryos/treatment, 150ml/dish) for 4 days with a media change once per day, and all embryos hatched and survived in this stage. At 5 days, the fish were transferred to 3.75L stainless steel tanks for a period of 5–21 dpf with the water changed once every three days. At 21 dpf, 30 larvae in each group were separated into three replicate tanks with a total of 10 fish per tank. Exposure solutions were renewed every three days. Each tank was checked daily for morbid fish and on each water change day for the water quality, including pH, ammonia, and nitrite levels. Feeding was initiated on day 5. Between 5–14 dpf, fish were fed three times a day with zebrafish larval diet (Aquatic Habitats, Apopka Florida). During 15–120 dpf they were fed on freshly hatched live artemia, three times a day during 15–95 dpf and two times a day after 96 dpf.
2.3. Adult behavior of F0 zebrafish exposed to PFOS
To evaluate sensory responses, computer-assisted video monitoring of swimming behavior was assessed by modifying the method of (Eddins et al. 2010). Adult behavior tests were carried out with males and females separated into two different test groups due to size and body type differences. For each test, the zebrafish (n=12) from each group (control, 1–21 dpf, 21–120 dpf, and 1–120 dpf) were placed in individual 1.75-L tanks containing about 1.5-L of fish water. Tanks were set inline on shelves with the broadside facing the camera, and were separated by dividers to isolate individual fish. Tanks were backed with blank white paper and were evenly backlit. The room temperature was controlled at 28°C. Trials were recorded using a Sony HD camcorder (Sony Handycam HDR-SR11). Startle stimulus to each fish was generated using an electro-magnetic solenoid attached to the bottom of each tank, which was controlled by a manual switch to hit each tank simultaneously. Fish were fasted for the duration of the behavior trials and were given 2 hr to acclimate prior to beginning the trials. Fish activity was recorded for a total of 30 minutes, of which, the last 16 minutes were analyzed including 12 minutes of background activity following by a 4 minutes of in response to a startle stimulus. Average movement speed in before or after tapping was used to evaluate the fish movement mode under tapping stimulus. Before tapping define as the first 12.5 minutes and after tapping define as 13.5–16.5 minutes. Analysis of the recorded tracks was performed with Noldus Etho-Vision XT V 7.0 software (Leesburg, VA, USA). Position of each fish in the tank was recorded every 0.2 seconds from which distance moved was calculated, which was later averaged for each 30 second intervals.
2.4. PFOS residues and developmental evaluation of F1 embryos and larvae
When chronic PFOS exposed zebrafish began to spawn eggs (at approximately three months of age), breeding trials with a sex ratio of 1:1 were carried out to produce F1 offspring. F1 embryos were monitored for developmental progression, malformations and mortality until 8 dpf with feeding starting from 5 dpf, in the absence of PFOS. Embryo monitoring was carried out in 6 well plates containing embryo media (5 ml/well). The experiment was repeated 5 times using 20 embryos per group during each replication. PFOS concentrations in whole body tissue of F1 embryos were determined with ACQUITY UPLC combined Micromass Quattro Premier XE (Waters Corp, USA) based on a protocol described by (So et al. 2006). Samples were prepared using method as described elsewhere (Huang et al. 2010). A total of 40 embryos were pooled as a single sample, and measurements were replicated 3 times for each group.
2.5. Larvae behavior of F1 offspring derived from parents exposed to PFOS
Embryos derived from PFOS chronic exposed parents were allowed to develop in the absence of PFOS, and surviving larvae at 4 dpf with normal morphology were further subjected to a behavioral assessment in response to alternating light and dark stimulus as described earlier (Chen et al. 2011). The test was performed in a ZebraLab behavior monitoring station (ViewPoint Life Sciences, Inc. Montreal, Canada). Briefly, the larvae were allowed to acclimatize for 20 minutes in the testing chamber before the test began. The lighting parameters used was alternating 10 minutes of light (visible light) and 10 minutes of dark (infrared light), with intervals repeated until 50 minutes of total elapsed time. Behavioral response to light stimulus was analyzed in 24 well plates each plate containing six fish from each treatment group including control. Average activity of a total of 20 fish for each group in light and dark condition was evaluated, respectively.
2.5. Data analysis
A one-way ANOVA was performed to determine statistical significance followed by the Dunnett T3 test as a post hoc test to independently compare each treated group. All results were reported as means ± standard error (SEM) unless otherwise stated. All statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL, USA) and p<0.05 was set as the significance level.
3. Results
3.1. Chronic PFOS exposure altered F0 adult behavior
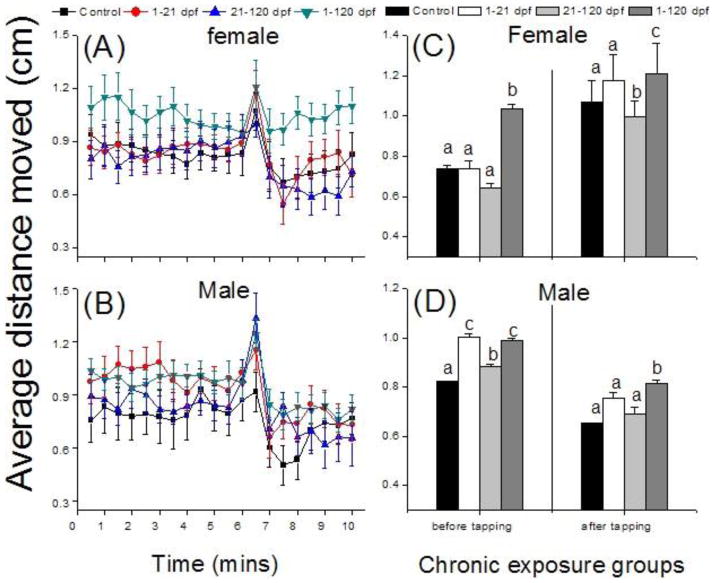
Exposure water conditions, including pH, ammonia, and nitrite, were measured throughout the whole study. The pH was well maintained between 6.8 and 7.6. The ammonia concentration was between 0–2 mg/L. Before 60 dpf, the nitrite concentration was low, 0.073±0.116 mg/L. After 60 dpf, its concentration increased and the averaged 1.02±0.651 (mg/L). Chronic PFOS exposure impaired the adult zebrafish behavior mode as measured by the average distance moved in response to the tapping stimulus (Fig. 1). The movement speed of 1–120 dpf exposed fish was significantly increased compared with control, while 1–21 and 21–120 dpf exposed groups were not severely affected (Fig. 1A, B). Fish in 1–120 dpf group had significantly (F>1; p<0.05) greater movements as compared with the other three groups both (Fig. 1C, D). The movement of fish in the 21–120 dpf group was significantly decreased (F=44.59; p=0.032) for female and increased (F=6.62; p=0.96) for male as compared with the control group, following the tap stimulus (Fig. 1C, D). Compared with control, male fish in 1–21 dpf group have significant higher movement speed before tapping (F=61.70; p=0.0001), while no different after tapping (F=6.62; p=0.27) (Fig. 1D).
Fig. 1.
Adult behavior analysis of four months 0.5μM PFOS chronic treated zebrafish during different life span. Female and male fish were separated to two different sets. After subtracting the time for rest period, activity of fish were recorded for 12 minutes (only 6 mins were showed) after which their response to the tap was measured in terms of change in movement (A and B). Average movement speed showed in 12.5 mins before and 4 mins after tapping (C and D). Bars of the same pattern sharing the same letter indicate no significant difference at P = 0.05 compared with control group.
3.2. Chronic exposure to PFOS affects PFOS residues in F1 embryos
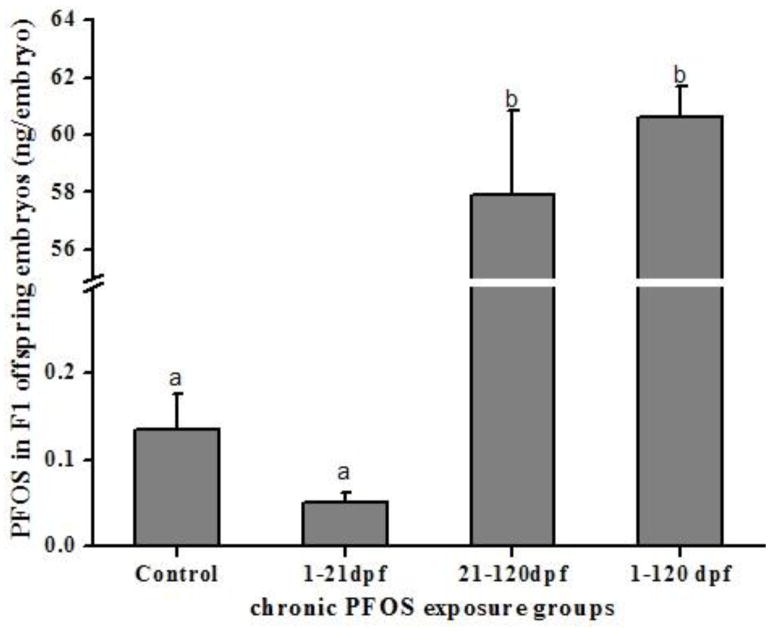
The F1 embryos in both the 21–120 dpf and 1–120 dpf groups had significantly higher PFOS residues transferred from parental exposed zebrafish as compared with those in the control and 1–21 dpf groups (F=2455; p<0.0001; Fig. 2). PFOS residues were not significantly different between the 21–120 dpf and 1–120 dpf groups (F=2455; p= 0.509; Fig. 2) and between the control and 1–21 dpf groups (p=0.088; Fig. 2).
Fig. 2.
PFOS residues in F1 offspring derived from PFOS exposed F0 parental zebrafish. N=4 with 40 eggs per replicate. Bars of the same pattern sharing the same letter indicate no significant difference at P = 0.001 compared with control group.
3.3. Chronic window of PFOS exposure affects F1 embryo development
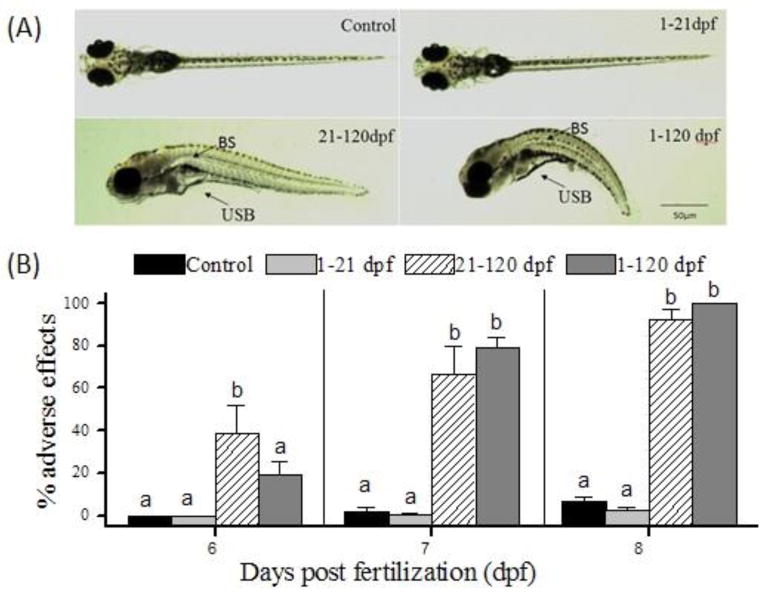
As compared with the control and 1–21 dpf groups, embryos derived from fish in the 21–120 dpf and 1–120 dpf groups showed obvious malformations, such as an uninflated swim bladder (USB) and bent spine (BS). Representative images of the four groups’ F1 embryos at 144 hpf are shown in Fig. 3A. At 6 dpf, the percentage of malformed embryos derived from the 21–120 dpf group were significantly higher than those of embryos derived from the control and 1–21 dpf groups (F=6.3; p=0.006). The percentage of malformation were significantly (F>1; p<0.001) elevated in F1 larvae at 7 and 8 dpf derived from 21–120 dpf and 1–120 dpf exposed F0 groups as compared with the control group (Fig. 3B).
Fig. 3.
PFOS induced adverse effects of F1 offspring embryos. (A) Representative photos of 144 hpf larvae showing physical effects of PFOS chronic exposure. (B) Adverse effects of F1 offspring embryos at 6, 7 and 8 dpf. BS, bent spine; USB, uninflated swim bladder. Bars of the same pattern sharing the same letter indicate no significant difference at P = 0.001 compared with control group.
3.4. PFOS affected the larval behavior of F1 embryos
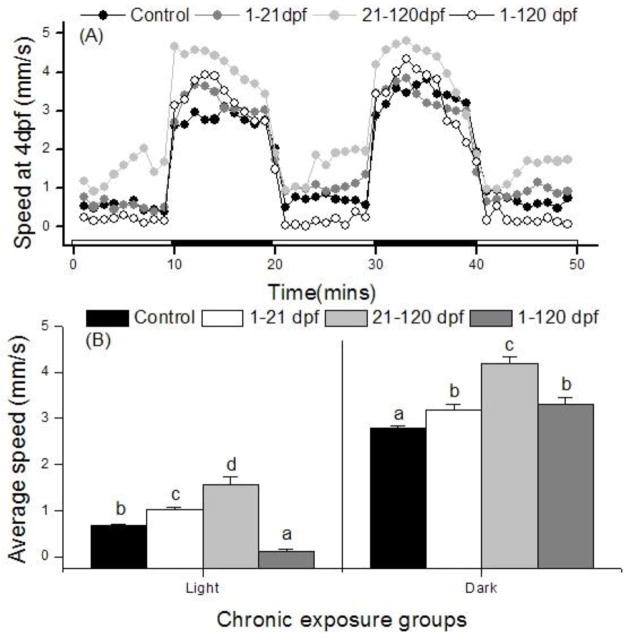
Larvae at 4 dpf with normal morphology derived from the four groups of adult fish were subjected to the light stimulation motor behavior test. A rapid transition from light to dark resulted in a similar, brief burst of swimming among fish in all groups (Fig. 4A). The parental 1–21 dpf and 21–120 dpf exposed groups elicited a higher basal swim rate (F>1; p<0.05) than the control group in both the light and dark periods (Fig. 4B). Embryos derived from the 1–120 dpf group showed lower speed in the light (F=52.6; p = 0.001) period and higher speed in the dark period (F=26.8; p = 0.028) as compared with the control group (Fig. 4B). Overall, the average speed displayed an inverted U-shape among the control, 1–21 dpf, 21–120 dpf, and 1–120 dpf groups (Fig. 4B).
Fig. 4.
The locomotor activity of F1 larvae at 4 dpf when subjected to a 50-min dark-light photoperiod stimulation. Dots represent the mean speed in 60s intervals of 10 larvae (A). Treatments referred to parental 0.5μM PFOS exposure period. Average speed in light or dark period was shown (B). Bars of the same pattern sharing the same letter indicate no significant difference at P = 0.05 compared with control group.
Discussion
In this study, we report for the first time that chronic exposure to low dose PFOS during different life stage results in behavioral anomalies in adult zebrafish and their offspring larvae. Additionally, as compared with the control and 1–21 dpf groups, the F1 embryos derived from the 21–120 and 1–120 dpf groups showed obvious malformations, such as an uninflated swim bladder (USB) and bent spine (BS), which is possibly related with the PFOS residues from the maternal transfer of PFOS to eggs. This finding is consistent with our previous study finding that decreased larval survival in F1 offspring is directly correlated with the PFOS body burden, and larval lethality and abnormality were due to maternal transfer of PFOS to the eggs (Wang et al. 2011)
Ours is the first study in where zebrafish were exposed to PFOS for varying duration and then raised in fish water until evaluation of behavior and F1 offspring development. The most vulnerable age period is expected to be early in life, 21 dpf was chosen as the early and late life span exposure cut-off point based on the zebrafish sex determination period beginning at 21 dpf (von Hofsten and Olsson 2005). Prenatal or neonatal exposure to PFOS at high doses in rats or mice leads to abnormal spontaneous behavior, increased motor activities, and reduced habituation (Butenhoff et al. 2009a; Johansson et al. 2009; Johansson et al. 2008). In contrast, adult animals exposed to PFOS have shown no or only slight neurobehavioral effects (Fuentes et al. 2007; Sato et al. 2009). By utilizing the stimulus to evoke startle response, we tested the effects of PFOS on somatosensory perception of the adult zebrafish. The ability to sense a tap is achieved through the hair cells on the lateral line of teleosts (Crispino 1983). Further studies will be required to determine the extent to which the nervous system, vision, sensory-motor perception, or more than one target organ contributes to the observed adult behavioral phenotypes.
The zebrafish larvae behavioral analysis often serves as a more sensitive tool for detecting chemical exposure effects (Kane et al. 2004). In this study, embryos derived from the 1–21 dpf PFOS treatment group developed morphologically indistinguishably form the control group. However, the F1 larvae behavior was significantly affected in all three PFOS treated groups. Zebrafish have a biorhythm and larvae become active after exposure to sudden darkness and then slow down (Hurd and Cahill 2002; Prober et al. 2006). The 4 dpf F1 offspring derived from PFOS exposed parents swam at a faster and then slower speed among the 1–21, 21–120, and 1–120 dpf groups, but elicited a higher basal activity in response to light-to-dark photoperiod stimulation. This trend is consistent with the PFOS residue burden, where lower dose PFOS increases the speed and a higher dose decreases the speed, as found in a previous study (Huang et al. 2010). The mechanisms at the physiological or biochemical levels underlying locomotion behaviors in response to light-to-dark stimulation are still not completely understood. Clearly there is a significant involvement of motor neurons and skeletal muscle in overall locomotor behavior (Drapeau et al. 1999; Levin et al. 2009). The histological examination of acute PFOS exposed larvae showed disordered and loosened arrays in the muscle fibers (Huang et al. 2010). Parental PFOS exposure led to disordered and loosened array in the slow muscle fiber of F1 larvae, and the primary motor neurons also exhibited slower development (Wang et al. 2011). The next step should be examining the molecular events underlying the observed changes in behavior.
Though there is little PFOS residue in 1–21 dpf group, the adverse behavioral effects on both adult and F1 larvae imply that the first 21 dpf low dose PFOS exposure can induce long-term adulthood and genetic F1 neurobehavior toxicity. This is consistent with recent studies that prenatal or neonatal exposure to PFOS correlated with behavioral anomalies in rats or mice and delayed neuromotor maturation (Butenhoff et al. 2009a; Johansson et al. 2009), and these behavioral modifications appear to persist into adulthood (Johansson et al. 2008). Since PFOS exposure has an effect on neurodevelopment, exposure in early childhood may cause more damage to the nervous system than exposure at any other stage of development. However, the reason for the F1 larvae behavior changes under parental PFOS exposure during 1–21 dpf still need further experimental exploration.
In conclusion, chronic exposure to low dose PFOS resulted in behavioral anomalies among adult zebrafish and their offspring larvae. The F1 embryos derived from 21–120 and 1–120 dpf groups showed obvious malformations, such as uninflated swim bladder (USB) and bent spine (BS). Though there is little PFOS residue in 1–21 dpf group, the adverse behavioral effects on both adult and F1 larvae imply that the first 21 dpf low dose PFOS exposure can induce long-term neurobehavior toxicity. Our findings demonstrate that chronic exposure to low dose PFOS in different life stage adversely impacts adult behavior, subsequent offspring malformation, and larval behavior.
Acknowledgments
This work was supported in part by funding from National Environmental Protection Public Welfare Science and Technology Research Program of China (No.200909089), the Research Program of Department of Education of Zhejiang Province (Y201010056), the International Collaboration Project from Wenzhou City Government (No.H20100062), the Natural Science Foundation of Zhejiang Province (Y2110659)., and the National Institute of Environmental Health Sciences Grant #P30 ES000210.
References
- Butenhoff JL, Ehresman DJ, Chang S-C, Parker GA, Stump DG. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: Developmental neurotoxicity. Reproductive Toxicology. 2009a;27(3–4):319–330. doi: 10.1016/j.reprotox.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Ehresman DJ, Chang SC, Parker GA, Stump DG. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: developmental neurotoxicity. Reprod Toxicol. 2009b;27(3–4):319–30. doi: 10.1016/j.reprotox.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang C, Zheng L, Simonich M, Bai C, Tanguay R, Dong Q. Trimethyltin chloride (TMT) neurobehavioral toxicity in embryonic zebrafish. Neurotoxicology and Teratology. 2011;33(6):721–726. doi: 10.1016/j.ntt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino L. Modification of responses from specific sensory systems in midbrain by cerebellar stimulation: experiments on a teleost fish. Journal of Neurophysiology. 1983;49(1):3–15. doi: 10.1152/jn.1983.49.1.3. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Ali DW, Buss RR, Saint-Amant L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. Journal of Neuroscience Methods. 1999;88(1):1–13. doi: 10.1016/s0165-0270(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicology and Teratology. 2010;32(1):99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Vicens P, Colomina MT, Domingo JL. Behavioral effects in adult mice exposed to perfluorooctane sulfonate (PFOS) Toxicology. 2007;242(1–3):123–9. doi: 10.1016/j.tox.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Grasty RC, Wolf DC, Grey BE, Lau CS, Rogers JM. Prenatal window of susceptibility to perfluorooctane sulfonate-induced neonatal mortality in the Sprague-Dawley rat. Birth Defects Res B Dev Reprod Toxicol. 2003;68(6):465–71. doi: 10.1002/bdrb.10046. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Knapen D, Meyer IJ, van der Ven K, Hoff P, De Coen W. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio) Aquatic Toxicology. 2008;88(3):155–163. doi: 10.1016/j.aquatox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Han J, Fang Z. Estrogenic effects, reproductive impairment and developmental toxicity in ovoviparous swordtail fish (Xiphophorus helleri) exposed to perfluorooctane sulfonate (PFOS) Aquatic Toxicology. 2010;99(2):281–290. doi: 10.1016/j.aquatox.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, Tanguay RL, Dong Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS) Aquatic Toxicology. 2010;98(2):139–147. doi: 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MW, Cahill GM. Entraining Signals Initiate Behavioral Circadian Rhythmicity in Larval Zebrafish. Journal of Biological Rhythms. 2002;17(4):307–314. doi: 10.1177/074873002129002618. [DOI] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, Viberg H. Neonatal Exposure to PFOS and PFOA in Mice Results in Changes in Proteins which are Important for Neuronal Growth and Synaptogenesis in the Developing Brain. Toxicological Sciences. 2009;108(2):412–418. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. NeuroToxicology. 2008;29(1):160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Kane AS, Salierno JD, Gipson GT, Molteno TCA, Hunter C. A video-based movement analysis system to quantify behavioral stress responses of fish. Water Research. 2004;38(18):3993–4001. doi: 10.1016/j.watres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Key BD, Howell RD, Criddle CS. Fluorinated Organics in the Biosphere. Environmental Science & Technology. 1997;31(9):2445–2454. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lein NPH, Fujii S, Tanaka S, Nozoe M, Tanaka H. Contamination of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in surface water of the Yodo River basin (Japan) Desalination. 2008;226(1–3):338–347. [Google Scholar]
- Levin ED, Aschner M, Heberlein U, Ruden D, Welsh-Bohmer KA, Bartlett S, Berger K, Chen L, Corl AB, Eddins D, et al. Genetic aspects of behavioral neurotoxicology. NeuroToxicology. 2009;30(5):741–753. doi: 10.1016/j.neuro.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y, Sato I, Tsuda S. Detection of Genotoxicity and Neurotoxicity of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic (PFOA) in Paramecia. Proceedings of the 138th Congress of Japanese Society of Veterinary Science; 2004. p. 198. [Google Scholar]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Developmental Dynamics. 2009;238(12):2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden-Adams MM, Keller JM, EuDaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of Humoral Immunity in Mice following Exposure to Perfluorooctane Sulfonate. Toxicological Sciences. 2008;104(1):144–154. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF. Hypocretin/Orexin Overexpression Induces An Insomnia-Like Phenotype in Zebrafish. The Journal of Neuroscience. 2006;26(51):13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner R. Growing Concern Over Perfluorinated Chemicals. Environmental Science & Technology. 2001;35(7):154A–160A. doi: 10.1021/es012317k. [DOI] [PubMed] [Google Scholar]
- Sato I, Kawamoto K, Nishikawa Y, Tsuda S, Yoshida M, Yaegashi K, Saito N, Liu W, Jin Y. Neurotoxicity of perfluorooctane sulfonate (PFOS) in rats and mice after single oral exposure. The Journal of Toxicological Sciences. 2009;34(5):569–574. doi: 10.2131/jts.34.569. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Mayack D, Roblee K, Yamashita N, Kannan K. Occurrence of Perfluoroalkyl Surfactants in Water, Fish, and Birds from New York State. Archives of Environmental Contamination and Toxicology. 2006;50(3):398–410. doi: 10.1007/s00244-005-1188-z. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116(6):716–22. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PKS. Health Risks in Infants Associated with Exposure to Perfluorinated Compounds in Human Breast Milk from Zhoushan, China. Environmental Science & Technology. 2006;40(9):2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2012;155(2):269–274. doi: 10.1016/j.cbpc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten J, Olsson PE. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reproductive biology and endocrinology : RB&E. 2005;3:63. doi: 10.1186/1477-7827-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen J, Lin K, Chen Y, Hu W, Tanguay RL, Huang C, Dong Q. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environmental Toxicology and Chemistry. 2011:n/a–n/a. doi: 10.1002/etc.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield O. A prescription for hospital safety: treating workplace violence. Healthc Facil Manag Ser. 1995:1–8. [PubMed] [Google Scholar]
- Wilhelm M, Angerer J, Fromme H, Hölzer J. Contribution to the evaluation of reference values for PFOA and PFOS in plasma of children and adults from Germany. International Journal of Hygiene and Environmental Health. 2009;212(1):56–60. doi: 10.1016/j.ijheh.2007.11.002. [DOI] [PubMed] [Google Scholar]