Abstract
Activation-induced cytidine deaminase (AID) initiates antibody class switch recombination (CSR) in activated B cells resulting in exchanging the IgH constant region and improved antibody effector function. During CSR, AID instigates DNA double strand break (DSB) formation in switch (S) regions located upstream of constant region genes. DSBs are necessary for CSR, but improper regulation of DSBs can lead to chromosomal translocations that can result in B cell lymphoma. The protein kinase ATM is an important proximal regulator of the DNA damage response (DDR), and translocations involving S regions are increased in its absence. ATM phosphorylates H2AX, which recruits other DNA damage response (DDR) proteins, including MDC1 and 53BP1, to sites of DNA damage. As these DDR proteins all function to promote repair and recombination of DSBs during CSR, we examined whether mouse splenic B cells deficient in these proteins would show alterations in S region DSBs when undergoing CSR. We find that in atm−/− cells Sµ DSBs are increased, whereas DSBs in downstream Sγ regions are decreased. We also find that mutations in the unrearranged Sγ3 segment are reduced in atm−/− cells. Our data suggest that ATM increases AID targeting and activity at downstream acceptor S regions during CSR and that in atm−/− cells Sµ DSBs accumulate as they lack a recombination partner.
INTRODUCTION
Activation of B cells by antigen and co-stimulatory signals from dendritic cells, follicular dendritic cells, and from T cells initiates two processes of antibody diversification. Somatic hypermutation (SHM) introduces mutations in the variable region genes, which, in conjunction with antigen selection, increases antibody affinity, while class switch recombination (CSR) enables B cells to diversify the constant (CH) region and thereby the effector function of the antibody, while maintaining the same antigen-binding specificity (1). CSR occurs by an intrachromosomal deletional recombination between switch (S) region sequences located upstream of the CH region genes. During CSR, DSBs are introduced into S regions and are necessary for CSR, but if not properly regulated and recombined, DSBs can lead to chromosomal translocations that cause cellular transformation, leading to B cell lymphoma.
Activation-induced cytidine deaminase (AID) is induced in B cells by a variety of B cell activators (2), and is essential for both SHM and CSR (3, 4). AID initiates CSR by deaminating cytosines, converting them to uracils, which are then excised by the uracil DNA glycosylase UNG, leaving abasic sites that are nicked by AP endonuclease (APE), forming single-strand breaks (SSBs) (1, 5, 6). Nearby SSBs (on opposite DNA strands) form DSBs required for the deletional recombination occurring during CSR. In addition, Msh2 and Msh6 help convert distal SSBs to DSBs during CSR (7).
DSBs are repaired by two prominent mechanisms, non-homologous end joining (NHEJ) and homologous recombination (HR) (8). NHEJ is the pathway of choice for repairing breaks that occur in G1 phase, and in switching B cells S region DSBs are introduced and repaired/recombined during G1 phase (7, 9). Repair of DSBs occurs by a complex process. The Mre11-Rad50-Nbs1 (MRN) complex is recruited within seconds to a DSB, where it functions to recruit the protein kinase ATM (Ataxia-telangiectasia mutated), which is the chief mobilizer of the cellular response to this form of DNA damage (10); (11). The MRN complex is involved in the repair of AID-generated DSBs as MRN deficiency in B cells confers a strong CSR defect (12), and Nbs1 is found at AID-dependent IgH DSBs (9) and at AID-dependent off-target DSBs (13). After phosphorylating itself at multiple sites (14), ATM phosphorylates numerous other proteins, including H2AX (15), which plays a central role in the recruitment of other DNA damage response (DDR) proteins to the sites of DNA damage (16, 17). One of these proteins is Mediator of Damage Checkpoint protein (MDC1), which binds phosphorylated H2AX (γH2AX) at DSBs (18–23) and mediates retention of the MRN complex to the sites of DNA damage via binding of Nbs1 to phosphorylated MDC1 (24–28). Once phosphorylated, MDC1 then serves as a platform for recruiting additional DDR proteins such as the ubiquitin ligase RNF8, which leads to the recruitment of 53BP1, BRCA1 and RAP80 to damage sites via ubiquitinated γH2AX (23, 29, 30). 53BP1 has been found to protect DNA ends from resection, resulting in the repair of DSBs by NHEJ rather than by HR (31). Oligomerization of 53BP1 has recently been shown to be required for a proper DDR (32).
Consistent with their roles in the DDR pathway, ATM, H2AX, MDC1, and 53BP1 have been shown to contribute to CSR and antibody responses. ATM has been shown to be required for efficient CSR (33, 34), mice lacking ATM display increased AID-dependent chromosomal translocation events leading to B cell leukemias and lymphomas (35). A recent study shows that ATM-deficiency impairs AID phosphorylation as well as its interaction with APE1 in B cells induced to undergo CSR (36). H2AX also contributes to CSR (37), and in its absence AID-dependent DSBs accumulate and progress to translocations involving the IgH locus (38). 53bp1−/− mice have greatly decreased CSR and an increase in internal Sµ recombinations (39–41). Mdc1−/− cells also have impaired CSR (42). AID expression and S region germline (GL) transcription are not affected in activated B cells from atm−/−, h2ax−/−, or 53bp1−/− mice (33, 34,36,37,39, 40).
As these 4 DDR proteins all function to promote repair and recombination of DSBs, we asked if splenic B cells deficient in these proteins would show alterations in frequencies of S region DSBs when undergoing CSR. In this study, we show that ATM plays a unique role in the regulation of S region DSBs compared to other DDR proteins. Our data suggest that ATM is important for the ability of AID to target downstream acceptor S regions during CSR.
MATERIALS AND METHODS
Mice
All mouse strains were extensively (≥8 generations) backcrossed to C75BL/6. AID-deficient mice were obtained from T. Honjo (Kyoto University, Kyoto, Japan) (3). ATM-deficient mice (43), Jackson Lab stock 002753, were provided by S. Jones (UMass Medical School, Worcester, MA). MDC1-deficient mice and 53BP1-deficient mice were previously published (42, 44), and H2AX-deficient mice (45) were obtained from A. Nussenzweig (National Institute of Health, Bethesda, MD). Atm−/−aid−/− mice were obtained by mating heterozygous mice. Mice were housed in the Institutional Animal Care and Use Committee-approved specific pathogen-free facility at the University of Massachusetts Medical School; these mice were used according to the guidelines from University of Massachusetts Medical School Animal Care and Use committee.
B cell purification and cultures
Mouse splenic B cells were isolated as previously described, by T cell depletion with antibody and complement (46). B cells were cultured at 2 × 105 cells/ml in 5% CO2 for 3 days for CSR assays and 2 days for LM-PCR assays. Lipopolysaccharide (LPS) (25 µg/ml) (Sigma-Aldrich) and human BAFF/BLyS (50 ng/ml) (Human Genome Sciences) were added to all cultures. IL-4 (20 ng/ml) was added to induce switching to IgG1; IFN-γ (20 pg/ml) and anti-δ-dextran (10 ng/ml) for IgG2a; TGFβ (2 ng/ml) for IgG2b; anti-δ-dextran (10 ng/ml) for IgG3, and to induce IgA switching, TGF-β (2 ng/ml), IL-5 (1.5 ng/ml), anti-δ-dextran (10 ng/ml) and retinoic acid (10 nM) were added. In some experiments we treated WT cells with the ATM inhibitor KU55933 (5 µM) (Calbiochem) and the control cells with equivalent amount of DMSO carrier. Fixing of cells and staining of cell surface Abs were performed as described previously (47). The results were analyzed by FlowJo software.
Antibodies
Chromatin immunoprecipitation (ChIP) was performed using the following antibodies from Millipore: Histone H3, clone A3S (05-928), Histone H4, Clone 62-141-13 (05-858), acetyl-Histone H4 (06-866), acetyl-Histone H3 (06-599), and trimethyl-Histone H3 (Lys4) (07-473); and from Santa Cruz Biotechnology, anti-ER (sc-8002X, lot K1809). For immunoblotting, primary antibodies were ER (sc-8002X) and GAPDH (sc-25778) and secondary antibodies were goat-anti-rabbit (sc-2004) or donkey anti-mouse-horseradish peroxidase (sc-2020) (all from Santa Cruz Biotechnology).
Retroviral constructs
pMX-PIE-AID-FLAG-ER-IRES-GFP-puro (48) was received from Drs V. Barretto and M. Nussenzweig (The Rockefeller University, NY). The control retrovirus pMX-PIE-ER-IRES-GFP, the production of retroviruses in Phoenix-E cells, and the retroviral infection of B cells were previously described (49).
Cell cycle sorting
Splenic B cells were cultured for 2 days as described above, then cell density was adjusted to 1 × 106 cells/ml and cells were incubated for 90 min at 37°C with 3.5 µg/ml Hoechst 33342 (Invitrogen Life Technologies) in RPMI containing 10% FCS. Cells were then resuspended in 2 ml of HBSS with 2% FCS and sorted by flow cytometry based on DNA content using a UV laser-equipped FACSAria II Cell sorter (BD Biosciences).
ChIP
Live cells were isolated by flotation on Lympholyte M (Cedar Lane, Ontario Canada) 2 days after initiation of culture or 1 day after retroviral transduction. After recovery and washing twice, 2×107 live cells were resuspended in SBSS, and were cross-linked with formaldehyde (1%) for 5 min at 37°C. Cross-linking was stopped by adjustment to 125 mM glycine and incubation for 5 min at room temp. Cross-linked cells were sonicated on ice for 200 sec. Sonication was performed in twenty 10 sec bursts; the sample was incubated for 15 sec in ice between each burst. 2×106 cell equivalents were used per IP, and 2×105 cells were used for the input samples. Lysates were incubated with the appropriate antibodies overnight at 4° C. Protein A or G Dynabeads (Invitrogen) were added to samples for 2 hours, which were then washed with low salt (150mM NaCl), high salt (500mM NaCl) and LiCl salt buffers, and finally with TE. The complexes were then eluted overnight at 65° C in the presence of 1 mg/ml RNAse A (Sigma). The following day, the samples were incubated twice with 10 mg/ml Proteinase K (Sigma). Following phenol:chloroform, and chloroform extractions, DNA was precipitated using 1 mg/ml glycogen (Roche) and 95% ethanol overnight at −20° C. ChIP results were assayed by real time PCR using Sybr Green (Applied Biosystems). Two or 3 PCRs were performed per ChIP. Primers for Sµ were: (forward) Sµ2 5’- CAG GTC GGC TGG ACT AAC TC-3’, (reverse) Sµ2 5’- CCC AGC TTT GCT TAC CTC AG-3’; Primers for Cµ were: (forward) DK46 5’-GTC AGT CCT TCC CAA ATG TCT TCC-3’, (reverse) DK47 5’-CTG GAA TGG GCA CAT GCA GAT CTT T-3’. Primers for Sγ3 were: (forward) 5’-ACA GGG TCC CAG GTT ATG CAG-3’, (reverse) 5’-GTC ACT CAC ACT GGC TTC CC-3’. Statistical significance was determined by a two-tailed Student’s T test or single factor ANOVA.
Genomic DNA preparation and linker ligation-mediated PCR (LM-PCR)
Viable cells were isolated by flotation on Lympholyte M, cells were imbedded in low melt agarose plugs, DNA isolated and LM-PCR performed as described (50), with slight modifications. Ligated DNA samples were heat inactivated at 70°C for 10 min, diluted 3× in dH20, and then heated to 70° C for an additional 20 min. The samples were then assayed for gapdh or mb1 DNA by PCR to adjust DNA input prior to LM-PCR. Three-times more template DNA was used in each lane for Sγ assays than for Sµ assays, as there are more DSBs at Sµ than at acceptor Sγ regions. Semi-quantitative assessment of DSB frequency was achieved by densitometry scanning of autoradiographs, combining all 3 dose titration lanes for each mouse.
PCR amplification, cloning, and sequencing of Sµ and Sγ3 GL sequences
Genomic DNA was isolated from purified splenic B cells after culturing for 4 days. S region GL sequences were amplified from genomic DNA by PCR using the Expand Long Template Taq and Pfu polymerase mix (Roche, Piscataway NJ) and the primers 5µ3 5’-AATGGATACCTCAGTGGTTTTTAATGGTGGGTTTA-3’ and 3µ2 5’ AGAGGCCTAGATCCTGGCTTCTCAAGTAG-3’ for Sµ; or using Pfu Ultra II HS polymerase (Stratagene) and the primers g3.2 Fwd 5’- GCG AAT TCT TGC AAC TCC TAA GAG GAA AGA TCC C-3’ and g3.2 Rev 5’- GCG GAT CCA GCC TGG TCC CTA CAC TCC TAA CAA C-3’ (51) for Sγ3. PCR products were cloned into the vector pCR4-TOPO (Invitrogen, Carlsbad, CA). For measurement of the mutation frequency and spectrum within recombined Sµ-Sγ3 segments, only clones whose Sµ or Sγ3 segments were completely sequenced were included.
RESULTS
DDR proteins differentially affect CSR
To directly compare the effects of DDR proteins on CSR in our cultures, we activated splenic B cells lacking atm, 53bp1, mdc1, or h2ax genes for 3 days with LPS, human BLyS and the appropriate cytokines (see Methods) to induce switching to IgG1, IgG2a, IgG2b, IgG3 and IgA. While all 4 mutants showed defects in CSR (Fig 1), 53bp1−/− cells had the greatest defect, switching ~20% as well as WT cells, while h2ax−/− and atm−/− cells retained about 40% of the WT switching levels. mdc1−/− cells appeared to be the least affected and maintained ~50% switching compared to WT cells. We monitored cell division by CFSE-dilution. There was no effect on proliferation except in atm−/− cells, where there was a small reduction, which, however, did not account for the reduction in CSR (not depicted), as reported previously (34). Our results are in agreement with previously reported switching defects in 53bp1−/− (40, 41), h2ax−/− (38), atm−/− (33), and mdc1−/− cells (42). Thus, although these 4 DDR proteins interact with each other at DSBs, they have different quantitative effects on CSR.
FIGURE 1. DDR proteins differentially affect CSR.
Purified splenic B cells from atm−/−, 53bp1−/−, mdc1−/− and h2ax−/− mice were induced with the appropriate cytokines (see Methods) to class switch to the indicated Ig isotypes and were analyzed by FACS after 3 days in culture. Data from 2 mice, 2 cultures each, were normalized to the percent of switching in splenic B cells from WT littermates in the same experiment, indicated as 100%. Mean percentages (+range) of switching for the different isotypes are shown.
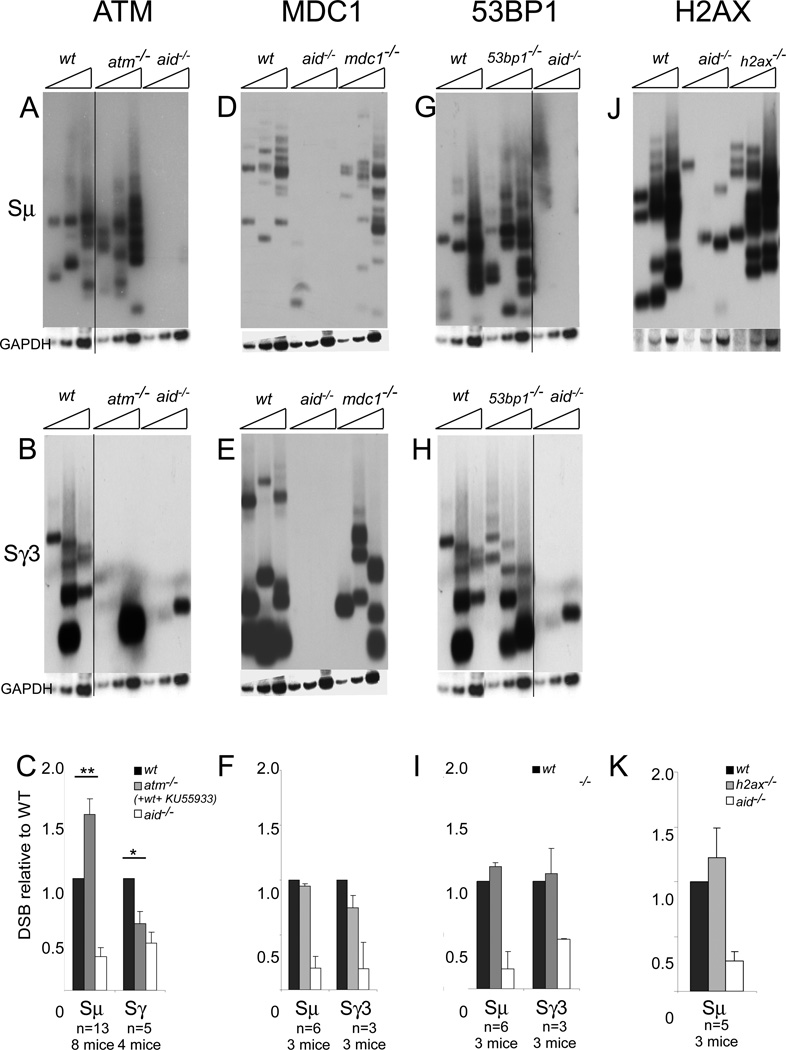
Atm−/− cells have more DSBs at the donor Sµ region and fewer DSBs at the acceptor Sγ regions compared to WT cells
We next investigated whether these proteins have differential effects on the occurrence of DSBs in IgH S regions. Splenic B cells were activated in culture for 2 days under IgG3 switching conditions, and S region DSBs were assayed by ligation-mediated PCR (LM-PCR) followed by Southern blotting. To quantitate the DSBs, the autoradiographs were scanned to measure total signal intensity for each genotype, and the results were normalized to the intensities of WT bands in each experiment (Fig 2C, F, I, K). Mdc1−/− (Fig 2D, E) and 53bp1−/− (Fig 2G, H and Fig 3) cells displayed no difference in the quantity of DSBs at Sµ and Sγ3 compared to WT cells. Also, there was no difference in breaks at Sµ in h2ax−/− cells compared to WT, but we were unable to obtain a conclusive result at Sγ3. In contrast, atm−/− cells have more DSBs at Sµ and fewer DSBs at Sγ3 compared to WT cells. The results observed using the IgG3 CSR conditions in atm−/− cells were confirmed when we used the IgG2a switching conditions (Fig 4A). Thus, none of the DDR genes affect the quantity of Sµ or Sγ DSBs detected by LM-PCR, except for ATM. As shown in Fig 2C, ATM-deficient cells have a ~60% increase in DSBs at Sµ and a 40% decrease in DSBs at Sγ3 and Sγ2a regions. This effect is not due to a long term effect of ATM-deficiency on B cells (perhaps by affecting B cell development), as we found that treatment of cells with an ATM inhibitor (KU55933) that acutely inhibits the ATM kinase function has similar effects (Fig 4C, 4D. These results are included in the quantitative data shown in Fig 2C). Furthermore, we confirmed that the DSB phenotype observed in atm−/− cells was aid-dependent by analyzing DSBs in aid−/−atm−/− cells. The quantity of DSBs in aid−/−atm−/− cells was similar to that observed in aid−/− cells, indicating that the DSB phenotype of atm−/− cells is indeed aid-dependent (not depicted). We conclude that ATM-deficiency leads to increased DSBs at the donor Sµ region and a decrease in DSBs at acceptor Sγ regions, whereas the absence of Mdc1, 53BP1 or H2AX does not alter the quantities of S region DSBs detected.
FIGURE 2. LM-PCR experiments reveal that Sµ DSBs are increased and Sγ3 DSBs are reduced in activated splenic B cells from atm−/− mice but not from mdc1−/−, 53BP1−/− and h2ax−/− mice.
Sµ and Sγ3 LM-PCR were performed on 3-fold dilutions of DNA isolated from cells stimulated for 2 days with LPS+anti-δ-dextran (IgG3). As there are more DSBs at Sµ than at acceptor S regions, we use 3 times more template DNA when assaying Sγ DSBs than when assaying Sµ DSBs. Blunt DSBs in Sµ (A,D,G,J) and Sγ3 (B,E,H) were assessed by use of gene specific primers in combination with a linker specific primer. PCR products were blotted and hybridized with an internal Sµ or Sγ3 probe. The gapdh gene was amplifed as an internal control. The vertical lines on some of the blots indicate where irrelevant lanes were removed. C,F,I,K: histograms depict means (+SEM) of densitometry measurements of autoradiographic films, normalized to WT, which was set at 1.0. For F,I,K, the results for Sµ DSBs are from cells induced to class switch to IgG1 or IgG3. For C, the results are from atm−/− cells induced to class switch to IgG1, IgG2a and IgG3, as well as from WT cells treated with the ATM inhibitor KU55933. All 3 titration lanes were scanned together. Significance was determined by 2-tailed paired Student’s t-test (**, p<0.005; *, p<0.05).
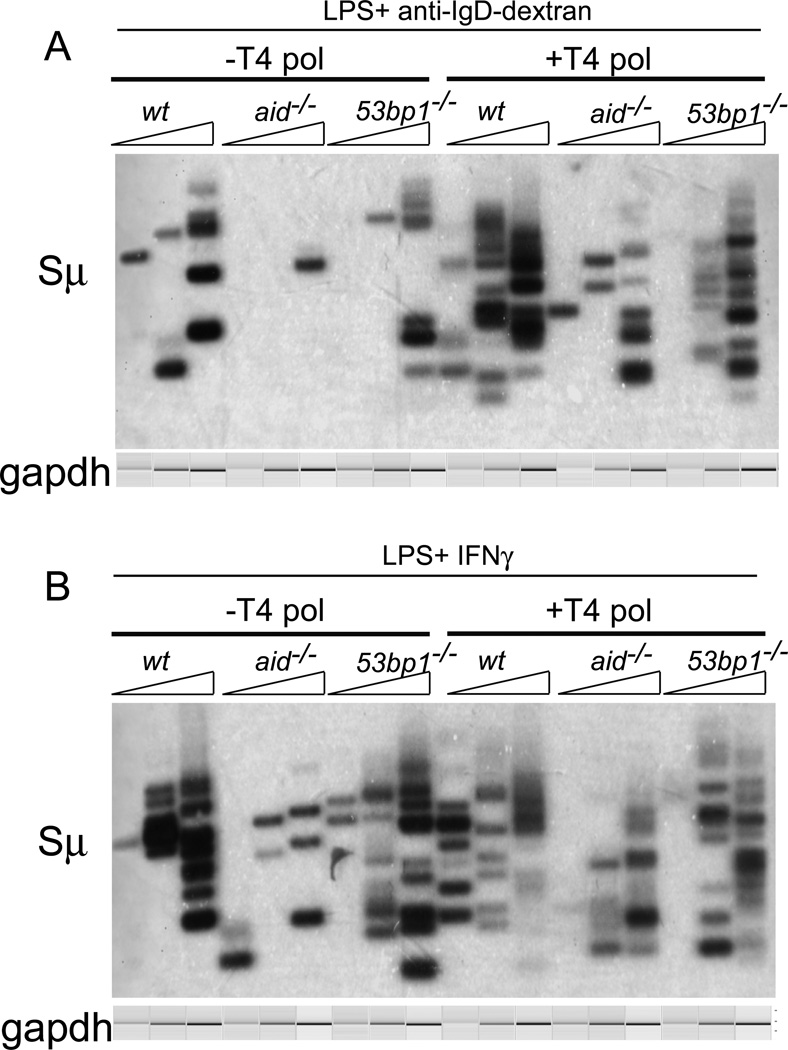
FIGURE 3. Both blunt and staggered Sµ DSBs are unaffected by the absence of 53BP1.
Sµ LM-PCRs were performed on 3-fold dilutions of DNA isolated from splenic B cells that had been stimulated to switch to IgG3 (A) or to IgG2a (B). As indicated, DNA was untreated or treated with T4 polymerase prior to linker ligation to identify staggered DSBs. Gels shown are from two independent experiments. The mb-1 PCR bands shown were obtained by electrophoretic analysis on a QIAxcel Advanced instrument, which subjects each sample to electrophoresis in a capillary, and provides an image and quantitation of each lane.
FIGURE 4. LM-PCRs show increased Sµ DSBs and fewer Sγ DSBs in atm−/− cells and in WT cells treated with an ATM-inhibitor.
Sµ (A,C) Sγ2a (B) and Sγ3 (D) LM-PCRs were performed on 3-fold dilutions of DNA isolated from splenic B cells that had been stimulated with LPS+IFNγ (IgG2a) (A,B) or LPS+anti-δ-dextran (IgG3) (C,D). (C,D) WT and aid−/− cells were treated with 5 µM ATM inhibitor KU55933. Blunt DSBs were assessed by use of gene specific primers in combination with a linker specific primer. PCR products were blotted and hybridized with an internal Sµ, Sγ2a or Sγ3 probe. Gels shown are representative of two or more independent experiments.
ATM-dependent DSBs at Sµ do not persist into the S/G2/M phases of the cell cycle
In WT cells Sµ DSBs are predominantly detected in the G1 phase of the cell cycle (7, 9). As ATM activates the G1-S phase checkpoint in response to DSBs (52), we asked if the increased Sµ DSBs in atm−/− cells might be due to their lack of repair/recombination in G1 phase, and their escape into S phase. Splenic B cells were activated for two days to induce IgG3 CSR; cells were then stained with Hoechst 33342, and sorted into G1 and S/G2/M populations based on DNA content. As shown in Fig 5A, C, in WT cells Sµ DSBs are found predominantly in G1 phase. This is also true in atm−/− cells, although it is less pronounced. This could be due to the fact that atm−/− cells are unable to repair breaks as efficiently as WT cells before proceeding to S phase.
FIGURE 5. LM-PCR experiments show that Sµ DSBs are mostly introduced and repaired/recombined during G1 phase in WT and atm−/− cells.
Splenic B cells were activated for 2 days, stained with Hoechst 33342, and sorted into G1 and S/G2/M populations. LM-PCR was performed on DNA from viable sorted cells activated to switch to IgG3. Three-fold dilutions of DNA were amplified using a 5’ Sµ primer and a linker specific primer. DNA was untreated (A) to identify blunt DSBs or treated (B) with T4 polymerase prior to linker ligation to identify staggered DSBs. Histograms depict means (+SEM for C, +Range for D) of densitometry measurements of autoradiographic films, normalized to WT (G1 subpopulation), which was set at 1.0. The mb-1 gene was amplified as an internal control for template dose. All 3 titration lanes were scanned together. Significance was determined by 2-tailed paired Student’s t-test (** p<0.01; * p<0.05).
As AID should induce mostly staggered DSBs and also because S region DSBs might be resected during S phase, we treated genomic DNA with T4 DNA polymerase prior to linker-ligation in order to detect staggered DSBs. In these experiments, we again observed an increase in the DSBs in both G1 and S/G2/M cells in atm−/− cells compared to WT cells (Fig 5B, D). However, the DSBs in both genotypes were clearly much enriched in G1 cells compared to S/G2/M phase cells. Taken altogether, most of the increased Sµ DSBs in atm−/− cells do not appear to be explained by the lack of a cell-cycle checkpoint. Instead, we hypothesize that Sµ DSBs accumulate in atm−/− cells due to a lack of acceptor Sγ DSBs with which Sµ can recombine.
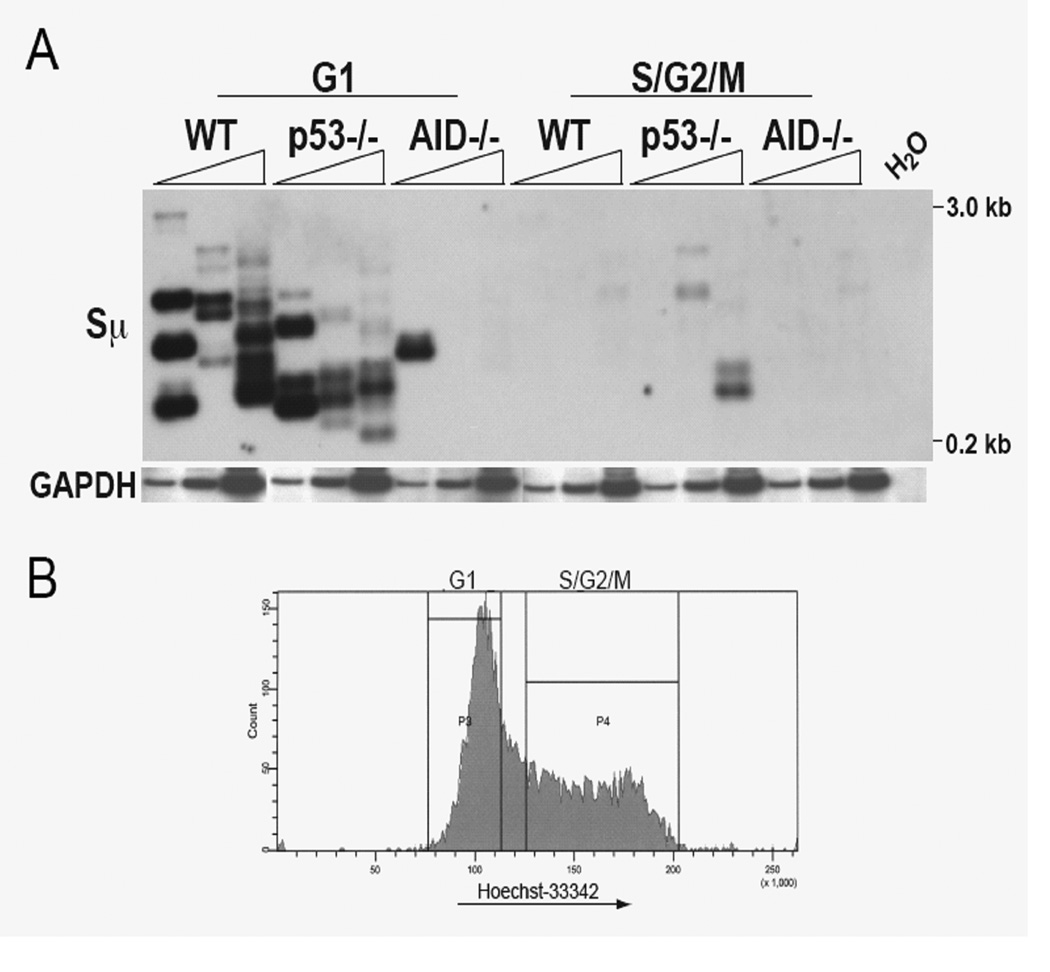
The apparent lack of a strong effect of ATM on the G1-S phase checkpoint in these cultures agrees with results obtained when we analyzed the cell cycle distribution of Sµ DSBs in p53-deficient B cells induced to switch, as we found that the great majority of DSBs are still restricted to G1 phase (Fig 6), suggesting that during induction of CSR in cultured splenic B cells, the G1-S phase checkpoint is not active. Thus, it appears that DSBs are introduced and rapidly repaired or recombined during G1 phase, independent of a checkpoint response. Consistent with this, Chk2-deficiency has no detectable effect on CSR and cell viability in these cultures (53, 54).
FIGURE 6. LM-PCR assays show that DSBs are restricted to the G1 phase in WT cells and in p53−/− cells.
Splenic B cells were activated for 2 days, stained with Hoechst 33342, and sorted into G1 and S/G2/M populations. (A) LM-PCR was performed on DNA from viable sorted cells. PCR amplification of the gapdh gene is shown below the blot as an internal control for template input. (B) Representative sorting profile.
ATM increases AID-dependent mutations at the downstream Sγ3 region
AID induces mutations in S regions, even those that have not undergone recombination in B cells induced to switch (9, 51, 55, 56). Interestingly, the mutation frequency is 3–4 fold greater at the unrearranged or germline (GL) Sµ region than at downstream GL Sγ3 or Sγ1 regions (51, 55), which has been interpreted to suggest that AID attacks Sµ before attacking the downstream/acceptor S region (55). Although the mutation frequency is higher at GL Sµ than at GL Sγ3, it is not higher at the Sµ side of Sµ-Sγ3 junctions, indicating that the low mutation frequency at GL Sγ3 is not simply due to the presence of fewer AID targets in acceptor S regions.
To determine if the ability of AID to deaminate the downstream S regions might depend upon ATM, we analyzed mutation frequencies within unrearranged GL Sµ and GL Sγ3 segments in B cells activated to switch for 4 days. We used Expand Long Template Taq and Pfu polymerase mix (Roche) to amplify the entire ~3 kb GL Sµ fragment and Pfu polymerase to amplify the 1.4 kb GL Sγ3 fragment from WT and atm−/− cells, and sequenced the 5’ 800 bp of Sµ and ~1.4 kb of Sγ3. There was no difference in mutation frequency at Sµ between WT and atm−/− cells (Fig 7A, D), similar to previously reported results (33, 34). Interestingly, atm−/− cells had a ~2-fold reduction in mutation frequency at Sγ3 compared to WT cells (Fig 7B, D). The Sγ3 mutation frequency in WT cells was significantly higher than the Pfu background (p<0.001), but in atm−/− cells the Sγ3 mutation frequency was not significantly different from the Pfu background at the 99% confidence level. In contrast, the mutation frequency at Sγ3 is the same in 53bp1−/− cells and their WT littermates, indicating that the reduced mutation frequency at Sγ3 is specific to ATM (Fig 7C, D). Both the mutation and LM-PCR data suggest that ATM enhances the activity of AID at downstream Sγ regions, perhaps by stimulating the enzymatic activity of AID specifically at downstream S regions, or alternatively by increasing accessibility of downstream S regions to AID.
FIGURE 7. ATM increases mutations in the GL Sγ3 region.
Mutation frequency at GL Sµ (A) and GL Sγ3 (B,C) was analyzed using Expand long-template PCR system and Pfu II HS Ultra, respectively. One pair of mice was used for A and C, and 3 pairs of mice were used for B. Total number of nucleotides and number of mutations analyzed are summarized in D. As different enzymes were used to amplify Sµ and Sγ3, and the sizes of the amplified fragments differ, their mutation frequencies cannot be compared in these experiments.
ATM does not significantly alter histone modifications at S regions
Certain histone modifications, e.g. acetylation and methylation, have been shown to increase the accessibility of chromatin to enzymes and other proteins and these activating modifications are found at S regions that are undergoing CSR in activated splenic B cells (57–60). To address the possibility that the ATM-dependence of AID activity at the acceptor S regions might be due to regulation of chromatin accessibility, we performed ChIP assays using antibodies against H3Ac, H4Ac, and H3K4me3. We hypothesized that these activating histone modifications might be increased at Sµ and reduced at Sγ3 in the absence of ATM. We also performed ChIP assays using H3 and H4 antibodies (Fig 8D, E) for the purpose of normalizing the histone modification data. As shown in Fig 8A–C, we did not observe a significant change in the activating modifications in the absence of ATM at either Sµ or Sγ3. Surprisingly, the amount of H4 at Sµ was markedly decreased in atm−/− cells, compared to WT and aid−/− cells (p<0.01), and there might also be a reduction in H3 binding in atm−/− cells, although the results were not significant (p=0.09). This apparent loss of nucleosomes might be caused by resection of the Sµ DSBs due to their delayed recombination. This possibility is consistent with the increased lengths of microhomology reported at S-S junctions in atm−/− cells (34, 61). As we found no evidence that ATM regulates the activating histone modifications at S regions, our data suggest that ATM does not regulate the accessibility of S region chromatin to AID.
FIGURE 8. ChIP analysis of histone modifications at the IgH locus.
Histone modifications H3Ac (A), H3K4me3 (B), H4Ac (C), and total histones H3 (D) and H4 (E) were determine by ChIP-qPCR at Sµ, Sγ3 and Cµ. Values for % input for histone modifications were normalized to H3 binding (A,B) or to H4 binding (C). The no antibody controls were subtracted from each ChIP result. N= number of individual IPs. The numbers of mice were 3 (A), 2 (B), 6–10 (C), 5 (D), and 6 (E). Means (+SEM) are shown and significance was established using a paired two-tailed Student’s t-test (* p<0.05; ** p<0.01). We also analyzed the H4 data by single factor ANOVA, and the atm−/− results were significantly different from WT and aid−/− (p=0.002).
The recruitment of AID to S regions is not affected by the absence of ATM
To ask whether ATM regulates AID recruitment to S regions, we performed ChIP for AID at Sµ and Sγ3. As our AID antibody does not provide a good ChIP signal, we transduced aid−/− and aid−/−atm−/− cells with retroviruses expressing ER-tagged AID or the ER tag alone and used anti-ER antibody to immunoprecipitate the chromatin-bound AID-ER. This is not an over-expression system, as we have found using qRT-PCR that the expression of mRNAs for endogenous AID and AID-ER are similar in these transduction experiments (Ucher et al., submitted). Also, the expression of AID-ER protein was similar in the aid−/− and aid−/−atm−/− cells (Fig 9B). As shown in Fig 9A, there was no difference in the recruitment of AID to Sµ in aid−/− cells compared to aid−/−atm−/− cells. Unfortunately, we could not detect AID binding at Sγ3 in these experiments (Fig 9A). These data are supported by the recent report that ATM does not affect the binding of endogenous AID to Sµ or Sγ1 in cells undergoing IgG1 CSR (36).
FIGURE 9. ChIP demonstrates that binding of AID to S regions is not altered in the absence of ATM.
(A) Anti-ER ChIP of chromatin from aid−/− and aid−/−atm−/− splenic B cells transduced with RV-AID-ER or RV-ER. ChIPs were analyzed by qPCR; % input was calculated, and % input in absence of antibody was subtracted. These values were normalized to WT values, which was set at 1.0. Means (+SEM) of 3 independent ChIPs (one IP per mouse) for Sµ, Sγ3 and Cµ are shown. (B) Western blots of whole cell extracts from transduced aid−/−- and aid−/−atm−/− mouse splenic B cells show equivalent levels of expression of AID-ER. GAPDH is the loading control.
DISCUSSION
In this study we have discovered a novel role for ATM in the regulation of S region DSBs during CSR. While ATM, 53BP1, MDC1 and γH2AX are recruited to sites of DSBs and are required for optimal CSR, only ATM affects the quantities of DSBs detected at S regions by LM-PCR. The results suggest that AID-induced Sµ DSBs increase the ability of AID to induce lesions specifically in the acceptor Sγ regions during CSR, and that this is dependent upon ATM. We hypothesize that Sµ DSBs accumulate in atm−/− cells due to an inability of AID to attack the acceptor S region, resulting in a lack of acceptor Sγ DSBs with which Sµ can recombine. In addition, it is likely that Sµ DSBs are poorly repaired in atm−/− cells and thus accumulate. However, we found that most of the Sµ DSBs in atm−/− cells are generated and repaired during G1 phase, as in WT cells. Thus, the accumulation of DSBs at Sµ in atm−/− cells does not appear to be explained by the lack of a cell-cycle checkpoint. Instead, it is likely that in WT and atm−/− splenic B cells undergoing CSR, DSBs accumulate only during G1 phase and either are repaired without recombining with an acceptor S region or are recombined with an acceptor S region prior to or at the beginning of S phase. Further suggesting that ATM does not regulate a G1-S phase checkpoint in these cultures, atm−/− B cells induced to undergo CSR in culture have been reported to proliferate similarly to WT B cells (33), or only slightly less well (34). The finding that most S region DSBs do not persist into S phase in atm−/− B cells undergoing CSR differs from results in developing B cells undergoing V(D)J recombination, as it has been demonstrated that RAG-dependent DSBs in atm−/− cells persist for several cell generations and even into B cell maturity (62).
In order to identify the mechanism to explain this phenotype of atm−/− cells, we asked whether ATM modifies the S regions’ accessibility to AID in cells undergoing CSR. However, we did not find an ATM-dependent difference in histone modifications that are associated with accessible chromatin (H3Ac, H3K4me3, H4Ac) at Sµ or Sγ3 regions. Furthermore, the binding of AID to S regions was not altered in atm−/− cells, consistent with a recent report (36). These results suggest that ATM does not increase the accessibility of the acceptor Sγ regions to AID. These results are consistent with previous reports that Kap-1, a protein involved in activation of NuA4, a complex that acetylates H4 in response to DSBs, has an ATM-independent role in augmenting CSR (17, 63).
One hypothesis to explain our data is that the ability of AID to induce DNA breaks at the downstream switch region is stimulated by ATM. Indeed, it has been recently reported that ATM deficiency leads to impaired protein kinase A (PKA)-dependent phosphorylation of AID at Ser38. This phosphorylation is necessary for optimal CSR (36). Splenic B cells expressing AID with the S38A mutation have very few Sµ and Sγ DSBs, similar to those detected in aid−/− cells, and greatly reduced CSR. The PKA-Cα catalytic subunit has Ser-Gln at position 35, a consensus motif for phosphorylation by ATM. Thus, it is possible that ATM could be activating AID via phosphorylation of PKA, which in turn would phosphorylate AID at Ser38. The S38 phosphorylation has been shown to cause APE1 to associate with AID, and this could result in recruitment of APE1 to AID-induced lesions, thereby increasing DNA breaks (36). In this same report, AID was shown to be phosphorylated at Sµ and Sγ1 in response to DSBs, and this was proposed as a feed-forward mechanism to increase AID activity locally at S regions to generate sufficient DSBs for CSR. Our data suggest this feed-forward mechanism might be important for generating AID-induced lesions and DSBs at acceptor S regions. Our data also suggest that DSBs in acceptor S regions might be a rate limiting step for CSR.
An additional hypothesis to explain our results is that ATM might increase synapsis/association between Sµ and acceptor S regions. Chromosome-conformation capture (3C) experiments suggest that association of Sµ and Sγ1 regions prior to CSR in cells treated to switch to IgG1 is dependent on IL-4, but also partially dependent upon AID (64). It is thought that the role of IL-4 is to induce GL transcripts, and ATM has been shown to have no effect on GL transcripts (33, 34). If AID contributes to bringing Sµ and an acceptor S region in proximity, the most straight-forward explanation for this would be that AID-induced DSBs promote the association. See model in Fig 10. AID-induced DSBs would activate ATM, and then ATM could promote interaction of Sµ and an acceptor S region. We propose that the breaks accumulate in atm−/− cells at the upstream S region because of the lack of a proximal downstream S region partner. Association of Sµ and an acceptor Sγ region might be necessary for AID to target the acceptor S region. This mechanism could help to prevent aberrant recombinations between S regions and other chromosomes, because AID would likely preferentially act at acceptor S region sites proximal to Sµ. In fact, in the absence of ATM there is a great increase in translocations between Sµ and c-myc and also of other types of aberrant events involving the IgH locus in splenic B cells undergoing CSR in culture (65, 66). Interestingly, in human Burkitt lymphomas the IgH-MYC translocations mostly involve Sµ, and not other S regions, suggesting that Sµ is the most frequently broken and aberrantly recombined S region (67). Thus, it is possible that targeting of the acceptor S regions depends upon ATM activation by Sµ DSBs that in turn cause association between Sµ and Sγ. This explanation for the role of ATM in allowing targeting of the acceptor S region is not mutually exclusive with the hypothesis that ATM-activated PKA increases the ability of AID to deaminate cytosines and to induce DSBs in acceptor S regions.
FIGURE 10. Proposed model for how ATM increases the ability of AID to target acceptor S regions.
(A) Upon induction of CSR, AID introduces DSBs at Sµ. (DSBs are indicated by the white boxes and arrows within the S regions.) (B) AID-induced Sµ DSBs activate ATM, which leads to ATM-dependent PKA-mediated S38 phosphorylation of AID, and S38 phosphorylation increases the ability of AID to introduce DSBs at Sµ (36). (C) The ATM response to AID-dependent DSBs results in closer association of Sµ and an acceptor Sγ region. (D) ATM activity also increases the ability of AID to induce mutations and DSBs in the acceptor S region.
In the absence of ATM, an increase in the lengths of microhomologies at Sµ-Sα and Sµ-Sγ junctions in human B cells and at Sµ-Sγ1 junctions in mouse B cells was observed (34, 61), although another group did not detect increased microhomology at mouse Sµ-Sγ1 junctions (33). ATM has been shown to inhibit end resection by Mre11 nuclease at DSBs in transfected plasmids in fibroblasts, therefore resulting in increased usage of microhomology-mediated end-joining (MMEJ) in atm−/− cells (68), similar to what has been observed at S-S junctions from atm−/− cells. However, B cells expressing a nuclease-deficient Mre11 do not show increased Sµ-Sγ1 junctional microhomology (12). Thus, the ability of ATM to inhibit Mre11-mediated end-resection does not explain the increased lengths of microhomologies at S-S junctions in atm−/− cells. We hypothesize that the delayed recombination caused by a lack of DSBs in the acceptor S region and/or poor synapsis of Sµ with the acceptor S region favors the generation of resected DSBs and thus the use of MMEJ during S-S recombination. Poorly regulated synapsis and the ensuing delay in recombination is consistent with the finding of increased frequency of translocations involving Sµ in atm−/− cells, and the fact that these translocations occur by MMEJ (69).
H2AX, Mdc1, and 53BP1 are among the numerous proteins phosphorylated by ATM in response to DSBs, and subsequently γH2AX and Mdc1 are important for recruiting 53BP1 to DSBs (17, 70). However, as discussed above, the effects of ATM and 53BP1-deficiencies on CSR are not identical. For example, increased microhomologies at S-S junctions are not found in 53bp1−/− cells (41). Also, unlike 53BP1-deficient cells, atm−/− cells do not show an increase in internal Sµ deletions in B cells undergoing CSR (33). Although 53BP1 has been shown to be important for interactions between distal sites on the chromosome during V(D)J recombination (71), our results indicate that 53BP1 is not required for the ability of AID to induce mutations and DSBs in the acceptor Sγ3 region.
The finding that ATM increases the phosphorylation of AID at S regions and its interaction with APE1 suggests that ATM should increase the ability of AID to generate DSBs at both Sµ and Sγ3 (36), unlike what we observed. Thus, although ATM appears to decrease Sµ DSBs, this could be due to their rapid recombination with DSBs in acceptor S regions in ATM-sufficient cells, perhaps due to the other possible activity of ATM, increasing the association of Sµ with an acceptor S region. As AID has greater deaminase activity at Sµ than at Sγ regions during CSR, as demonstrated by their relative mutation frequencies in ung−/−msh2−/− cells (51), it is possible that Sγ DSBs are limiting for CSR. Thus, the ability of ATM to increase AID activity at acceptor S regions might be very important for CSR efficiency and for prevention of translocations involving Sµ.
ACKNOWLEDGMENTS
We thank Stephen Jones and Joseph Gosselin (Department of Cell Biology, Univ of Massachusetts Medical School) for the atm+/− mice. We thank Andre Nussenzweig (NCI, NIH) for the h2ax+/− mice. We thank Vasco Barretto and Michel Nussenzweig (Rockefeller University) for the AID-ER retroviral expression plasmid. We thank Jayanta Chaudhuri (Memorial Sloan-Kettering Cancer Center) for helpful comments on the manuscript. We thank the Department of Animal Medicine, Univ of Massachusetts Medical School, for help with obtaining the mice and for their care. We thank the Flow Cytometry core facility, Univ of Massachusetts Medical School, for help with cell sorting.
LK was supported by a Fellowship from the Lauri Strauss Leukemia Foundation. JEJG was supported by a Fellowship from the Cancer Research Institute. CES was supported by RO3-AI092528. The research was supported by NIH RO1-AI023283 and R21-AI088578 (to JS).
Abbreviations used
- 53BP1
p53 binding protein
- AID
activation induced cytidine deaminase
- APE
apurinic-apyrimidinic endonuclease
- ATM
ataxia telangiectasia mutated
- C-NHEJ
classical non-homologous end-joining
- DSB
double strand break
- CSR
class switch recombination
- DDR
DNA damage response proteins
- GL
germline or unrearranged gene
- HR
homologous recombination
- Mdc1
mediator of DNA damage checkpoint 1
- MMEJ
microhomology-mediated end-joining
- SHM
somatic hypermutation
- SSB
single-strand break
- UNG
uracil-N-glycosylase
REFERENCES
- 1.Stavnezer J, Guikema JEJ, Schrader CE. Mechanism and regulation of class switch recombination. Ann Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran TH, Nakata M, Suzuki K, Begum NA, Shinkura R, Fagarasan S, Honjo T, Nagaoka H. B cell-specific and stimulation-responsive enhancers derepress Aicda by overcoming the effects of silencers. Nat Immunol. 2010;11:148–154. doi: 10.1038/ni.1829. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Rada C, Williams GT, Nilsen H, Barnes DE, Lindahl T, Neuberger MS. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 6.Guikema JE, Linehan EK, Tsuchimoto D, Nakabeppu Y, Strauss PR, Stavnezer J, Schrader CE. APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination. J Exp Med. 2007;204:3017–3026. doi: 10.1084/jem.20071289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrader CE, Guikema JE, Linehan EK, Selsing E, Stavnezer J. Activation-induced cytidine deaminase-dependent DNA breaks in class switch recombination occur during G1 phase of the cell cycle and depend upon mismatch repair. J Immunol. 2007;179:6064–6071. doi: 10.4049/jimmunol.179.9.6064. [DOI] [PubMed] [Google Scholar]
- 8.Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair. 2006;5:1021–1029. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Petersen S, Casellas R, Reina-San-Martin B, Chen HT, Difilippantonio MJ, Wilson PC, Hanitsch L, Celeste A, Muramatsu M, Pilch DR, Redon C, Ried T, Bonner WM, Honjo T, Nussenzweig MC, Nussenzweig A. AID is required to initiate Nbs1/gamma-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 11.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 12.Dinkelmann M, Spehalski E, Stoneham T, Buis J, Wu Y, Sekiguchi JM, Ferguson DO. Multiple functions of MRN in end-joining pathways during isotype class switching. Nat Struct Mol Biol. 2009;16:808–813. doi: 10.1038/nsmb.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staszewski O, Baker RE, Ucher AJ, Martier R, Stavnezer J, Guikema JE. Activation-induced cytidine deaminase induces reproducible DNA breaks at many non-Ig Loci in activated B cells. Mol Cell. 2011;41:232–242. doi: 10.1016/j.molcel.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 15.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 16.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 17.Price BD, D'Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421:961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 19.Lou Z, Minter-Dykhouse K, Wu X, Chen J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature. 2003;421:957–961. doi: 10.1038/nature01447. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D, Bartek J, Jackson SP. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003;421:952–956. doi: 10.1038/nature01445. [DOI] [PubMed] [Google Scholar]
- 21.Lou Z, Chen BP, Asaithamby A, Minter-Dykhouse K, Chen DJ, Chen J. MDC1 regulates DNA-PK autophosphorylation in response to DNA damage. J Biol Chem. 2004;279:46359–46362. doi: 10.1074/jbc.C400375200. [DOI] [PubMed] [Google Scholar]
- 22.Lou Z, Chini CC, Minter-Dykhouse K, Chen J. Mediator of DNA damage checkpoint protein 1 regulates BRCA1 localization and phosphorylation in DNA damage checkpoint control. J Biol Chem. 2003;278:13599–13602. doi: 10.1074/jbc.C300060200. [DOI] [PubMed] [Google Scholar]
- 23.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukas C, Melander F, Stucki M, Falck J, Bekker-Jensen S, Goldberg M, Lerenthal Y, Jackson SP, Bartek J, Lukas J. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004;23:2674–2683. doi: 10.1038/sj.emboj.7600269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Bosch M, Bree RT, Lowndes NF. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 2003;4:844–849. doi: 10.1038/sj.embor.embor925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spycher C, Miller ES, Townsend K, Pavic L, Morrice NA, Janscak P, Stewart GS, Stucki M. Constitutive phosphorylation of MDC1 physically links the MRE11-RAD50-NBS1 complex to damaged chromatin. J Cell Biol. 2008;181:227–240. doi: 10.1083/jcb.200709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Luo K, Lou Z, Chen J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc Natl Acad Sci U S A. 2008;105:11200–11205. doi: 10.1073/pnas.0802885105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers KC, Shen F, Sierra Potchanant EA, Phipps EA, Hickey RJ, Malkas LH. Phosphorylation: the molecular switch of double-strand break repair. Int J Proteomics. 2011;2011:373816. doi: 10.1155/2011/373816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bothmer A, Rommel PC, Gazumyan A, Polato F, Reczek CR, Muellenbeck MF, Schaetzlein S, Edelmann W, Chen PL, Brosh RM, Jr, Casellas R, Ludwig T, Baer R, Nussenzweig A, Nussenzweig MC, Robbiani DF. Mechanism of DNA resection during intrachromosomal recombination and immunoglobulin class switching. J Exp Med. 2013;210:115–123. doi: 10.1084/jem.20121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lottersberger F, Bothmer A, Robbiani DF, Nussenzweig MC, de Lange T. Role of 53BP1 oligomerization in regulating double-strand break repair. Proc Natl Acad Sci U S A. 2013;110:2146–2151. doi: 10.1073/pnas.1222617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J Exp Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumsden JM, McCarty T, Petiniot LK, Shen R, Barlow C, Wynn TA, Morse HC, 3rd, Gearhart PJ, Wynshaw-Boris A, Max EE, Hodes RJ. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J Exp Med. 2004;200:1111–1121. doi: 10.1084/jem.20041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbiani DF, Nussenzweig MC. Chromosome translocation, B cell lymphoma, and activation-induced cytidine deaminase. Annu Rev Pathol. 2012;8:79–103. doi: 10.1146/annurev-pathol-020712-164004. [DOI] [PubMed] [Google Scholar]
- 36.Vuong BQ, Herrick-Reynolds K, Vaidyanathan B, Pucella JN, Ucher AJ, Donghia NM, Gu X, Nicolas L, Nowak U, Rahman N, Strout MP, Mills KD, Stavnezer J, Chaudhuri J. A DNA break- and phosphorylation-dependent positive feedback loop promotes immunoglobulin class-switch recombination. Nat Immunol. 2013;14:1183–1189. doi: 10.1038/ni.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reina-San-Martin B, Difilippantonio S, Hanitsch L, Masilamani RF, Nussenzweig A, Nussenzweig MC. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J Exp Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco S, Gostissa M, Zha S, Lombard DB, Murphy MM, Zarrin AA, Yan C, Tepsuporn S, Morales JC, Adams MM, Lou Z, Bassing CH, Manis JP, Chen J, Carpenter PB, Alt FW. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Manis JP, Morales JC, Xia Z, Kutok JL, Alt FW, Carpenter PB. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 40.Ward IM, Reina-San-Martin B, Olaru A, Minn K, Tamada K, Lau JS, Cascalho M, Chen L, Nussenzweig A, Livak F, Nussenzweig MC, Chen J. 53BP1 is required for class switch recombination. J Cell Biol. 2004;165:459–464. doi: 10.1083/jcb.200403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reina-San-Martin B, Chen J, Nussenzweig A, Nussenzweig MC. Enhanced intra-switch region recombination during immunoglobulin class switch recombination in 53BP1−/− B cells. Eur J Immunol. 2007;37:235–239. doi: 10.1002/eji.200636789. [DOI] [PubMed] [Google Scholar]
- 42.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, Alt FW, Chen J. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 43.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 44.Minter-Dykhouse K, Ward I, Huen MS, Chen J, Lou Z. Distinct versus overlapping functions of MDC1 and 53BP1 in DNA damage response and tumorigenesis. J Cell Biol. 2008;181:727–735. doi: 10.1083/jcb.200801083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrader CE, Edelmann W, Kucherlapati R, Stavnezer J. Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J. Exp. Med. 1999;190:323–330. doi: 10.1084/jem.190.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guikema JE, Stavnezer J, Schrader CE. The role of Apex2 in class-switch recombination of immunoglobulin genes. Int Immunol. 2010;22:213. doi: 10.1093/intimm/dxq003. author reply-4. [DOI] [PubMed] [Google Scholar]
- 48.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell. 2003;12:501–508. doi: 10.1016/s1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 49.Ranjit S, Khair L, Linehan EK, Ucher AJ, Chakrabarti M, Schrader CE, Stavnezer J. AID binds cooperatively with UNG and Msh2-Msh6 to Ig switch regions dependent upon the AID C terminus. J Immunol. 2011;187:2464–2475. doi: 10.4049/jimmunol.1101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrader CE, Linehan EK, Mochegova SN, Woodland RT, Stavnezer J. Inducible DNA breaks in Ig S regions are dependent upon AID and UNG. J Exp Med. 2005;202:561–568. doi: 10.1084/jem.20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue K, Rada C, Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. J Exp Med. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 53.Guikema JE, Schrader CE, Brodsky MH, Linehan EK, Richards A, El Falaky N, Li DH, Sluss HK, Szomolanyi-Tsuda E, Stavnezer J. p53 Represses Class Switch Recombination to IgG2a through Its Antioxidant Function. J Immunol. 2010;184:6177–6187. doi: 10.4049/jimmunol.0904085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jankovic M, Robbiani DF, Dorsett Y, Eisenreich T, Xu Y, Tarakhovsky A, Nussenzweig A, Nussenzweig MC. Role of the translocation partner in protection against AID-dependent chromosomal translocations. Proc Natl Acad Sci U S A. 2010;107:187–192. doi: 10.1073/pnas.0908946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrader CE, Bradley SP, Vardo J, Mochegova SN, Flanagan E, Stavnezer J. Mutations occur in the Ig Sµ region but rarely in Sγ regions prior to class switch recombination. Embo J. 2003;22:5893–5903. doi: 10.1093/emboj/cdg550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T. Activation-induced Deaminase (AID)-directed Hypermutation in the Immunoglobulin Sµ Region: Implication of AID Involvement in a Common Step of Class Switch Recombination and Somatic Hypermutation. J Exp Med. 2002;195:529–534. doi: 10.1084/jem.20012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Whang N, Wuerffel R, Kenter AL. AID-dependent histone acetylation is detected in immunoglobulin S regions. J Exp Med. 2006;203:215–226. doi: 10.1084/jem.20051774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II,and histone modifications create chromatin accessibility during class switch recombination. J Exp Med. 2009;206:1817–1830. doi: 10.1084/jem.20081678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuang FL, Luo Z, Scharff MD. H3 trimethyl K9 and H3 acetyl K9 chromatin modifications are associated with class switch recombination. Proc Natl Acad Sci U S A. 2009;106:5288–5293. doi: 10.1073/pnas.0901368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniel JA, Santos MA, Wang Z, Zang C, Schwab KR, Jankovic M, Filsuf D, Chen HT, Gazumyan A, Yamane A, Cho YW, Sun HW, Ge K, Peng W, Nussenzweig MC, Casellas R, Dressler GR, Zhao K, Nussenzweig A. PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science. 2010;329:917–923. doi: 10.1126/science.1187942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan-Hammarstrom Q, Lahdesmaki A, Zhao Y, Du L, Zhao Z, Wen S, Ruiz-Perez VL, Dunn-Walters DK, Goodship JA, Hammarstrom L. Disparate roles of ATR and ATM in immunoglobulin class switch recombination and somatic hypermutation. J Exp Med. 2006;203:99–110. doi: 10.1084/jem.20050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callen E, Jankovic M, Difilippantonio S, Daniel JA, Chen HT, Celeste A, Pellegrini M, McBride K, Wangsa D, Bredemeyer AL, Sleckman BP, Ried T, Nussenzweig M, Nussenzweig A. ATM Prevents the Persistence and Propagation of Chromosome Breaks in Lymphocytes. Cell. 2007;130:63–75. doi: 10.1016/j.cell.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, Alt FW, Losson R, Reina-San-Martin B. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. 2011;208:1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wuerffel R, Wang L, Grigera F, Manis J, Selsing E, Perlot T, Alt FW, Cogne M, Pinaud E, Kenter AL. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, Eisenreich TR, Chen J, Dickins RA, Lowe SW, Nussenzweig A, Nussenzweig MC. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JH, Gostissa M, Yan CT, Goff P, Hickernell T, Hansen E, Difilippantonio S, Wesemann DR, Zarrin AA, Rajewsky K, Nussenzweig A, Alt FW. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature. 2009;460:231–236. doi: 10.1038/nature08159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guikema JE, Schuuring E, Kluin PM. Structure and consequences of IGH switch breakpoints in Burkitt lymphoma. J Natl Cancer Inst Monogr. 2008:32–36. doi: 10.1093/jncimonographs/lgn020. [DOI] [PubMed] [Google Scholar]
- 68.Rahal EA, Henricksen LA, Li Y, Williams RS, Tainer JA, Dixon K. ATM regulates Mre11-dependent DNA end-degradation and microhomology-mediated end joining. Cell Cycle. 2010;9:2866–2877. doi: 10.4161/cc.9.14.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, Rajewsky K, Alt FW. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 70.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Difilippantonio S, Gapud E, Wong N, Huang CY, Mahowald G, Chen HT, Kruhlak MJ, Callen E, Livak F, Nussenzweig MC, Sleckman BP, Nussenzweig A. 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature. 2008;456:529–533. doi: 10.1038/nature07476. [DOI] [PMC free article] [PubMed] [Google Scholar]