Abstract
Prions are the proteinaceous infectious agents responsible for the transmission of prion diseases. The main or sole component of prions is the misfolded prion protein (PrPSc), which is able to template the conversion of the host’s natively folded form of the protein (PrPC). The detailed mechanism of prion replication and the high-resolution structure of PrPSc are unknown. The currently available information on PrPSc structure comes mostly from low-resolution biophysical techniques, which have resulted in quite divergent models. Recent advances in the production of infectious prions, using very pure recombinant protein, offer new hope for PrPSc structural studies. This review highlights the importance of, challenges for and recent progress toward elucidating the elusive structure of PrPSc, arguably the major pending milestone to reach in understanding prions.
Transmissible spongiform encephalopathies (TSEs) are infectious disorders characterized by motor and cognitive impairments, extensive brain damage and neuronal dysfunction. After typically long incubation periods, individuals affected by TSEs deteriorate rapidly and progressively once the clinical symptoms arise, with lethal consequences in all cases. TSEs were first described in sheep exhibiting such abnormal behavior as erratic involuntary movements, ataxia and excessive scratching, and the disease was called scrapie1.
In humans, the most common TSE is Creutzfeldt-Jakob disease (CJD), which appears sporadically at a rate of one new case per million people per year2. Another human TSE is Kuru, first reported in 1954 among members of the Fore tribe in Papua New Guinea2, whose practice of cannibalism was thought to be a determinant for spreading the disease3. In fact, the infectious nature of TSEs quickly became evident, but early efforts to isolate the underlying agent were unsuccessful. The infectious agent was found to have unusual features, such as small size and resistance to procedures that inactivate nucleic acids4. More recently, an outbreak of TSE affecting cows (termed BSE) destined for human consumption raised worldwide concerns regarding potential transmission to humans5. This concern proved correct when a new variant form of CJD was identified and strongly linked to interspecies transmission from BSE6,7.
The nature of the infectious entity associated with TSEs has been a matter of debate for years8. In 1967, John Griffith proposed that the scrapie infectious material was a self-replicating protein9. Decades later, experiments in animal models of TSE showed that infectivity was associated with a glycosylphosphatidylinositol (GPI)-anchored membrane protein termed prion protein (PrP)10,11. It soon became clear that PrP exists in two forms: the normal protein present in healthy individuals, termed PrPC, for cellular PrP, and the protein found in infected animals, named PrPSc after scrapie-associated PrP. There are no chemical differences between PrPC and PrPSc, and their distinction is at the level of the structure and aggregation of the protein12,13. Today the widely accepted prion hypothesis states that the infectious agent associated with TSE is a self-propagating protein in an aberrant or ‘misfolded’ conformation14,15. Weissmann and co-workers achieved an important breakthrough for the prion hypothesis by showing that PrP knockout mice were completely resistant to scrapie16. Other supporting evidence came from experiments showing that transgenic mice expressing PrP mutations associated with fatal familial insomnia or modifications that rendered the loop at positions 166–175 more rigid develop spontaneous disease that is transmissible to wild-type animals17,18. Perhaps the most important evidence came from the generation of infectious material in the test tube by in vitro conversion and replication of PrPC of both mammalian and recombinant origin19–21. One argument often used against the prion hypothesis is the existence of prion strains8, a phenomenon difficult to reconcile with an exclusively proteinaceous infectious agent. However, recent findings have shown that strain properties can be propagated in vitro, suggesting that all elements enciphering prion strains are encoded on the PrPSc structure22.
Despite the clear involvement of PrPSc in TSE pathogenesis, the mechanisms by which the misfolded protein causes brain damage and disease are for the most part unknown. The reasons for the disease’s lethal outcome are the extensive synaptic damage, neuronal loss and widespread spongiform degeneration, but how PrPSc is implicated in these processes is unclear. The current thinking on TSE, as well as other neurodegenerative diseases associated with protein misfolding and aggregation (Box 1), is that small oligomers of the misfolded protein are mainly responsible for neurotoxicity23. The relationship between PrPSc polymer size and infectivity has been investigated using field-flow fractionation24 and sedimentation velocity25, which showed that per mass of PrP monomer, the most infectious particles are small oligomers with 12–24 monomers.
BOX 1. Protein misfolding in other diseases and expansion of the prion concept.
TSEs are not the only diseases associated with misfolded proteins; some of the most common neurodegenerative diseases (for example, Alzheimer’s and Parkinson’s diseases) and many systemic disorders (for example, type 2 diabetes and secondary systemic amyloidosis) are associated with the accumulation of misfolded protein aggregates in different organs23,102. There is experimental evidence for prion-like mechanisms of transmission in various protein misfolding disorders (for reviews, see refs. 98–101). Indeed, recent studies have shown that the pathological hallmarks of various diseases, including Alzheimer’s, Parkinson’s and Huntington’s diseases, and some forms of systemic amyloidosis, can be induced by administration of tissue homogenates carrying the respective misfolded proteins. Confirmation of these observations by human epidemiological data would indicate that the prion mechanism could be responsible for various protein misfolding disorders, a concept with broad-ranging implications for understanding disease mechanisms and for the development of strategies in disease prevention and intervention.
The widespread involvement of protein misfolding in different pathologies (Box 1) indicates a more ubiquitous phenomenon underlying protein folding regulation at the cellular level. It is likely that other diseases not yet associated with prions may have similar roots, particularly given that the ability to form the highly structured supramolecular protein arrangements called amyloids is a trait encoded within the backbone of most, if not all, proteins23. In addition, the prion phenomenon seems to have non-pathogenic roles in certain organisms (Box 2).
BOX 2. Prions as genes.
The prion paradigm of transmission of biological information by propagation of protein misfolding has been proposed as a new mechanism for non-Mendelian inheritance103,104. The discovery of self-propagating proteins associated with protein-based conformational inheritance in yeast and other fungal species opened up new avenues for studying prions. Although the potential role of different yeast prions in the regulation of cellular processes is still under debate, their discovery raises the question of whether prions are more than a rarity in nature105 and points to the possibility that cells may use the prion principle to propagate functional changes through auto-catalytic replication of protein-folding alterations. Indeed, a recent study has demonstrated that prions occur with a surprisingly high frequency in wild yeast and provide beneficial phenotypes under selective conditions106. However, the analogies between fungal and mammalian prions are not straightforward. Fungal prions do not produce disease; moreover, they have structural features associated with highly organized, β-sheet–rich protein aggregates termed amyloids, whereas PrPSc usually has a rather amorphous supramolecular organization. In both cases, the existence of different prion ‘strains’ arising from the same primary sequence has led to the hypothesis that a prion can adopt multiple conformations that can themselves self-propagate through protein-protein interactions107,108.
Elucidating PrPSc structure: importance and challenges
The structure of natively folded PrPC became available in 1996 (ref. 26), but the high-resolution three-dimensional structure of the abnormal form of PrP has remained elusive, along with mechanistic details of PrPSc self-propagation. These are arguably the major remaining challenges in the prion field. Elucidating the structure of PrPSc is essential to fully understand the mechanism of prion replication, just as the discovery of DNA structure enabled us to understand the process of genetic information transmission. Furthermore, the PrPSc structure should clarify the molecular basis of the species barrier and could allow predicting which species or strains of PrPSc can convert which PrPC sequences. Finally, the availability of the PrPSc structure will provide a great incentive for the development of drugs to treat prion diseases.
The available evidence indicates that PrPSc is a polymer composed of PrP monomers organized in an intermolecular β-sheet structure. Prion replication probably follows a seeding-nucleation model, in which PrPSc acts as a seed to template the conversion of PrPC, incorporating it into the growing polymer27–29. The spontaneous (unseeded) formation of PrPSc would be thermodynamically unfavorable, which may explain the low frequency of sporadic disease.
Solving the high-resolution structure of PrPSc faces many, so far insurmountable, obstacles:
PrPSc consists of a large collection of interconvertible polymers of different sizes in dynamic equilibrium in solution24,25. Any particular prion strain is known to exist as a diverse population of PrP species with different buoyancy-associated densities25, which may correspond to different degrees of polymerization of an as-yet-unknown basic PrPSc molecular unit.
PrPSc aggregates typically have a high molecular weight30. Although most infective PrP species seem to be rather small oligomers, these are still in the 400- to 600-kDa (ref. 24) range, which creates a substantial hurdle for classical biophysical analysis.
PrPSc aggregates are mainly hydrophobic. Like other amyloid-like aggregates, prions are water-insoluble particles31, and under the conditions required for structural studies, PrPSc forms non-crystalline aggregates that cannot be efficiently solubilized by any detergent tested31. Some degree of solubilization has been achieved with combinations of chemicals and heat treatments, but the resulting samples show a pronounced decrease in the infectivity titer, indicating that these procedures can change prion structure and its ability to self-propagate32,33. More recent reports have described the isolation of partially detergent-soluble infectious PrPSc oligomers that are markedly more protease sensitive than classical prions and may prove a suitable substrate for biophysical characterization34.
PrPSc particles probably contain a mixture of PrP molecules with different degrees of glycosylation (di-, mono- and non-glycosylated)35.
Despite various protocols to purify PrPSc, its sticky nature results in the capture of many contaminants inside the prion particle, including other proteins, lipids and nucleic acids36.
Current techniques to produce infectious prions in vitro have relatively low yield, which makes it difficult to generate sufficient material for biophysical studies.
Production of synthetic prions for structural studies
The difficulty in obtaining PrPSc for structural studies by using brain-derived material from diseased animals has led to attempts to produce synthetic PrPSc with the biochemical, biological and infectious properties of bona fide prions. Such efforts included either chemically or physically altering the conformation of recombinant PrP produced in bacteria (recPrP)37–41. Whereas in vitro generation of amyloid-like aggregates by using recPrP is relatively straightforward, those aggregates typically lack infectivity42.
The first landmark in synthetic prion generation was achieved by Prusiner and colleagues in 2004, who reported that in vitro–assembled recPrP amyloid fibrils could produce prion-like symptoms when injected into transgenic mice overexpressing a truncated form of PrP, after long incubation times43. However, the same fibrils lacked infectivity when injected into wild-type mice, raising justified concerns, given that transgenic animals overexpressing PrP have a well-known propensity to spontaneously develop prion-like diseases44–46. Follow-up studies showed that disease can be transmitted to wild-type mice after multiple passaging in transgenic mice47. Moreover, aggregates prepared under various different conditions resulted in distinct strain properties upon serial passages in wild-type mice47,48. Using a similar approach, Baskakov and colleagues reported that recPrP amyloid fibrils produced infectious prions after two successive passages in wild-type mice49. Although the animals did not show any symptoms upon direct injection of fibrils, a PrPSc-like protease-resistant signal was detected by immunoblot analysis in several brains, after long incubation times.
Although these results are encouraging, the very long incubation periods and the need for various in vivo passages before the agent showed the typical properties of prions point to differences between in vitro–produced recPrP aggregates and in vivo–generated PrPSc. This is further supported by studies using X-ray fiber diffraction, hydrogen exchange and atomic force microscopy, showing that recPrP fibrils and PrPSc appear to have substantially different cross– β-spine architectures50–52.
At least three scenarios can explain the differences between in vitro–generated recPrP aggregates and in vivo–generated PrPSc (ref. 50): (i) recPrP aggregates may correspond to an ‘immature’ conformation that undergoes specific structural rearrangements in vivo toward a more infectious form that is equivalent to PrPSc; (ii) in vitro aggregation of recPrP results in a highly heterogeneous mixture of structures, of which only a minority has the folding and properties of infectious PrPSc; and (iii) some of the recPrP aggregates may inhibit replication of bona fide PrPSc, resulting in the reduction of infectivity, increase of the incubation period and the inability to infect wild-type animals in the first passage. Regardless of which scenario is correct, at this point, it is clear that recPrP aggregates are not a suitable model to study the structure of infectious PrPSc.
A more successful approach to generate PrPSc in vitro has been to mimic prion replication in the test tube templated by brain-isolated PrPSc. Initial attempts led to the cell-free conversion assay, developed by Caughey and colleagues53, in which radioactively labeled PrPC was incubated with a molar excess of PrPSc, usually in the presence of a chaotrophic denaturing reagent. This resulted in small amounts of newly converted misfolded PrP that was resistant to proteolytic degradation, but its infectivity could not be tested, owing to the low efficiency of the system and the inability to distinguish newly formed PrPSc from the original PrPSc inoculum.
More recently, an efficient in vitro prion-replication system was developed, termed the protein misfolding cyclic amplification (PMCA) assay54. In this system, prions are replicated by mixing minute amounts of brain homogenates containing PrPSc with healthy brain homogenates harboring PrPC. The replication of PrPSc can be amplified exponentially, as PrPSc polymers are fragmented by sonication, multiplying the number of seeds for conversion54. The newly converted PrPSc has physicochemical properties identical to those of brain-derived PrPSc and, more importantly, is highly infectious in wild-type animals19. PMCA allows faithful replication of prion strain properties22, including complex characteristics such as species barrier, strain adaptation and strain memory55,56.
PMCA has become a powerful tool to culture prions in vitro, providing information on the nature of the infectious agent and the mechanism of prion replication, and serving as a highly sensitive prion-detection system. However, the use of brain homogenates limits its usefulness to provide structural information on the conversion process. Using highly purified PrPC from healthy brains as a substrate for PMCA, Supattapone and colleagues generated infectious prions with only the addition of synthetic polyanions20. This was the first time prions were generated from pure components, but the need for polyanionic molecules and the presence of co-purifying lipids raised questions about the involvement of non-PrP components during conversion. Although initial attempts to use recPrP as a substrate for PMCA were unsuccessful57, Wang and co-workers have reported the formation of prions from recPrP that were highly infectious to wild-type mice21. Notably, PrPSc formation required not only recPrP but also synthetic lipids and mouse-isolated total RNA. The same authors reported that endogenous RNA can be replaced by synthetically produced RNA polynucleotide58. In other studies, recPrP prions were generated by PMCA, using only a combination of buffers and detergents59, but these showed a low-infectivity titer, reflected in highly variable attack rates (proportion of animals showing clinical symptoms) and long incubation times.
Altogether, these findings clearly indicate that non-protein components participate in prion replication, at least in vitro. The questions then are what specific functions do these non-PrP molecules have and which molecules fulfill these functions in vivo15,60,61. Cofactor molecules can influence PrP misfolding through at least two different mechanisms (Fig. 1). In the first model, the cofactor may act as a catalytic molecule that binds both the normal and misfolded PrP forms and brings them together, lowering the activation energy for the conversion process (Fig. 1a). Upon binding, the cofactor may also induce conformational changes in PrPC and/or PrPSc that facilitate the interaction and conversion process. In the second model, the infectious PrPSc conformation would be stabilized by the cofactor (Fig. 1b). In biological terms, the main difference is whether the cofactor is a molecule provided by the host or a component of the infectious particle. In the latter case, the infectious agent would not be ‘protein-only’ but rather would consist of a complex between PrPSc and the cofactor. This difference is not only important for clarifying the nature of the infectious agent, it is also crucial for the elucidation of the PrPSc structure.
Figure 1.
Potential roles of non-PrP cofactor molecules during conversion of PrPC into PrPSc. (a) Template-based conversion of PrPC (blue triangles) into PrPSc (red triangles) requires surpassing a large energetic barrier that may preclude efficient misfolding during experimental timescales. In the presence of certain cofactor molecules (red line), the conversion will be greatly enhanced by reduction in the free energy of activation (ΔΔG‡), as in typical surface-catalyzed chemical reactions. (b) The formation of an infection-competent misfolded PrP conformation depends on permanent binding of a cofactor molecule (blue hexagon) to PrPSc, leading to the stabilization of this structure. The resulting complex is able to propagate and produce disease upon in vivo transmission, whereas in the absence of this molecule, PrPSc-only aggregates (blue trapezoids) are unable to propagate in vivo.
Although far from conclusive, the available evidence leans toward a scaffolding role for the cofactor (model 1). Negatively charged molecules (particularly nucleic acids, lipid particles and heparin sulfate proteoglycans) have long been proposed as PrP partners during conversion62–64, and aggregation of PrP in the presence of DNA or RNA is well known62,65,66. In addition, infectious prions form nuclease- and protease-resistant protein–nucleotide complexes in vitro. The scaffolding role to catalyze prion replication is also consistent with the observation that short-length nucleotides are highly inefficient in PMCA assays that are run with pure components67. PrPSc-templated conversion of pure PrPC by PMCA in the presence of light-cleavable nucleotides generated infectious PrP that showed no differences in titer and strain properties when the nucleotides were hydrolyzed after conversion68, suggesting that polyanions act during conversion and do not need to be part of the infectious agent. Finally, though many molecules can be found associated with PrPSc particles, no specific molecules are present in high quantity in the infectious material.
It is therefore likely that polyanionic molecules act as two-dimensional catalytic scaffolds that efficiently gather PrPC and PrPSc, increasing the likelihood of conversion63. Still, the lack of high-resolution structural data makes it impossible to rule out the stabilizing role of a cofactor as an integral part of the infectious agent. In addition, cofactors could be involved in prion conversion through alternative pathways, as described elsewhere15,60.
Probing the prion structure with low-resolution techniques
The unique properties of prion aggregates pose challenges for X-ray crystallization and NMR. Similar obstacles also exist for most amyloid systems, yet remarkable breakthroughs have been achieved with short peptides that form amyloid fibrils and are amenable to crystallization69. Those structures revealed unique peptide arrangements called steric zippers, that is, pairs of β-sheets that are stabilized by tight interdigitation69,70. However, extrapolating these observations to full-length proteins is not trivial. The structure of the prion-forming domain of fungal prion Het-S has been solved71, revealing cross-β in-register amyloid-like structures. The relevance of these findings to mammalian prions is unclear, as infectious PrPSc aggregates are typically not amyloid fibrils42.
As an alternative, several groups have used low-resolution biophysical and biochemical techniques to gather structural information on PrPSc. Although these approaches do not provide information about tertiary contacts and the overall arrangement of PrPSc, they can provide useful information for building structural models.
Initial efforts relied on classical spectroscopic techniques such as CD spectroscopy and FTIR spectroscopy, and revealed the predominantly β-sheet composition of PrPSc isolated from diseased brains, in contrast to the mainly α-helical nature of normally folded PrP13,72,73. Indeed, characteristic IR spectral bands between 1,615 and 1,636 cm−1 associated with β-sheet structures are typically observed in prion samples. These findings were corroborated by FTIR for PrPSc from many different strains and species74–76. These studies have also uncovered prion strain–associated differences in the secondary structure of PrPSc75,76. However, the presence of complex glycans attached to PrPSc, and its C-terminal GPI anchor, added considerable interference to the data. Recently, GPI-less transgenic mice able to replicate and produce infectious anchorless PrPSc (ref. 77) were developed, and the PrPSc obtained was also mostly non-glycosylated. FTIR analyses of this material showed no differences between wild-type and anchorless PrPSc of a particular strain52,78, indicating that glycans and GPI do not affect the overall prion structure.
Limited proteolysis has also provided structural information on PrPSc. As early as the time of the first identification of PrP in the infectious material, it was clear that PrPSc was substantially resistant to proteolysis11. Proteinase K treatment removes a fragment of about 12 kDa from the N terminus of PrPSc (ref. 79), resulting in a truncated form that retains infectious properties80 and is often referred to as PrP27-30 because of the apparent size of the monomer in western blots. These observations suggest that the N-terminal region of PrP (up to around amino acid 90) is not essential for self-propagation. Experiments using transgenic mice expressing different PrP truncations confirmed that the minimal region required for sustaining PrPSc in vivo propagation starts from residue ~90 all the way up to the C-terminal part of PrP81. Interestingly, distinct prion strains show different resistance to proteolytic degradation, and the cleavage site can also vary between distinct strains82. The latter has been used to argue that the folding and packing of PrP associated with distinct strains is different.
Antibody mapping studies have examined a panel of monoclonal antibodies with known epitopes in recognizing PrPSc untreated or treated with denaturing agents, to investigate the accessibility of those sequences within the polymer. These studies showed that the region spanning residues ~90 to ~120 is not accessible to antibodies unless PrPSc is completely denatured83, whereas segments located C-terminal to this region, such as the sequences 152–163 and 225–231, are accessible84.
The ultrastructural features of prions have been studied by transmission electron microscopy (TEM)30,85 and more recently by atomic force microscopy86. Brain-isolated PrPSc molecules usually appear as amorphous aggregates of heterogeneous sizes. Upon exhaustive purification procedures, including prolonged protease treatment, the aggregates acquire more defined structures called prion rods. Rods are typically in the range of 10- to 100-nm long and 5-nm wide and are usually shorter than classical amyloid fibrils47. TEM analyses do not show appreciable differences between distinct strains. However, sedimentation velocity experiments have shown that size-distribution patterns differ between distinct strains, and the size of the polymers tends to correlate with infectivity properties25. This agrees with the observation that strains containing higher proportions of oligomeric species self-propagate quickly and more efficiently in animal models24. Yet, prion strain isolates composed of larger aggregates were recently shown to propagate better in vivo87. The explanation was that larger aggregates may be more prone to fragmentation and may therefore spread prion seeds at higher rates. On the other hand, extensive sonication of PrPSc aggregates right before intracerebral inoculation did not alter any of the strain properties of a particular isolate (263K), including the incubation period88. A plausible compromise interpretation is that prion strain differences lie within unique secondary and/or tertiary structural elements that give rise to strain-specific quaternary arrangements upon in vivo spreading. Therefore, the size distribution of a particular strain will be faithfully recovered upon injection even with low amounts of highly disrupted material. It is also important to consider that extensive manipulation of prions, including simple brain extraction in mild detergents, may well yield changes in the size distribution of aggregates. Finally, the highly dynamic interconversion of aggregates makes it difficult to evaluate the biological properties of isolated aggregates.
Although PrPSc isolated from the brain of diseased animals does not form crystals amenable to X-ray crystallography, low-resolution diffraction patterns can be obtained by X-ray fiber diffraction89. This technique relies on the quasi-symmetrical scattering of fiber-like macromolecular aggregates upon X-ray bombardment. The data obtained are useful to study the packing of the core regions and the overall organization of the aggregates. This technique has been widely used to study the fiber-like organization of amyloids, and has revealed a motif called the cross–β-sheet, in which parallel β-sheets are stacked perpendicularly to the fiber axis. A similar motif was identified in PrPSc. The fiber diffraction data of fibrils formed with protease-treated PrPSc (PrP27-30) showed a sharp, albeit weak, meridional 4.72-Å cross–β-reflection, typical of amyloid aggregates, but the equatorial 10-Å reflection typical of amyloids was absent in PrPSc, replaced by a weak and broad 8-Å signal50. These data suggest that PrPSc has a structure with cross–β-packing similar to that in amyloid fibrils but with considerable differences.
Electron crystallography has also provided some clues about PrPSc structural organization. This technique proves useful when very small crystals (usually with 2D spatial arrangements) are available, as electrons can interact more strongly with the protein crystal lattice than X-rays in thin samples, producing better beam diffraction. Using such an approach, combined with computational threading, Govaerts et al. produced one of the first structural models for PrPSc, the β-helix structure90 (discussed in the next section).
Alternative methods to obtain residue-level structural constraints can also yield information on the PrPSc structure. EPR relies on the use of paramagnetic probes attached to certain amino acids in the protein that can report about site-specific structures as well as intra- and intermolecular distances91. EPR studies based on labeled recPrP subjected to in vitro misfolding showed evidence for a parallel, in-register β-sheet arrangement, similar to that of classical amyloids92, but the lack of infectivity of these samples raises questions about the extrapolation of these findings to the PrPSc structure. Hydrogen-deuterium exchange coupled to either MS (HX-MS) or NMR (HX-NMR) also provides residue-specific structural constraints by means of the degree of accessibility to water hydrogens within specific regions. Recently, the use of HX-MS was successful in showing that brain-isolated prions have a highly water-inaccessible core composed mainly of β-sheets and small loops spanning from residue ~90 to the C-terminal52. Moreover, several different strains had subtle differential exchanges in the region of residues 90 to 140, suggesting that specific conformational differences may be involved in prion strains52.
Structural models for PrPSc
The information obtained from low-resolution biophysical techniques has been used to develop structural models for PrPSc, with several proposed in the last decade. Here we describe and discuss some of these models.
The β-helix
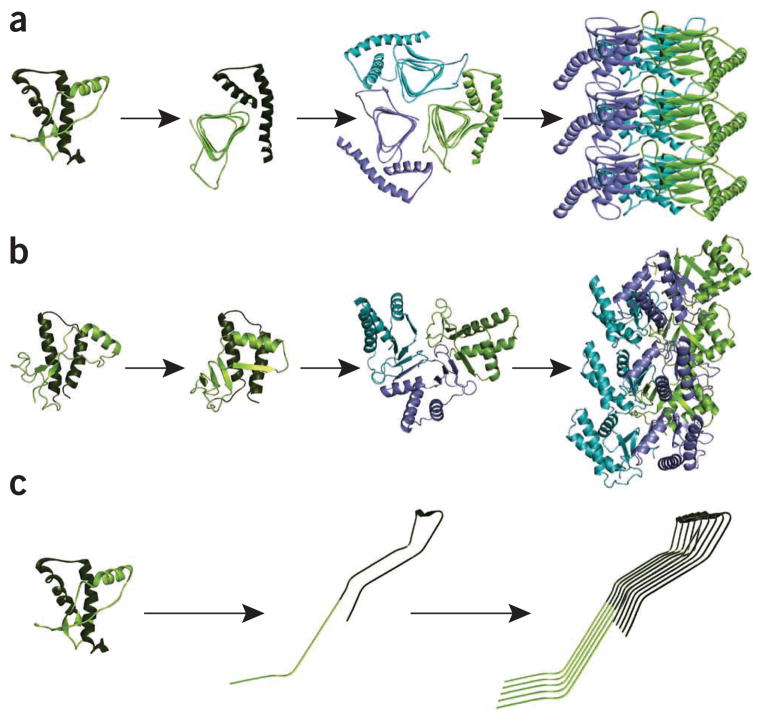
This is one of the most popular models that was proposed based on EM data on 2D crystals90. The authors found that a left-handed β-helix would best fit the experimental data. A model was then constructed by threading the PrP sequence through a known β-helix motif (Fig. 2a). In this model, a trimeric arrangement constitutes the basic symmetrical unit for PrPSc, with the N-terminal residues of PrP27-30 (~90–175) forming left-handed β-helices that are horizontally stacked and include a long unstructured loop encompassing residues 145–163 (Fig. 2a). Larger aggregates are formed by vertically stacking PrP trimers along the β-helical axis. In addition to a major refolding within the N-terminal region of PrP27-30, the model is characterized by only minor structural rearrangements in the C-terminal part of the protein, which retains most of its native secondary structure, except for the first small α-helix that switches to a loop (Fig. 2a). Interestingly, the β-helical motif has been observed in other proteins that exhibit biochemical features reminiscent of PrPSc, such as partial resistance to protease degradation and aggregation propensity93,94. Remarkably, the fungal prion HET-s was shown to form a β-solenoid arrangement of β-sheets that is structurally similar to β-helices71.
Figure 2.
Alternative models proposed for the structure of PrPSc. (a) In the β-helical model, a major refolding of the N-terminal region of PrP27-30 into a β-helix motif from residues 90 to 177 (light green) is proposed. The C-terminal region (residues 178–230, dark green) maintains the α-helical secondary structure organization, as in PrPC. (b) The β-spiral model developed by molecular dynamics simulation consists of a spiraling core of extended sheets comprising short β-strands, spanning residues 116–119, 129–132, 135–140 and 160–164. In this model, the three α-helices in PrPC maintain this conformational motif. (c) The parallel in-register extended β-sheet model of PrPSc proposes a thorough refolding of PrPC into a structure composed mainly of β-sheets. To facilitate comparison, the same color assignment for structural motifs has been used in all panels. The figure for the spiral model was kindly provided by W. Chen and V. Daggett.
The β-spiral
This model was proposed by Daggett and colleagues based on molecular dynamics simulations of PrP conformational fluctuations under amyloidogenic conditions (low pH), using the natively folded structure as the starting point95 (Fig. 2b). The model consists of a spiraling core of extended sheets, comprising three short β-strands (spanning residues 116–119, 129–132 and 160–164) and an isolated strand (residues 135–140) (Fig. 2b). The advantage of this model is that the structural scaffold was not chosen arbitrarily; instead, the model is the result of a putative conversion pathway from the monomer to the misfolded oligomer. As with the β-helical model, formation of β-strands involves the natively unfolded N-terminal region of PrP27-30, whereas most of the C-terminal remains intact, preserving the three α-helices characteristic of PrPC. Fibrils are symmetrically arranged in a way that resembles spiral-like amyloid organization (Fig. 2b). This model satisfies many of the observations obtained by low-resolution techniques, except perhaps the proteolysis and the HX-MS data.
The extended in-register β-sheet
In this radically different model, proposed by Surewicz and colleagues, PrPSc is represented as a stack of parallel β-sheets that form an in-register arrangement, allowing for indefinite growth of the fibrils (Fig. 2c). This model is based on structural constraints obtained by HX-MS studies from recPrP fibrils92 and with brain-derived PrPSc (ref. 52). In the latter study, the authors used PrP27-30 isolated from prion-infected transgenic mice expressing mostly non-glycosylated PrP lacking the GPI anchor77 to avoid interference from these post-translational modifications in the HX-MS studies. In this model, PrPSc consists of β-strands and relatively short turns and/or loops, with no α-helices present (Fig. 2c). Therefore, PrP conversion would involve refolding of the entire protein, and PrPSc would not preserve any of the structural motifs of PrPC. The overall structure of the aggregates would resemble that of typical amyloid assemblies.
It is difficult to determine which of these three models is a closer representation of the PrPSc structure, as they are all based on data from low-resolution biophysical experiments. Nevertheless, the fact that these models are so substantially different reflects how little we know about the structural details of PrPSc.
A point of contention is the structural fate of the C-terminal domain, which is globular in PrPC, with well-defined and stable α-helices. In both the β-helical and the β-spiral models, the C-terminal domain retains most of its structure upon misfolding, whereas in the extended in-register β-sheet model, the entire protein refolds into a mainly β-sheet conformation. The latter model fits the proteolysis data better, as it is difficult to understand the high resistance to proteolytic degradation of the C-terminal part of PrPSc if its structure is not substantially different from PrPC, in which this region is easily cleaved by proteases. Indeed, in both the β-helix and spiral models, the α-helical domains face the outside of the polymer, hence they should be at least partially accessible to proteases.
On the other hand, the extended in-register β-sheet model is in conflict with CD and FTIR studies indicating a substantial amount of α-helical structure in PrPSc. Indeed, different groups have consistently reported that PrPSc is 15–35% α-helical13,19,72–76. The majority of these experiments were conducted with FTIR, in which peaks at ~1,556–1,661 cm−1 were attributed to α-helices. However, this assignment is not always straightforward because other structures, including turns, loops and unordered segments, can also give rise to amide I bands in this frequency range96. Indeed, bands around 1,656–1,658 cm−1 have been observed in FTIR spectra of proteins that had no α-helices, according to X-ray crystallography or NMR spectroscopy data96,97. Moreover, a report using FTIR to analyze both wild-type and GPI anchorless PrPSc has cast doubt on the presence of α-helices78. Finally, the extended in-register β-sheet model has many similarities to the high-resolution structures of short peptides aggregated into amyloid fibrils and of yeast prions, suggesting that this is a plausible model for misfolded aggregates that have the ability to self-propagate. Nevertheless, these similarities represent a double-edged sword because, as discussed above, the infectious folding of PrPSc seems to be different from that of classical amyloid fibrils.
Conclusions and perspectives
The ability of proteins to self-propagate specific conformations and associated biological functions continues to fascinate researchers. The once heretical hypothesis that a protein can act as an infectious agent to propagate disease is now widely accepted, and the prion principle is being extended to other degenerative diseases associated with the accumulation of misfolded proteins98–101. However, several key questions about prion biology, including the mechanisms of toxicity, the molecular basis of in vivo prion propagation and the detailed PrPSc structure, remain unanswered. Moreover, it is possible that new findings about the role of as-yet-unidentified cofactor molecules may undermine this already controversial hypothesis by demonstrating the participation of an essential non-protein component in PrP self-replication and infectivity.
From the key unanswered questions in the prion field, determination of the high-resolution structure of PrPSc will undoubtedly be a major step in understanding the mechanism by which proteins can propagate biological information. The structure of PrPSc should also reveal the mysterious relationship between prion strains and PrPSc conformation and enable the rational design of much-needed treatments for these devastating diseases.
Acknowledgments
We are very grateful to V. Daggett and W. Chen from the University of Washington in Seattle for kindly providing a model for the β-spiral structure of PrPSc. We also want to thank W. Surewicz from Case Western Reserve University for providing high-resolution pictures for the extended in-register β-sheet model and H. Wille from University of California, San Francisco, for providing structural coordinates for the β-helix model. This work was funded in part by US National Institutes of Health grant R01NS041973 to C.S.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Delez AL, Gustafson DP, Luttrell CN. Some clinical and histological observations on scrapie in sheep. J Am Vet Med Assoc. 1957;131:439–446. [PubMed] [Google Scholar]
- 2.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Alper T. Scrapie agent unlike viruses in size and susceptibility to inactivation by ionizing or ultraviolet radiation. Nature. 1985;317:750. doi: 10.1038/317750a0. [DOI] [PubMed] [Google Scholar]
- 4.Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- 5.Hope J, Ritchie L, Farquhar C, Somerville R, Hunter N. Bovine spongiform encephalopathy: a scrapie-like disease of British cattle. Prog Clin Biol Res. 1989;317:659–667. [PubMed] [Google Scholar]
- 6.Will RG, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 7.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet. 1999;354:317–323. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 8.Soto C, Castilla J. The controversial protein-only hypothesis of prion propagation. Nat Med. 2004;10:S63–S67. doi: 10.1038/nm1069. [DOI] [PubMed] [Google Scholar]
- 9.Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 10.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 11.McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 12.Stahl N, et al. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993;32:1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- 13.Pan KM, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto C. Prion hypothesis: the end of the controversy? Trends Biochem Sci. 2011;36:151–158. doi: 10.1016/j.tibs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Büeler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 17.Jackson WS, et al. Spontaneous generation of prion infectivity in fatal familial insomnia knockin mice. Neuron. 2009;63:438–450. doi: 10.1016/j.neuron.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurdson CJ, et al. De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci USA. 2009;106:304–309. doi: 10.1073/pnas.0810680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Deleault NR, Harris BT, Rees JR, Supattapone S. From the cover: formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castilla J, et al. Cell-free propagation of prion strains. EMBO J. 2008;27:2557–2566. doi: 10.1038/emboj.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 24.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tixador P, et al. The physical relationship between infectivity and prion protein aggregates is strain-dependent. PLoS Pathog. 2010;6:e1000859. doi: 10.1371/journal.ppat.1000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riek R, et al. NMR structure of the mouse prion protein domain PrP(121–321) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 27.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 28.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinley MP, Braunfeld MB, Bellinger CG, Prusiner SB. Molecular characteristics of prion rods purified from scrapie-infected hamster brains. J Infect Dis. 1986;154:110–120. doi: 10.1093/infdis/154.1.110. [DOI] [PubMed] [Google Scholar]
- 31.Gabizon R, McKinley MP, Prusiner SB. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci USA. 1987;84:4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riesner D, et al. Disruption of prion rods generates 10-nm spherical particles having high alpha-helical content and lacking scrapie infectivity. J Virol. 1996;70:1714–1722. doi: 10.1128/jvi.70.3.1714-1722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wille H, Zhang GF, Baldwin MA, Cohen FE, Prusiner SB. Separation of scrapie prion infectivity from PrP amyloid polymers. J Mol Biol. 1996;259:608–621. doi: 10.1006/jmbi.1996.0343. [DOI] [PubMed] [Google Scholar]
- 34.Pastrana MA, et al. Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochemistry. 2006;45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]
- 35.Cobb NJ, Surewicz WK. Prion diseases and their biochemical mechanisms. Biochemistry. 2009;48:2574–2585. doi: 10.1021/bi900108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diringer H, et al. Highly infectious purified preparations of disease-specific amyloid of transmissible spongiform encephalopathies are not devoid of nucleic acids of viral size. Intervirology. 1997;40:238–246. doi: 10.1159/000150553. [DOI] [PubMed] [Google Scholar]
- 37.Leffers KW, et al. Assembly of natural and recombinant prion protein into fibrils. Biol Chem. 2005;386:569–580. doi: 10.1515/BC.2005.067. [DOI] [PubMed] [Google Scholar]
- 38.Cobb NJ, Apetri AC, Surewicz WK. Prion protein amyloid formation under native-like conditions involves refolding of the C-terminal alpha-helical domain. J Biol Chem. 2008;283:34704–34711. doi: 10.1074/jbc.M806701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskakov IV, Legname G, Baldwin MA, Prusiner SB, Cohen FE. Pathway complexity of prion protein assembly into amyloid. J Biol Chem. 2002;277:21140–21148. doi: 10.1074/jbc.M111402200. [DOI] [PubMed] [Google Scholar]
- 40.Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrPSc. J Mol Biol. 2005;346:645–659. doi: 10.1016/j.jmb.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 41.Jackson GS, et al. Reversible conversion of monomeric human prion protein between native and fibrilogenic conformations. Science. 1999;283:1935–1937. doi: 10.1126/science.283.5409.1935. [DOI] [PubMed] [Google Scholar]
- 42.May BC, Govaerts C, Prusiner SB, Cohen FE. Prions: so many fibers, so little infectivity. Trends Biochem Sci. 2004;29:162–165. doi: 10.1016/j.tibs.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Legname G, et al. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 44.Westaway D, et al. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell. 1994;76:117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 45.Chiesa R, Piccardo P, Ghetti B, Harris DA. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- 46.Nazor KE, et al. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J. 2005;24:2472–2480. doi: 10.1038/sj.emboj.7600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colby DW, et al. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colby DW, et al. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makarava N, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wille H, et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci USA. 2009;106:16990–16995. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piro JR, et al. Seeding specificity and ultrastructural characteristics of infectious recombinant prions. Biochemistry. 2011;50:7111–7116. doi: 10.1021/bi200786p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smirnovas V, et al. Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol. 2011;18:504–506. doi: 10.1038/nsmb.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kocisko DA, et al. Cell-free formation of protease-resistant prion protein. Nature. 1994;370:471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- 54.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 55.Castilla J, et al. Crossing the species barrier by PrPSc replication in vitro generates unique infectious prions. Cell. 2008;134:757–768. doi: 10.1016/j.cell.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyerett C, et al. In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology. 2008;382:267–276. doi: 10.1016/j.virol.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Kim JI, Surewicz K, Gambetti P, Surewicz WK. The role of glycophosphatidylinositol anchor in the amplification of the scrapie isoform of prion protein in vitro. FEBS Lett. 2009;583:3671–3675. doi: 10.1016/j.febslet.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, et al. Genetic informational RNA is not required for recombinant prion infectivity. J Virol. 2012;86:1874–1876. doi: 10.1128/JVI.06216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JI, et al. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem. 2010;285:14083–14087. doi: 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supattapone S. Biochemistry. What makes a prion infectious? Science. 2010;327:1091–1092. doi: 10.1126/science.1187790. [DOI] [PubMed] [Google Scholar]
- 61.Abid K, Morales R, Soto C. Cellular factors implicated in prion replication. FEBS Lett. 2010;584:2409–2414. doi: 10.1016/j.febslet.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425:717–720. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- 63.Cordeiro Y, Silva JL. The hypothesis of the catalytic action of nucleic acid on the conversion of prion protein. Protein Pept Lett. 2005;12:251–255. doi: 10.2174/0929866053587138. [DOI] [PubMed] [Google Scholar]
- 64.Caughey B. Protease-resistant PrP accumulation and scrapie agent replication: a role for sulphated glycosaminoglycans? Biochem Soc Trans. 1994;22:163–167. doi: 10.1042/bst0220163. [DOI] [PubMed] [Google Scholar]
- 65.Cordeiro Y, et al. DNA converts cellular prion protein into the beta-sheet conformation and inhibits prion peptide aggregation. J Biol Chem. 2001;276:49400–49409. doi: 10.1074/jbc.M106707200. [DOI] [PubMed] [Google Scholar]
- 66.Adler V, et al. Small, highly structured RNAs participate in the conversion of human recombinant PrP(Sen) to PrP(Res) in vitro. J Mol Biol. 2003;332:47–57. doi: 10.1016/s0022-2836(03)00919-7. [DOI] [PubMed] [Google Scholar]
- 67.Geoghegan JC, et al. Selective incorporation of polyanionic molecules into hamster prions. J Biol Chem. 2007;282:36341–36353. doi: 10.1074/jbc.M704447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piro JR, Harris BT, Supattapone S. In situ photodegradation of incorporated polyanion does not alter prion infectivity. PLoS Pathog. 2011;7:e1002001. doi: 10.1371/journal.ppat.1002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson R, Eisenberg D. Structural models of amyloid-like fibrils. Adv Protein Chem. 2006;73:235–282. doi: 10.1016/S0065-3233(06)73008-X. [DOI] [PubMed] [Google Scholar]
- 70.Nelson R, et al. Structure of the cross-β spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wasmer C, et al. Amyloid fibrils of the HET-s(218–289) prion form a β solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 72.Safar J, Roller PP, Gajdusek DC, Gibbs CJ., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993;268:20276–20284. [PubMed] [Google Scholar]
- 73.Caughey BW, et al. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 74.Baldwin MA, et al. Spectroscopic characterization of conformational differences between PrPC and PrPSc: an α-helix to β-sheet transition. Phil Trans R Soc Lond B. 1994;343:435–441. doi: 10.1098/rstb.1994.0041. [DOI] [PubMed] [Google Scholar]
- 75.Aucouturier P, Kascsak RJ, Frangione B, Wisniewski T. Biochemical and conformational variability of human prion strains in sporadic Creutzfeldt-Jakob disease. Neurosci Lett. 1999;274:33–36. doi: 10.1016/s0304-3940(99)00659-x. [DOI] [PubMed] [Google Scholar]
- 76.Caughey B, Raymond GJ, Bessen RA. Strain-dependent differences in β-sheet conformations of abnormal prion protein. J Biol Chem. 1998;273:32230–32235. doi: 10.1074/jbc.273.48.32230. [DOI] [PubMed] [Google Scholar]
- 77.Chesebro B, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 78.Baron GS, et al. Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry. 2011;50:4479–4490. doi: 10.1021/bi2003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parchi P, et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 1996;39:767–778. doi: 10.1002/ana.410390613. [DOI] [PubMed] [Google Scholar]
- 80.Cronier S, et al. Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J. 2008;416:297–305. doi: 10.1042/BJ20081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Supattapone S, et al. Prion protein of 106 residues creates an artifical transmission barrier for prion replication in transgenic mice. Cell. 1999;96:869–878. doi: 10.1016/s0092-8674(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 82.Bessen RA, Marsh RF. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peretz D, et al. A conformational transition at the N terminus of the prion protein features in formation of the scrapie isoform. J Mol Biol. 1997;273:614–622. doi: 10.1006/jmbi.1997.1328. [DOI] [PubMed] [Google Scholar]
- 84.Williamson RA, et al. Mapping the prion protein using recombinant antibodies. J Virol. 1998;72:9413–9418. doi: 10.1128/jvi.72.11.9413-9418.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merz PA, Somerville RA, Wisniewski HM, Manuelidis L, Manuelidis EE. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983;306:474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- 86.Sim VL, Caughey B. Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol Aging. 2009;30:2031–2042. doi: 10.1016/j.neurobiolaging.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 87.Ayers JI, et al. The strain-encoded relationship between PrP replication, stability and processing in neurons is predictive of the incubation period of disease. PLoS Pathog. 2011;7:e1001317. doi: 10.1371/journal.ppat.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deleault AM, Deleault NR, Harris BT, Rees JR, Supattapone S. The effects of prion protein proteolysis and disaggregation on the strain properties of hamster scrapie. J Gen Virol. 2008;89:2642–2650. doi: 10.1099/vir.0.2008/002303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eanes ED, Glenner GG. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968;16:673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- 90.Govaerts C, Wille H, Prusiner SB, Cohen FE. Evidence for assembly of prions with left-handed β-helices into trimers. Proc Natl Acad Sci USA. 2004;101:8342–8347. doi: 10.1073/pnas.0402254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serag AA, Altenbach C, Gingery M, Hubbell WL, Yeates TO. Arrangement of subunits and ordering of β-strands in an amyloid sheet. Nat Struct Biol. 2002;9:734–739. doi: 10.1038/nsb838. [DOI] [PubMed] [Google Scholar]
- 92.Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK. Molecular architecture of human prion protein amyloid: a parallel, in-register β-structure. Proc Natl Acad Sci USA. 2007;104:18946–18951. doi: 10.1073/pnas.0706522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schuler B, Rachel R, Seckler R. Formation of fibrous aggregates from a non-native intermediate: the isolated P22 tailspike β-helix domain. J Biol Chem. 1999;274:18589–18596. doi: 10.1074/jbc.274.26.18589. [DOI] [PubMed] [Google Scholar]
- 94.Junker M, et al. Pertactin β-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc Natl Acad Sci USA. 2006;103:4918–4923. doi: 10.1073/pnas.0507923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeMarco ML, Daggett V. From conversion to aggregation: protofibril formation of the prion protein. Proc Natl Acad Sci USA. 2004;101:2293–2298. doi: 10.1073/pnas.0307178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Surewicz WK, Mantsch HH, Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993;32:389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- 97.Wilder CL, Friedrich AD, Potts RO, Daumy GO, Francoeur ML. Secondary structural analysis of two recombinant murine proteins, interleukins 1 alpha and 1 beta: is infrared spectroscopy sufficient to assign structure? Biochemistry. 1992;31:27–31. doi: 10.1021/bi00116a006. [DOI] [PubMed] [Google Scholar]
- 98.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aguzzi A. Cell biology: beyond the prion principle. Nature. 2009;459:924–925. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- 101.Westermark GT, Westermark P. Prion-like aggregates: infectious agents in human disease. Trends Mol Med. 2010;16:501–507. doi: 10.1016/j.molmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 103.Wickner RB, et al. Prions: proteins as genes and infectious entities. Genes Dev. 2004;18:470–485. doi: 10.1101/gad.1177104. [DOI] [PubMed] [Google Scholar]
- 104.Uptain SM, Lindquist S. Prions as protein-based genetic elements. Annu Rev Microbiol. 2002;56:703–741. doi: 10.1146/annurev.micro.56.013002.100603. [DOI] [PubMed] [Google Scholar]
- 105.Halfmann R, Alberti S, Lindquist S. Prions, protein homeostasis, and phenotypic diversity. Trends Cell Biol. 2010;20:125–133. doi: 10.1016/j.tcb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halfmann R, et al. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–368. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toyama BH, Kelly MJ, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–237. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 108.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]