Abstract
Prions, the proteinaceous infectious agent responsible for prion diseases, can be detected with high sensitivity by protein misfolding cyclic amplification (PMCA) technology. Here we describe a quantitative PMCA procedure to calculate the concentration of very low levels of prions in biological samples. Using this procedure, we determined the quantities of misfolded prion protein (PrPSc) in brain, spleen, blood and urine of scrapie-affected hamsters.
Prion diseases are transmissible neurodegenerative disorders affecting humans and various animals1. The infectious agent, prion, is composed by a misfolded form of the prion protein (PrPSc) that propagates in the absence of nucleic acid. The unprecedented nature of the infectious agent and the recent appearance of new forms of transmissible prion diseases with devastating consequences, pose a considerable risk for human health1. One important objective in prion research is to detect and quantify small amounts of PrPSc present in various samples, for both research and practical applications2,3. Quantification of prions will allow estimation of the concentration of infectious material in diverse samples, which will be important for risk assessment and implementation of regulatory measures to prevent prion spreading.
Prions accumulate in large quantities in the brain, but small amounts of PrPSc exist in many tissues and biological fluids even at early stages of the presymptomatic period1–4. PrPSc can be detected with high sensitivity by PMCA5. PMCA is a cyclical process, conceptually analogous to PCR, that takes advantage of the nucleation-dependent prion conversion to accelerate the process by using ultrasound waves to multiply the number of replication nuclei5. Prion replication can be started with the equivalent of one molecule of PrPSc (ref. 6). This high level of sensitivity has enabled detection PrPSc in blood and urine of experimentally inoculated animals7–11.
Here we establish a quantitative PMCA (qPMCA) procedure to estimate the PrPSc concentration in various samples. The method is based on the observation that there is a direct relationship between the quantity of PrPSc in a given sample and the number of PMCA cycles and rounds necessary for its detection. To establish the method and minimize variability owing to the presence of other components in the tissue or fluid sample, we first partially purified PrPSc using a procedure involving precipitation in the presence of sarkosyl, as described previously12. Using this protocol, more than 90% PrPSc is recovered and corresponds to full-length PrPSc. To estimate the PrPSc concentration in our stock solution, we deglycosylated partially purified PrPSc and determined its concentration by comparison to the signal for known quantities of recombinant prion protein via western blotting and enzyme-linked immunosorbent assay (ELISA) (data not shown). Then we spiked various amounts of partially purified PrPSc (1 × 10−8 to 1 × 10−19 g) into normal hamster brain homogenate and subjected the samples to serial rounds of 144 PMCA cycles. We determined the number of PMCA rounds required to produce a PrPSc signal detectable by western blot. For the signal to be considered ‘positive’ it had to be at least five times greater than the densitometric signal of the background. We then plotted the quantity of PrPSc added to the tube versus the number of PMCA rounds needed for detection (Fig. 1). By extrapolating the number of PMCA rounds required to detect an unknown sample, we could estimate the quantity of PrPSc originally present in the sample.
Figure 1.
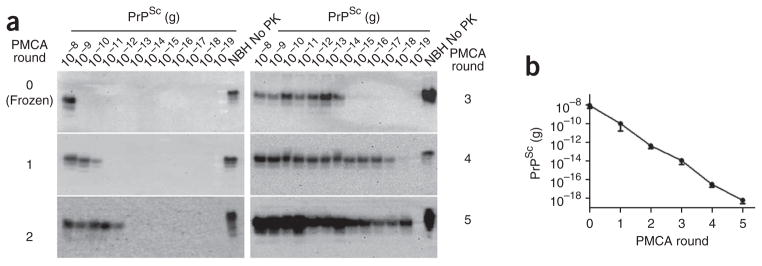
Relationship between PrPSc concentration and PMCA rounds required for detection. (a) Aliquots with the indicated amounts of PrPSc were subjected to serial rounds of PMCA (144 cycles each) using standard conditions, and PrPSc was detected by western blotting using the 3F4 antibody. All samples, except the normal brain homogenate (NBH) used as a migration control were digested with proteinase K (PK). (b) Western blots from five independent experiments (as the example shown in a) were analyzed by densitometry, and the last detectable signal after each PMCA round was plotted, yielding a standard calibration curve to estimate PrPSc amounts in hamster samples. Error bars, s.e.m. (n = 5).
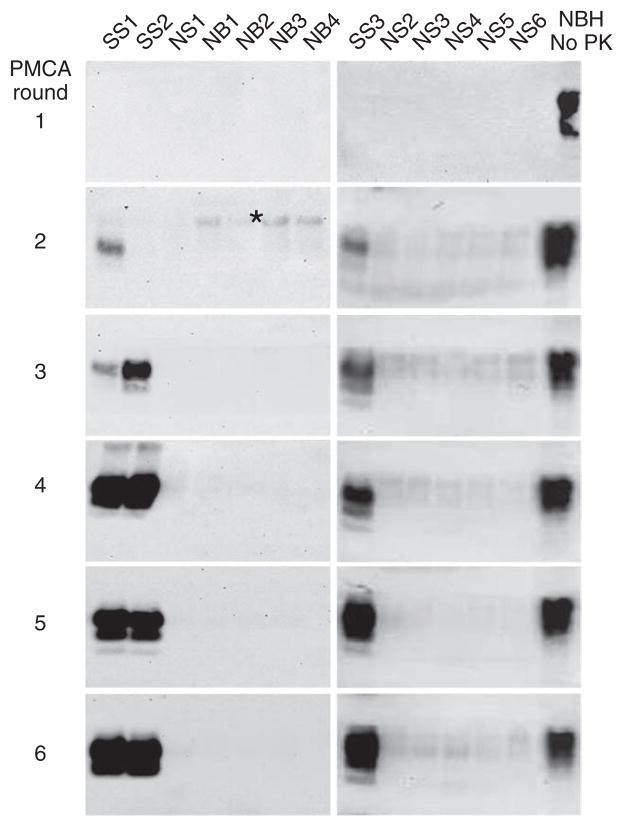
We used the qPMCA methodology to estimate PrPSc concentration in various tissues and biological fluids of scrapie-affected hamsters. We collected samples from brain, spleen, blood and urine from five hamsters exhibiting the clinical signs of the disease after intraperitoneal inoculation with strain 263K prions. As described above, we partially purified PrPSc by sarkosyl precipitation to remove components that may affect PMCA efficiency. After centrifugation, we resuspended the pellets directly in healthy hamster brain homogenate and subjected them to serial rounds of 144 PMCA cycles. From three positive spleen samples, PrPSc was detectable after two rounds of PMCA for two samples and after the third round for the third sample (Fig. 2). Extrapolation from the calibration curve (Fig. 1b) enabled us to estimate that the average concentration of PrPSc in the ‘symptomatic’ spleen was 20 pg g−1 of tissue (Table 1). As controls, we subjected various samples of spleen and brain from healthy hamsters to the same procedure. We observed no PrPSc signal after six rounds of PMCA in any of the control samples (Fig. 2), indicating that under the experimental conditions used, we detected no spontaneous generation of PrPSc.
Figure 2.
Detection of PrPSc in spleen of scrapie-affected hamsters. PrPSc was partially purified from spleen (homogenized in PBS) by sarkosyl precipitation. The equivalent to half of the spleen homogenate was resuspended in normal hamster brain homogenate and subjected to serial PMCA with subsequent detection of PrPSc by western blot (scrapie spleen, samples SS1–3). Control samples of normal (noninfected) spleen homogenate (samples NS1–6) and brain homogenate (samples NB1–4) were subjected to the same PMCA procedure to assess the rate of spontaneous appearance of PrPSc reactivity. Normal brain homogenate (NBH) not digested with proteinase K (PK) was used as a migration control. *, incomplete digestion of normal cellular prion protein (PrPC) with proteinase K, which sometimes occurs after PMCA and is distinguishable from PrPSc by the unchanged molecular weight.
Table 1.
PrPSc concentration in scrapie-affected hamsters
| Source | PrPSc concentration in tissues (g g−1) and fluids (g ml−1) |
|---|---|
| Brain | 2.3 × 10−5 ± 6.8 × 10−6 |
| Spleen | 2.0 × 10−11 ± 1.1 × 10−11 |
| Buffy coat | 2.6 × 10−13 ± 2.4 × 10−13 |
| Plasma | 1.3 × 10−14 ± 1.1 × 10−14 |
| Urine | 2.0 × 10−16 ± 1.7 × 10−16 |
PrPSc concentration in tissues and fluids was estimated by determining the number of PMCA rounds required to detect the signal by western blots and obtaining the concentration from the standard curve. Indicated values are means ± s.e.m. for five hamsters.
We also analyzed PrPSc concentrations in other tissues and fluids (Table 1). As expected, the brain contained the highest amount of PrPSc, which was readily detectable by western blotting without the need for PMCA (data not shown). We separated blood samples into plasma and buffy coat; using qPMCA we calculated a concentration of PrPSc of 13 and 260 fg ml−1 of blood, respectively (Table 1). Finally, we estimated PrPSc concentration in urine as 0.2 fg ml−1. These results indicate that spleen, buffy coat, plasma and urine from symptomatic hamsters respectively contain approximately 106, 108, 2 × 109 and 1011 times less PrPSc than the brain. These proportions, however, likely change dramatically at different stages of the disease. We are currently using qPMCA to assess the dynamic changes in PrPSc concentration in various tissues and fluids during the progression of the disease from the initial infection to the onset of the clinical disease. The principle of qPMCA is applicable to prions from any strain or species, but the calibration curve and the dynamic range of detection will likely be different and have to be empirically determined for specific samples.
PMCA has contributed to understanding the mechanism of prion replication, the nature of the infectious agent and the detection of small quantities of PrPSc in biological samples13. qPMCA may have many scientific and practical applications to estimate PrPSc concentration. The technology may be useful to diagnose disease, develop prion decontamination procedures, identify drugs to prevent the formation and eliminate PrPSc, quantify the extent of prion contamination in medical or environmental materials and may be used to guide regulatory measures to prevent further spread of prion diseases.
ONLINE METHODS
Sample preparation
Syrian hamsters were intraperitoneally inoculated with strain 263K prions and monitored for the appearance of clinical signs, using a standard scale, as previously described14. When disease was confirmed, hamsters were killed by CO2 inhalation, and brains, spleen and blood were collected. Before killing the hamsters, urine was collected using metabolic cages, as described previously11. Brain or spleen homogenates were prepared at 10% (wt/vol) in PBS (pH 7.2) plus Complete cocktail of protease inhibitors (Roche). The samples were clarified by a 45-s, low-speed centrifugation (450g). Blood samples were obtained directly from the heart in tubes containing citrate. Plasma and buffy coat were separated by centrifugation in ficoll gradient, as described7. The samples of normal brain homogenate used for PMCA substrate were obtained after perfusing hamsters with PBS and 5 mM EDTA. Solutions of 10% normal brain homogenate were made in conversion buffer (PBS without Ca2+ and Mg2+ with 150 mM NaCl, 1.0% triton X-100 and Complete protease inhibitors). Debris was removed by a 45-s, low-speed centrifugation (450g) in an Eppendorf centrifuge.
PrPSc partial purification by sarkosyl precipitation
To minimize interferences in PMCA from other components present in tissues or fluids, PrPSc was partially enriched by sarkosyl precipitation, as previously described12. Briefly, samples were incubated with 1 volume of 20% sarkosyl for 10 min at room temperature (22–25 °C) and centrifuged at 100,000g for 1 h at 4 °C. Supernatants were discarded and pellets were resuspended into two volumes of 10% sarkosyl. The centrifugation process was repeated, and pellets were resuspended directly in 10% normal brain homogenate prepared in conversion buffer. Following this protocol, PrPSc was recovered in the pellet fraction with a yield higher than 90%.
PMCA procedure
PMCA was performed as described previously13,15. Briefly, samples were loaded onto 0.2-ml PCR tubes. Tubes were positioned on an adaptor placed on the plate holder of a microsonicator (Misonix model 4000), and samples were subjected to cycles of 30 min incubation at 37 °C followed by a 20 s pulse of sonication set at an amplitude of 75. Samples were incubated without shaking immersed in the water of the sonicator bath. Standard PMCA rounds consisted of 144 cycles. After each round of cycles, a 10 μl aliquot of the amplified material was diluted into 90 μl of normal brain homogenate and a new round of PMCA cycles was performed.
PrPSc detection
Samples were first digested with 50 μg ml−1 of proteinase K at 37 °C for 1 h, and the reaction was stopped by adding NuPAGE LDS sample buffer. The proteins were then fractionated using 4–12% SDS-PAGE, electroblotted into Hybond ECL nitrocellulose membrane and probed with the 3F4 antibody (Covance) (dilution 1:5,000). The immunoreactive bands were visualized by ECL Plus western blotting detection system and quantified by densitometry using a UVP Bioimaging system EC3 apparatus. qPMCA is compatible with any PrPSc detection procedure (for example, ELISA, conformation-dependent immunoassay and others). In this study, we used the western blot because it is the most standard and widely used assay in the field. Additionally, western blot has the important advantage over other techniques, such as ELISA, in that it can be used to distinguish bona fide PrPSc (PrPSc protease-resistant fragment after proteinase K treatment) from incomplete digestion of normal cellular prion protein (PrPC) (Fig. 2).
Acknowledgments
We thank D. Gonzalez-Romero (University of Texas Houston Medical School) for participating in the PMCA experiments with urine, R. Diaz-Espinoza (University of Texas Houston Medical School) for providing recombinant PrP and D. Zhao (China Agricultural University) for suggestions and providing support to B.C. This research was supported in part by US National Institutes of Health grants R01NS049173 and P01AI077774 to C.S.
Footnotes
AUTHOR CONTRIBUTIONS
B.C. performed most of the experiments, analyzed the data and prepared the figures; R.M. helped with experimental design and data analysis; M.A.B. performed the PMCA experiments in blood; and C.S. developed the hypothesis, designed and analyzed all data, wrote the manuscript and supervised the project.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemethods/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Aguzzi A, Calella AM. Physiol Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 2.Soto C. Nat Rev Microbiol. 2004;2:809–819. doi: 10.1038/nrmicro1003. [DOI] [PubMed] [Google Scholar]
- 3.Brown P. Neurology. 2008;70:713–722. doi: 10.1212/01.wnl.0000302186.10596.f6. [DOI] [PubMed] [Google Scholar]
- 4.Wadsworth JD, et al. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 5.Saborio GP, Permanne B, Soto C. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 6.Saa P, Castilla J, Soto C. J Biol Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 7.Castilla J, Saa P, Soto C. Nat Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 8.Saa P, Castilla J, Soto C. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 9.Thorne L, Terry LA. J Gen Virol. 2008;89:3177–3184. doi: 10.1099/vir.0.2008/004226-0. [DOI] [PubMed] [Google Scholar]
- 10.Murayama Y, et al. J Gen Virol. 2007;88:2890–2898. doi: 10.1099/vir.0.82786-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. FEBS Lett. 2008;582:3161–3166. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales R, et al. Biochem Biophys Res Commun. 2008;377:373–378. doi: 10.1016/j.bbrc.2008.09.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castilla J, et al. Methods Enzymol. 2006;412:3–21. doi: 10.1016/S0076-6879(06)12001-7. [DOI] [PubMed] [Google Scholar]
- 14.Castilla J, Saá P, Hetz C, Soto C. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Saa P, Castilla J, Soto C. Methods Mol Biol. 2005;299:53–65. doi: 10.1385/1-59259-874-9:053. [DOI] [PubMed] [Google Scholar]