Abstract
Salmonella organisms are gram-negative and facultative anaerobic bacteria that cause typhoid fever in humans. In this study, we evaluated LPS-specific adaptive immunity in innate immune-deficient mice after oral administration of attenuated S. enterica serovar Typhymurium (S. Typhimurium) strains. Of interest, identical levels of LPS-specific IgG and IgA antibodies were elicited in the systemic (i.e., serum and spleen) and mucosal (i.e., fecal extract and small intestine) compartments of wild-type, TLR4−/−, and MyD88−/− mice following oral vaccination with recombinant attenuated S. Typhimurium (RASV). Depletion of CD4+ T cells during RASV vaccination completely abrogated the generation of LPS-specific antibodies in MyD88−/− mice. In addition, mRNA expression levels of a B-cell activating factor of the TNF family (BAFF) were significantly increased in the spleens of MyD88−/− mice after oral administration, implying that T cell–independent B cell switching might be also enhanced in the MyD88 signal-deficient condition. Of most interest, orally vaccinated MyD88−/− mice that possessed high levels of LPS-specific IgG and IgA, which had neutralizing effect against Salmonella, died earlier than non-vaccinated wild-type mice following lethal oral challenge with virulent Salmonella species. These results suggest that innate immunity mediated by MyD88 signal is dispensable for induction of LPS-specific antibody responses following oral administration of attenuated Salmonella strains but indispensable for efficient protection.
Keywords: Mucosa, Vaccination, Salmonella, MyD88, innate immunity
Introduction
Oral infection with Salmonella species in contaminated food and water causes wasting diseases including typhoid fever and gastroenteritis in humans and other animals (1, 2). The disease manifestation depends on both host susceptibility and the infectious S. enterica serovar. In humans, serovars Typhi, Paratyphi, and Sendai cause enteric fever, while most serovars cause enterocolitis and/or diarrhea (3). A typhoid-like disease caused by infection of susceptible inbred mice with S. Typhimurium has been shown to be a good model in which to study human immune responses to S. typhi (4)
Mucosal cells, whether of the digestive, respiratory, or reproductive tracts, are constantly exposed to antigens of microbial, environmental, or food origin and require an effective defense system (5, 6). Immune cells stimulated at one mucosal surface induce both systemic and local protection, thus providing the potential for vaccines to be used for a broad spectrum of infectious diseases (7). As an alternative to vaccination by injection, mucosal vaccination offers obvious safety advantages, since it eliminates the risks of blood-borne infections from unsterile needles (7). The Secretory IgA (SIgA) antibody in the mucosal surfaces make bacterial antigen cannot adhere to mucus, and neutralize and eliminate them (8). Previous study indicated that SIgA is crucial against secreted bacteria toxins which might inhibit early colonization of bacteria, but is not essential for protection against reinfection with S. Typhimurium or Citrobactirium (C.) rodentium (9). On the other hand, a recent study clearly supports the crucial role of innate SIgA antibody to protect against S. Typhimurium infection (10).
Innate immunity is the first line of defense against infection provoked by exogenous antigens including bacteria, viruses, and allergens (11, 12). It is now thought that to elicit an effective immune response, microorganisms must interact with pattern-recognition receptors (PRRs), both within and outside of cells. There are two major families of PRRs in the intestines: toll-like receptors (TLRs) and the intracellular nucleotide-binding and oligomerization domain (NOD) family (11, 13). Recognized TLR signals are transferred to adaptor molecules such as the myeloid differentiation primary response gene 88 (MyD88) or the toll/interleukin-1 receptor (TIR)-containing adaptor-inducing IFN-β (TRIF). Then the terminal point of signal through the TLR pathway can activate the level of gene coding pro-inflammatory cytokines such as nuclear factor κB (NF-κB) (11, 12). This signaling causes production of nonspecific defense mediators, which activate T and B cells (11, 12). Hence, several cytokines through TLR signaling can directly enhance the early stages of the adaptive immune response by up-regulation of co-stimulatory molecules and activation of dendritic cells (DCs) (14). In view of these findings, innate immunity including MyD88 signaling may be essential in the immune system as a front line defense against pathogens and also play a critical role with B cell immunity (15). However, controversial issues remain to be resolved such as whether TLR signal is necessary to link innate and adaptive immune systems (16).
Salmonella organisms express a variety of toxin-caused molecules including lipopolysaccharides (LPS), flagellin, the peptidoglycan layer, and lipoprotein (11, 17). Once these bacteria enter the host, TLRs can recognize a variety of microbial products including bacterial cell wall components and endocytosed nucleic acids, thereby triggering innate immune responses (11). Among those LPS is generally accepted as a protective antigen of Salmonella and also as a virulence factor (17). Although Salmonella LPS is an agonist of TLR4 in vivo and in vitro (18–20), it remains unclear whether TLR-mediated innate immunity is involved in the induction of LPS-specific acquired immunity in vivo. In this study, to clarify the role of TLR-mediated innate immunity for the induction of LPS-specific acquired immunity, we employed a live recombinant attenuated S. Typhimurium vaccine (RASV) strain to observe B cell responses (21, 22).
Our findings demonstrate that LPS-specific B cell responses are highly enhanced without the help of TLR4- or MyD88-mediated innate immunity following oral administration of RASV strain; however, the MyD88 signal is indispensable for protection against lethal Salmonella challenge.
Materials and Methods
Bacteria strain
The recombinant attenuated Salmonella vaccine (RASV) strain, S. Typhimurium χ9241 [ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198:: araCPBADlacI (ATG)TT containing pYA3620], used in this study (23). For challenge experiments, wild-type virulent S. Typhimurium (UK-1) strain (1×107 CFU per dose) was given orally 14 days following the final oral vaccination. Both RASV and UK-1 strains were grown at 37°C in Luria-Bertani (LB) broth without shaking. The actual administered bacterial dose was confirmed by plating serial dilutions onto MacConkey agar (Becton, Dickinson, MD) plates.
Animals
Female BALB/c and C57BL/6 (B6) mice were purchased from Charles River Laboratories (Orient Co., Sungnam, Korea) and B cell–deficient (B6.µMT) mice were purchased from Jackson Laboratory (Bar Harbor, ME). TLR4−/− and MyD88−/− mice were kindly provided by Prof. Shizuo Akira (Research Institute for Microbial Diseases, Osaka Univ., Osaka, Japan). To generate the PP-null mice, pregnant mice were injected i.v. with 600 µg of anti-IL-7R mAb on gestational day 14. The progeny was adopted for the vaccination experiment as PP-null mice (24). All mice were maintained under specific pathogen-free conditions in the animal facility at the International Vaccine Institute (Seoul, Korea) where they received sterilized food (certified diet MF; Oriental Yeast) and water ad libitum. The experiments were approved by the Institutional Animal Care and Use Committee of the International Vaccine Institute.
Enzyme-linked immunosorbent assay (ELISA)
The plates (Falcon, USA) were coated with 5 µg/ml of S. Typhimurium–derived LPS (Sigma-Aldrich, St. Louis, MO) in 50 mM sodium bicarbonate and incubated overnight at 4°C. After a blocking step, two-fold serially diluted samples (starting at 1:64 for serum, 1:4 for fecal extract) were applied onto plates and incubated for 2 hr at 37°C. HRP-conjugated goat anti-mouse IgG and IgA antibody (Southern Biotechnology Associates, Birmingham, AL) (1:3000 in 0.1% BSA in PBS plus 0.1% Tween 20) was added to each well at 37°C. For color development, the substrate solution (TMB; Moss, INC) was added. Color development was stopped by adding 0.5 N HCl. The color development was measured at 450 nm on an ELISA reader (Microplate spectrophotometer; Molecular Devices) and endpoint titers were expresses as reciprocal log2 titers.
Enzyme-linked immunosorbent spot (ELISPOT) assay
The 96-well nitrocellulose microplates (Millipore) were coated with 5 µg/ml of S. Typhimurium-derived LPS (Sigma-Aldrich) in 50 mM sodium bicarbonate and incubated overnight at 4°C as described elsewhere (25). After blocking with RPMI media including 10% FBS, serially diluted MNCs were plated and incubated for 4 hr at 37°C in a 5% CO2 incubator. For color development, peroxidase substrates (AEC kit; Moss, INC) were adopted and the number of ASCs was counted with the aid of a stereomicroscope (SZ2-ILST; Olympus, Tokyo, Japan).
Neutralizing antibodies
Hybridomas producing anti-CD4 (GK1.5) antibody were purchased from ATCC (Rockville, MD) and obtained from ascites. Mice were injected intraperitoneally (i.p.) with 150 µg of depleting anti-CD4 or isotype control rat IgG (Sigma-Aldrich) antibodies 1 day before vaccination and every 3 or 4 days thereafter.
Adoptive transfer
To reconstitute wild-type mice with sera or B cells obtained from RASV-immunized BALB/c wild-type and MyD88−/− mice, mice were orally administered 109 cfu of RASV twice at 2-week intervals. The B cells were isolated from the spleen and MLN of the immunized mice using a B cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Groups of BALB/c wild-type mice were i.v. transferred with 500 µl of sera or 2 × 107 cells of B cells, and the next day challenged with UK-1 strain. To assess the role of MyD88−/− B cells for the LPS-specific antibody responses, µMT mice of B6-background were transferred with 2 × 107 cells of B cells from wild-type B6 or B6-background MyD88−/− mice 1 day before RASV oral administration. Mice were immunized twice with two weeks interval, and sera and feces were obtained 14 days after the final boosting for the LPS-specific ELISA.
cDNA synthesis and real-time PCR
Samples of the PP, MLN, and spleen of wild-type and MyD88−/− mice were taken at 0, 24, and 48 hr following RASV oral administration and then homogenized after a wash with nuclease-free water. Total RNA was extracted with TRIzol reagent (Invitrogen Life Technologies) and 5 µg of RNA was converted into cDNA using Superscript II Reverse Transcriptase (Invitrogen Life Technologies). The products were used as a template for each cytokine-specific real-time PCR set for the amplification of GAPDH, BAFF, and APRIL. The amplification reactions were performed with 100–200 ng of cDNA (TaqMan Universal PCR Master mix; Applied Biosystems) and each of the TaqMan primer-probe sets for each gene (Applied Biosystems). Then gene expression was quantified using an ABI PRISM Sequence Detection System instrument (Applied Biosystems). The levels of mRNA expression were displayed as the expression units of each target gene relative to the expression units of expressed GAPDH.
Data and statistical analysis
The Kaplan-Meier method was used to determine the statistical significance of differences in survival time. We performed the Log-Rank test (Mantel-Cox), using SPSS 12.0K for Windows. To compare the differences between two experimental groups, we used the Student t-test. To compare multiple groups, we carried out one-way ANOVA followed by the Tukey-HSD post hoc test. Each experiment was repeated at least three times using five to ten mice per group. P values of <0.05, <0.01 and <0.001 were assumed to be statistically significant.
Results
Oral administration with recombinant attenuated S. Typhimurium vaccine (RASV) strain elicits high levels of LPS-specific IgG and secretory IgA responses in serum and intestine in a Peyer’s patch (PP)-dependent manner
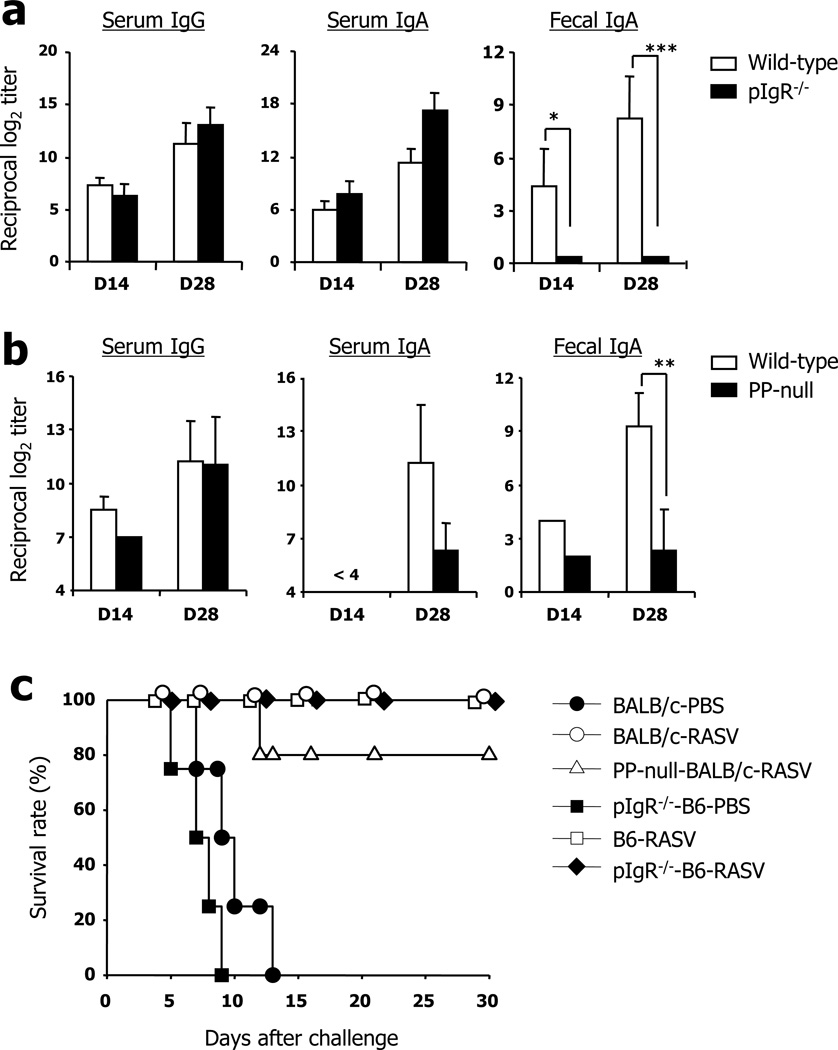
By use of RASV strain, we first investigated LPS-specific antibody responses to oral immunization in systemic (i.e., serum) and mucosal (i.e., fecal extract) compartments (Fig. 1a). High levels of serum IgG and IgA were determined 14 days after oral administration of RASV strain, and the antibody levels were much increased after boosting. The levels of fecal IgA responses were significantly enhanced at day 14 after boosting compared with those after priming. No LPS-specific IgG and IgA responses were detected in serum and fecal extracts isolated from PBS-vaccinated control mice (data not shown). To assess whether IgA antibodies in fecal extract are in secretory form, we adopted polymeric immunoglobulin receptor (pIgR)−/− mice lacking the IgA secretion pathway (8). These mice showed complete loss of IgA responses in fecal extract but high levels of IgG and IgA were maintained in the serum 14 days after priming and boosting with RASV strain when compared with wild-type mice (Fig. 1a). These results indicate that LPS-specific IgA antibody in mucosal compartments, induced by oral vaccination with RASV strain, is secretory in form.
FIGURE 1.
Levels of LPS-specific antibody responses following oral administration of recombinant attenuated Salmonella vaccine (RASV) strain. (a) Wild-type (WT; white bars) and poly immunoglobulin receptor (pIgR)−/− mice (black bars) were orally immunized with RASV strain (109 CFU per head) twice at 2-week intervals. At 14 days after 1st (D14) and 2nd (D28) immunization, the levels of LPS-specific IgG and IgA antibodies were measured by ELISA in mouse sera and fecal extracts. * ; p<0.05, *** ; p<0.001. (b) Peyer’s patch (PP)-null mice of BALB/c background were produced by in utero treatment with anti-IL-7R antibodies and orally immunized twice with RASV. ** ; p<0.01. (c) Immunized WT, pIgR−/− and PP-null mice of B6 or BALB/c background were challenged with virulent Salmonella UK-1 strain (107 CFU/head) 1 month after boosting and monitored for survival. Data are representative of ≥2 independent experiments.
Although PP is a known potential inductive site of oral Salmonella challenge, the exact role of PP on the induction of LPS-specific B cell responses remains poorly understood. In order to clarify the role of PP following oral vaccination with RASV strain, we produced PP-null BALB/c mice by in utero treatment with anti-IL-7R monoclonal antibody (mAb) (24, 26). PP-null mice had significantly defective levels of LPS-specific IgA responses in the serum and fecal extract compared with those of PP-intact mice (Fig. 1b). In contrast, identical levels of serum IgG were determined in PP-intact wild-type and PP-null mice. These results clearly indicate that PP plays a crucial role for induction of LPS-specific IgA antibody responses following oral vaccination with RASV.
We next assessed whether vaccination with RASV could confer protection against virulent Salmonella in immunized wild-type mice. In support of previous reports (22), the mice vaccinated with RASV were completely protected against a lethal dose of virulent UK-1 strain (Fig. 1c). To address a role of SIgA for protection, pIgR−/− mice of B6 background were adopted. Unexpectedly, pIgR−/− of B6 background mice as well as PP-null mice of BALB/c background which possess low levels of LPS-specific SIgA in intestines survive more than 30 days against virulent Salmonella UK-1 challenge (Fig. 1c). These results indicating that other factors rather than SIgA could be involve for generation of protective immunity against Salmonella.
LPS-specific B cell responses after oral administration with RASV strain in TLR4−/− and MyD88−/− mice
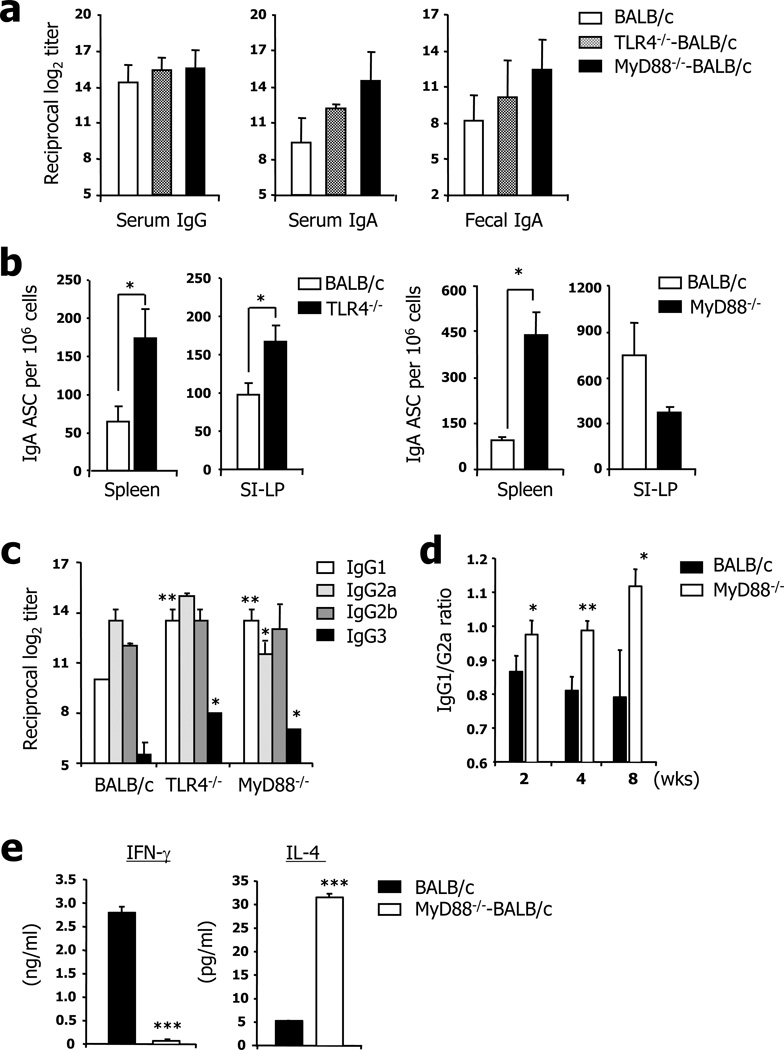
To address the role of TLR-mediated innate immunity on the induction of humoral immunity, BALB/c background wild-type, TLR4−/−, and MyD88−/− mice were orally administered RASV strain (109 CFU/head) and their LPS-specific antibody levels were determined by ELISA. Of note, identical levels of LPS-specific IgG and IgA antibodies were found in the serum and mucosal secretions (i.e., fecal extract) of wild-type, TLR4−/−, and MyD88−/− mice following two times oral vaccinations with RASV strain (Fig. 2a). To further confirm the induction levels of LPS-specific B cells in the systemic and mucosal tissues following oral administration with RASV, mononuclear cells were isolated from spleen and SI-LP of wild-type, TLR4−/−, and MyD88−/− mice and numbers of LPS-specific IgA antibody-secreting cells (ASCs) were determined by ELISPOT (Fig. 2b). Interestingly, much higher numbers of LPS-specific IgA ASCs were determined in the spleen of TLR4−/− and MyD88−/− mice than in wild-type mice. Furthermore, compared to the number of LPS-specific IgA ASCs elicited in the WT mice, similar or significantly higher numbers of LPS-specific IgA ASCs were elicited in the SI-LP of MyD88−/− or TLR4−/− (Fig. 2b) mice, respectively, after oral vaccination with RASV. To clarify the Th1/Th2 balance in those mice, we measured LPS-specific IgG isotypes in serum after boosting. The ratio of IgG1 (Th2) to IgG2a (Th1) was higher in the TLR4−/− and MyD88−/− mice than in wild-type mice, indicating that predominantly Th2-type responses were elicited in the TLR4 and MyD88-deficient condition (Fig. 2c). To further confirm the kinetics of IgG1/G2a ratio after RASV vaccination, the sera from immunized wild-type or MyD88−/− mice were analyzed for the LPS-specific IgG1 and IgG2a at 2, 4, and 8 wks after immunization (Fig. 2d). The elevated IgG1/G2a ratio was continued for at least 8 wks after immunization, which means the increased IgG1/G2a Ab response in MyD88−/− mice after RASV vaccination are not simply due to the altered kinetics of antibody production. These data suggest that oral RASV immunization induces LPS-specific systemic and mucosal antibodies without the assistance of innate immunity-especially that mediated by TLR4 and MyD88 signals.
FIGURE 2.
Identical levels of LPS-specific antibodies in wild-type (WT), TLR4−/− and MyD88−/− mice. Groups of mice were orally given RASV strain twice at 2-week intervals. Levels of LPS-specific antibodies were measured in sera and fecal extracts (a) and serum isotypes (c and d) were measured by ELISA on day 28. b; IgA antibody-secreting cells (ASCs) were measured in the spleen and lamina propria of the small intestine (SI-LP) of TLR4−/− and Myd88−/− mice by ELISPOT assay. d, The ratios of LPS-specific IgG1 versus IgG2a at 2, 4, and 8 weeks after RASV vaccination in wild-type and MyD88−/− were shown. e, IL-4 and IFN-γ secretion after 24 hrs in vitro culture of total splenocytes obtained 28 days following two immunization of wild-type or MyD88−/− mice with RASV. * ; p<0.05, ** ; p<0.01 and *** ; p<0.005. Values given represent mean ± SD. Data are representative of ≥3 independent experiments.
To directly check whether the deficiency of MyD88 signaling contribute to Th2-dominant response, mononuclear cells obtained from spleen of wild-type or MyD88−/− mice at 28 days following two times immunization with RASV were co-cultured for 24 hrs in the presence of killed Salmonella strain (10 cfu/cell) and analyzed for the cytokines secretion such as IL-4 and IFN-γ (Fig. 2e). Although splenocytes from RASV-immunized wild-type mice secreted high level of IFN-γ, those from MyD88−/− mice fail to release IFN-γ upon 24 hrs in vitro restimulation. On the contrary, the level of IL-4 was higher in splenocytes from MyD88−/− mice compared to those from wild-type mice. This data together with IgG1/IgG2a ratio results strongly suggests that Th2-dominant responses were elicited in the MyD88−/− mice following oral administration with RASV.
Role of CD4+ T cells in LPS-specific antibody responses after oral administration of RASV strain
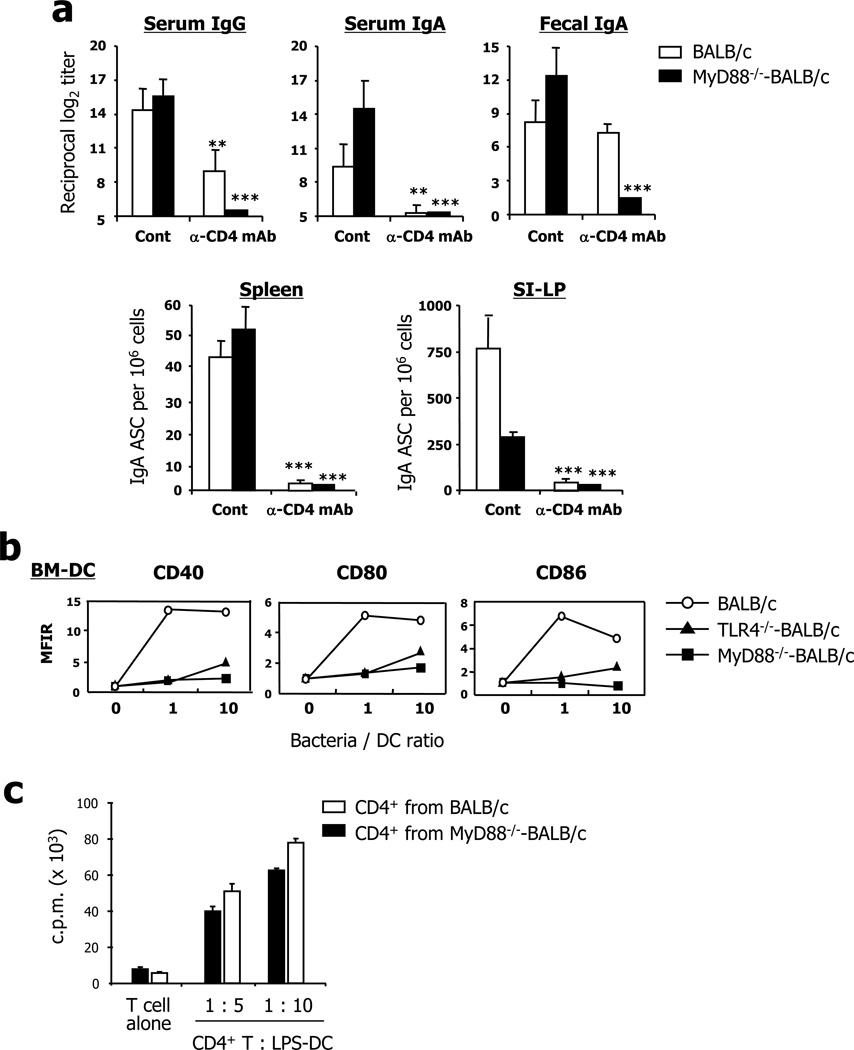
To clarify whether CD4+ T cells enable the induction of LPS-specific antibodies, we used CD4+ T cell–depleted mice treated with neutralizing anti-CD4 mAbs (27). Although LPS is a well-known T cell–independent antigen, depletion of CD4+ T cells reduced IgG and IgA antibody levels in the serum following oral vaccination with RASV strain (Fig. 3a). In addition, much lower levels of secretory IgA antibody were found in the fecal extract of MyD88−/− mice than in those of wild-type mice in the absence of CD4+ T cells. In parallel, the CD4+ T cell–depleted mice had fewer IgG and IgA ASCs in both systemic (i.e., spleen) and mucosal compartments (i.e., SI-LP) than did wild-type mice following oral vaccination with RASV (Fig. 3a). These results suggest that CD4+ T cells play a crucial role for induction of LPS-specific IgG and IgA responses in both systemic and mucosal tissues after oral administration of RASV strain. Next we looked for the mechanism that activated CD4+ T cells for LPS-specific antibody production in MyD88−/− mice. When we assessed the activation of DCs after in vitro stimulation with RASV, we could not detect any increase in the expression level of costimulatory molecules, including CD40, CD80, and CD86, on bone marrow–derived DCs from MyD88−/− mice compared to those of wild-type mice (Fig. 3b). However, CD4+ T cells from RASV-immunized MyD88−/− mice proliferated efficiently to the same extent as those from wild-type mice co-cultured with LPS-stimulated DCs of naïve mice (Fig. 3c). Thus, it seems likely that factors other than activated DCs stimulate CD4+ T cells for the generation of Salmonella-specific humoral immune responses after oral RASV administration in MyD88−/− mice.
FIGURE 3.
CD4+ T cells play a crucial role for induction of LPS-specific antibody responses in both systemic and mucosal compartments. (a) CD4+ T cells were depleted using anti-CD4 depleting mAbs during vaccination to assess a role for CD4+ T cells for induction of LPS-specific B cell responses. At 1 week after the 2nd vaccination with RASV strain, the levels of LPS-specific antibodies were measured in sera and fecal extracts, and numbers of LPS-specific antibody-secreting cells (ASC) were measured in the spleen and lamina propria of the small intestine (SI-LP). * ; p<0.05, ** ; p<0.01, *** ; p<0.001 vs. isotype control antibody-treated mice. (b) Bone marrow–derived dendritic cells (BM-DC) from wild-type (WT), TLR4−/−, and Myd88−/− mice were co-cultured with RASV strain for 48 hr in vitro. After incubation, DCs were stained for co-stimulatory molecules (CD40, CD80, CD86) and analyzed by flow cytometry. (c) CD4+ T cells isolated from spleen of RASV-vaccinated WT and MyD88−/− mice were co-cultured with LPS-stimulated DCs from naïve mice spleens for 90 hr, including a final 18-hr pulse with [3H] thymidine. Cells were harvested and [3H] thymidine incorporation measured. Values given represent mean ± SD. Data are representative of ≥2 independent experiments.
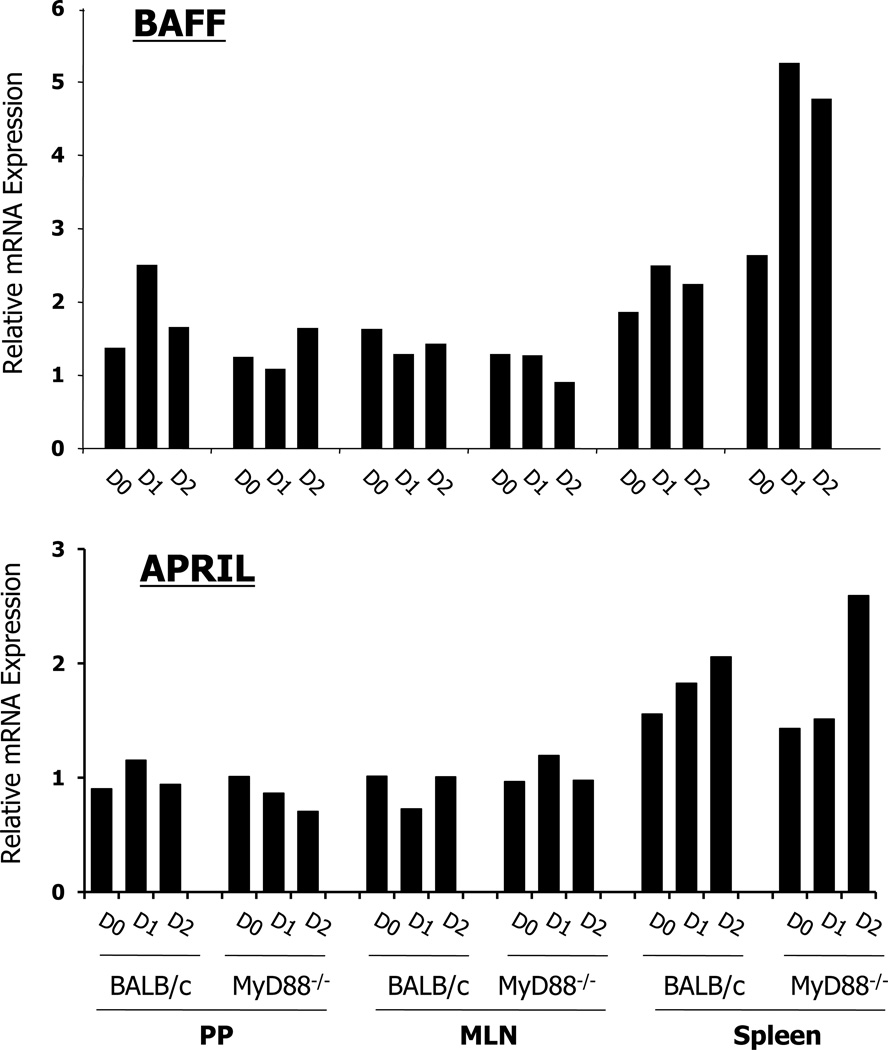
Enhanced expression levels of BAFF and APRIL in the spleen of MyD88−/− mice at early time points after oral vaccination with RASV strain
Two members of the tumor necrosis factor (TNF) family (i.e., BAFF [B-cell activating factor of the TNF family] and APRIL [a proliferation-inducing ligand]) are involved in stimulating class switch recombination (CSR) to IgG and IgA production independent of CD4+ T cells and CD40-CD40L engagement (28). Accordingly, we further examined expression levels of both molecules after oral vaccination. Each tissue (i.e., PP, mesenteric lymph node [MLN], and spleen) were isolated at 0, 1, and 2 days following oral administration with RASV strain and whole tissue homogenates were used for real-time PCR. Interestingly, highly enhanced expression levels of BAFF and APRIL mRNA were determined in the spleen of MyD88−/− mice at an early time point after oral administration of RASV strain (Fig. 4). In contrast, no significant difference was found in PP and MLN of both wild-type and MyD88−/− mice. Overall, BAFF- and APRIL-mediated CSR appear to be necessary for induction of LPS-specific B cell responses in the MyD88 signaling-deficient condition.
FIGURE 4.
Relative level of BAFF and APRIL mRNA determined by real-time PCR. cDNA was constructed using RNA isolated from Peyer’s Patches (PP), mesenteric lymph node (MLN), and spleen of wild-type (WT) and MyD88−/− mice at 0, 1, and 2 days after oral RASV administration. Expression level of BAFF and APRIL mRNA was normalized by GAPDH. Data are representative of 3 independent experiments.
MyD88 signal protects against oral challenge with virulent S. Typhimurium strain
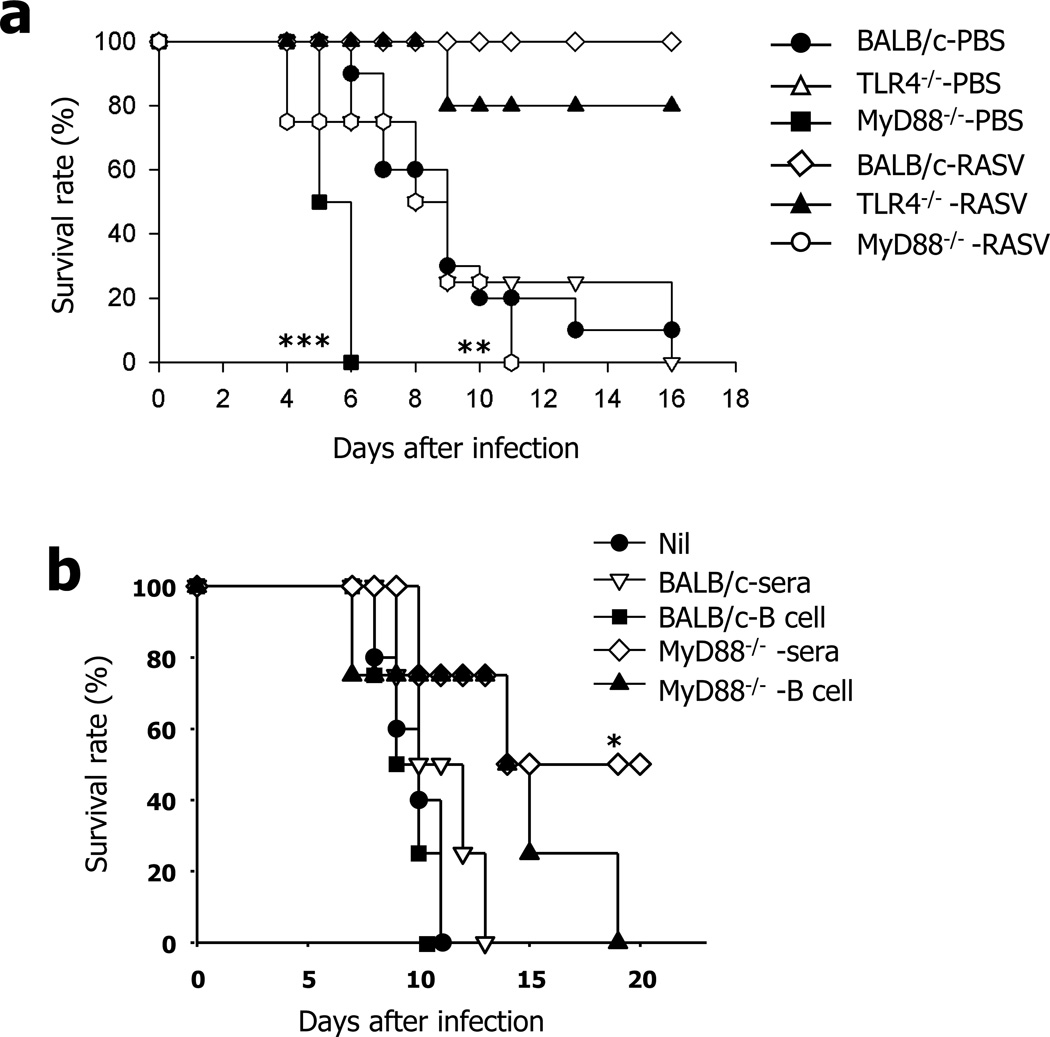
To determine if highly enhanced LPS-specific IgG and IgA antibodies (after oral administration of RASV strain) possess neutralizing effects, we measured the protective efficacy against oral Salmonella infection. Groups of mice were orally challenged with a lethal dose of virulent Salmonella UK-1 strain (1×107 CFU/head) 28 days following a second vaccination with PBS or RASV strain and body weight changes (data not shown) and survival rates were measured (Fig. 5a). As expected, all groups of PBS-vaccinated wild-type, TLR4−/−, or MyD88−/− mice died within 2 weeks after oral challenge with a lethal dose of UK-1 strain (Fig. 5a). Of note, PBS-vaccinated MyD88−/− mice died much earlier than PBS-vaccinated wild-type and TLR4−/− mice. The fact that RASV-vaccinated wild-type and TLR4−/− mice survived without loss of body weight after oral challenge with UK-1 strain indicates that LPS-specific antibodies derived by oral administration with RASV possessed strong neutralizing effects against oral S. Typhimurium infection. However, vaccinated MyD88−/− mice, which possessed brisk levels of LPS-specific IgG and IgA antibodies in both systemic and mucosal compartments, died at early time points after oral challenge compared with RASV-vaccinated wild-type or TLR4−/− mice (Fig. 5a). These results demonstrate that LPS-specific antibodies induced by oral RASV possess strong neutralizing effects that are elicited without assistance of TLR-mediated innate immunity but that MyD88 signal is indispensable for protection against oral S. Typhimurium infection.
FIGURE 5.
Characteristic features of neutralizing antibodies and B cells of MyD88−/− mice following oral administration of RASV. (a) At 1 month after second immunization, groups of mice were challenged orally with a lethal dose of Salmonella UK-1 strain (1×107 CFU per head) and survival monitored. ** ; p<0.01, *** ; p<0.001 vs. wild-type (WT) mice. (b) Sera and B cells were isolated from MyD88−/− mice 14 days after 2nd oral vaccination with RASV and reconstituted to naïve BALB/c mice. At 1 day after reconstitution, groups of mice were orally challenged with a lethal dose of virulent UK-1 strain and survival was monitored. * ; p<0.05, ** ; p<0.05 and *** ; p<0.001 vs. mice in non-treated group (nil).
To directly assess the functional role of antibodies, sera and splenic B cells from RASV-vaccinated MyD88−/− mice were reconstituted in naïve BALB/c mice. One day later, recipient mice were orally challenged with a lethal dose of Salmonella UK-1 strain (Fig. 5b). About half of the recipient mice given sera from RASV-vaccinated MyD88−/− mice survived whereas all mice given reconstituted by B cells died within 20 days of oral challenge (Fig. 5b). However, sera obtained from BALB/c mice immunized with RASV could not confer significant protection against a lethal dose of Salmonella UK-1 strain after transferring into naïve BALB/c mice (Fig. 5b), indicating that other factors including activated phagocytes seems to be required for the efficient protection against Salmonella infection.
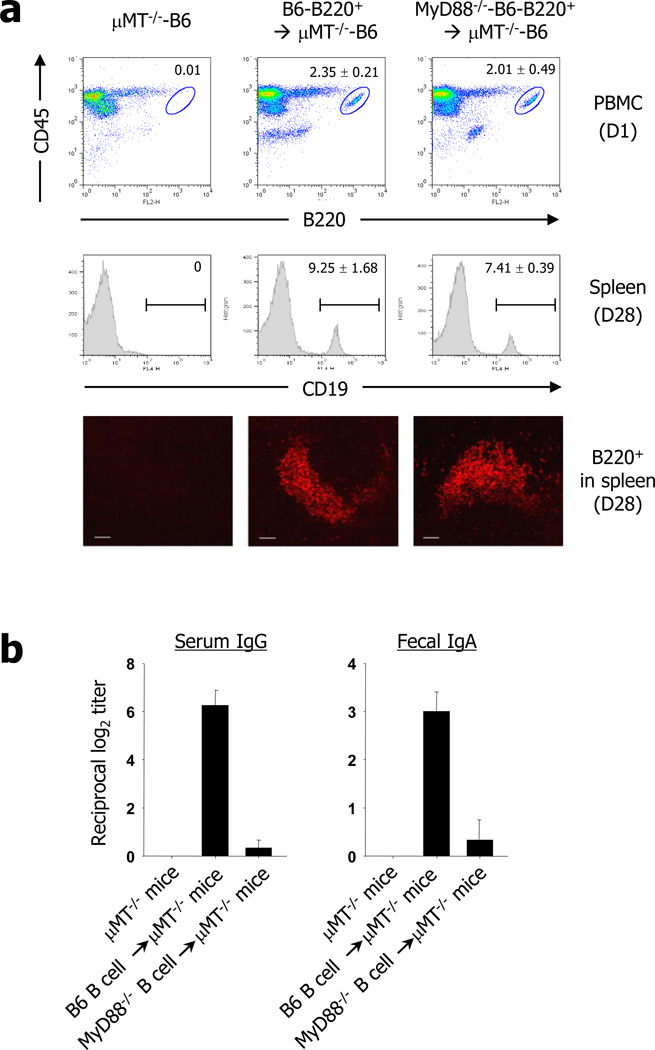
To further clarify the functional role of MyD88−/− B cells for induction of LPS-specific immunoglobulin responses, B220+ cells were isolated from naïve wild-type and MyD88−/− mice of B6 background and reconstituted in µMT−/− mice of B6 background. One day post transfer, proximally 2% of donor-derived B220+ B cells were detected in CD45+ PBMC in all recipient µMT−/− mice which transferred by B220+ cells of wild-type or MyD88−/− mice (Fig. 6a). Mice were then immunized with RASV strain orally. Since the portion of B220+ PBMC were decreased to 1.74±0.53 (wild-type B cells) and 0.75±0.46 (MyD88−/− B cells) at day 14 (data not shown), it seems likely that there was no severe homeostatic expansion of B cells in the recipient µMT−/− mice after adoptive transfer. Further, survival rate of MyD88−/− B cells were slightly less than MyD88+/+ B cells. At 4 wks post vaccination, groups of mice were sacrificed and the splenic B cells were analyzed by both flow cytometry and immunohistochemistry. There were similar level of B cells in the spleen of recipient µMT−/− mice in wild-type B cells-transferred (9.25 ± 1.68) or MyD88−/− B cells-transferred (7.41 ± 0.29) mice (Fig. 6a). Further, both of wild-type and MyD88−/− B cells in µMT−/− mice formed B cell follicle-like lymphoid architecture in spleen (Fig. 6a). It seems likely that there were no defects in the reconstitution of wild-type or MyD88−/− B cells into µMT−/− mice. However, the µMT−/− mice given the wild-type B cells had high levels of LPS-specific serum IgG and fecal IgA LPS-specific IgG and IgA antibody levels when determined in the sera and fecal extracts of recipient µMT−/− mice after RASV vaccination (Fig. 6b). On the contrary, µMT−/− mice given MyD88−/− B cells had no detectable antibodies in systemic and mucosal compartments (Fig. 6b). These results clearly suggest defective function of MyD88−/− B cells for induction of LPS-specific IgG and IgA antibodies following oral administration of RASV strain. Taken together, the LPS-specific antibody production in MyD88−/− mice after oral RASV administration can be ascribed to the Th2-dominant environment in MyD88−/− mice and to the MyD88 signal in other cells rather than to neutralizing antibodies indispensible for the protective role of Salmonella.
FIGURE 6.
Functional role of MyD88−/− B cells for induction of LPS-specific immunoglobulin responses. (a) The B cells were isolated from naïve WT and MyD88−/− mice and reconstituted to µMT−/− mice (4×107 cells/mouse). One day after B cells transfer, B220+ B cells in CD45+ PBMC were analyzed by flowcytometry Then, the recipient µMT−/− mice were immunized with RASV and splenic B cells were analyzed at 4 wks after vaccination by flow cytometry and immunohistochemistry. b, At 1 day after reconstitution, groups of mice were orally administered RASV twice at 2-week intervals and LPS-specific IgG in sera and IgA in fecal extracts measured. Values are mean ± SD. Data are representative of ≥2 independent experiments.
Discussion
To clarify the role of innate immunity for induction of antigen-specific B cell responses following oral vaccination with a bacteria-based vaccine possessing several TLR ligands, we studied TLR4−/− and MyD88−/− mice given recombinant attenuated Salmonella vaccine (RASV) strain. Our findings show that LPS-specific IgG and IgA antibodies were induced after oral administration of RASV strain through a PP-dependent, CD4+ T cell-dependent, and TLR4-mediated MyD88 signaling-independent pathway. Most importantly, although innate immunity mediated by MyD88 signal is not essential for production of LPS-specific antibodies, it is indispensable for survival against oral infection by S. Typhimurium.
The surface of gram-negative bacteria such as salmonellae express several TLR ligands such as LPS (for TLR4), flagellin (for TLR5), and lipoprotein (for TLR2) (11). Previous studies clearly demonstrated that Salmonella LPS is an agonist of TLR4 in vivo and in vitro (18–20). Thus, we speculated that TLR4-mediated MyD88 signal might be crucial for development of LPS-specific B cell responses following oral vaccination with attenuated Salmonella vaccine. However, predominant levels of LPS-specific IgG and IgA antibodies in systemic and mucosal compartments were elicited in TLR4−/− and MyD88−/− mice following oral vaccination with RASV strain, indicating that TLR4-mediated MyD88 signaling is not essential for induction of LPS-specific antibody response. Further, Th2-skewed responses (IgG1 > IgG2a) were elicited in the MyD88−/− mice but not in wild-type mice (IgG1 < IgG2a) following oral RASV (Fig. 2c). When MyD88−/− mice were immunized systemically with KLH-CFA, predominant levels of IL-4 but not IFN-γ were produced by CD4+ T cells isolated from draining lymph nodes in the presence of APC (29). Thus, DCs from wild-type mice induced allogenic T cells to secrete IFN-γ but DCs from MyD88−/− mice skewed T cells to secrete IL-4 instead of IFN-γ in response to LPS (29). Overall, findings from our current study and from earlier works indicate that TLRs in the MyD88-independent pathway can confer APC with the ability to polarize Th2-type immunity, which might be responsible for induction of humoral immunity.
One of our interesting results was that MyD88−/− mice vaccinated with RASV strain, which possessed brisk levels of LPS-specific IgG and IgA antibodies, died at early time points similar to those of non-vaccinated MyD88−/− mice following oral challenge with a virulent Salmonella strain. Both RASV-vaccinated and PBS-vaccinated MyD88−/− mice died without loss of body weight. These results provide convincing evidence that the MyD88 signal is more essential for induction of effective protection against oral Salmonella infection than the enhanced LPS-specific B cell responses induced by TLRs in an independent manner. MyD88−/− mice are susceptible to bacterial challenge and after infection with C. rodentium exhibit bacteremia, gangrenous mucosal necrosis, severe colitis, and death (30). Signals recognized by TLRs are transferred to sub-molecules such as MyD88 and then to the terminal point of signal through the TLR pathway, which can cause production of non-specific defense mediators such as nitric oxide and subsequent secretion of cytokines including IL-6 and TNF-α (11, 12). Alternatively, as suggested in a recent study, MyD88 may have a crucial role in killing gram-negative bacteria (e.g., Escherichia coli and attenuated S. Typhimurium) through NADPH oxidase activity in phagocytic cells, such as macrophages (31). Thus, although an adaptive response occurs, impaired pro-inflammatory cytokines and/or phagocytosis regulated by MyD88-dependent signaling are necessary for efficient clearance of the pathogen.
We further investigated which immune cells play a critical role for induction of acquired immunity following oral administration of an attenuated Salmonella vaccine strain. We found DCs, including important professional APCs, did associate with Salmonella infection by the TLR4 and MyD88 signaling-dependent manner under in vitro culture conditions (Fig. 3b); however, DCs function was not changed at 1, 2, and 5 days following oral administration of RASV strain (data not shown). These results imply there might be alternative cell subsets that play a critical role as APCs in vivo after oral administration of attenuated Salmonella vaccine strains. One recent study suggested accumulation and function of monocyte subsets in the blood, PP, and MLN during oral Salmonella infection (32). The data show that CD68highGr-1intermediate monocytes, along with CD68intermediateGr-1high neutrophils, rapidly accumulate in PP and MLN (32). Further, the monocytes but not neutrophils and DCs have increased MHC-II and costimulatory molecule expression and produce inducible NO synthase after oral Salmonella infection (32). Of note, even though MyD88-deficient B cells were significantly impaired, there were significant levels of neutralizing antibodies in MyD88−/− mice (Fig. 5b and c). Thus, we are now investigating which APC are essential for induction of acquired immunity following oral vaccination of RASV strain in the MyD88-deficient condition.
Since there were no significant differences in B cell reconstitution in µMT−/− mice between wild-type and MyD88−/− B cells (Fig. 6a), we presume that there might be other factors in MyD88−/− mice contributing enhanced B cells response against RASV vaccination. In this regards, we found increased numbers of Gr-1+ myeloid cells in the secondary lymphoid organs (e.g., spleen and mesenteric LN) of MyD88−/− mice after RASV vaccination compared to wild-type mice (data not shown). Thus, it might be possible to speculate that increased Gr-1+ cells under the MyD88 signal-deficient circumstance directly help to secrete Ab production by MyD88−/− B cells in situ. This speculation is supported by others that Gr-1+ IL-4-producing innate cells produced by alum or Schistosoma mansoni egg have shown to induce Th2-biased immune response (33). Alternatively, Gr-1+ cells could derive CD4+ T cells activation to help B cells more efficiently in MyD88−/− mice comparing in wild-type mice. Overall, this kind of expansion of unique cell population in situ under MyD88 signal-deficient circumstance could make difference of B cell function in vitro.
The results of the current study clearly indicate the irrelevance of innate immunity-mediated MyD88 signal with acquired immunity that provides protective efficacy against oral Salmonella infection. This result contributes to our understanding of the relationship between innate- and acquired immunity and will facilitate development of efficacious mucosal vaccines targeting enteric bacterial infection.
Acknowledgements
We thank Dr. Masafumi Yamamoto (Nihon University, Matsudo, Japan) for generous gift of anti-IL-7R mAb and helpful discussion.
Abbreviation used
- RASV
recombinant attenuated S. enterica serovar Typhymurium vaccine
- MyD88
myeloid differentiation primary response gene 88
- BAFF
a B-cell activating factor of the TNF family
- pIgR
polymeric immunoglobulin receptor
- PP
Peyer’s Patches
Footnotes
This work was supported by the Bill & Melinda Gates Foundation (No. 37863); NIH R01-AI056289 and by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (No. 2007-04213 and R01-2008-000-10649-0)
Conflict of Interest
The authors have no conflicting financial interests.
References
- 1.Pang T, Bhutta ZA, Finlay BB, Altwegg M. Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 1995;3:253–255. doi: 10.1016/s0966-842x(00)88937-4. [DOI] [PubMed] [Google Scholar]
- 2.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 3.Fierer J, Guiney DG. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J Clin Invest. 2001;107:775–780. doi: 10.1172/JCI12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stecher B, Macpherson AJ, Hapfelmeier S, Kremer M, Stallmach T, Hardt WD. Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin. Infect Immun. 2005;73:3228–3241. doi: 10.1128/IAI.73.6.3228-3241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–577. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Kiyono H, Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 8.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 9.Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol. 2005;35:180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- 10.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Inohara Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 15.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 16.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtiss R, 3rd, Kelly SM, Hassan JO. Live oral avirulent Salmonella vaccines. Vet Microbiol. 1993;37:397–405. doi: 10.1016/0378-1135(93)90038-9. [DOI] [PubMed] [Google Scholar]
- 18.Poltorak A, He X, Smirnova I, Liu MY, Huffel CVan, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 19.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 21.Mahoney RT, Krattiger A, Clemens JD, Curtiss R., 3rd The introduction of new vaccines into developing countries. IV: Global Access Strategies. Vaccine. 2007;25:4003–4011. doi: 10.1016/j.vaccine.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Kang HY, Srinivasan J, Curtiss R., 3rd Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect Immun. 2002;70:1739–1749. doi: 10.1128/IAI.70.4.1739-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zekarias B, Mo H, Curtiss R., 3rd Recombinant attenuated Salmonella enterica serovar typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin Vaccine Immunol. 2008;15:805–816. doi: 10.1128/CVI.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha+ CD3− cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 25.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashizume T, Togawa A, Nochi T, Igarashi O, Kweon MN, Kiyono H, Yamamoto M. Peyer's patches are required for intestinal immunoglobulin A responses to Salmonella spp. Infect Immun. 2008;76:927–934. doi: 10.1128/IAI.01145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson AJ, MaCoy KD, Johansen F-E, Brandzaeg P. The immune geography of IgA induction and function. Mucosal Immunology. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 29.Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support Th2 cell differentiation. Int Immunol. 2002;14:695–700. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- 30.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 31.Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 32.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 33.McKee AS, MacLeod M, White J, Crawford F, Kappler JW, Marrack P. Gr1+IL-4-producing innate cells are induced in response to Th2 stimuli and suppress Th1-dependent antibody responses. Int Immunol. 2008;20:659–669. doi: 10.1093/intimm/dxn025. [DOI] [PMC free article] [PubMed] [Google Scholar]