Abstract
Skeletal muscles are commonly exposed to radiation for diagnostic procedures and the treatment of cancers and heterotopic bone formation. Few studies have considered the impact of clinical doses of radiation on the ability of satellite cells (myogenic stem cells) to proliferate, differentiate and contribute to recovering/maintaining muscle mass. The primary objective of this study was to determine whether the proliferation of irradiated satellite cells could be rescued by manipulating NO levels via pharmacological approaches and mechanical stretch (which is known to increase NO levels). We used both SNP (NO donor) and PTIO (NO scavenger) to manipulate NO levels in satellite cells. We observed that SNP was highly effective in rescuing the proliferation of irradiated satellite cells, especially at doses less than 5 Gy. The potential importance of NO was further illustrated by the effects of PTIO, which completely inhibited the rescue effect of SNP. Mechanical cyclic stretch was found to produce significant increases in NO levels of irradiated satellite cells, and this was associated with a robust increase in satellite cell proliferation. The effects of both radiation and NO on two key myogenic regulatory factors (MyoD and myogenin) were also explored. Irradiation of satellite cells produced a significant increase in both MyoD and myogenin, effects that were mitigated by manipulating NO levels via SNP. Given the central role of myogenic regulatory factors in the proliferation and differentiation of satellite cells, the findings of the current study underscore the need to more fully understand the relationship between radiation, NO and the functionality of satellite cells.
INTRODUCTION
Satellite cells are myogenic stem cells that initially were discovered by Mauro (1), who observed their presence directly outside the cell membrane of individual muscle fibers (cells). Satellite cells play a fundamental role in dictating the growth of skeletal muscle during development, the regeneration of skeletal muscle in response to myopathies or trauma, and growth in the adult state as might occur after muscle atrophy or in response to resistance training (2–6).
There are over 600 muscles in the human body, and they collectively represent ~40% of the body's mass. Satellite cells play a fundamental role in shaping the body and its physiology. It follows then that any factor negatively influencing the biology of satellite cells also has the potential to produce negative effects both locally (muscle specific) and systemically. In this context, skeletal muscles are commonly exposed to radiation from diagnostic procedures and for the treatment of cancers and heterotopic bone formation (7–11). While skeletal muscle fibers (cells) are postmitotic and as a result are thought to be highly radioresistant, few studies have considered the impact of clinical doses of radiation on the ability of satellite cells to proliferate, differentiate and contribute to recovering/maintaining muscle mass. This is an important consideration because a number of studies (12–19) have noted that muscle mass and function can be negatively affected after procedures involving radiation (e.g., treatment of breast/bone cancers).
We performed a series of baseline studies in a previous study (20) in which we examined the effects of clinical doses of γ radiation on satellite cell proliferation, cell cycle regulation, DNA double-strand breaks, oxidative stress and NO levels. The effect of γ radiation on satellite cell NO levels was of particular interest because Anderson (21) and Allen and colleagues (22–24) demonstrated that the proliferation of satellite cells was dependent on elevations in NO, acting through an MMP2/HGF/c-met-mediated pathway. In our earlier study, we observed that both 1 and 5 Gy reduced NO levels in satellite cells by approximately 50–55% and that this corresponded to large decreases in satellite cell proliferation (30 and 70% decreases, respectively). Our novel observation that radiation exposure leads to a reduction in NO levels is likely to be significant given the central role of NO in mediating a range of biological responses, including satellite cell proliferation.
We hypothesized that satellite cell proliferation might be effectively rescued from the harmful effects of c radiation using an NO donor. Our analyses included measures of NO levels, satellite cell proliferation, the response to mechanical stretch (which is known to increase NO levels), and regulation of key myogenic regulatory factors (MRFs) involved in proliferation and differentiation. Collectively, the findings of the current study demonstrate that NO donors can be used to rescue satellite cells from the harmful effects of γ radiation. However, such effects appear to be limited to doses less than 5 Gy and raise important mechanistic and clinical issues.
METHODS
Satellite Cell Isolation and Tissue Culture
Satellite cells were isolated from male Sprague-Dawley rats weighing approximately 150 g according to the protocol of Allen et al. (25). In brief, muscle groups from the hind limb were excised, trimmed of fat and connective tissue, minced with scissors, and digested with 1.25 mg/ml protease type XIV (Sigma, St. Louis, MO) for 1 h at 37°C. Cells were separated from muscle fibers and tissue debris by differential centrifugation and plated on Corning plastic flasks coated with matrigel in a 1:100 dilution with PBS-minus. Cells were grown in the presence of low-glucose Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA) containing 20% fetal bovine serum (Hyclone, Logan, UT), 1× penicillin-streptomycin (Gibco), and 40 μg/ml of G418 (Sigma). Cells were maintained in a humidified atmosphere of 95% air/5% CO2 at 37°C. They were kept in exponential growth and passaged twice weekly. Experiments were conducted according to guidelines set forth by the Institutional Review Board at the University of California, Irvine.
Irradiation
Exponentially growing cultures of satellite cells were exposed to γ radiation (1, 2 or 5 Gy) using a 137Cs irradiator (J. L. Shepherd and Associates Mark I) at a dose rate of 2.2 Gy/min.
NO Production Assay
Satellite cells were seeded at 10,000 cells per well of a 96-well clear-bottom black plate overnight. Prior to the assays, the satellite cell medium containing phenol red was removed and replaced with Krebs Ringer buffer (118 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgSO4, 1 mM KH2PO4, 2.5 mM CaCl2, 25 mM Hepes, 5 mM glucose) containing 10 μM DAF (4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate, Invitrogen, Carlsbad, CA) and either 1 or 100 μM SNP (Sigma-Aldrich) with or without 5 μM of the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO, Sigma-Aldrich). The fluorescence of DAF (excitation 495 nM, emission 515 nM) was measured on a Synergy MX plate reader (Biotek, Winooski, VT), preheated to and maintained at 37°C, every 8 min for 100 min. The relative fluorescence unit (RFU) values were plotted and used to determine the instantaneous change in RFU per minute as an indicator of NO production as detected by the increase in DAF fluorescence over time.
Proliferation Assays with SNP and/or PTIO
Satellite cells were plated onto 24-well Costar plastic plates coated with matrigel in a 1:100 dilution with PBS-minus. Cells from passages 1–3 were used, and 2500 cells were plated per well in 1 ml of medium.
The cells were γ-irradiated with 0, 1, 2 and 5 Gy 1 day after plating. SNP (Calbiochem, La Jolla, CA) and/or PTIO (MP Biomedicals, Irvine, CA) were added immediately after irradiation at concentrations of 0, 0.3, 1, 3 and 30 μM SNP and 0 and 5 μM PTIO. Daily medium changes with fresh drug were performed over the course of 72 and 96 h.
Cell Counting
Satellite cells were counted in 24-well plates using the SynergyMx plate reader. After the medium was removed from each well, the cells were washed once with PBS-minus and placed in a −80°C freezer for a minimum of 30 min to facilitate cell lysis. Cell lysis was completed using a mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA) containing 1× SYBRGreen I dye (Invitrogen) to quantify nucleic acids. Fluorescence intensity was detected at an excitation wavelength of 497 ± 9 nm and an emission wavelength of 520 ± 13.5 nm. The mean RFU obtained from the plate reader were calibrated against a standard curve to determine absolute cell counts.
FACs Analysis of MyoD and Myogenin Expression
Satellite cells from passage 1 were grown on matrigel-coated T75 flasks. The cells were γ-irradiated with 0, 1, 2 and 5 Gy. Fresh medium ± 1 mM SNP was added immediately after irradiation, and daily medium changes with fresh SNP were performed over 96 h. At 96 h, the cells were harvested and fixed as single cells in 4% paraformaldehyde in PBS-minus. The cells were then incubated with primary MyoD and myogenin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 1:20 in an antibody diluent (Dako, Carpinteria, CA) at 4°C overnight. After incubation with immunofluorescent secondary antibodies for 1 h, the cells were immediately subject to FACS analysis.
Manipulating NO Levels Using Continuous Cyclic Mechanical Stretch
Satellite cells were plated on BioFlex amino-culture plates (Flexcell International, McKeesport, PA). These plates have flexible membranes whereby vacuum pressures can be used to impose various patterns of strain (both static and dynamic) on cultured cells. The cells were irradiated with 5 Gy and then were subjected to cyclic strain (20.5% strain) at a frequency of 0.1 Hz (once every 10 s) for 24 h, using the Flexcell FX-4000 system. The proliferation and NO levels in these irradiated + stretched cells were then compared with those in cells that were only irradiated with 5 Gy. The cyclic stretch protocol used in this study was modeled after that of Tatsumi et al. (23, 24), who observed that this protocol produced an increase in satellite cell proliferation that was mediated by NO.
Statistics
All data are reported as means ± SE. The NO data for satellite cells shown in Fig. 1 were analyzed using three-way ANOVA (SNP, PTIO, time). The cell count data shown in Figs. 2 and 3 were analyzed using two-way ANOVA. The MyoD, myogenin and mechanical stretch data were analyzed using one-way ANOVA. All statistical analyses were performed using Systat 10.2 (Chicago, IL). Statistical significance was defined as P < 0.05.
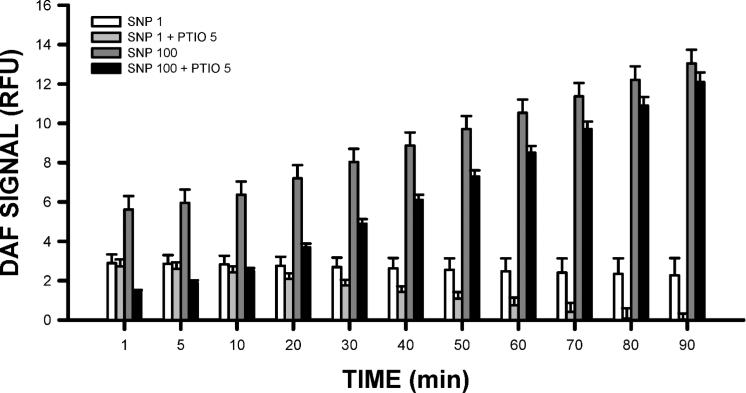
FIG. 1.
The effects of an NO donor (SNP) and scavenger (PTIO) on nitric oxide-based DAF fluorescence in satellite cells. Measures of DAF signal intensity were also made under conditions in which neither SNP nor PTIO was present. The DAF signal intensity under this null condition was zero and is not shown. Note that 1 μM of SNP produced a significant (P < 0.001) increase in NO levels that remained elevated throughout the 90-min experiment. When PTIO was added in combination with 1 μM of SNP, there was an initial rise in NO levels, but over 90 min the NO levels progressively declined to baseline levels (P < 0.001). When SNP was increased to 100 μM, there was a large and continuous increase in NO levels (P < 0.001). Although PTIO was initially effective in suppressing the effects of 100 μM SNP, at the later times PTIO did not quench the SNP effect. Each data point represents the mean ± SE.
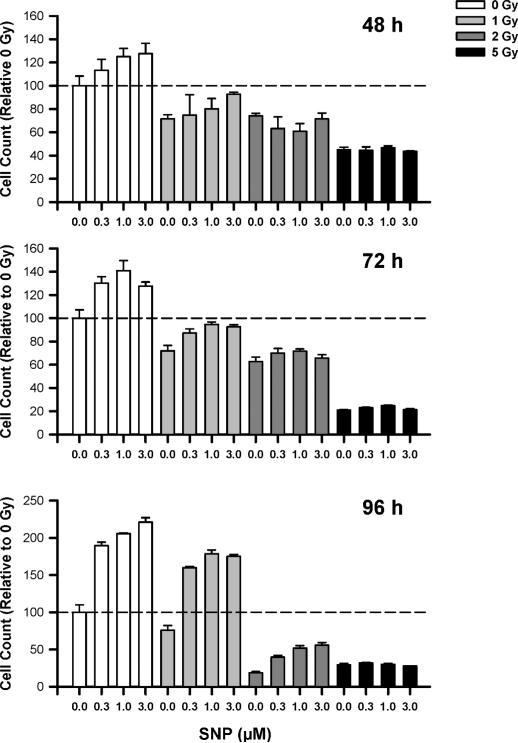
FIG. 2.
Rescue of satellite cells from the effects of γ radiation. Cell count data were collected 48, 72 and 96 h after irradiation. All data were normalized to the cell count measured under control conditions (first bar in each panel; dashed horizontal line; 0 Gy; 0 μM SNP). SNP significantly increased cell counts at each time. Furthermore, SNP was capable of rescuing proliferation in satellite cells receiving <5 Gy. All data are reported as means + SE. Data for each time were analyzed using two-way ANOVA with group variables of radiation dose and SNP concentration. The main effects (radiation dose, SNP concentration and interaction effect) were all significant at P < 0.05 at each time.
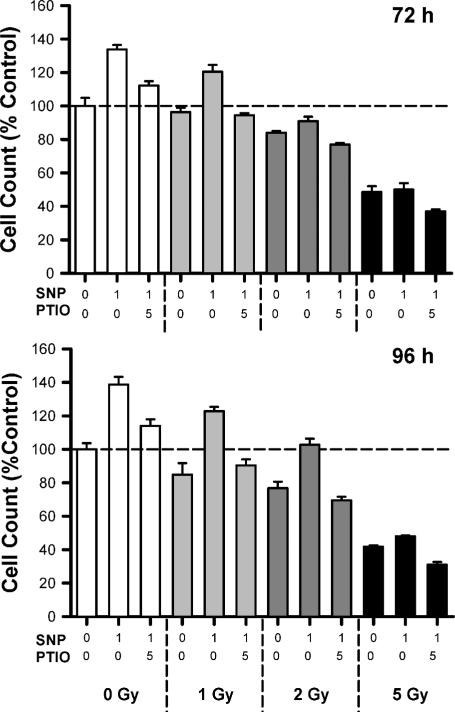
FIG. 3.
The rescue effect of NO can be blocked using the NO scavenger PTIO. Data were collected 72 and 96 h after γ irradiation. The effects of SNP shown at each time are consistent with the concept that NO donors can be used to rescue satellite cell proliferation after irradiation. However, to provide further evidence of the importance of NO, we used the NO scavenger PTIO to blunt/block the rescue effect of SNP. At each time and each dose of γ radiation, PTIO was highly effective in blocking the rescue effect of SNP. A PTIO-only treated group was also included at each time as a negative control, and the mean value was only 2% greater than the control cell counts (data not shown). All results are reported as means ± SE. Data for each time and each dose of γ radiation were analyzed using two-way ANOVA. The interaction effect (SNP × PTIO main effect) was highly significant (P = 5 0.001) at each dose and time.
RESULTS
Modulating NO Levels Using SNP and PTIO
As noted above, we used DAF signal intensity as a measure of NO levels. In pilot studies (data not shown), we examined the effects of three different NO donors (SNP, SIN-1, NO-sulindac) at various concentrations. Based on the reliability and consistency of the DAF and proliferation data we obtained, SNP was chosen as the NO donor for these studies. To further validate our ability to manipulate NO levels, we used the NO-specific scavenger PTIO to reduce NO levels and quench DAF fluorescence.
The use of 1 μM SNP produced a rapid elevation in DAF levels of satellite cells, which was observed at the earliest time (1 min; Fig. 1). DAF levels remained elevated throughout the 90 min of observation. When SNP was used in combination with the NO scavenger PTIO (5 μM), DAF fluorescence was attenuated, indicating the ability of PTIO to reduce NO levels in satellite cells. The efficiency of scavenging increased over time, with DAF levels reduced to background levels by 70 min in the presence of PTIO (Fig. 1).
To determine the dependence of DAF fluorescence on NO generation, a second, higher concentration of SNP (100 μM) was used. The presence of 100 μM of SNP produced significantly higher DAF levels in satellite cells compared to the lower concentration of 1 μM (Fig. 1) and continued to rise throughout the 90-min time course (Fig. 2). The presence of PTIO under elevated SNP concentrations was still found to attenuate DAF fluorescence (Fig. 1). Initially, this attenuation led to substantial reductions in the DAF signal induced by SNP (≤30 min). However, the signal intensity of DAF gradually increased over time, and after 60 min, no significant differences in DAF intensity were found in the presence of PTIO, suggesting that the higher SNP levels generated saturating levels of NO, overcoming the scavenging capacity of PTIO under the stated conditions.
Rescuing Satellite Cells from the Harmful Effects of γ Radiation: The Role of NO
The primary focus of this study was to determine whether a NO donor could be used to rescue satellite cells from the harmful effects of γ radiation. To address this issue, we performed a time-course study (48, 72 and 96 h) that examined the interactive effects of different concentrations of SNP (0, 0.3, 1 and 3 μM) and doses of γ radiation (0, 1, 2 and 5 Gy).
All three concentrations of SNP were effective at increasing satellite cell proliferation in nonirradiated cells, even at the earliest time examined (48 h; Fig. 2). At 96 h, all three concentrations of SNP produced increases in cell counts that were at least 90% greater than the control value (Fig. 2, bottom panel).
When satellite cells were irradiated with 1 Gy, the cell count was reduced approximately 25–28% (at 48, 72 and 96 h). At the earliest time, only the highest concentration of SNP appeared to be effective in rescuing satellite cell proliferation. However, at 96 h, each concentration of SNP was highly effective at rescuing satellite cell proliferation. For instance, 0.3 μM SNP more than doubled satellite cell proliferation (~160% of the control condition) above the 1-Gy condition (75% of the control condition, Fig. 1 bottom panel). SNP concentrations of 1.0 and 3.0 μM produced similar increases in proliferation that were ~2.3-fold higher than cells exposed to 1 Gy in the absence of SNP.
The impact of SNP on proliferation progressively declined at higher doses of γ radiation. Progressive and significant reductions in proliferation were found in satellite cells irradiated with 2 Gy (Fig. 2). At 96 h, cell counts were reduced by ~80% compared to unirradiated controls. As noted above, the ability of SNP to promote proliferation was evident at the earliest time (48 h) in cells irradiated with 1 Gy. At higher doses, this effect was markedly attenuated, and the ability of SNP to stimulate proliferation was delayed and was evident only at 96 h (Fig. 2). Treatment with SNP was only able to partially rescue satellite cell proliferation. For example, while the counts of cells treated with 1 and 3 μM SNP were ~2.9 times greater than those cells receiving 2 Gy, SNP treatment elevated cell counts to levels that were only ~50% of control values (0 Gy). SNP was ineffective at rescuing cell counts for those cells exposed to 5 Gy, suggesting a possible threshold for NO-mediated rescue of satellite cell proliferation.
Inhibiting the Cellular Response to NO Using Specific NO Scavengers
To provide further evidence regarding the potential importance of NO in mediating the effects of γ radiation on satellite cell proliferation, a series of follow-up studies were performed in the presence of the NO scavenger PTIO. Consistent with our prior findings (Fig. 2), cell proliferation was found to depend on the presence of NO derived from SNP. Satellite cell proliferation was fully restored by SNP 96 h after irradiation with 1 or 2 Gy (Fig. 3). As noted above, SNP was incapable of rescuing satellite cell proliferation at 5 Gy. The boost in proliferation afforded by the SNP was completely abolished in the presence of PTIO at either 72 or 96 h postirradiation (Fig. 3). Cells subjected to PTIO alone were relatively unaffected, and they exhibited a modest 2% increase in proliferation compared to untreated controls (data not shown). Thus these experiments show that under clinically relevant doses (≤2 Gy), cell proliferation depends on NO levels and can be manipulated in a predictable way by interventions that directly modulate NO.
Effects of Cyclic Stretch on Cell Counts and NO Production in Irradiated Satellite Cells
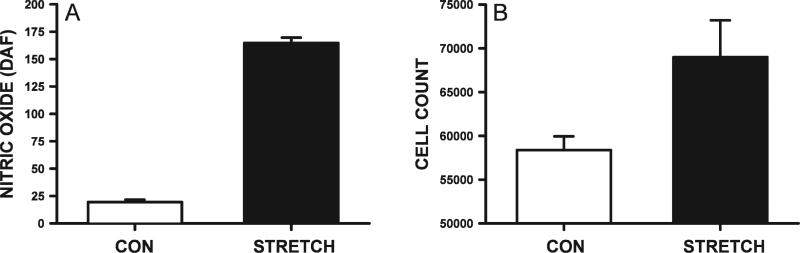
To determine whether an in vitro model of mechanical stretch could elicit similar cellular changes in the absence of SNP, satellite cells were irradiated with 5 Gy and subjected to the Flexcell system involving 24 h of cyclic stretch. Mechanical stretch of the satellite cells over this period produced a large and highly significant (P < 0.001) increase in NO levels as reflected by the increased DAF fluorescence (Fig. 4). The increased NO caused by mechanical stretch translated to enhanced proliferation; a significantly (P < 0.02) higher number of cells (~20%) were found after 5 Gy compared to irradiated satellite cells cultured identically but in the absence of stretching.
FIG. 4.
Effects of mechanical stretch on satellite cell proliferation after γ irradiation. Satellite cells exposed to 5 Gy were subjected to a cyclic stretch over 24 h. Assessment of NO levels and proliferation showed that mechanical stretch resulted in significantly higher levels of NO (P < 0.01) and increased numbers of cells (P < 0.01) compared to unstretched controls. Cells were assayed in triplicate, and results are shown as means ± SE.
Effects of γ Radiation and NO on Myogenic Regulatory Factors (MRFs)
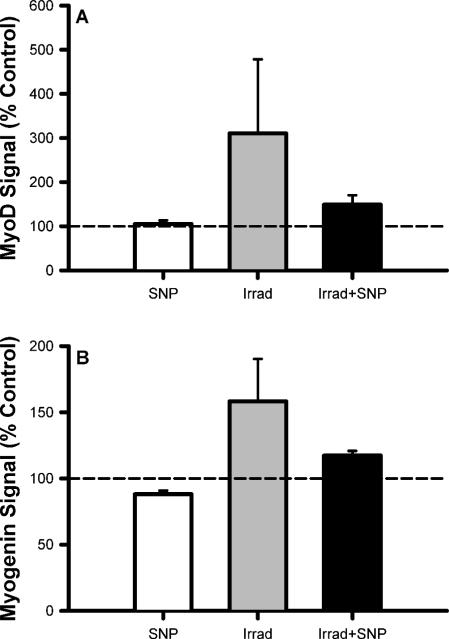
To determine in part how γ radiation and SNP impact key cellular mediators of proliferation and differentiation, satellite cells were analyzed for changes in the levels of two MRFs, MyoD and myogenin, after exposure to these agents. SNP alone had little effect on MyoD and myogenin (Fig. 5). In contrast, when satellite cells were treated with 2 Gy of γ radiation, there were large and consistent increases in both MyoD and myogenin. Both the MyoD and myogenin levels approached baseline levels when irradiated cells were also given 1 μM SNP (Fig. 5).
FIG. 5.
Effects of 2 Gy γ radiation and SNP on expression of MyoD and myogenin. All results are expressed relative to the null condition (0 Gy and 0 μmM SNP; horizontal dashed line). SNP did not affect either MyoD or myogenin levels. A dose of 2 Gy produced significant increases in MyoD (P < 0.01) and myogenin levels (P < 0.01). This radiation effect on these myogenic regulatory factors was consistently suppressed when irradiated cells were treated with SNP. All assays were run in triplicate. All results are reported as means ± SE.
DISCUSSION
Skeletal muscles are frequently injured due to trauma, ischemia or unusually large loading forces. Typically, such injuries produce a robust regenerative response that is highly dependent on the proliferative capacity of satellite cells. Unlike these overt forms of injury, irradiation of skeletal muscle elicits more subtle effects that can impair the impressive regenerative capacity of skeletal muscle by adversely impacting the activation and proliferation of satellite cells. In this context, we observed previously (20) that clinically relevant doses of γ radiation produced significant reductions in satellite cell proliferation. We also observed that γ radiation of satellite cells produced a reduction in their NO levels. This latter observation was of particular interest given that Anderson et al. (21) and Tatsumi et al. (24, 26) have published a significant amount of data demonstrating that NO plays a central role in mediating satellite cell proliferation. The primary objective of the present study was to test the idea that NO donors can be used to rescue the proliferation of irradiated satellite cells. The findings of this study are unique in at least four ways. First, we observed that NO donors were effective in rescuing the proliferation of irradiated satellite cells, with an apparent threshold for restoration limited to doses <5 Gy. Second, we found that an NO scavenger was effective in blocking the pro-proliferative effects of NO donors, further demonstrating the potential importance of NO. Third, mechanical stretch of satellite cells produced significant increases in NO levels and was more effective than SNP at rescuing proliferation of irradiated satellite cells (i.e., enhanced proliferation was still found after 5 Gy in cultures subjected to stretching). Finally, radiation alone was found to produce large increases in key myogenic regulatory factors, an effect that could be reversed by scavenging NO through the use of PTIO after γ irradiation.
The Proliferation of Irradiated Satellite Cells Can be Affected by Manipulating NO Levels
The cellular effects of NO have been described in several publications, and while a preponderance of evidence implicates the importance of the MMP2/ HGF/c-met pathway in mediating the biological responses of satellite cells to physiological stress, other pathways may exert an influence over satellite cell proliferation (27–30). In other cell types, elevated proliferation has been found to depend upon the sustained activation of PI3K-AKT signaling, driven by nitric oxide synthase activation and NO production that stimulates the Ras family members (27). Inflammation has also been found to trigger similar pro-proliferative responses that depend upon PI3K signaling and NO production (28). Thus exogenous NO used in these studies may act in part by short-circuiting normal regulatory channels to stimulate proliferation through the prolonged activation of PI3K-Akt signaling. Key discoveries during the past decade have led to the current view that NO plays a central role in regulating the proliferation of satellite cells. In particular, Anderson (21) demonstrated that the repair of injured muscles was impaired when NOS was pharmacologically inhibited in iNOS knockout mice. Complementary studies by Allen and his colleagues have shown that NO controls satellite cell proliferation by acting through an MMP2/ HGF/c-met pathway (22, 23, 25, 31, 32). The evidence supporting this view can be summarized as follows: (1) HGF has been shown to robustly increase satellite cell proliferation (25); (2) anti-neutralizing HGF antibodies have been shown to prevent satellite cell proliferation (23); (3) NO has been shown to release bound HGF from MMP2 (32); and (4) inhibitors of MMP2 completely block the proliferative response of satellite cells to stretch (33).
As noted above, we recently observed that γ radiation reduces NO levels in satellite cells (20). This observation combined with those of Anderson (21) and Allen and colleagues (22–25, 31–33) suggests that the impaired proliferation in irradiated satellite cells might be amenable to rescue by manipulation of NO levels through pharmacological or mechanotransduced interventions. Therefore, in this study, we manipulated NO levels in irradiated cells using two different but complementary approaches. The first involved treating irradiated satellite cells with a well-known NO donor, SNP. As shown in Figs. 2 and 3, satellite cell proliferation was rescued using SNP donors. The sparing effect of SNP on proliferation became more pronounced at 96 h, suggesting that further beneficial effects might be found at more protracted times postirradiation. To further emphasize the importance of NO in rescuing irradiated satellite cells, a subsequent set of experiments was conducted to determine whether an NO scavenger (PTIO) could inhibit the pro-proliferative effects of an NO donor. The results clearly demonstrated that PTIO could attenuate the proliferative gain afforded by the SNP, providing further evidence to support the functional relevance of NO in regulating satellite cell proliferation.
Mechanical Stretch Can be Used to Rescue the Proliferation of Irradiated Satellite Cells
In previous studies (24, 32), it was shown that mechanical stretch of satellite cells results in marked increases in proliferation that are dependent on NO. Thus we performed an additional study to assess the feasibility of rescuing the proliferation of irradiated satellite cells using mechanical stretch. Mechanical stretch was found to markedly increase NO levels in irradiated satellite cells and, more importantly, to significantly increase proliferation compared to the control cells. Noteworthy too was the capability of mechanical stretch to promote the proliferation of satellite cells irradiated with 5 Gy. The inability of SNP to spare the loss of proliferation after a dose of 5 Gy suggests some potential differences in the efficiency and/or mechanisms by which NO exerts its influence. While the precise mechanism of action is unclear, data suggest that exogenous (pharmacologically derived) NO derived from SNP was not as efficacious at promoting proliferation in irradiated satellite cells because the NO derived endogenously through mechanotransduction. Thus our in vitro model of “physical rehabilitation” may have important clinical ramifications, since many cancer patients require interventions involving radiation and subsequently develop muscle atrophy and loss of function. Our results suggest that physical therapy involving cyclical stretch may mitigate the harmful effects of γ radiation on satellite cells and consequently minimize loss of muscle mass and function to hasten the recovery of the irradiated tissue bed.
Irradiation of Satellite Cells Produces an Increase in Key Myogenic Transcription Factors That Can be Reversed Using NO
Myogenic regulatory factors (MRFs) are a subfamily of basic helix-loop-helix transcription factors. The primary MRFs include MyoD, myogenin, Myf-5 and MRF4, and they have been shown to control the development of muscle (5, 6). MyoD−/− satellite cells have a severely impaired proliferative capacity, and this strongly suggests that MyoD plays a central role in the proliferation/differentiation of satellite cells (34).
The effects of genotoxic and cytotoxic agents on the expression and regulation of MRFs in satellite cells have been poorly studied, and thus a consensus regarding the impact of such agents on MRFs is lacking. The need for further research in this area is emphasized by the small number of conflicting studies, the small number of genotoxic/cytotoxic agents examined thus far, and the novel observations of the current study. The primary focus has been on oxidative stress. However, while Lee et al. (35, 36) observed that increased oxidative stress via manipulation of glutathione peroxidase leads to significant elevations in both MyoD (~18-fold) and myogenin (~4-fold) expression in myoblasts, Zaccagnini et al. (36) observed that increased oxidative stress via H2O2 produced a decrease in both MyoD and myogenin. We observed that γ radiation produced an increase in both MyoD and myogenin, an effect that could be blunted by treating cells with the NO donor SNP. While it is possible that the effects of radiation and SNP-derived NO are toggling satellite cells between states of proliferation and differentiation, further speculation regarding the impact of both γ radiation and NO on the expression of MRFs should await further experimentation.
Summary
The ubiquitous distribution of skeletal muscle predis-pose it as an unintended target during radiotherapy and related clinical interventions and underscores the need for a more complete understanding of radiation effects in this tissue. The present findings suggest that manipulating NO levels in satellite cells can modulate certain harmful effects of γ radiation. Elevating NO levels in irradiated satellite cells effectively rescues their proliferation, a finding that provides additional impetus for understanding further the biology of NO, its downstream effectors, and the impact of γ radiation on this system.
ACKNOWLEDGMENTS
This work was supported by grants from the California Institute for Regenerative Medicine no. RS1-00413 (CLL and VJC), the National Space Biomedical Research Institute through NASA NCC 9-58 (BPT and CLL), and a pilot project (VJC) supported through NIH Grant HD50837 to the National Skeletal Muscle Research Center at the University of California, San Diego.
REFERENCES
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J. Appl. Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- 5.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson JR, Evarts CM, Hubbard LF. Radiation therapy in the prevention of heterotopic ossification after total hip arthroplasty. Hip. 1982:211–227. [PubMed] [Google Scholar]
- 8.Lo TC, Healy WL, Covall DJ, Dotter WE, Pfeifer BA, Torgerson WR, Wasilewski SA. Heterotopic bone formation after hip surgery: prevention with single-dose postoperative hip irradiation. Radiology. 1988;168:851–854. doi: 10.1148/radiology.168.3.3136510. [DOI] [PubMed] [Google Scholar]
- 9.Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J. Bone Joint. Surg. Am. 1981;63:201–208. [PubMed] [Google Scholar]
- 10.Ashton LA, Bruce W, Goldberg J, Walsh W. Prevention of heterotopic bone formation in high risk patients post-total hip arthroplasty. J. Orthop. Surg. (Hong Kong) 2000;8:53–57. doi: 10.1177/230949900000800210. [DOI] [PubMed] [Google Scholar]
- 11.Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:1289–1299. doi: 10.1016/j.ijrobp.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res. Treat. 2005;94:1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 13.Johansson K. Is physiotherapy useful to the breast cancer patient? Acta Oncol. 2005;44:423–424. doi: 10.1080/02841860510029914. [DOI] [PubMed] [Google Scholar]
- 14.Keramopoulos A, Tsionou C, Minaretzis D, Michalas S, Aravantinos D. Arm morbidity following treatment of breast cancer with total axillary dissection: a multivariated approach. Oncology. 1993;50:445–449. doi: 10.1159/000227227. [DOI] [PubMed] [Google Scholar]
- 15.Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44:449–457. doi: 10.1080/02841860510029905. [DOI] [PubMed] [Google Scholar]
- 16.Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res. Treat. 2008;110:19–37. doi: 10.1007/s10549-007-9710-9. [DOI] [PubMed] [Google Scholar]
- 17.Senkus-Konefka E, Jassem J. Complications of breast-cancer radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2006;18:229–235. doi: 10.1016/j.clon.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, Sugden E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res. Treat. 2007;106:19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 19.Sugden EM, Rezvani M, Harrison JM, Hughes LK. Shoulder movement after the treatment of early stage breast cancer. Clin. Oncol. (R. Coll. Radiol.) 1998;10:173–181. doi: 10.1016/s0936-6555(98)80063-0. [DOI] [PubMed] [Google Scholar]
- 20.Caiozzo VJ, Giedzinski E, Baker M, Suarez T, Izadi A, Lan M, Cho-Lim J, Tseng B, Limoli CL. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat. Res. 2010;174:582–589. doi: 10.1667/RR2190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol. Biol. Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve. 2004;30:654–658. doi: 10.1002/mus.20114. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol. Biol. Cell. 2002;13:2909–2918. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am. J. Physiol. Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 25.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi R, Wuollet AL, Tabata K, Nishimura S, Tabata S, Mizunoya W, Ikeuchi Y, Allen RE. A role for calcium-calmodulin in regulating nitric oxide production during skeletal muscle satellite cell activation. Am. J. Physiol. Cell Physiol. 2009;296:C238–C252. doi: 10.1152/ajpcell.00471.2008. [DOI] [PubMed] [Google Scholar]
- 27.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheu ML, Chao KF, Sung YJ, Lin WW, Lin-Shiau SY, Liu SH. Activation of phosphoinositide 3-kinase in response to inflammation and nitric oxide leads to the up-regulation of cyclooxygenase-2 expression and subsequent cell proliferation in mesangial cells. Cell Signal. 2005;17:975–984. doi: 10.1016/j.cellsig.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Hickok JR, Thomas DD. Nitric oxide and cancer therapy: the emperor has NO clothes. Curr. Pharm. Des. 2010;16:381–391. doi: 10.2174/138161210790232149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int. J. Biochem. Cell Biol. 2008;40:2183–2191. doi: 10.1016/j.biocel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Yamada M, Tatsumi R, Kikuiri T, Okamoto S, Nonoshita S, Mizunoya W, Ikeuchi Y, Shimokawa H, Sunagawa K, Allen RE. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve. 2006;34:313–319. doi: 10.1002/mus.20601. [DOI] [PubMed] [Google Scholar]
- 34.Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin. Cell Dev. Biol. 2005;16:575–584. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Shin HS, Shireman PK, Vasilaki A, Van Remmen H, Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic. Biol. Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Zaccagnini G, Martelli F, Magenta A, Cencioni C, Fasanaro P, Nicoletti C, Biglioli P, Pelicci PG, Capogrossi MC. p66(ShcA) and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. J. Biol. Chem. 2007;282:31453–31459. doi: 10.1074/jbc.M702511200. [DOI] [PubMed] [Google Scholar]