Abstract
Background/Aims
Greater responsiveness of emotional arousal circuits in relation to delivered visceral pain has been implicated as underlying central pain amplification in Irritable Bowel Syndrome (IBS), with females showing greater responses than males.
Methods
Functional MRI was used to measure neural responses to an emotion recognition paradigm, using faces expressing negative emotions (fear and anger). Sex and disease differences in the connectivity of affective and modulatory cortical circuits were studied in 47 IBS (27 premenopausal females) and 67 healthy controls (HCs; 38 premenopausal females).
Results
Male subjects (IBS+HCs) showed greater overall brain responses to stimuli than female subjects in prefrontal cortex, insula, and amygdala. Effective connectivity analyses identified major sex and disease related differences in the functioning of brain networks related to prefrontal regions, cingulate, insula, and amygdala. Males had stronger connectivity between anterior cingulate subregions, amygdala, and insula, whereas females had stronger connectivity to and from the prefrontal modulatory regions (medial/dorsolateral cortex).
Conclusions
Male IBS demonstrate greater engagement of cortical and affect related brain circuitry compared to male controls and females, when viewing faces depicting emotions previously shown to elicit greater behavioral and brain responses in male subjects.
Keywords: Irritable bowel syndrome (IBS), sex differences, emotion recognition
1.0.0 INTRODUCTION
Sex-related differences in the structure and function of the human brain [36] have paralleled sex-specific prevalence rates of chronic pain disorders [19,47,54]. Sex based differences in the brain’s response to symptom related affective and cognitive stimuli may be important for understanding the pathophysiology of these disorders – in terms of increased susceptibility to develop these disorders, and in order to develop more effective individualized therapies [67].
Irritable Bowel Syndrome (IBS) occurs with a slightly greater prevalence in females [10,19,43,50], and sex related differences in visceral perception, autonomic nervous system and brain responses to visceral stimuli have been reported [11,39]. Male subjects show greater sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis responses to certain types of stress, whereas females show reduced vagal tone and greater visceral hypersensitivity [11,70]. Brain imaging studies in rodents [78] and in IBS subjects [44–46,73,77] during aversive visceral stimulation, and expectation of such stimuli, demonstrate greater engagement of cortical regions (insula [INS] and dorsal prefrontal cortex [PFC]) in males, and greater engagement of affective brain regions and related circuits in females (amygdala [AMYG], subgenual cingulate cortex [sgACC]) [40,53]. These findings suggest that in response to gut (and disease) related stimuli, IBS subjects show sex-related differences in brain activation and functional connectivity.
In response to emotion-related stimuli (including faces, images, words, odors, music) female subjects generally show greater brain activation related to emotions of sadness, disgust and unpleasantness [67], whereas men demonstrate greater neural responses to emotions such as anger, fear, and guilt [29,67]. Differences in brain responses to the viewing of faces expressing different emotions have been used to measure differences in the engagement of emotion-related brain circuits and their cortical modulation [13,20,22,58,66], as well as disease [18,56,63,66,76] and sex-related [21,29,36,67] differences in these circuits. Even though differences in amygdala responsiveness and cortical modulation of such responses when viewing fear related faces have been demonstrated, this paradigm is not associated with changes in subjective emotions or autonomic responses [15,23].
In the current study, we used the paradigm of viewing of negative affective (fear and anger) and neutral faces to test sex and IBS related differences in brain response associated with cognitive processes, i.e. processing negative emotions. We studied a large sample of male and predominantly premenopausal female IBS subjects and matched HCs by monitoring brain responses to negative facial emotions (fear and anger) in the NimStim paradigm [71], which is a variation on the Ekman faces [16,71]. We aimed to test the following hypotheses: 1) Greater brain responses in affective regions, and less recruitment of prefrontal inhibitory regions will be observed in IBS compared to HCs, in male compared to female subjects, in male IBS compared to female IBS, and in male HCs compared to female HCs. 2) Greater regional brain activation by the emotional faces paradigm will be accompanied by changes in the effective connectivity of the emotion related circuit and its cortical modulatory input.
2.0.0 MATERIALS AND METHODS
2.1.0 Participants
This study was approved by the institutional review board of the University of California, Los Angeles. All subjects provided written informed consent to participate. IBS subjects were recruited through the UCLA Digestive Disease Clinic and from community advertisements. The diagnosis of IBS was confirmed using Rome III [14] criteria during a clinical examination by a gastroenterologist or nurse practitioner experienced in functional GI disorders. IBS is defined as recurrent abdominal pain or discomfort for at least 3days/month in the last 3 months and is associated with two or more of the following: 1) Improvement in defecation. 2) Onset associated with a change in frequency of stool. 3) Onset associated with a change in form (appearance) of stool. Healthy control subjects (HCs) were recruited by advertisement and screened via medical exam for absence of functional pain disorders. Inclusion criteria for all subjects included the absence of current or past psychiatric illness or substance abuse disorder and the absence of major medical or neurological conditions. No subjects were taking medications for 30 days prior to scanning. 14 healthy controls from another onsite imaging study took a placebo medication on the day of scanning. In order to determine if the HC subjects taking placebo could be combined with the HC subjects not taking placebo, an independent sample t-test was applied using Statistical Parametric Mapping V8 [51] and indicated no statistically significant differences in brain response to stimuli on the faces paradigm between the two groups, supporting the combination of the two groups to create a larger sample size of HC subjects.
2.2.0 Questionnaires
Subjects completed the UCLA Bowel Symptom Questionnaire (BSQ) [49] to measure symptom severity, and anxiety was measured using the HAD Anxiety measure and depression was measured using the HAD Depression [81]. For female subjects, menopause status was assessed by a self-report question, which categorized the subjects as either premenopausal or postmenopausal, and the majority of the studies were done during the follicular phase of the menstrual cycle. The majority of female subjects in the study (82%) were not taking any oral contraceptives.
2.3.0 fMRI Data Acquisition
fMRI was performed using a 3.0T MRI scanner (Siemens Trio; Siemens, Erlangen, Germany). A high resolution structural image was acquired from each subject with a magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2300 ms, echo time (TE) = 2.85 ms, 256 slices, 160*240 matrix, 3 mm voxel size. Functional blood oxygen-level dependent (BOLD) images were acquired (TR = 3000 ms, TE = 28 ms, flip angle = 90°, 38 slices, slice thickness = 3 mm) while subjects completed two runs of the emotional faces tasks. Stimuli were presented via MRI-compatible goggles using Superlab 4.0 software (Cedrus Corp, San Pedro, CA). Subjects responded using an MRI-compatible button box by pressing one of two buttons with the right hand.
2.4.0. fMRI Imaging Task: Faces Paradigm
One of the most commonly used experimental paradigms for fMRI studies has been the viewing of images with negatively valenced facial expressions [24,27,31]. Paradigms using negative emotional facial expressions have also been used in several imaging genetics studies demonstrating increased hyperresponsiveness of emotion related networks (including the amygdala) in healthy control subjects with increased harm avoidance and SERT gene polymorphisms [25,26,34], and within several psychiatric disorders including posttraumatic stress disorder [18,63], autism [76], trait anxiety [56,66], and Parkinson’s disease [61].
During this fMRI study, brain responses to the NimStim Emotional Faces Viewing task [71] were measured. During matching emotions (ME), subjects viewed a target face depicting an angry or fearful expression and were asked to select one of two other faces that expressed the same emotion. During matching form (MF), a condition controlling for the sensory-motor aspects of the ME task, subjects viewed a target circular shape (approximately the same size as a human face) and were asked to select one of two other shapes that best matched the target. Participants also viewed the same target faces as in the matching condition, but had to judge which of two linguistic labels, such as angry or afraid, best described the emotion (identifying emotion (IDE)). As a control task, the subjects viewed the same target faces, but labeled the faces based on their gender, either male or female, and not on affect (identifying gender (IDG)). We did not analyze the results of the linguistic labeling task in this manuscript. Stimuli were shown with randomized sequences counter-balanced across 2 runs. Each condition was presented as a block of 6 images, with each image presented for 3 sec, with a total block length of 18 sec. In each run, each condition (i.e., match forms) was randomly presented. An instruction cue was presented for 3 sec prior to each block and a rest period of 6 sec followed each block. Each run began with a 30 second anticipatory baseline.
2.5.0 Data Analysis: Image Processing and Data Analysis
Preprocessing
The first two volumes were discarded to allow for stabilization of the magnetic field. The remaining functional images were slice-time and motion corrected, spatially normalized to the MNI template, and spatially smoothed with an 8 mm3 Gaussian kernel using SPM8 (Welcome Department of Cognitive Neurology, London, UK). Brain activity during the Emotional Faces Viewing task was estimated for each subject with first-level fixed effects general linear models specifying the 30 second anticipatory baseline (from 2 runs, 3sec inter-stimulus intervals, 3sec cues, ME, MF, IDE, and IDG. Brain activity was measured by contrasting brain responses during ME and MF tasks.
Statistical Parametric Mapping
Sex differences during the matching tasks (ME-MF) were tested in apriori specified regions comprising emotional arousal, cortico-modulatory and homeostatic afferent circuitry by applying a second-level random effects general linear model specifying group (Male IBS, Female IBS, Male HC, and Female HC) as a factor via the full factorial model option in SPM8. Here the group factor represents the interaction of disease (IBS, HC) and sex (male, female). After thresholding whole brain activity maps at p<.005 uncorrected, each region of interest was tested using linear contrast analysis and small volume correction in SPM which corrects for the number of voxels comprising each region of interest. Furthermore, we applied false discovery rate (FDR) to correct the voxel-corrected probability values to control for the number of ROIs tested. Cluster level significance was considered at q<.05 [3,4,28].
Linear contrasts
For each region of interest we specified contrasts to test the main effects of disease (IBS vs. HC) and sex (Males vs. Females). Furthermore, we explicitly tested two global (omnibus) interaction contrasts to determine whether differences in sex depend upon disease, e.g., [HC(Male-Female) - IBS(Male-Female)] and whether disease differences depend on sex [Female(HC-IBS) - Male(HC-IBS)]. We also tested four a priori linear contrasts: 1) Are there sex differences in brain activity within IBS? (Male IBS vs. Female IBS), 2) Are differences in brain activity in males due to disease? (Male IBS vs. Male HC), and 3) Are differences in brain activity in females due to disease (Female IBS vs. Female HC), 4) Are there sex differences in brain activity within HC? (Male HC vs. Female HC), To examine whether anxiety and depression contribute to group differences, we calculated two additional models as described above entering anxiety and depression as a covariate.
Regions of Interest
Based on prior research, sixteen regions of interest (ROIs) were chosen a priori and consisted of emotion related brain areas including amygdala (AMYG), cingulate cortex subregions (anterior mid cingulate cortex [aMCC], subgenual cingulate cortex [sgACC], pregenual cingulate cortex [pACC], posterior cingulate cortex [PCC], anterior insula [aINS], hippocampus [HIPP], hypothalamus [HYPO], dorsal pons [PAG], and nucleus accumbens [NACC]) [37,38,59] as well prefrontal regions involved in cognitive control processes during detection and regulation of affectively salient stimuli (ventrolateral prefrontal cortex [vlPFC], ventromedial prefrontal cortex [vmPFC], dorsal medial prefrontal cortex [dmPFC], and dorsolateral prefrontal cortex [dlPFC]) [57,60]. We also examined the involvement of the posterior insula (pINS) as it provides interoceptive signals to the anterior insula (aINS) and mid insula (mINS) for integration with cortical and affective signals to provide the basis for feeling and self [12].
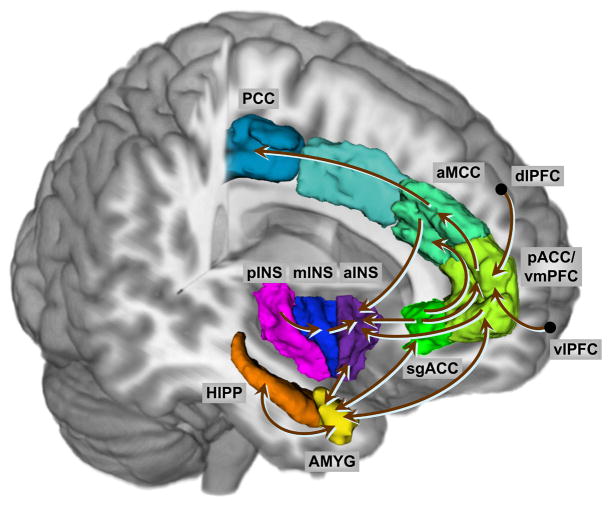
Effective connectivity analysis was applied to test for hypothesized group differences (IBS vs. HC, Male vs. Female, Male IBS vs. Female IBS) in the engagement of a stress-related emotional arousal circuits during the viewing of specific negative emotional faces (anger and fear). Described previously [38,59,65], the affective network is characterized by core inhibitory circuitry comprising pACC/vmPFC, AMYG and sgACC in addition to extended connectivity/interactions with the HIPP, aMCC, aINS, mINS, pINS, dlPFC, and vlPFC. In line with known anatomical connectivity in the macaque [74], a priori connections between nodes of the network were specified to test for differences in the effective connectivity of the a) the core inhibitory circuit (pACC/vmPFC to AMYG) b) the influence of top-down cognitive control influences (dlPFC, vlPFC) and extended affective system connectivity with the aMCC, PCC, HIPP, and INS. Given the limitations in specifying all potential bilateral connections we specified bidirectional AMYG connectivity only (Figure 1).
Figure 1. Cortical-Affective Circuit Effective Connectivity Model.
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; vlPFC, ventro-lateral prefrontal cortex
For each region comprising the nodes of the circuit, brain activity was extracted from the spatial location of the peak voxels identified from the results of the primary SPM analyses. After specifying the structural model, path analysis using a structural equation modeling (SEM) framework was performed with Amos 18.0 conducting full information likelihood estimation. Standard errors for parameter estimates (unstandardized betas) were obtained via 500 bootstrapped samples and used to calculate bias-corrected 95% confidence intervals based on the normal distribution. Residual variances, representing external input into the system (e.g. unspecified regions, psychological characteristics), were fixed at 35% [48] of the observed regional variances within group (IBS, HC) and sex (Male, Female) conditions. Group differences (Males IBS, Female IBS, Male HC, Female HC) in the effective connectivity of the cortico-limbic network in IBS and HCs were tested using specific multi-group tests for invariance [32,48]. Given our hypotheses, we specified three a priori contrasts examining sex and disease effects: 1) Male IBS vs. Female IBS, 2) Male IBS vs. Male HC, and 3) Female IBS vs. Female HC. Critical values for the multigroup significance tests were based on a one degree of freedom chi-square distribution where critical values where χ2Δ=3.84, p=.05 and χ2Δ= 6.64, p=.01. However, false discovery rate (FDR) was applied to control for the number of paths tested per group comparison (n=19) and significance was considered at q<.05 [3,4,28]. For interpretation of results, unstandardized betas, which represent effect size in terms of standard deviation units, were interpreted as weak (β=0 to.30), moderate (β=30 to.80), or strong (β= >.80).
Data analysis of non-imaging data
Differences in clinical and demographics variables were examined using the general linear model in SPSS version 19 by specifying a 2×2 independent group ANOVA.
3.0.0 Results
3.1.0 Clinical and behavioral characteristics
Table 1 summarizes clinical and personality characteristics of the four study groups (Male IBS, Female IBS, Male HC, Female HC). Although mostly within normal clinical ranges, 18% of all subjects (IBS+HCs) had anxiety scores above the clinical cutoff and <1% of all subjects had depression scores above the clinical cutoff. Compared to HCs, IBS subjects as a group showed significantly higher anxiety symptoms (F=14.58, p<0.001), with male IBS subjects showing slightly higher levels than female IBS (F=4.26, p=0.041). Small group differences were also observed for depression symptoms between IBS and HCs (F=21.43, p<0.001) and similarly between males and females (F=6.62, p=0.011). The female sample was predominantly premenopausal (93% of female HCs and 92% of female IBS subjects).
Table 1.
Study Demographics and Characteristics
| IBS | CONTROL | F | p | |||
|---|---|---|---|---|---|---|
| MALES | FEMALES | MALES | FEMALES | |||
| N | 20 | 27 | 29 | 38 | - | - |
|
| ||||||
| PREMENOPAUSAL | - | 25 | - | 35 | - | - |
|
| ||||||
| AGE (yrs) | 36.05 (12.56) | 31.41 (10.35) | 35.61 (12.46) | 31.74 (13.19) | a) 0.00 b) 3.39 |
a) .100 b) .068 |
|
| ||||||
| IBS Symptom Severity | 3.05 (0.76) | 3.00 (0.62) | 1.03 (0.19) | 1.03 (0.16) | a) 27.35 b) 0.44 |
a) <.001** b) .508 |
|
| ||||||
| ANXIETY (HAD) | 6.80 (4.51) | 5.59 (3.54) | 2.83 (2.62) | 3.63 (2.50) | a) 14.58 b) 4.26 |
a) <.001** b) .041* |
|
| ||||||
| DEPRESSION (HAD) | 3.70 (3.7) | 3.23 (2.55) | 1.90 (2.09) | 0.95 (1.11) | a) 21.43 b) 6.62 |
a) <.001** b) .011* |
Measures: IBS Symptom Severity: 1=None; 2=Mild; 3=Moderate; 4=Severe; 5=Very Severe
HAD: Hospital Anxiety and Depression Measure
Premenopausal status was determined using a question and female subjects were classified as premenopausal and postmenopausal
Groups: IBS, Irritable Bowel Syndrome; HC, Healthy Controls
a) IBS vs. HC; b) Males vs. Females
Significance levels:
p<.05,
p<.001
3.2.0 Disease and sex-related differences in brain responses
3.2.1 Disease related differences
No statistically significant differences in the response of emotional arousal regions were found between all IBS subjects (N=47) and all HCs (N=67) while viewing negative emotional faces.
3.2.2 Sex related differences
When comparing all female subjects (IBS+HC; n=65) with all male subjects (IBS+HC; n= 49), significant differences in emotional arousal regions were observed. During the viewing of negative faces, male subjects showed greater activation compared to females in subregions of the PFC (mPFC, left dlPFC), of the cingulate cortex (including PCC, sgACC, and aMCC,) and of the INS (mINS, pINS), as well as the NACC and the HIPP (Table 2, Figure 2a). Female subjects showed greater activity compared to males in the right dlPFC and PAG while viewing negative faces (Figure 2b).
Table 2.
Peak MNI Coordinates of Significant Activation for Males vs. Females for the Matched Emotions versus Matched Forms Contrast (ME-MF)
| Region | Hemisphere | Cluster Size | p value | q valu(FDR)e | Z | X mm | Y mm | Z mm | |
|---|---|---|---|---|---|---|---|---|---|
| ME-MF | |||||||||
|
| |||||||||
| Males-Females | |||||||||
| mINS | Left | 180 | .007 | .010 | 5.503 | −38 | 0 | 10 | |
| mINS | Right | 281 | .002 | .003 | 4.826 | 36 | 4 | 6 | |
| pINS | Left | 528 | .001 | .001 | 4.925 | −34 | −26 | 14 | |
| pINS | Right | 432 | .001 | .002 | 4.866 | 36 | −24 | 4 | |
| mPFC | Left | 860 | .001 | .001 | 5.618 | −6 | 44 | −2 | |
| mPFC | Right | 215 | .012 | .017 | 4.327 | 2 | 48 | 4 | |
| dlPFC | Left | 312 | .023 | .028 | 5.521 | −40 | 2 | 10 | |
| aMCC | Left | 112 | .026 | .031 | 4.216 | 0 | 28 | 18 | |
| aMCC | Right | 127 | .022 | .028 | 4.362 | 2 | 30 | 18 | |
| pACC | Left | 995 | .001 | .001 | 5.618 | −6 | 44 | −2 | |
| pACC | Right | 589 | .001 | .001 | 4.823 | 0 | 34 | −4 | |
| sgACC | Left | 116 | .012 | .015 | 5.492 | −4 | 34 | −4 | |
| sgACC | Right | 134 | .010 | .014 | 4.823 | 0 | 34 | −4 | |
| HIPP | Left | 18 | .049 | .050 | 3.333 | −28 | −28 | −14 | |
| NACC | Left | 3 | .036 | .040 | 4.748 | −8 | 16 | −4 | |
| NACC | Right | 7 | .029 | .033 | 3.742 | 10 | 12 | −4 | |
| PCC | Left | 256 | .001 | .001 | 4.260 | −2 | −36 | 38 | |
| PCC | Right | 256 | .001 | .002 | 4.520 | 2 | −24 | 38 | |
| Females-Males | |||||||||
| dlPFC | Right | 749 | .001 | .001 | 4.48 | 46 | 20 | 22 | |
| PAG | Left | 5 | .022 | .022 | 3.48 | 0 | −28 | −4 | |
| PAG | Right | 7 | .027 | .027 | 4.27 | 4 | −30 | −6 | |
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, HYPO, hypothalamus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; pACC, pregenual cingulate cortex; PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; NACC, nucleus accumbens;
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME-MF, matching emotions versus matching forms
Coordinates are represented in MNI (Montreal Neurologic Institute) space with x, y, z coordinates
FDR: False discovery rate (correction for multiple comparisons)
Figure 2. Sex Differences (IBS+HC) for (ME-MF).
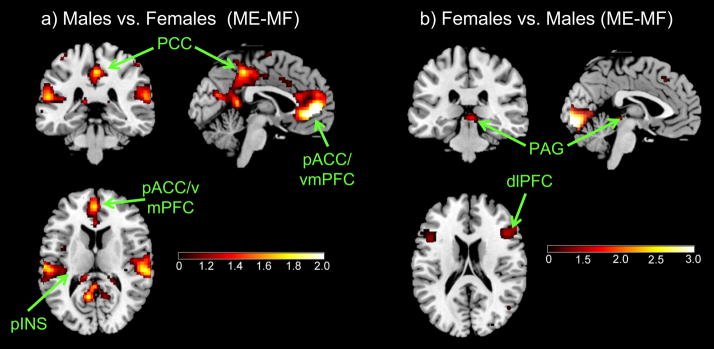
a) Males vs. Females (ME-MF)
b) Females vs. Males (ME-MF)
IBS: Irritable bowel syndrome, HC: Healthy control
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; mPFC, medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; PAG, dorsal pons; NACC, nucleus accumbens.
3.2.3 Interaction between sex and disease
Interaction contrasts examining the differences between males and females related to disease [(Male IBS-Female IBS) – (Male HC-Female HC)] indicated greater differences in brain responses at the set level between male and female IBS subjects as opposed to male and female HCs for the response of right amygdala, bilateral insula, bilateral pregenual cingulate cortex, and right mPFC (Table 3, Figure 3). No other interaction effects were observed.
Table 3.
Peak MNI Coordinates of Significant Activation for the Interaction between Sex and Disease [(Male IBS-Female IBS) – (Male HC-Female HC)] Effects for the Matched Emotions versus Matched Forms Contrast (ME-MF)
| Region | Hemisphere | Set p value | q value (FDR) | Number of Set Clusters | Cluster Size | Z | X mm | Y mm | Z mm | |
|---|---|---|---|---|---|---|---|---|---|---|
| ME-MF | ||||||||||
| [(Male IBS – Female IBS) – (Male HC – Female HC)] | ||||||||||
| pINS | Left | <.001 | .001 | 4 | 2 | 2.773 | −36 | −4 | −18 | |
| <.001 | .001 | 2 | 2.763 | −34 | −10 | −4 | ||||
| <.001 | .001 | 1 | 2.721 | −36 | −16 | −10 | ||||
| <.001 | .001 | 1 | 2.692 | −34 | −12 | −8 | ||||
| pINS | Right | .039 | .045 | 2 | 7 | 3.099 | 38 | −2 | −16 | |
| .039 | .045 | 19 | 2.962 | 36 | −12 | −2 | ||||
| mPFC | Right | .024 | .030 | 3 | 38 | 3.095 | 12 | 50 | 16 | |
| .024 | .030 | 22 | 2.901 | 20 | 60 | 28 | ||||
| . 024 | .030 | 5 | 2.754 | 2 | 40 | 12 | ||||
| pACC | Left | .001 | .002 | 4 | 27 | 2.812 | 0 | 40 | 10 | |
| .001 | .002 | 1 | 2.768 | −18 | 48 | 6 | ||||
| .001 | .002 | 3 | 2.746 | −2 | 30 | 8 | ||||
| .001 | .002 | 1 | 2.634 | −8 | 34 | 6 | ||||
| pACC | Right | .013 | .017 | 3 | 35 | 3.096 | 12 | 50 | 16 | |
| .013 | .017 | 28 | 2.812 | 0 | 40 | 10 | ||||
| .013 | .017 | 1 | 2.697 | 0 | 30 | 8 | ||||
| AMYG | Right | .005 | .007 | 2 | 22 | 3.159 | 32 | −2 | −18 | |
| .005 | .007 | 11 | 3.063 | 18 | −6 | −18 | ||||
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, HYPO, hypothalamus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; pACC, pregenual cingulate cortex; PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; NACC, nucleus accumbens;
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME-MF, matching emotions versus matching forms
Coordinates are represented in MNI (Montreal Neurologic Institute) space with x, y, z coordinates
FDR: False discovery rate (correction for multiple comparisons)
Figure 3. Interaction between sex and disease for (ME-MF).
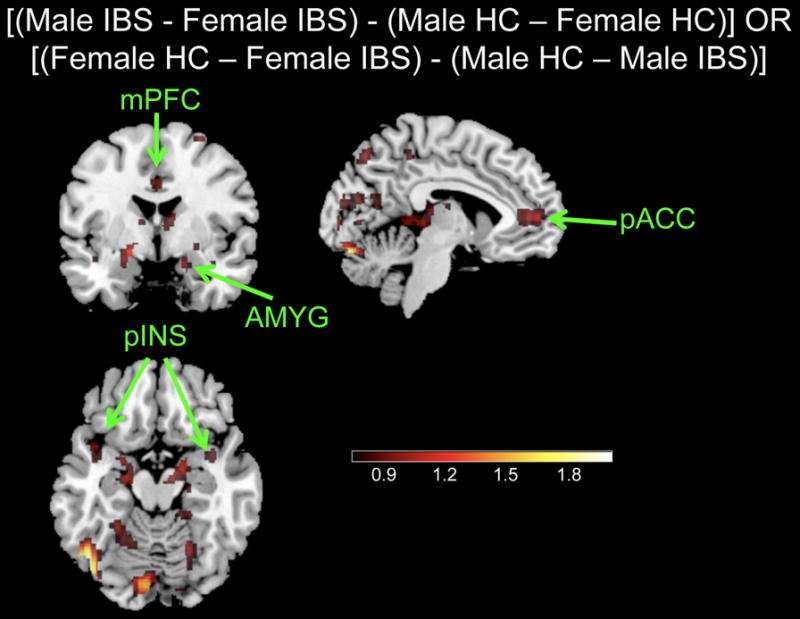
[(Male IBS-Female IBS) – (Male HC-Female HC)] or [(Female HC-Female IBS) – (Male HC-Male IBS)]
IBS: Irritable bowel syndrome, HC: Healthy control
Regions: AMYG, amygdala; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus; HYPO, hypothalamus; aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; NACC, nucleus accumbens.
3.2.4 Sex and disease related differences
Are there sex differences in brain activity within IBS? As shown in Table 4, male IBS subjects (N=20) compared to female IBS (N=27) showed greater activation in several subregions of the PFC (mPFC, dlPFC), ACC (pACC, sgACC, aMCC), PCC, INS subregions (mINS, pINS), as well as HIPP, HYPO and NACC (Table 4, Figure 4a).
Are differences in brain activity in males due to disease? Male IBS subjects compared to male HCs showed greater activation in the AMYG (Table 4, Figure 4b).
Are differences in brain activity in females due to disease? No significant differences were seen for female IBS subjects compared to female HCs (Table 4).
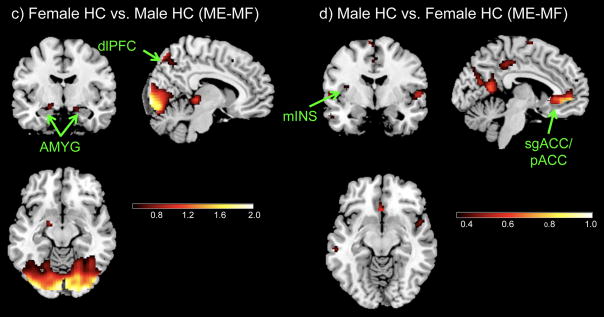
Are there sex differences in brain activity within HC? Female HCs compared to male HCs (Table 4, Figure 4c) showed greater activation in the cognitive modulatory regions of the dlPFC and the PAG. Male HCs (N=29) compared to female HCs (N=38) (Table 4, Figure 4d) showed greater activation in several subregions of the ACC (pACC, sgACC), mINS and NACC. For specific within group ROI activity (activations and deactivations) see supplemental Table 1.
Table 4.
Peak MNI Coordinates of Significant Activation for the Sex and Disease Effects for the Matched Emotions versus Matched Forms Contrast (ME-MF)
| Region | Hemisphere | Cluster Size | p value | q value (FDR) | Z | X mm | Y mm | Z mm | |
|---|---|---|---|---|---|---|---|---|---|
| ME-MF | |||||||||
|
| |||||||||
| Male IBS-Female IBS | |||||||||
| mINS | Left | 149 | .001 | .015 | 4.663 | −42 | 0 | 8 | |
| mINS | Right | 263 | .001 | .005 | 4.099 | 40 | −4 | 14 | |
| pINS | Left | 286 | .001 | .004 | 4.128 | −36 | −22 | 16 | |
| pINS | Right | 414 | .001 | .002 | 4.333 | 38 | −24 | 6 | |
| mPFC | Left | 736 | .001 | .001 | 4.715 | −6 | 46 | 2 | |
| mPFC | Right | 347 | .005 | .005 | 3.775 | 2 | 48 | 8 | |
| dlPFC | Left | 330 | .019 | .026 | 5.092 | −40 | 2 | 10 | |
| aMCC | Left | 95 | .033 | .041 | 3.922 | 0 | 26 | 18 | |
| aMCC | Right | 131 | .021 | .026 | 3.993 | 2 | 30 | 16 | |
| pACC | Left | 972 | .001 | .001 | 4.715 | −6 | 46 | 2 | |
| pACC | Right | 654 | .001 | .001 | 4.043 | 0 | 46 | 8 | |
| sgACC | Left | 107 | .012 | .018 | 3.876 | −4 | 32 | −2 | |
| sgACC | Right | 104 | .015 | .021 | 4.160 | 6 | 24 | −2 | |
| HIPP | Left | 31 | .001 | .018 | 3.340 | −28 | −28 | −14 | |
| HYPO | Left | 1 | .001 | .048 | 2.939 | −10 | −2 | −2 | |
| NACC | Left | 3 | .036 | .042 | 3.869 | −8 | 16 | −4 | |
| NACC | Right | 1 | .038 | .042 | 2.812 | 8 | 18 | −4 | |
| PCC | Left | 863 | .001 | .002 | 3.864 | −12 | −18 | 46 | |
| PCC | Right | 863 | .001 | .002 | 4.115 | 8 | −30 | 48 | |
| Male HC–Female HC | |||||||||
| mINS | Left | 44 | .047 | .050 | 2.772 | −34 | −4 | 14 | |
| pACC | Left | 236 | .008 | .014 | 4.113 | −4 | 34 | −4 | |
| pACC | Right | 132 | .027 | .041 | 3.5557 | 0 | 32 | −6 | |
| sgACC | Left | 101 | .013 | .022 | 4.113 | −4 | 34 | −4 | |
| sgACC | Right | 84 | .020 | .031 | 3.628 | 0 | 26 | −8 | |
| NACC | Left | 2 | .038 | .049 | 2.772 | −10 | 18 | −4 | |
| NACC | Right | 5 | .031 | .046 | 3.157 | 10 | 12 | −4 | |
| Female HC–Male HC | |||||||||
| dlPFC | Left | 1059 | .001 | .001 | 5.648 | −44 | 14 | 24 | |
| PAG | Left | 6 | .021 | .026 | 3.707 | 0 | −28 | −4 | |
| PAG | Right | 7 | .027 | .032 | 4.157 | 4 | −30 | −6 | |
| Male IBS–Male HC | |||||||||
| AMYG | Right | 11 | .045 | .050 | 2.830 | 30 | 0 | −22 | |
| AMYG | Right | 22 | .047 | .079 | 3.159 | 32 | −2 | −18 | |
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, HYPO, hypothalamus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; pACC, pregenual cingulate cortex; PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; NACC, nucleus accumbens;
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME-MF, matching emotions versus matching forms
Coordinates are represented in MNI (Montreal Neurologic Institute) space with x, y, z coordinates
FDR: False discovery rate (correction for multiple comparisons)
Figure 4. Disease and Sex Differences for (ME-MF).
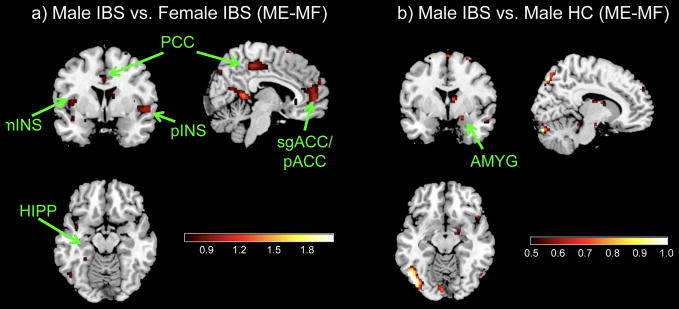
a) Male IBS vs. Female IBS
b) Male IBS vs. Male HC
c) Female HC vs. Male HC
d) Male HC vs. Female HC
IBS: Irritable bowel syndrome, HC: Healthy control
Regions: AMYG, amygdala; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus; HYPO, hypothalamus; aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; NACC, nucleus accumbens.
When controlling symptoms of anxiety and depression, results remained. In addition male IBS compared to female IBS had greater activity in the HYPO (MNI -10,-2,-2; Z=2.9; k=3; p=0.045) when accounting for anxiety and greater activity in the aINS (MNI -42,0,-4; Z=4.1; k=266, p=0.002) when accounting for depression.
3.3.0 Effective connectivity in the stress related cortical-affective circuitry during the matched emotions versus the matched forms contrast (ME-MF)
Widespread group differences were observed in the connectivity of prefrontal and hippocampal modulatory inputs to AMYG and aINS, as well as the connectivity between INS subregions, and cingulate subregions. The signified chi-square values for the paths specified in the model survived FDR correction. A complete list of these differences are shown in Tables 5a and 5b, and Figure 5.
Table 5a.
Beta weights and Confidence Intervals for Disease and Sex Effects for the Matched Emotions versus the Matched Forms Contrast (ME-MF).
| Matching Emotions –Matching Forms (ME-MF) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Male HC | Female HC | Male IBS | Female IBS | |||||
| Beta | CI (L-U) | Beta | CI (L-U) | Beta | CI (L-U) | Beta | CI (L-U) | |
| A. Affective Limbic Circuit | ||||||||
|
| ||||||||
| sgACC → AMYG | −0.13 | −0.67 | −0.18 | −0.58 | 0.25 | −0.51 | −0.24 | −0.86 |
| 0.25 | 0.14 | 0.77 | 1.01 | |||||
|
| ||||||||
| AMYG → sgACC | 0.13 | −0.14 | −0.19 | −0.84 | 0.24 | −0.26 | −0.23 | −0.70 |
| 0.42 | 0.14 | 1.00 | 0.51 | |||||
|
| ||||||||
| sgACC → pACC/vmPFC | 0.51 | 0.08 | 0.54 | 0.26 | 0.48 | 0.11 | 1.39 | 0.53 |
| 1.00 | 1.03 | 1.21 | 2.17 | |||||
|
| ||||||||
| pACC/vmPFC → AMYG | −0.20 | −0.61 | 0.11 | −0.50 | −0.39 | −1.38 | 0.24 | −0.75 |
| 0.21 | 0.52 | 0.49 | 0.71 | |||||
|
| ||||||||
| pACC/vmPFC → aINS | 0.16 | −0.33 | −0.13 | −0.41 | 0.58 | −0.59 | 0.25 | −0.09 |
| 0.57 | 0.37 | 1.39 | 0.71 | |||||
|
| ||||||||
| sgACC → aMCC | 0.16 | −0.14 | 0.01 | 0.23 | −0.13 | 0.58 | −0.44 | −1.04 |
| 0.43 | 0.23 | 0.92 | 0.00 | |||||
|
| ||||||||
| aMCC → aINS | 0.82 | 0.25 | 0.42 | 0.01 | 0.99 | … | 0.41 | 0.07 |
| 1.71 | 0.74 | 1.45 | 0.81 | |||||
|
| ||||||||
| AMYG → aINS | −0.20 | −0.76 | 0.01 | −0.36 | 0.09 | −0.48 | 0.02 | −0.55 |
| 0.43 | 0.42 | 0.97 | 0.53 | |||||
|
| ||||||||
| aINS → AMYG | 0.52 | −0.01 | 0.40 | −0.22 | 0.39 | … | 0.78 | −0.35 |
| 0.83 | 0.91 | 0.58 | 1.37 | |||||
|
| ||||||||
| B. Prefrontal and Hippocampal Modulatory Input to Affective Limbic Circuit | ||||||||
|
| ||||||||
| dlPFC →pACC/vmPFC (to amyg) | −0.12 | −0.59 | −0.22 | −0.62 | 0.03 | −0.28 | 0.78 | −0.59 |
| 0.18 | 0.15 | 0.73 | 1.89 | |||||
|
| ||||||||
| vlPFC → pACC/vmPFC (to amyg) | 0.06 | −0.62 | −0.05 | −0.74 | 0.24 | … | −0.01 | −0.69 |
| 1.22 | 0.39 | 0.59 | 0.77 | |||||
|
| ||||||||
| HIPP → AMYG | 0.78 | −0.09 | 0.32 | −0.25 | −0.03 | −0.84 | −0.14 | −1.06 |
| 1.54 | 1.05 | 1.00 | 2.18 | |||||
|
| ||||||||
| AMYG → HIPP | 0.16 | −0.01 | 0.04 | −0.29 | 0.16 | −0.18 | 0.64 | −0.79 |
| 0.39 | 0.25 | 0.54 | 1.49 | |||||
|
| ||||||||
| C. Intrainsular Connectivity | ||||||||
|
| ||||||||
| pINS → mINS | 0.41 | 0.24 | 0.57 | 0.22 | 0.60 | 0.20 | 0.57 | 0.15 |
| 0.56 | 1.09 | 1.14 | 1.08 | |||||
|
| ||||||||
| mINS → aINS | 0.89 | −0.01 | −0.04 | −0.58 | 0.29 | −0.86 | 0.12 | −0.53 |
| 1.95 | 0.33 | 1.48 | 0.79 | |||||
|
| ||||||||
| sgACC → aINS | −0.28 | −0.59 | −0.04 | −0.41 | −0.43 | −1.47 | −0.27 | −0.69 |
| 0.11 | 0.22 | 0.59 | 0.22 | |||||
|
| ||||||||
| D. Intracingulate Connectivity | ||||||||
|
| ||||||||
| AMYG → pACC/vmPFC | −0.01 | −0.45 | 0.07 | −0.30 | −0.46 | −0.87 | −0.24 | −0.94 |
| 0.53 | 0.57 | … | 2.25 | |||||
|
| ||||||||
| pACC/vmPFC → aMCC | −0.19 | −0.45 | 0.00 | −0.42 | 0.69 | −0.27 | 0.38 | −0.30 |
| 0.13 | 0.35 | 1.66 | 0.63 | |||||
|
| ||||||||
| aMCC → PCC | 0.00 | −0.46 | 0.21 | −0.27 | 0.51 | −0.29 | 0.72 | 0.29 |
| 0.82 | 0.83 | 0.79 | 1.12 | |||||
Critical Values: Uncorrected: χ2=6.63, p < .01, χ2=3.84, p < .05; significance after FDR corrected:
p < .01 corrected,
p < .05 corrected (Male IBS vs. Female IBS, Male HC vs. Female HC, df=1)
Bootstrapped CI(L-U): confidence interval- lower to upper limit, 95% bias corrected
Beta weights: weak (β=0 to.30), moderate (β=30 to.80), or strong (β= >.80).
… When AMOS was unable to estimate the upper or lower bound confidence limits
Regions: AMYG, amygdala; aINS, mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; vlPFC, ventro-lateral prefrontal cortex
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME, matching emotions; MF, matching forms
Table 5b.
χ2 differences Disease and Sex Effects for the Matched Emotions versus the Matched Forms Contrast (ME-MF).
| Matching Emotions-Matching Forms (ME-MF) | |||
|---|---|---|---|
| Male IBS vs. Female IBS X2 difference |
Male IBS vs. Male HC X2 difference |
Female IBS vs. Female HC X2 difference |
|
| A. Affective Limbic Circuit | |||
| sgACC → AMYG | 3.9 | 2.5 | 0.0 |
| AMYG → sgACC | 3.2 | 0.2 | 0.0 |
| sgACC → pACC/vmPFC | 13.5** | 0.0 | 11.7** |
| pACC/vmPFC → AMYG | 5.6 | 0.5 | 0.4 |
| pACC/vmPFC → aINS | 0.6 | 0.9 | 7.6** |
| sgACC → aMCC | 1.9 | 2.3 | 7.2** |
| aMCC → aINS | 4.4 | 0.3 | 0.0 |
| AMYG → aINS | 0.1 | 0.9 | 0.0 |
| aINS → AMYG | 2.6 | 0.5 | 2.2 |
| B. Prefrontal and Hippocampal Modulatory Input to Affective Limbic Circuit | |||
| dlPFC → pACC/vmPFC (to amyg) | 5.9 | 1.0 | 8.2** |
| vlPFC → pACC/vmPFC (to amyg) | 0.9 | 0.5 | 0.0 |
| HIPP → AMYG | 0.2 | 4.5 | 3.8 |
| AMYG → HIPP | 9.3** | 0.0 | 13.0** |
| C. Intrainsular Connectivity | |||
| pINS → mINS | 0.0 | 1.0 | 0.0 |
| mINS → aINS | 0.2 | 1.5 | 0.6 |
| sgACC → aINS | 0.3 | 0.0 | 0.0 |
| D. Intracingulate Connectivity | |||
| AMYG → pACC/vmPFC | 0.4 | 2.4 | 1.0 |
| pACC/vmPFC → aMCC | 1.3 | 10.8** | 9.3** |
| aMCC → PCC | 1.3 | 6.5 | 6.9** |
Significance: Uncorrected: χ2=6.63, p < .01, χ2=3.84, p < .05; significance after FDR corrected:
p < .01 corrected,
p < .05 corrected (Male IBS vs. Female IBS, Male HC vs. Female HC, df=1)
FDR: False discovery rate (controlling false discovery rate at 5%)
Regions: AMYG, amygdala; aINS, mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; vlPFC, ventro-lateral prefrontal cortex
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME, matching emotions; MF, matching forms
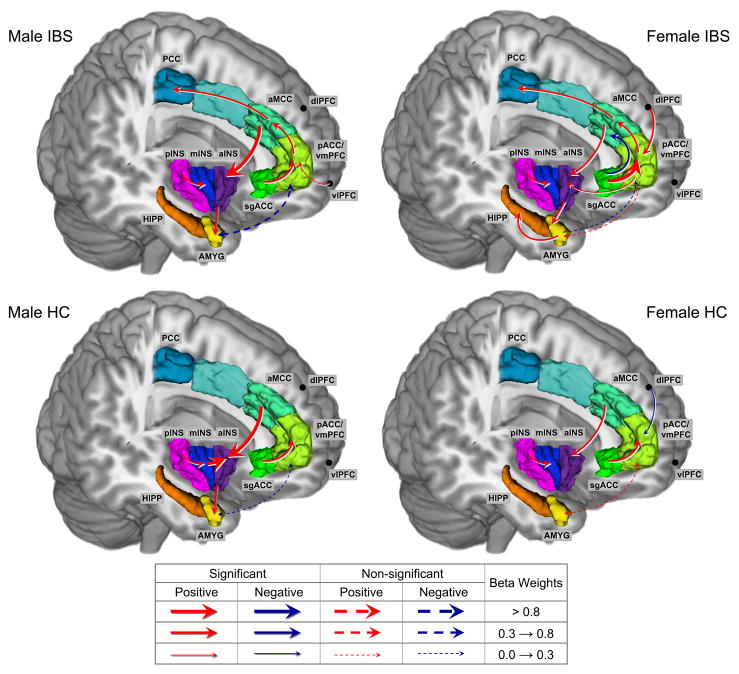
Figure 5. Effective Connectivity for (ME-MF) by Group (IBS Males, IBS Females, HC Males, HC Females).
IBS: Irritable bowel syndrome, HC: Healthy control
Regions: AMYG, amygdala; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; HIPP, hippocampus, aMCC, anterior mid cingulate cortex; sgACC, subgenual cingulate cortex; PCC, posterior cingulate cortex; pACC, pregenual cingulate cortex; vmPFC, ventro-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; dlPFC, dorsalateral prefrontal cortex
3.3.1 Connectivity within the emotional arousal circuit
Connectivity between AMYG and sgACC was weakly positive in males (HCs and IBS) and weakly negative in females (HCs and IBS), but these differences did not reach statistical significance. All groups showed moderate positive connectivity from sgACC to pACC/vmPFC, with female IBS showing the strongest connectivity (β=1.39) which was significantly higher than female HC (χ2Δ=11.7, p<.01) and male IBS (χ2Δ=13.5, p<.01). Female IBS showed a weak positive connectivity pACC/vmPFC to the AMYG, (β=.24) whereas male IBS subjects had moderate and negative connectivity (β=−.39) resulting in a significant group difference, χ2Δ=5.6, p<.05. Additionally, the input from pACC/vmPFC on aINS, showed moderate positive connectivity for IBS as a group with significant greater connectivity observed for female IBS compared to female HC, χ2Δ=7.6, p<.01. There was a significantly greater positive connectivity from aMCC to aINS for male IBS (β=.99) compared to female IBS (β=.41)(χ2Δ=4.4, p<.01). Weak inputs were observed for all groups from the AMYG to the aINS and moderate positive inputs from the aINS to the AMYG, without any significant group differences. Thus, significant group differences were observed for modulatory influences of anterior aspects of the cingulate cortex with the AMYG and the aINS, with male subjects showing greater positive connectivity between aMCC and aINS and greater moderate negative connectivity from pACC/vmPFC to AMYG.
3.3.2 Prefrontal and hippocampal modulatory inputs to the emotional arousal circuit
Significant group differences were observed for the influence of dlPFC (but not vlPFC) on the pACC/vmPFC. Specifically, female IBS showed a strong moderate positive influence of the dlPFC on the pACC/vmPFC (β=.78). This connectivity was absent in male IBS (β=.03) and weakly negative in HC subjects. As such, significant sex differences were observed with female IBS having greater connectivity between these cortical regions, compared to male IBS (χ2Δ=5.9, p<.05) and female HCs (χ2Δ=8.2, p<.01).
The HIPP showed a weak negative connectivity to AMYG in the IBS group, whereas connectivity in HCs was positive with male HC showing the strongest connectivity (β=.78) followed by female HC (β=.32). This resulted in significant group differences in lesser engagement of this circuit for male IBS compared to male HC (χ2Δ=4.5, p<.05), and lesser for female IBS compared to female HC (χ2Δ=3.8, p<.05). Significant differences were also observed for AMYG to HIPP with female IBS having a moderate positive connectivity (β=.64) compared to weak positive connectivity in the other 3 groups (β’s <=.16). Statistical significant differences were observed for female IBS greater than female HC (χ2Δ=13.0, p<.01) and male IBS (χ2Δ=9.3, p<.01). Thus, disease related group differences were observed in the reciprocal connectivity between HIPP and AMYG, with HCs having stronger positive connections than IBS with the male HCs showing the strongest connection; and with IBS having stronger positive connections than HCs in the AMYG to the HIPP, with female IBS showing the strongest connection.
4.0.0 Discussion
The aim of the study was to identify disease and sex related differences in brain responses to an emotion recognition paradigm unrelated to gastrointestinal (GI) stimuli or symptoms. The paradigm was limited to images of negative emotions (anger and fear), which have shown to elicit greater brain responses in males compare to females [8,29]. The main findings of the current study were: 1) Functional activity during the viewing of emotional faces did not differ when IBS subjects as a group were compared to HCs. 2) Male subjects showed greater functional activations in PFC, INS, NACC and HIPP, than female subjects regardless of disease group. 3) Male IBS subjects compared to female IBS showed greater activations in subregions of the PFC, ACC, and INS, as well as the PCC, HIPP, HYPO and NACC. Male IBS subjects showed greater activations in the AMYG compared to male HCs 4) Effective connectivity analyses identified major sex and disease related differences within the emotional arousal circuit, and in the modulatory influences of prefrontal and hippocampal regions on this circuitry. These findings for the first time demonstrate differential brain activity and connectivity of male IBS compared to female IBS and male HCs to a disease unrelated stimulus. Similar to the published greater responsiveness of an emotional arousal circuit to an IBS related stimulus in female IBS [69,72], the current findings in males demonstrate that greater IBS-related engagement of emotional arousal and cognitive modulatory brain circuitry can be demonstrated in male subjects. However, different stimuli are required in male and female subjects to elicit these disease and sex related differences.
4.1.0 Sex and disease related differences in brain activation in response to the emotion recognition task
4.1.1 Group differences between IBS and HCs are sex dependent
We found no statistically significant differences between IBS compared to HCs. These results differ from reports using controlled rectal balloon distension, where female IBS subjects had greater engagement of emotional arousal circuits, male IBS had greater engagement of homeostatic afferent and endogenous pain modulation regions, and HCs showed greater engagement of cognitive modulatory regions [69]. Our findings suggest that the failure to detect a disease-related difference was due to sex-related differences in these responses: When comparing male IBS subjects with male HCs, subjects showed greater amygdala responses to the emotion recognition paradigm, while no such difference was observed between female IBS compared to female HCs.
4.1.2 Greater brain responses in male subjects in viscerosensory, affective and cognitive brain regions
When comparing all males with all females, we found greater activation in mid and posterior subregions of the insula, subregions of the PFC, amygdala, as well as subgenual and posterior cingulate cortical subregions. Male IBS subjects showed similar differences in activating brain regions as the male group as a whole (IBS and HC), but also showed greater activity in HIPP, HYPO and NACC. These findings of greater cortical responses in males are consistent with previous findings using abdominal pain related tasks in both humans [5,17,30] and in rodents [68,79], where greater reactivity was observed in the prefrontal cortex and the mid and posterior insula. However, greater amygdala activity in males has not been reported in previous IBS samples. When viewed together with previous reports, the current findings suggest a generalized sex and IBS disease difference in brain responses to a variety of emotionally salient stimuli, including threat of pain, aversive visceral stimuli and viewing of negative emotional faces [20,67,75]. One may speculate about the observed greater responsiveness in male subjects to this particular stimulus. Recognition of emotions of anger and fear in a potential adversary can be seen as early correlates of the fight and flight response, a context in which males show greater hypothalamic-pituitary-adrenal (HPA) axis and sympathetic/sacral parasympathetic nervous system responses [2,55,64,80].
4.1.3. Greater responses in female subjects in regions of cortical-pontine circuits
Female HCs showed a greater response than male HCs in the right dlPFC and the PAG. This sex difference was not observed in the IBS group. These findings may be related to a specific, female response to the recognition of certain emotions [33], which is not engaged in IBS.
Group differences based on disease and sex were observed in symptoms of anxiety and depression, with IBS subjects and males as a group (IBS+HCs) showing slightly higher levels on both measures. When anxiety and depression were statistically controlled these differences remained, with the exception of increased activity in male IBS compared to female IBS in the hypothalamus for anxiety and in the anterior insula for depression. This suggests that the majority of the observed differences in the brain’s response to the emotion recognition faces task were not explained by the differences in underlying differences in mood and affect, but reflect fundamental differences in the brain’s responsiveness to this stimulus.
4.2.0 Sex and disease differences in effective connectivity of brain circuits related to emotional arousal and cortical modulation
4.2.1 Sex related similarities and differences
Both male IBS and male HCs showed strong positive connectivity between the aMCC and the aINS, compared to weak connectivity between these regions in females, possibly related to the greater engagement of the INS seen in male subjects seen in this and previous studies [6,52]. Both the aMCC and the aINS are also regions of a salience network, supporting the greater relevance of the fear and anger stimuli for males [62]. Male compared to female IBS subjects showed significant differences in the connectivity within the emotional arousal circuit, including stronger connectivity between the pACC/vmPFC to the AMYG and modulatory input of PFC subregions to pACC/vMPFC. While male IBS showed weak positive connectivity from the vlPFC, female IBS showed moderately strong input from dlPFC. Overall, greater engagement of emotional arousal circuit was observed in males.
4.2.2 Disease related similarities and differences
Both male and female IBS differed from their respective control groups in the connectivity between sgACC to pACC/vmPFC, and in the connectivity between aMCC and PCC, suggesting these differences to be IBS related. Male IBS subjects differed from male HCs in the connectivity between pACC/vmPFC to aMCC, which was stronger in IBS. Together with the stronger activation of the involved cingulate subregions, and the positive connectivity between aMCC and aINS, the pACC/vmPFC to aMCC to aINS pathway may underlie the greater engagement of the INS in male subjects, previously reported in IBS [7,52]. Greater positive connectivity from HIPP to AMYG was also observed in male IBS versus male HCs, consistent with the greater engagement of the AMYG in male IBS. Female IBS differed from female controls in the connectivity between dlPFC and pACC/vmPFC, which was negative in HCs and positive in IBS.
4.3.0 Limitations
There are several limitations to the study: 1) Although we were able to show clear disease and sex related differences in the AMYG to a non-IBS related stimulus, there are some limitations to the emotional recognition task used. The faces task has been shown to elicit an immediate response in brain circuits involved in attention, cognition, and emotion [1,35], highlighting the highly adaptive social trait involved when recognizing and responding to emotional states of another individual [9]. While initially proposed to quantify individual differences in emotional reactivity [20], the task is not associated with subjective emotional feelings or autonomic nervous system responses, and is better viewed as an emotion recognition task which engages both cognitive and affective brain circuits [15,23,41,42]. 2) We were unable to compare the specific brain responses associated with fear and anger respectively, which could have highlighted sex-specific responses associated with differing negative emotions. 3) We were unable to assess possible effects of female sex hormones on brain responses in our sample of predominantly premenopausal females, scanned during the follicular phase of the menstrual cycle.
4.4.0 Summary and conclusions
When viewed together with previously published studies in female IBS subjects using GI specific stimuli [69], the current findings confirm the general concept that IBS subjects show sex-specific increased responsiveness to emotionally valenced stimuli, which are not fully accounted for by anxiety. This increased brain responsiveness to emotionally-valenced stimuli, may play a role in the process of central sensory amplification of visceral and non-visceral stimuli, characteristic for this patient population [70]. The fact that in males altered brain responsiveness could be elicited by a stimulus without any relevance to IBS or pain symptoms, is consistent with the concept that brain alterations play a role in IBS pathophysiology, and that IBS is not a gut specific disorder. Increased perception of visceral stimuli appears to be just one of several manifestations of this brain hyper-responsiveness. The findings emphasize the importance of taking sex-related differences into account, when evaluating disease differences and when assessing the importance of symptom related stressors in the clinic. While aversive pelvic stimuli or their expectation may be more salient to women (the majority of whom have experienced physiological pain in this area related to menstrual cycle and delivery), negative personal interactions involving recognition of potential adversaries may be more salient in males to elicit greater responses in cognitive affective brain circuits, and contribute to symptom variations.
Supplementary Material
Regions: aINS, anterior insula; mINS, mid insula; pINS, posterior insula; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; omPFC. orbitomedial prefrontal cortex; aMCC, anterior mid cingulate cortex; pACC, pregenual cingulate cortex; sgACC, subgenual cingulate cortex; AMYG, amygdala; HIPP, hippocampus, HYPO, hypothalamus, NACC, nucleus accumbens; PAG, dorsal pons
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME-MF, matching emotions versus matching forms
Within Group Activity: A, Activations; D, Deactivations; NS, No significant activity
Summary.
Sex differences in the engagement of brain networks in response to negative emotional faces was investigated. Male IBS demonstrated differential engagement of emotional arousal circuits.
Acknowledgments
Funding Sources: This research was supported in part by grants from the National Institute of Digestive Diabetes and Kidney Diseases (NIDDK) DK48351 (EAM), R24 AT002681 (EAM), and the US Department of Veterans Affairs VA Merit Review (BN).
Abbreviations
- IBS
Irritable bowel syndrome
- HC
Healthy Control
- M
male
- F
female
- ME
match emotions
- MF
match forms
- ROI
region-of-interest
- AMYG
amygdala
- ACC
anterior cingulate cortex
- aMCC
anterior mid cingulate cortex
- sgACC
subgenual cingulate cortex
- pACC
pregenual cingulate cortex
- PCC
posterior cingulate cortex
- HIPP
hippocampus
- HYPO
hypothalamus
- PAG
dorsal pons
- NACC
nucleus accumbens
- PFC
prefrontal cortex
- vlPFC
ventrolateral prefrontal cortex
- vmPFC
ventromedial prefrontal cortex
- dmPFC
dorsomedial prefrontal cortex
- dlPFC
dorsolateral prefrontal cortex
- INS
insula
- aINS
anterior insula
- mINS
mid insula
- pINS
posterior insula
- BSQ
UCLA Bowel Symptom Questionnaire
- HAD
Hospital Anxiety and Depression Index
- SPM
Statistical Parametric Mapping software
- MNI
Montreal Neurological Institute
Footnotes
Disclosures: No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams RB, Franklin RG, Nelson AJ, Gordon HL, Kleck RE, Whalen PJ, Ambady N. Differentially tuned responses to restricted versus prolonged awareness of threat: A preliminary fMRI investigation. Brain and Cognition. 2011;77(1):113–119. doi: 10.1016/j.bandc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery fate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25(1):60–83. [Google Scholar]
- 4.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. [Google Scholar]
- 5.Benson S, Rosenberger C, Schedlowski M, Forsting M, Gizewski ER, Elsenbruch S. Cognitive and Emotional Modulation of Visceral Pain Processing in Ibs Patients and Healthy Subjects. Int J Behav Med. 2010;17:98–98. [Google Scholar]
- 6.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain-London. 2000;4(2):157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 7.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol-Reg I. 2006;291(2):R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 8.Biele C, Grabowska A. Sex differences in perception of emotion intensity in dynamic and static facial expressions. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2006;171(1):1–6. doi: 10.1007/s00221-005-0254-0. [DOI] [PubMed] [Google Scholar]
- 9.Blechert J, Sheppes G, Di Tella C, Williams H, Gross JJ. See What You Think: Reappraisal Modulates Behavioral and Neural Responses to Social Stimuli. Psychological Science. 2012;23(4):346–353. doi: 10.1177/0956797612438559. [DOI] [PubMed] [Google Scholar]
- 10.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140(3):761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L, Heitkemper M. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 12.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 13.Demenescu LR, Renken R, Kortekaas R, van Tol MJ, Marsman JBC, van Buchem MA, van der Wee NJA, Veltman DJ, den Boer A, Aleman A. Neural correlates of perception of emotional facial expressions in out-patients with mild-tomoderate depression and anxiety. A multicenter fMRI study. Psychological Medicine. 2011;41(11):2253–2264. doi: 10.1017/S0033291711000596. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA. Introduction. The Rome Foundation and Rome III. Neurogastroent Motil. 2007;19(10):783–786. doi: 10.1111/j.1365-2982.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunsmoor JE, Mitroff SR, Labar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Memory. 2009;16(7):460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekman P. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- 17.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139(4):1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and Disconnected Insular-Amygdalar Blood Oxygenation Level- Dependent Response to Threat-Related Emotional Faces in Women with Intimate-Partner Violence Posttraumatic Stress Disorder. Biological psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroen Hepatol. 2011;26:110–115. doi: 10.1111/j.1440-1746.2011.06631.x. [DOI] [PubMed] [Google Scholar]
- 20.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- 21.Gard MG, Kring AM. Sex differences in the time course of emotion. Emotion. 2007;7(2):429–437. doi: 10.1037/1528-3542.7.2.429. [DOI] [PubMed] [Google Scholar]
- 22.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain Activation to Facial Expressions in Youth with Ptsd Symptoms. Depress Anxiety. 2012;29(5):449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grillon C, Charney DR. In the face of fear: Anxiety sensitizes defensive responses to fearful faces. Psychophysiology. 2011;48(12):1745–1752. doi: 10.1111/j.1469-8986.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 25.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: Perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in cognitive sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 28.Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of FMRI data. NeuroImage. 2006;33(2):599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- 29.Hess U, Adams RB, Grammer K, Kleck RE. Face gender and emotion expression: Are angry women more like men? J Vision. 2009;9(12) doi: 10.1167/9.12.19. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(35):12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. NeuroImage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Joreskog KG. Simultaneous Factor Analysis in Several Populations. Psychometrika. 1971;36(4):409. [Google Scholar]
- 33.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav R. 2001;25(7–8):669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 34.Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, Bueller JA, Suyenobu B, Jarcho JM, McRoberts JA, Niesler B, Mayer EA. The HTR3A polymorphism c-42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140(7):1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. NeuroImage. 2009;47(3):952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson M, Tillisch K, Craig A, Engström M, Labus J, Naliboff B, Lundberg P, Ström M, Mayer E, Walter S. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142(3):463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130(1):26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;11(3):468–480. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin M, Crane C. Cognition and the Body: Somatic Attributions in Irritable Bowel Syndrome. Behav Cogn Psychoth. 2003;31(1):13–31. [Google Scholar]
- 44.Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8(5):451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex Differences in the Brain: The Not So Inconvenient Truth. Journal of Neuroscience. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIntosh A, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Human brain mapping. 1994;2:2–22. [Google Scholar]
- 49.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112(1):55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 50.Mykletun A, Jacka F, Williams L, Pasco J, Henry M, Nicholson GC, Kotowicz MA, Berk M. Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS). An epidemiological population based study of women. Bmc Gastroenterology. 2010:10. doi: 10.1186/1471-230X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata T, Tsuyuguchi N, Uda T, Ohata K. Non-normalized individual analysis of statistical parametric mapping for clinical fMRI. Neurol India. 2011;59(3):339–343. doi: 10.4103/0028-3886.82714. [DOI] [PubMed] [Google Scholar]
- 52.Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124(7):1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 53.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131(2):352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrin. 2011;32(2):227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrino. 2012;37(8):1135–1157. doi: 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul ES, Pope SAJ, Fennell JG, Mendl MT. Social Anxiety Modulates Subliminal Affective Priming. Plos One. 2012;7(5) doi: 10.1371/journal.pone.0037011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulus MP, Yu AJ. Emotion and decision-making: affect-driven belief systems in anxiety and depression. Trends in cognitive sciences. 2012;16(9):476–483. doi: 10.1016/j.tics.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perlman SB, Almeida JRC, Kronhaus DM, Versace A, LaBarbara EJ, Klein CR, Phillips ML. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disorders. 2012;14(2):162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 60.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SCR, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. P Roy Soc B-Biol Sci. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pontieri FE, Assogna F, Stefani A, Pierantozzi M, Meco G, Benincasa D, Colosimo C, Caltagirone C, Spalletta G. Sad and happy facial emotion recognition impairment in progressive supranuclear palsy in comparison with Parkinson’s disease. Parkinsonism Relat D. 2012;18(7):871–875. doi: 10.1016/j.parkreldis.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 62.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiat. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 64.Smeets T, Dziobek I, Wolf OT. Social cognition under stress: Differential effects of stress-induced cortisol elevations in healthy young men and women. Horm Behav. 2009;55(4):507–513. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdalla connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 67.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 68.ter Horst J, de Kloet ER, Schachinger H, Oitzl MS. Relevance of Stress and Female Sex Hormones for Emotion and Cognition. Cell Mol Neurobiol. 2012;32(5):725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140(1):91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54(10):1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truong TT, Naliboff BD, Chang L. Novel techniques to study visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2008;10(4):369–378. doi: 10.1007/s11894-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 73.van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological psychiatry. 2009;66(7):649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Vogt P, Ferrari JR, Lookingbill TR, Gardner RH, Riitters KH, Ostapowicz K. Mapping functional connectivity. Ecol Indic. 2009;9(1):64–71. [Google Scholar]
- 75.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 76.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Social cognitive and affective neuroscience. 2007;2(3):227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, Bradesi S, Maarek JMI, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008;138(1):233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Guo YM, Bradesi S, Labus JS, Maarek JMI, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145(1–2):120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yim IS, Quas JA, Cahill L, Hayakawa CM. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrino. 2010;35(2):241–248. doi: 10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 81.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions: aINS, anterior insula; mINS, mid insula; pINS, posterior insula; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; omPFC. orbitomedial prefrontal cortex; aMCC, anterior mid cingulate cortex; pACC, pregenual cingulate cortex; sgACC, subgenual cingulate cortex; AMYG, amygdala; HIPP, hippocampus, HYPO, hypothalamus, NACC, nucleus accumbens; PAG, dorsal pons
Groups: IBS, Irritable bowel syndrome; HC, Healthy Control
Contrasts: ME-MF, matching emotions versus matching forms
Within Group Activity: A, Activations; D, Deactivations; NS, No significant activity