Abstract
Background:
Chronic nerve compression neuropathies result in decreased blood flow at the site of compression. Surgical decompression of the nerve often has variable postoperative results. The current study examines whether the timing of surgical intervention is an important variable in reversing the compression-induced ischemia and associated changes in biochemical markers.
Methods:
An established model of chronic nerve compression injury was created in 100 C57BL/6 mice, and serial electrophysiological examinations were used to confirm the creation of a chronic nerve compression injury. Laser speckle imaging was used to measure neural blood flow. Nerves in the animals that did not undergo decompression were harvested at two, four, and six weeks after injury and analyzed for hypoxia-inducible factor 1α (HIF1α), catalase, superoxide dismutase (SOD), and matrix metalloproteinases (MMPs) 2 and 9. Surgical decompression in other animals was performed at either an early (two-week) or late (six-week) time point after injury, with specimens harvested at multiple time points after decompression. One-way analysis of variance with Bonferroni correction was performed.
Results:
Chronic nerve compression injury initially induced hyperemia (1.37 ± 0.50 times that in the contralateral, uninjured nerve) followed by a decline in neural blood flow by four weeks (0.66 ± 0.14, p = 0.0313). In parallel, HIF1α, catalase, and SOD were elevated early after compression, whereas extracellular matrix-altering proteins were elevated later in the disease. Although early decompression yielded a return of blood flow to a hyperemic state (1.35 ± 0.16, p = 0.0057), late decompression did not result in reversal of the abnormal neurovascular flow. With late decompression, an MMP9-mediated structural alteration of the extracellular matrix was seen, producing irreversible changes in blood flow parameters. Although nerve conduction velocity measurements returned to normal two weeks after decompression irrespective of the timing of the surgical intervention, distal latency returned to normal only after early decompression (0.97 ± 0.06 msec compared with 1.22 ± 0.06 msec for late decompression, p = 0.009).
Conclusions:
Chronic nerve compression injuries decreased neurovascular flow and induced ischemia by upregulating HIF1α, catalase, and MMP9. Early surgical intervention offered better return to normal electrophysiological parameters compared with late intervention.
Clinical Relevance:
These data present a clinical correlate to the variable functional outcomes seen following surgical release of chronic nerve compression injuries and provide early support for using distal latency as a predictor of outcomes following surgical release.
Chronic nerve compression injuries such as carpal tunnel syndrome, cubital tunnel syndrome, and spinal nerve root stenosis result in morbidity with the possible loss of motor and sensory function, pain, paresthesia, and paralysis1-3. The effective therapeutic modalities for such injuries are currently strikingly limited to anti-inflammatory agents, corticosteroid injections, or surgical intervention4. Therefore, understanding the pathogenesis of such injuries is necessary to identify more successful treatment options.
Recent clinical data have shown that early surgical management of carpal tunnel syndrome has better efficacy and produces better long-term outcomes compared with more conservative medical approaches, such as injections, anti-inflammatory agents, and splinting4-7. If surgical decompression is to be performed, it has been suggested that the timing of the surgery is also an important parameter for optimizing functional recovery8. Although surgical decompression often ameliorates the symptoms of carpal tunnel syndrome when performed early in the disease course by reversing the sensory dysfunction, there is limited understanding of why surgical intervention is less effective when performed at later time points and has limited effect on reversing the motor atrophy that develops later in the disease course4,5,7,9.
Currently, electrophysiology is considered important for making the diagnosis of carpal tunnel syndrome10,11. In a study by Chandra et al., electrophysiological findings reversed after carpal tunnel release in both early surgery and delayed surgery groups8, but this is not always the case. In fact, many clinicians remain quite skeptical of the role of an electrophysiological examination in the management of patients suspected of having a compressive neuropathy. The American Academy of Orthopaedic Surgeons Clinical Guideline on the Diagnosis of Carpal Tunnel Syndrome provides a mixed endorsement of this examination on the basis of the varying levels of evidence supporting its use12.
Recent animal studies have provided data regarding the changes in blood flow following an acute nerve injury13,14. Xu et al. found that epineurial red blood cell flux was reduced within one hour after a nerve crush injury, with a subsequent rebound hyperemia13, and Igarashi et al. also found a decrease in blood flow after a spinal nerve crush14. Although neurovascular flow after acute nerve injury has been studied, similar studies after chronic nerve compression injury are limited. Previous studies have shown that such chronic injuries are distinct from acute nerve injuries and have a fundamentally different pathogenesis in which Wallerian degeneration is not seen early after injury15. Although axonal pathology and degeneration are absent early after a chronic nerve compression injury, Schwann cells undergo concurrent processes of proliferation and apoptosis15. The compressed nerve has increased vascular permeability and neural vascularity with an upregulation of vascular endothelial growth factor (VEGF) and its Schwann cell receptors (fetal liver kinase receptor and fms-like tyrosine kinase receptor) at early time points, followed by a dramatic decrease16-18. Recent data have revealed that hypoxia potentiates the demyelinating effect of hydrostatic compression in an in vitro model of nerve compression injury19, but the role that ischemia plays in triggering the pathological responses in such an injury still remains unknown.
Patients with carpal tunnel syndrome have decreased blood flow in the median nerve, which is restored after release of the transverse carpal tunnel ligament20. These observations have led to the hypothesis that chronic nerve compression alters neural blood flow and induces ischemia in a fashion different from that in other neural injuries. The present study assesses the extent of the ischemia induced by chronic nerve compression and the ensuing changes in biochemical markers over time in a mouse model. In addition, this study assesses the effect of the timing of surgical decompression (early or late) on restoration of neurovascular flow, biochemical marker levels, and electrophysiological parameters.
Materials and Methods
Animal Model
A previously described model of chronic nerve compression was utilized21. Fifty-four six-week-old, male C57BL/6 mice (Harlan Laboratories, United Kingdom) were anesthetized by intraperitoneal injection of ketamine and xylazine and were divided equally into groups to be assessed two, four, and six weeks after chronic nerve compression injury. A dorsal gluteal-splitting approach was used to isolate and dissect each sciatic nerve free from the surrounding tissue. A 3-mm-diameter biologically inert Silastic tube (Cole-Parmer, Vernon Hills, Illinois) was placed atraumatically around the ipsilateral nerve below the level of the sciatic notch. Prior to surgery, all tubing had been placed in a Petri dish and soaked in 70% ethanol overnight, then allowed to dry in a vacuum hood, to minimize the inflammatory response. As previously detailed21, this procedure creates a chronic nerve compression injury that is defined by a progressive decline in nerve conduction velocity that plateaus at the six-week time point.
Two additional surgical cohorts of eighteen mice each were treated with early decompression, at two weeks after an identical injury, or with late decompression, at six weeks after injury21. Previous data have indicated that nerve conduction velocity exhibits a small decrease at two weeks after this injury and a large decrease at six weeks before plateauing21. Therefore, as these two time points represent the earliest and final changes seen after the chronic nerve compression injury, they were chosen to define the time points for early and late decompression. Decompression was performed by atraumatically removing the inert tubing from the nerve.
A separate cohort of ten mice that received a crush injury served as positive controls for demonstrating a decrease in neural blood flow. The right sciatic nerve was carefully exposed, mobilized, and crushed immediately distal to its emergence from the gluteus maximus with use of hemostatic forceps for thirty seconds22.
Thus, nerves in six groups totaling 100 mice were used in the study (Table I). A power analysis was performed prior to the study to estimate the group sizes needed to achieve significance in the various experiments. All procedures involving living animals were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
TABLE I.
Surgical Groups*
| Group | N |
| Dissected, uninjured nerve | — |
| Crushed nerve | 10 |
| Two-week CNC injury | 18 |
| Four-week CNC injury | 18 |
| Six-week CNC injury | 18 |
| Decompression after two weeks of CNC injury | 18 |
| Decompression after six weeks of CNC injury | 18 |
CNC = chronic nerve compression.
Electrodiagnostic Evaluation
Nerve conduction velocity was measured in all animals preoperatively and serially at weekly time points, by a board-certified neurologist with clinical expertise in neuromuscular diseases and extensive research experience with rats and mice, until the nerves were harvested. Recordings of both the ipsilateral experimental and contralateral limbs were made in vivo under ketamine and xylazine anesthesia on an electromyography instrument (Sierra LT; Cadwell Laboratories, Kennewick, Washington). Motor conduction at the sciatic and tibial nerves was assessed by stimulation at the sciatic notch and knee with use of a monopolar needle electrode. The reference for the stimulating electrode was placed in the ipsilateral lumbar paraspinal muscle. The compound muscle action potential of the ankle dorsiflexion muscle (tibialis anterior) innervated by the tibial nerve was recorded by placing subdermal electroencephalographic electrodes in the muscle approximately 2 mm above the heel. The reference recording electrode was inserted into the dorsal aspect of the foot, and the amplitude of the compound muscle action potential and the motor nerve conduction velocity were measured.
Laser Speckle Imaging
Laser speckle imaging was used to measure neurovascular blood flow23. This is a previously described, noninvasive technique for studying the motion of optically scattering materials with high spatial and temporal resolution24. Light from a 785-nm continuous-wave diode laser (B&W Tek, Newark, Delaware) was delivered to the target with an optical fiber and diverged with a ground-glass diffuser (Thorlabs, Newton, New Jersey). Reflected speckle patterns were imaged with an 8-bit CCD (charge-coupled device) camera (A602f; Basler, Exton, Pennsylvania) equipped with a macro lens. Images were transferred to a laptop computer in real time via the FireWire 400 interface. Processing and rendering of the speckle flow index (SFI) images was performed with use of custom-written software developed in LabVIEW (National Instruments, Austin, Texas).
The laser speckle imaging was performed at two, four, and six weeks after injury in the no-decompression groups and at one, two, and three weeks after treatment in the decompression groups. Imaging in the positive controls that received a crush injury was performed at baseline, every twenty minutes for the first three hours, and at one day and one and two weeks.
Western Blotting
The markers of interest were hypoxia-inducible factor (HIF)1α , matrix metalloproteinases (MMPs) 2 and 9, superoxide dismutase (SOD), and catalase. The primary antibodies used were rabbit anti-HIF1α (1:1000 dilution; Novus Biologicals, Littleton, Colorado), mouse anti-MMP9 (1:5000 dilution; Abcam, Cambridge, Massachusetts), rabbit anti-MMP2 (1:5000 dilution; abcam), rabbit anti-superoxide dismutase (1:5000 dilution; Sigma-Aldrich, St. Louis, Missouri), mouse anti-catalase (1:5000 dilution; Sigma-Aldrich), mouse anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (1:50,000 dilution; Fitzgerald, Acton, Massachusetts), and mouse anti-tubulin (1:10,000 dilution, Sigma-Aldrich)19,25-31.
The western blotting procedure was performed at two, four, and six weeks after injury in the no-decompression groups as well as at two and six weeks after treatment in the decompression groups. Both the ipsilateral (experimental) nerve and the contralateral nerve were harvested and homogenized completely. Each sample was centrifuged at 14,000 rpm for ten minutes to remove fibrotic remnants of the nerve, and the protein concentration in the resultant whole-tissue lysate was determined with use of a BCA Protein Assay Kit (Thermo Scientific, Rockford, Illinois). Briefly, 75 to 100 μg of protein was separated by 8% or 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), transferred to nitrocellulose membrane, blocked with 5% dry skimmed milk, and incubated overnight at 4°C with primary antibodies. Donkey anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP, 1:10,000 dilution; EMD Millipore, Billerica, Massachusetts) or goat anti-rabbit secondary antibody conjugated with HRP (1:10,000 dilution) was used for detection19,25. Blot development was performed with Immobilon Western Chemiluminescent HRP Substrate (Millipore). Films were digitized with use of a color scanner and analyzed with use of MetaMorph image analysis software (Molecular Devices, Sunnyvale, California). Quantitative results were calculated by normalizing the densitometry data for each antibody with respect to that of β-tubulin or GAPDH on the same blot.
Statistical Analysis
Data are presented as the mean and standard error along with the 95% confidence interval (CI). One-way analysis of variance with Bonferroni post hoc comparison was performed unless otherwise indicated. A p value of <0.05 was considered significant.
Source of Funding
Funding for this study was provided by the National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (grant 2R01NS049203-06A1) and through the Laser Microbeam and Medical Program, a Biotechnology Resource Center supported by the National Institute of Biomedical Imaging and Bioengineering (grant P41EB015890).
Results
Sustained Decreases in Nerve Conduction Velocity After Injury
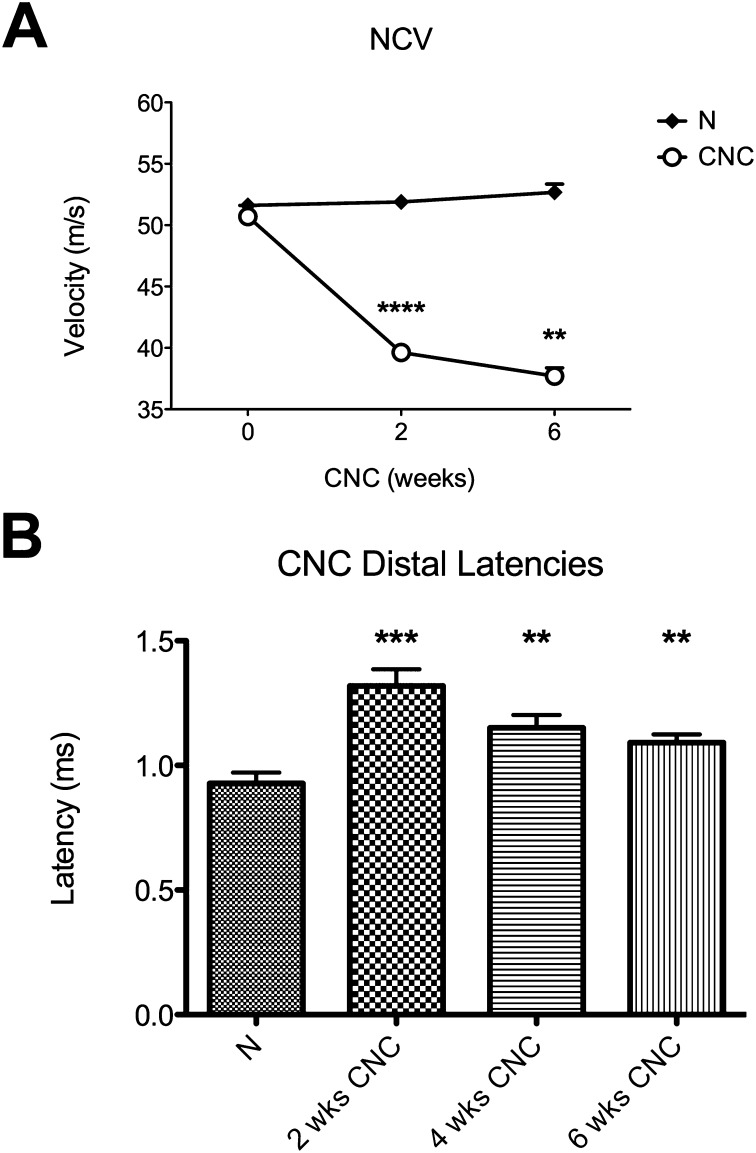
Normal and uninjured wild-type sciatic nerves that had been mobilized but not compressed maintained a baseline nerve conduction velocity of 51.5 ± 1.6 m/sec, consistent with that in previous studies21. After creation of a chronic nerve compression injury, the velocity declined progressively to 37.5 ± 2.5 m/sec at six weeks after injury (Fig. 1-A). The amplitude of the compound muscle action potential was assessed to confirm that this decline resulted primarily from demyelination of the sciatic nerves rather than from axonal pathology and damage21. Consistent with previous experiments21, no significant difference was observed between the amplitudes in compressed and uncompressed nerves. Distal latency increased as early as two weeks after creation of a chronic nerve compression injury and remained elevated (1.13 ± 0.05 msec at six weeks compared with 1.32 ± 0.07 msec [95% CI = 1.17 to 1.47 msec] at two weeks, p = 0.007) (Fig. 1-B).
Fig. 1.
Figs. 1-A and 1-B Electrophysiological studies of nerve conduction velocity (NCV) and distal latency after two to six weeks of chronic nerve compression (CNC) injury. The mean and standard error are plotted. N = normal (uninjured) nerve at 2 weeks. Fig. 1-A NCV in the nerves with a CNC injury showed a progressive decline until six weeks after injury; the normal nerve remained unchanged. Fig. 1-B Distal latency was prolonged after CNC injury. **P < 0.01. ***P < 0.001. ****P < 0.0001.
Decreased Neural Blood Flow After Injury
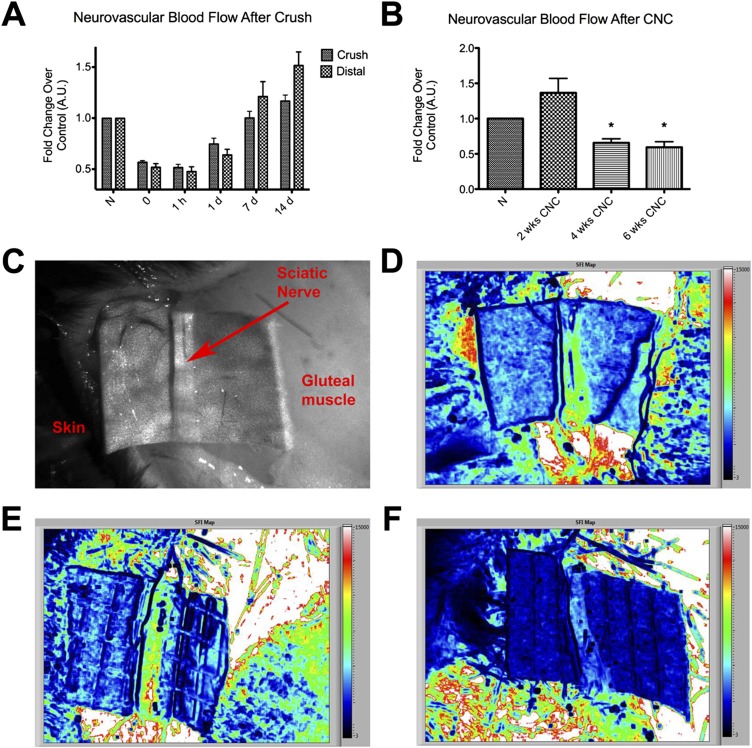
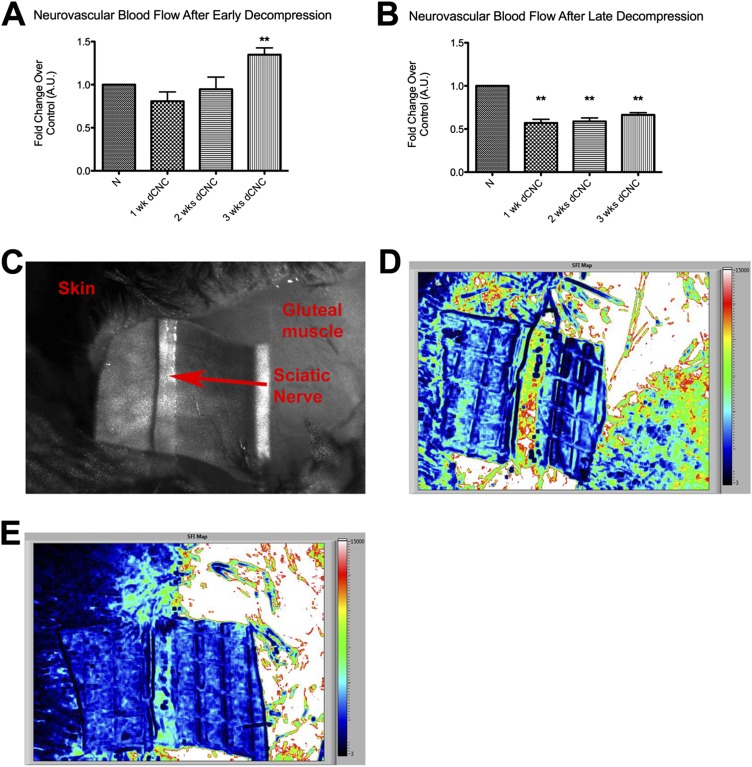
Laser speckle imaging was used to assess neural blood flow in the sciatic nerve. Imaging performed in mice that received a crush injury (the positive controls) confirmed the decline in neural blood flow; similar to previously reported results13, the blood flow in the sciatic nerve decreased immediately after the crush and returned to baseline by one week after injury (Fig. 2-A). In the mice in which a chronic nerve compression injury was created, baseline values of neural blood flow were measured in both the experimental and contralateral, control nerves prior to placement of the inert tube. The measurements were repeated at two, four, and six weeks after injury to determine the level of hypoxia that was induced. In contrast to the immediate decrease in blood flow observed after the neural crush injury, chronic nerve compression injury initially created a period of hyperemia (1.37 ± 0.50 times that in the contralateral, uninjured nerve at the same time point), although the difference between the treatments did not reach significance. However, this initial hyperemia was then followed by a drastic decline in blood flow through the nerve to 0.66 ± 0.14 (95% CI = 0.51 to 0.80) at four weeks (p = 0.0313), and the lower value was maintained over time (Fig. 2-B). Raw speckle images showing blood flow through the nerve are also shown in Figure 2.
Fig. 2.
Figs. 2-A through 2-F Use of laser speckle imaging to analyze sciatic nerve blood flow relative to that in the normal, uninjured nerve (N) after crush or chronic nerve compression (CNC) injury. The mean and standard error are plotted in Figs. 2-A and 2-B. *P < 0.05. A.U. = arbitrary units. Fig. 2-A After a crush injury, blood flow in the sciatic nerve (at the crush site as well as distal to it) decreased immediately and eventually returned to a hyperemic state after several days. Fig. 2-B CNC injury was followed by a distinctly different pattern of neurovascular changes, with an initial period of hyperemia followed by a marked decline in flow. Fig. 2-C Raw contrast image showing different areas within the gluteal split. Fig. 2-D Speckle flow index (SFI) map showing blood flow across a normal nerve; high flow is red and low flow is black. Fig. 2-E SFI map of a nerve after a two-week CNC injury. Fig. 2-F SFI map of a nerve after a six-week CNC injury revealing markedly decreased blood flow.
Alterations in Cellular Markers of Hypoxia After Injury
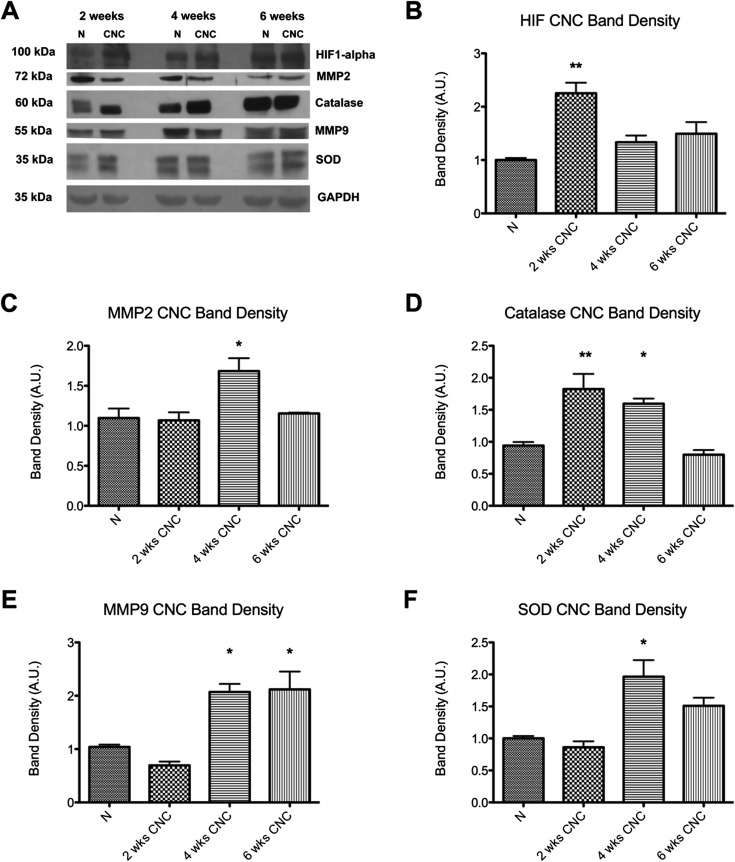
The level of HIF1α increased after two weeks of compression (from 1.00 ± 0.04 to 2.25 ± 0.20 [95% CI = 1.40 to 3.11], p = 0.0035), followed by a dramatic decline by six weeks (Figs. 3-A and 3-B). Catalase also increased after two weeks of compression (from 0.94 ± 0.06 to 1.82 ± 0.24 [95% CI = 1.26 to 2.39], p = 0.047) (Figs. 3-A and 3-D). However, SOD was elevated after four weeks of compression (from 1.00 ± 0.04 to 1.97 ± 0.26 [95% CI = 1.30 to 2.63], p = 0.0011) (Figs. 3-A and 3-F).
Fig. 3.
Figs. 3-A through 3-F Western blot analysis of normal, uninjured nerves (N) and nerves after two, four, and six weeks of chronic nerve compression (CNC) injury. The densitometry for the markers in Fig. 3-A is plotted in the remaining panels as the mean and standard error. *P < 0.05. **P < 0.01. A.U. = arbitrary units. Fig. 3-A Western blot bands for the cellular hypoxic markers and for the GAPDH reference. Fig. 3-B The HIF1α level was elevated early after CNC injury. Fig. 3-C The MMP2 level was elevated later after injury. Fig. 3-D The catalase level was elevated early after injury, and the elevation persisted longer than it did for HIF1α. Fig. 3-E The MMP9 level was elevated at four and six weeks after injury. Fig. 3-F The SOD level was elevated at four weeks after injury.
MMP9 was elevated after four weeks of compression (from 1.04 ± 0.04 to 2.07 ± 0.15 [95% CI =1.74 to 2.40], p < 0.05) and remained elevated at the six-week time point (2.12 ± 0.34, 95% CI = 1.38 to 2.86) (Figs. 3-A and 3-E). MMP2, which is involved in the regulation of vascularization, also showed an elevation after four weeks of compression (from 1.11 ± 0.12 to 1.68 ± 0.16 [95% CI = 1.33 to 2.04], p = 0.0313) (Figs 3-A and 3-C).
Early but Not Late Surgical Decompression Reversed Deleterious Hypoxic Changes
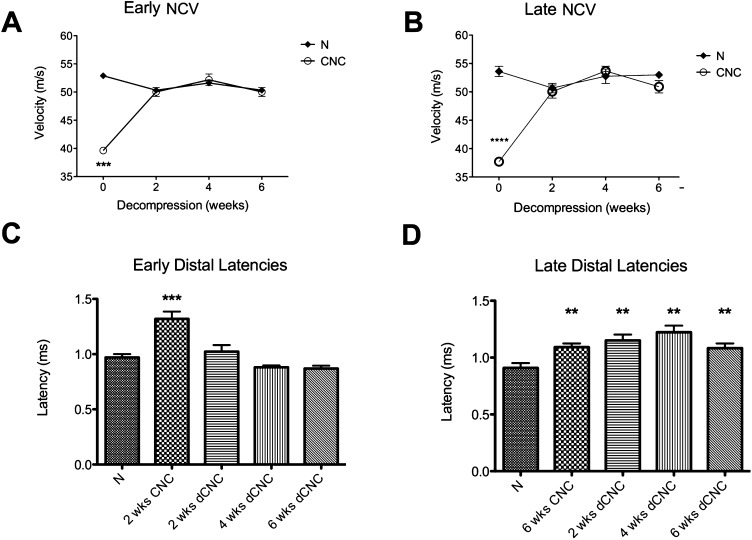
Nerve conduction velocity returned to baseline by two weeks after both early decompression (performed two weeks after injury) and late decompression (performed six weeks after injury) (52.90 ± 0.69 and 53.60 ± 0.90 m/sec, respectively) (Figs. 4-A and 4-B). Distal latency returned to baseline after early decompression was performed (0.97 ± 0.06 msec at four weeks) but not after late decompression (1.22 ± 0.06 msec [95% CI = 1.10 to 1.35 msec] at four weeks, p = 0.009) (Figs. 4-C and 4-D). Thus, even though nerve conduction velocity returned to normal after late decompression, distal latency did not.
Fig. 4.
Figs. 4-A through 4-D Nerve conduction velocity (NCV) and distal latency at two, four, and six weeks after early (left) and late (right) surgical decompression (dCNC) of a chronic nerve compression (CNC) injury. **P < 0.01. ***P < 0.001. ****P < 0.0001. N = normal (uninjured) nerve. The mean and standard error are plotted. Figs. 4-A and 4-B NCV returned to the normal range within two weeks after both early and late decompression. Figs. 4-C and 4-D Distal latency was prolonged after two weeks of CNC but subsequently returned to the normal range only after early decompression.
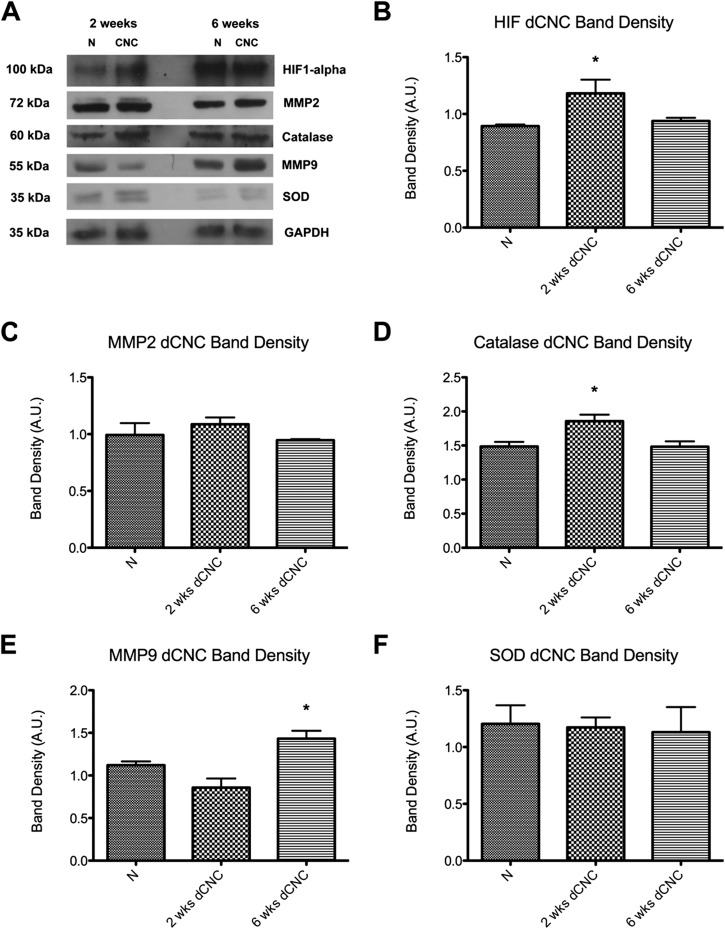
Neurovascular flow dynamics, as assessed with laser speckle imaging at one, two, and three weeks after decompression, showed a pattern similar to that of distal latency. Neural blood flow after early decompression showed a progressive rebound to a hyperemic state that exceeded the original baseline value within three weeks (1.35 ± 0.16 [95% CI = 1.10 to 1.60] times that in the contralateral nerve at the same time point, p = 0.0057) (Figs. 5-A, 5-C, and 5-D). However, neural blood flow after late surgical decompression failed to return to the baseline state by three weeks (0.66 ± 0.03 [95% CI = 0.59 to 0.73]) (Figs. 5-B and 5-D). Importantly, HIF1α and catalase were elevated after early decompression (HIF1α, 1.18 ± 0.1 [95% CI = 0.91 to 1.46] compared with 0.89 ± 0.01 in the normal nerve at six weeks after early decompression; catalase, 1.86 ± 0.10 [95% CI = 1.61 to 2.10] compared with 1.49 ± 0.07 in the normal nerve). However, HIF1α levels were not affected after late decompression was performed (Figs. 6-A, 6-B, and 6-D). In addition, MMP9 remained significantly elevated even after decompression if the late decompression was performed (1.43 ± 0.09 [95% CI = 1.24 to 1.63] compared with 1.12 ± 0.04 in the normal nerve).
Fig. 5.
Figs. 5-A through 5-E Neurovascular blood flow changes at one, two, and three weeks after surgical decompression (dCNC) of a chronic nerve compression injury. Figs. 5-A and 5-B Blood in the nerve rebounded to a hyperemic state within three weeks after early decompression but remained below the baseline value after late decompression was performed. The mean and standard error are plotted. **P < 0.01. N = normal (uninjured) nerve. Fig. 5-C Raw contrast image showing different areas within the gluteal split. Fig. 5-D Speckle flow index (SFI) map showing a return of blood flow after early decompression; high flow is red and low flow is black. Fig. 5-E Speckle flow index map after late decompression showing lack of blood flow resulting in permanent hypoxic changes within the nerve.
Fig. 6.
Figs. 6-A through 6-F Western blot analysis of normal, uninjured nerves (N) and nerves six weeks after early and late decompression (dCNC) of a chronic nerve compression (CNC) injury. The densitometry for the markers in Fig. 6-A is plotted in the remaining panels as the mean and standard error. *P < 0.05. A.U. = arbitrary units. Fig. 6-A Western blot bands for the cellular hypoxic markers HIF1α, catalase, SOD, MMP2, and MMP9 and for the GAPDH reference. Fig. 6-B HIF1α remained elevated after early decompression but not after late decompression. Fig. 6-C MMP2 levels were unchanged after decompression. Fig. 6-D Catalase levels followed a pattern similar to HIF1α, with elevation after early decompression. Fig. 6-E MMP9 remained elevated after late decompression. Fig. 6-F SOD levels were unchanged.
Discussion
Although recent data have shown patient satisfaction with surgical interventions to treat carpal tunnel syndrome32, there remains limited understanding regarding the biochemical changes that occur after surgical intervention. Moreover, surgical intervention often results in limited or no reversal of the related motor atrophy as well as variable changes in the electrophysiological parameters. The present study provided new data to better understand the cellular and molecular changes after chronic nerve compression injury. Although there is considerable evidence suggesting a dramatic decrease in blood flow immediately following a neural injury such as a crush injury13,14, the studies characterizing the changes in hypoxia seen after a chronic nerve compression injury are quite limited. The present study revealed that a chronic nerve compression injury results in a gradual decline in neurovascular blood flow to a new plateau, which contrasts with the changes seen after a crush injury. It also revealed attempts by the peripheral nerve to compensate for the decrease in blood flow caused by the mechanical forces and stresses resulting from the chronic nerve compression injury. These attempts are reflected by the elevated cellular levels of hypoxic markers such as HIF1α, catalase, and MMP9. HIF1α, a transcriptional regulator of hypoxia-inducible genes, induces angiogenesis to increase oxygen delivery when a low oxygen tension is present. MMP9 likely serves to break down the extracellular matrix and remodel tissue in the Schwann cell basal lamina to aid the peripheral nerve in establishing its new baseline blood flow after the chronic nerve compression injury.
Recent advancements in the understanding of compressive neuropathies suggest that a chronic nerve compression state may actually represent a form of chronic neural wound-healing33. Classically, wound-healing is a well-described biological phenomenon consisting of inflammation, cellular proliferation, angiogenesis, and connective tissue remodeling34. We previously demonstrated the prevalence of tissue inflammation, as indicated by upregulation of TNFα (tumor necrosis factor α), proliferation of Schwann cells and fibroblasts, altered neural vasculature, and connective tissue remodeling, during the progression of a chronic nerve compression injury33. The results of that study support the concept that chronic nerve compression creates a neural scar that alters the neural blood flow, as seen in the previous and present experiments.
Hypoxic conditions induce expression of VEGF and other molecules in the nervous system of adults rodents, both in vitro and in vivo35,36. Ischemia augments transcription via upregulation of transcriptional factors known as hypoxia-inducible factors. VEGF production results in improved oxygen delivery to the compressed region of the nerve. The presence of excess oxygen causes the formation of free radicals in the compressed nerve and its associated Schwann cells, and the free radicals are likely neutralized through the production of superoxide dismutases and catalase. HIF1α is located upstream of VEGF, superoxide dismutase, and catalase and induces their production once hypoxia occurs. Enhanced production of these molecules by Schwann cells has been reported previously and suggests that neovascularization occurs secondary to chronic nerve compression18. The elevation of HIF1α and catalase early in the disease process corresponds to the hyperemia seen initially. Furthermore, if these molecular markers are still elevated at the time of surgical decompression, it can be inferred that neurovascular blood flow can be restored by removal of the offending compression and increased angiogenesis. However, once the matrix metalloproteinases are activated, it appears that the hypoxic changes have progressed and permanent pathogenesis has begun, as evidenced by the data presented here.
Seiler et al. performed carpal tunnel release of the median nerve in patients and showed that median nerve blood flow became pulsatile and synchronized with the patient’s pulse within one minute after release of the transverse carpal ligament, implying that the blood flow in these patients had been compromised20. Although such early clinical data exist, we are aware of no relevant previous data regarding what happens to neural blood flow after the surgical intervention is completed. The present study of chronic nerve compression injury in a mouse model suggests that the deleterious hypoxic changes can be reversed if surgical decompression is performed at two weeks after the injury but not at six weeks; the changes in blood flow are permanent in the latter case, implying that there is a time point beyond which the surgical intervention may have limited value. Additional studies analyzing blood vessel formation are needed to understand the molecular mechanisms by which surgical decompression offers better outcomes than more conservative treatment options.
Performing an early surgical decompression offers the optimal treatment option for patients with a nerve compression injury8. Although the relevant time frames may differ between murine and human nerve injuries, the timing of the corresponding changes in molecular markers in human subjects can be used as a basis for establishing the proper time frame for clinical intervention. Furthermore, this approach should be applicable to compression of a variety of nerves including the median nerve, ulnar nerve, or spinal nerve roots in human patients, as the changes at the cellular and molecular levels are expected to be similar to those for compression of the sciatic nerve in our murine model.
Electrophysiological studies are a tool for making the diagnosis of carpal tunnel syndrome in patients10,11. As carpal tunnel syndrome represents a distal demyelination syndrome, prolongation of distal latency may be a more sensitive measure of carpal tunnel syndrome than median nerve conduction velocity, which may not be affected in the measured segment37-40. Our study revealed that distal latency persisted after surgical decompression performed at six weeks but actually returned to the normal range after decompression performed at two weeks. The data for the group treated with decompression at six weeks after injury suggest that distal latency may be a more sensitive and specific measure for predicting patient outcomes after surgical release than the routine use of nerve conduction velocity values alone. Most importantly, these data support previous reports of the importance of early surgical intervention in providing optimal treatment for nerve compression syndromes8.
Footnotes
Investigation performed at the Departments of Orthopaedic Surgery and Biomedical Engineering, University of California, Irvine, California
A commentary by Lindley B. Wall, MD, is linked to the online version of this article at jbjs.org.
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg Am. 2001May;26(3):460-6 [DOI] [PubMed] [Google Scholar]
- 2.von Schroeder HP, Botte MJ. Carpal tunnel syndrome. Hand Clin. 1996November;12(4):643-55 [PubMed] [Google Scholar]
- 3.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993November;75(11):1585-92 [DOI] [PubMed] [Google Scholar]
- 4.Gerritsen AAM, de Vet HCW, Scholten RJPM, Bertelsmann FW, de Krom MCTFM, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002September11;288(10):1245-51 [DOI] [PubMed] [Google Scholar]
- 5.Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552 Epub 2008 Oct 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, Hollingworth W, Kerrigan CL, Deyo RA. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009September26;374(9695):1074-81 [DOI] [PubMed] [Google Scholar]
- 7.Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001October;88(10):1285-95 [DOI] [PubMed] [Google Scholar]
- 8.Chandra PS, Singh PK, Goyal V, Chauhan AK, Thakkur N, Tripathi M. Early versus delayed endoscopic surgery for carpal tunnel syndrome: prospective randomized study. World Neurosurg. 2013May-Jun;79(5-6):767-72 Epub 2012 Sep 25 [DOI] [PubMed] [Google Scholar]
- 9.Scholten RJPM, Mink van der Molen A, Uitdehaag BMJ, Bouter LM, de Vet HCW. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905 Epub 2007 Oct 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arle JE, Zager EL. Surgical treatment of common entrapment neuropathies in the upper limbs. Muscle Nerve. 2000August;23(8):1160-74 [DOI] [PubMed] [Google Scholar]
- 11.Phillips LH 2nd, Juel VC. The role of electrodiagnostic testing in carpal tunnel syndrome. Neurosurg Focus. 1997July15;3(1):e2. [DOI] [PubMed] [Google Scholar]
- 12.Keith MW, Masear V, Chung KC, Maupin K, Andary M, Amadio PC, Watters WC 3rd, Goldberg MJ, Haralson RH 3rd, Turkelson CM, Wies JL, McGowan R. American Academy of Orthopaedic Surgeons clinical practice guideline on diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2009October;91(10):2478-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Q, Midha R, Zochodne DW. The microvascular impact of focal nerve trunk injury. J Neurotrauma. 2010March;27(3):639-46 [DOI] [PubMed] [Google Scholar]
- 14.Igarashi T, Yabuki S, Kikuchi S, Myers RR. Effect of acute nerve root compression on endoneurial fluid pressure and blood flow in rat dorsal root ganglia. J Orthop Res. 2005March;23(2):420-4 [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003June23;461(2):174-86 [DOI] [PubMed] [Google Scholar]
- 16.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg. 1977;11(3):179-87 [DOI] [PubMed] [Google Scholar]
- 17.Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood blow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981January;6(1):3-12 [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Gray M, Chao T, Bear D, Modafferi E, Mozaffar T. Schwann cells upregulate vascular endothelial growth factor secondary to chronic nerve compression injury. Muscle Nerve. 2005April;31(4):452-60 [DOI] [PubMed] [Google Scholar]
- 19.Lin MY, Frieboes LS, Forootan M, Palispis WA, Mozaffar T, Jafari M, Steward O, Gall CM, Gupta R. Biophysical stimulation induces demyelination via an integrin-dependent mechanism. Ann Neurol. 2012July;72(1):112-23 [DOI] [PubMed] [Google Scholar]
- 20.Seiler JG 3rd, Milek MA, Carpenter GK, Swiontkowski MF. Intraoperative assessment of median nerve blood flow during carpal tunnel release with laser Doppler flowmetry. J Hand Surg Am. 1989November;14(6):986-91 [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Nassiri N, Hazel A, Bathen M, Mozaffar T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve. 2012February;45(2):231-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Koning P, Brakkee JH, Gispen WH. Methods for producing a reproducible crush in the sciatic and tibial nerve of the rat and rapid and precise testing of return of sensory function. Beneficial effects of melanocortins. J Neurol Sci. 1986July;74(2-3):237-46 [DOI] [PubMed] [Google Scholar]
- 23.Moy AJ, White SM, Indrawan ES, Lotfi J, Nudelman MJ, Costantini SJ, Agarwal N, Jia W, Kelly KM, Sorg BS, Choi B. Wide-field functional imaging of blood flow and hemoglobin oxygen saturation in the rodent dorsal window chamber. Microvasc Res. 2011November;82(3):199-209 Epub 2011 Jul 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang O, Cuccia D, Choi B. Real-time blood flow visualization using the graphics processing unit. J Biomed Opt. 2011Jan-Feb;16(1):016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao T, Frump D, Lin M, Caiozzo VJ, Mozaffar T, Steward O, Gupta R. Matrix metalloproteinase 3 deletion preserves denervated motor endplates after traumatic nerve injury. Ann Neurol. 2013February;73(2):210-23 Epub 2012 Dec 31 [DOI] [PubMed] [Google Scholar]
- 26.Savaş S, Delibaş N, Savaş C, Sütçü R, Cindaş A. Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord. 2002May;40(5):224-9 [DOI] [PubMed] [Google Scholar]
- 27.Grigorian A, Hurford R, Chao Y, Patrick C, Langford TD. Alterations in the Notch4 pathway in cerebral endothelial cells by the HIV aspartyl protease inhibitor, nelfinavir. BMC Neurosci. 2008;9:27 Epub 2008 Feb 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Wang Y, Gao Z, Yun Z, Ye J. Hypoxia-inducible factor 1 activation from adipose protein 2-cre mediated knockout of von Hippel-Lindau gene leads to embryonic lethality. Clin Exp Pharmacol Physiol. 2012February;39(2):145-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006October13;281(41):30678-83 Epub 2006 Aug 22 [DOI] [PubMed] [Google Scholar]
- 30.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011March4;286(9):7468-78 Epub 2010 Dec 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 2010February16;107(7):3012-7 Epub 2010 Jan 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louie DL, Earp BE, Collins JE, Losina E, Katz JN, Black EM, Simmons BP, Blazar PE. Outcomes of open carpal tunnel release at a minimum of ten years. J Bone Joint Surg Am. 2013June19;95(12):1067-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang JR, Hahn P, Wang W, Nassiri N, Frump D, Mozaffar T, Gupta R Neural wound healing: why surgery may not be enough for carpal tunnel syndrome. Presented at the Annual Meeting of the American Society for Peripheral Nerve, Las Vegas, 2012.
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999September2;341(10):738-46 [DOI] [PubMed] [Google Scholar]
- 35.Kuo NT, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol (1985). 1999January;86(1):260-4 [DOI] [PubMed] [Google Scholar]
- 36.Sinor AD, Irvin SM, Cobbs CS, Chen J, Graham SH, Greenberg DA. Hypoxic induction of vascular endothelial growth factor (VEGF) protein in astroglial cultures. Brain Res. 1998November23;812(1-2):289-91 [DOI] [PubMed] [Google Scholar]
- 37.Kothari MJ, Rutkove SB, Caress JB, Hinchey J, Logigian EL, Preston DC. Comparison of digital sensory studies in patients with carpal tunnel syndrome. Muscle Nerve. 1995November;18(11):1272-6 [DOI] [PubMed] [Google Scholar]
- 38.Di Benedetto M, Mitz M, Klingbeil GE, Davidoff D. New criteria for sensory nerve conduction especially useful in diagnosing carpal tunnel syndrome. Arch Phys Med Rehabil. 1986September;67(9):586-9 [PubMed] [Google Scholar]
- 39.Macdonell RA, Schwartz MS, Swash M. Carpal tunnel syndrome: which finger should be tested? An analysis of sensory conduction in digital branches of the median nerve. Muscle Nerve. 1990July;13(7):601-6 [DOI] [PubMed] [Google Scholar]
- 40.Robinson LR, Micklesen PJ, Wang L. Optimizing the number of tests for carpal tunnel syndrome. Muscle Nerve. 2000December;23(12):1880-2 [DOI] [PubMed] [Google Scholar]