Abstract
Skeletal muscles are the organ of movement, and their growth, regeneration and maintenance are dependent in large part on a population of myogenic stem cells known as satellite cells. Skeletal muscles and these resident myogenic stem cells (i.e., satellite cells) are commonly exposed to significant doses of radiation during diagnostic procedures and/or during the radiotherapeutic management of cancer. The main objective of this study was to examine the effects of clinically relevant doses of γ radiation on satellite cell survival and proliferation, cell cycle regulation, apoptosis, DNA double-strand break repair, oxidative stress and NO production. Overall, our findings demonstrate that doses of γ radiation ≥5 Gy reduced satellite cell numbers by at least 70% due in part to elevated apoptosis and the inhibition of cell cycle progression. Radiation was also found to cause a significant and persistent increase in the level of reactive oxygen and nitrogen species. Interestingly, and within this backdrop of elevated oxidative stress, similar doses were found to produce substantial reductions in the levels of nitric oxide (NO). Proliferation of satellite cells has been shown to depend in part on the production of NO, and our findings give rise to the possibility that radiation-induced reductions in NO levels may provide a mechanism for the inhibition of satellite cell proliferation in vitro and possibly the regrowth of skeletal muscle exposed during clinical irradiation procedures.
INTRODUCTION
Skeletal muscles are the organ of movement. Their actions are essential not only for maintaining muscle mass and function but also for the health of other systems of the body such as that of the skeletal (i.e. bones, tendons and ligaments) and cardiovascular systems.
The health of skeletal muscle and its ability to regenerate and grow in adults is dependent on a special population of myogenic stem cells known as satellite cells. Mauro (1) was the first to identify these cells, and he named them satellite cells because they were located exclusively at the periphery of skeletal muscle fibers (cells). When activated, satellite cells proliferate and differentiate into myoblasts that fuse with the existing muscle fiber, increasing the population of myonuclei and, as a consequence, the transcriptional machinery of that cell (2). The importance of satellite cells in maintaining the health of skeletal muscle can be exemplified by their central role in the regeneration of skeletal muscle after injury induced by trauma, genetic defects or eccentric contractions, the growth of skeletal muscle during development and in response to resistance training, the recovery of muscle mass after atrophy, and maintaining muscle mass during aging (2-5).
Skeletal muscles and satellite cells are commonly exposed to significant doses of radiation during diagnostic procedures and/or during the radiotherapeutic management of cancer. Skeletal muscles and satellite cells are also irradiated during the treatment of heterotopic bone growth, which can occur in patients receiving joint replacements (~200,000/year) and in individuals who suffer traumatic injuries (e.g., soldiers injured due to explosive devices) (6-14). The treatment of various disorders (e.g., breast/bone cancer, trauma) often results in extensive muscle disuse and atrophy (15-21). The recovery of muscle mass appears to be dependent on the proliferation and differentiation of satellite cells. Herein lies a potentially significant clinical problem: the use of ionizing radiation in the management of certain cancers and heterotopic bone growth has the potential to damage satellite cells and, as a result, the health of skeletal muscle and that of the whole body.
Given the importance of satellite cells in maintaining the health of skeletal muscle, it is somewhat surprising that the effects of γ radiation on satellite cells have been poorly studied. Thus we performed a series of studies designed to test the hypothesis that clinically relevant doses of γ radiation negatively affect satellite cell proliferation. We examined satellite cell proliferation, cell cycle regulation, apoptotic and non-apoptotic modes of cell death, the presence of double-strand breaks (DSBs), oxidative stress and NO production. This collective set of analyses suggests that radiation damages satellite cells not only by direct means (i.e., cell killing) but also by indirect means that promote an oxidative microenvironment that further inhibits the growth and maturation of myogenic precursor cells, possibly via a pathway involving nitric oxide (NO).
METHODS AND MATERIALS
Isolation of Satellite Cells and Tissue Culture
Satellite cells were derived from female Sprague-Dawley rats (weight 250–300 g; from Taconic) using procedures similar to those described elsewhere (22-24). Muscle groups from the upper hind limb were excised, trimmed of fat and connective tissue, minced with scissors, and digested with 1.25 mg/ml protease type XIV (Sigma, St. Louis, MO) for 1 h at 37°C. Cells were separated from muscle fiber fragments and tissue debris by differential centrifugation and plated on Corning plastic flasks coated with Matrigel in a 1:100 dilution with PBS-minus. Cells were grown in the presence of F10 nutrient mixture (base medium, Gibco), containing 20% fetal bovine serum (Gibco), 1× penicillin/streptomycin (Gibco), and 40 μg/ml of G418 (Sigma). All cultures were grown on tissue culture treated plasticware. To demonstrate that the cells were multipotent, they were plated onto matrigel-coated chambered slides and maintained for 3 days. After 3 days, adherent cells were fixed and immunofluorescence was used to determine their phenotypic characteristics. The cell-specific antibodies used included MyoD (putative marker of satellite cell proliferation; Santa Cruz satellite cell-760), Pax 7 (Developmental Studies Hybridoma Bank), and myogenin (putative marker of differentiation; Santa Cruz satellite cell-576). When satellite cells are activated, they quickly express MyoD (25-27), whereas the expression of myogenin is thought to represent the initiation of myogenic differentiation (25, 27-29). Approval for animal experiments was obtained from our Institutional Review Board before the experiments were conducted.
Measurement of Apoptosis
Poly(ADP-ribose)polymerase (PARP) cleavage and annexin V assays were used to assess apoptosis. For the PARP analyses, cells were fixed in 4% paraformaldehyde and labeled with a preconjugated labeled antibody. Annexin V labeling was performed by harvesting cells, rinsing them in PBS, and then incubating the cells for 20 min at ambient temperature in limiting volumes (~ 0.2–0.5 ml) of binding buffer (BD Biosciences) containing FITC-conjugated annexin V. Cell suspensions were brought to 0.5 × 106 cells/ml in PBS and were immediately subjected to FACS analysis. Apoptosis assays were performed in triplicate and derived from independently irradiated cultures of cells. FACS data were also analyzed using the ModFit LT program to estimate the percentage of apoptotic cells from cell cycle histograms.
Cell Cycle Analysis
Exponentially growing cultures of satellite cells were either sham-irradiated or exposed to 5 Gy and at various times after irradiation (6, 12, 18, 24 and 48 h) were fixed in 70% ethanol and stored at −20°C. On the day of assay, samples were resuspended for 30 min at ambient temperature in isotonic phosphate-buffered saline (PBS) supplemented with RNase (50 U/ml) and propidium iodide (PI, 10 μg/ml). Then cells were assayed for DNA content by FACS analysis of PI fluorescence. Raw data were gated to eliminate debris and doublets and to calculate the distribution of cells throughout the cell cycle. A minimum of 10,000 cells were analyzed at each time using ModFit LT analysis software (Verity Software House, Topsham, ME). Reverse χ2 values were routinely under 5, indicating that the cell cycle data were within the parameters of the ModFit algorithm.
γ-H2AX Labeling of Cells
Immunofluorescence was performed to determine the nuclear distribution of γ-H2AX-positive foci in individual cells. Cells were grown on chambered slides for 1 day prior to γ irradiation, treated, fixed in 4% paraformaldehyde, permeabilized in a 0.2% solution of Triton X-100 in PBS, and blocked in 10% FBS/1% BSA for 1 h using a 1:1000 dilution of the fluorescein isothiocyanate (FITC)-labeled mouse monoclonal antibody against γ-H2AX (Millipore, CA). After overnight incubation at 4°C, slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in MP Prolong GOLD antifade (Invitrogen-Molecular Probes, Carlsbad, CA). Five hundred cells from each treatment were scored blind for the presence or absence of γ-H2AX nuclear foci visible against a DAPI counterstain.
Measurement of Reactive Oxygen and Nitrogen Species (ROS/RNS)
The detection of intracellular ROS (peroxides, hydroxyl radicals, superoxide) and RNS (peroxynitrite) was based on the ability of live cells to oxidize fluorogenic dyes to their corresponding fluorescent analog. Exponentially growing cultures were treated for 1 h at 37°C with two ROS sensitive dyes: 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (5 μM, CM-H2DCFDA, Molecular Probes, Eugene OR) and 0.5 μM MitoSOX (Invitrogen, Carlsbad, CA), then immediately harvested and subjected to FACS analysis. For each postirradiation time, measurements of ROS/RNS in irradiated and sham-irradiated control cultures were performed in parallel. All measurements were performed in triplicate and were derived from independently irradiated cell cultures.
Measurement of Nitric Oxide (NO)
The detection of intracellular NO was based on the ability of cells to oxidize the NO-sensitive fluorogenic dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF, Molecular Probes, Eugene, OR). At selected times postirradiation, exponentially growing cultures were treated for 1 h at 37°C with 5 μM of the dye, then harvested and subjected to FACS analysis. For each postirradiation time, measurements of NO in irradiated and sham-irradiated control cultures were performed in parallel. All measurements were performed in duplicate and were derived from independently irradiated cell cultures.
Data Analysis and Statistics
Significance of differences between data sets obtained by FACS analysis was determined by the Kolmogorov-Smirnov test (K-S test) provided with the Cell Quest™ software. Fluorescence values derived from FACS data are presented as relative fluorescence units (RFU). For all other data sets, means were calculated and assessed for significance (P ≤ 0.05) by analysis of variance (ANOVA).
RESULTS
Satellite Cell Proliferation and Viability
As shown in Fig. 1, we used the developmental marker MyoD to demonstrate that satellite cells were in a proliferative phase at the time of analysis. To gauge the relative sensitivity of satellite cells to ionizing radiation, cells were irradiated and analyzed by cell counting and by an XTT assay performed 3 days later. The effects of γ radiation on satellite cell proliferation and metabolic viability (i.e. XTT assay) are shown in Fig. 2. Satellite cell proliferation was significantly reduced at all doses examined (P < 0.01), reaching a relative maximum (~70% reduction) at doses over 5 Gy. The results of the XTT assay mirrored those seen for proliferation.
FIG. 1.
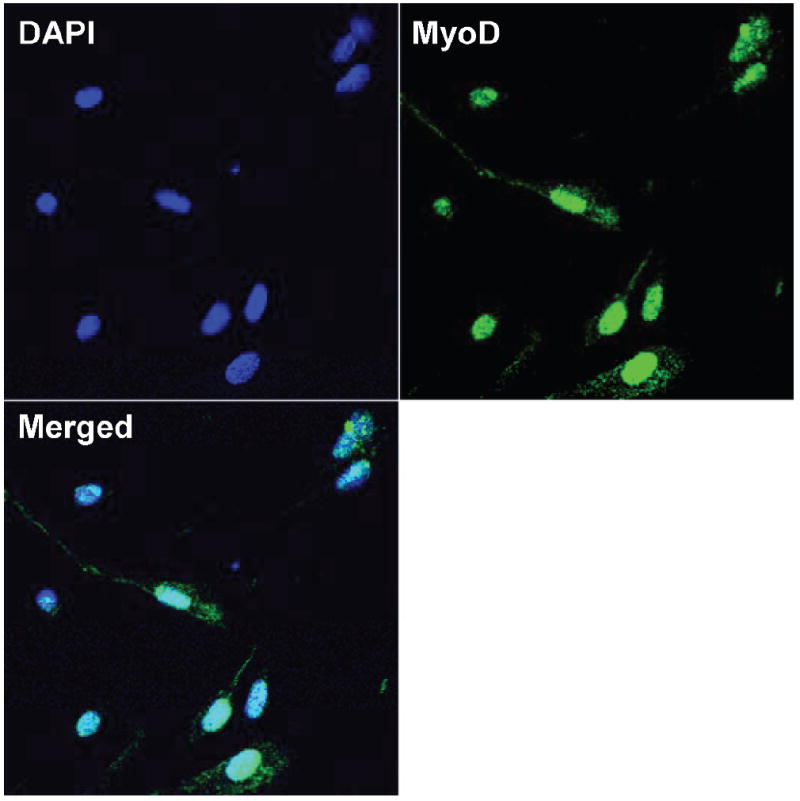
Images of cultured satellite cells labeled for the presence of nuclei (DAPI) and a developmental marker (MyoD) thought to be specific for satellite cells undergoing proliferation. Merged image shows that all cells (as determined by DAPI) were MyoD-positive.
FIG. 2.
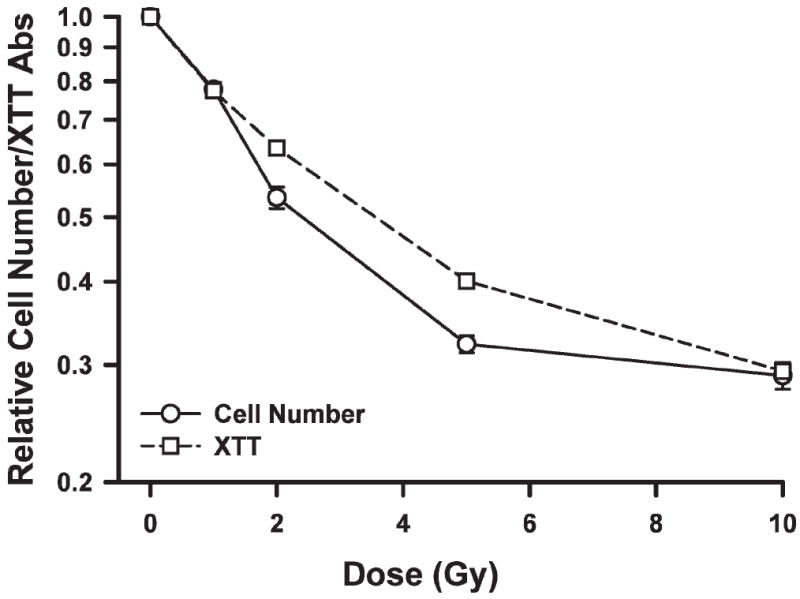
Relative change in satellite cell number or XTT absorbance as a function of dose. Points are means of triplicate measurements ± SE.
Radiation Induced Cell Cycle Checkpoints in Satellite Cells
To ascertain whether exposure to radiation led to the inhibition of satellite cell cycle progression, cells were given 5 Gy and assessed for DNA content using standard FACS methodologies. At 6 h after irradiation (Fig. 3), satellite cells showed a marked increase (2.5-fold) in cells at the G2/M border; the fraction of cells with a 4n diploid complement of DNA remained high over the course of the experiment (48 h; see Fig. 3). There was a steady decline in S-phase cells over the first 18 h after irradiation. The fraction of S-phase cells did not recover during the subsequent 30 h (see Fig. 3). As satellite cells gradually escaped the initial block at G2/M, they traversed into the block at G1/S by 12 h where they accumulated at ~1.3-fold higher levels. Collectively, these data show that a dose of 5 Gy produces the same pattern of cell cycle inhibition and arrest that has been observed for many other types of cells subjected to similar levels of irradiation.
FIG. 3.
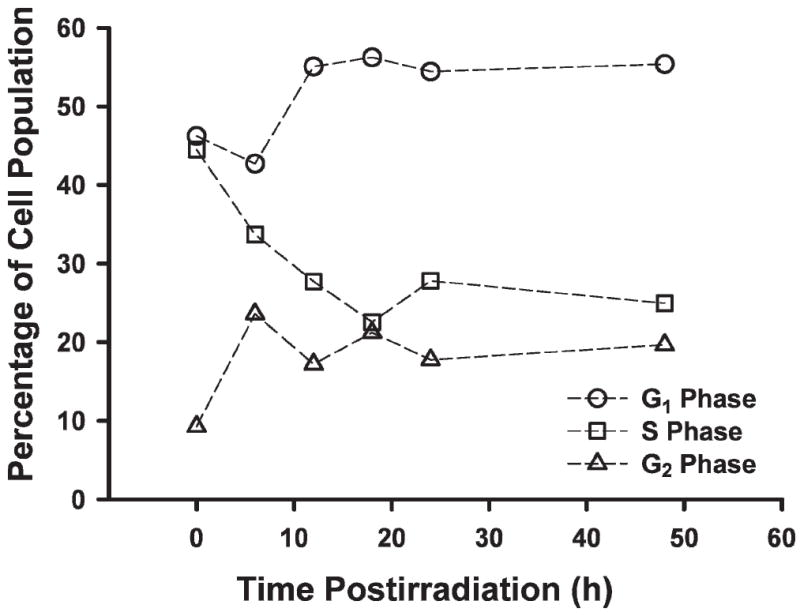
Satellite cells show G1/S and G2/M cell cycle checkpoints. Cells were irradiated with a dose of 5 Gy, fixed at the indicated times, and analyzed for DNA content by FACS. The ModFit LT algorithm was used to fit data and derive cell cycle distributions that minimized the reverse chi-square (RCS) value.
Kinetics of γ-H2AX
The formation and disappearance of DSBs after 5 Gy γ irradiation was tracked using the phosphorylation of H2AX. As shown in Fig. 4A and C, radiation produced a rapid and large increase in the proportion of γ-H2AX-positive cells. For example, approximately 90% of the satellite cells contained γ-H2AX-positive foci 20 min after irradiation (Fig. 4). The level of γ-H2AX-positive foci remained elevated for approximately 2 h, after which there was a rapid reduction in the relative proportion of DSBs as reflected by the disappearance of γ-H2AX-positive foci, approaching baseline values 6 h after irradiation (see Fig. 4B and C).
FIG. 4.
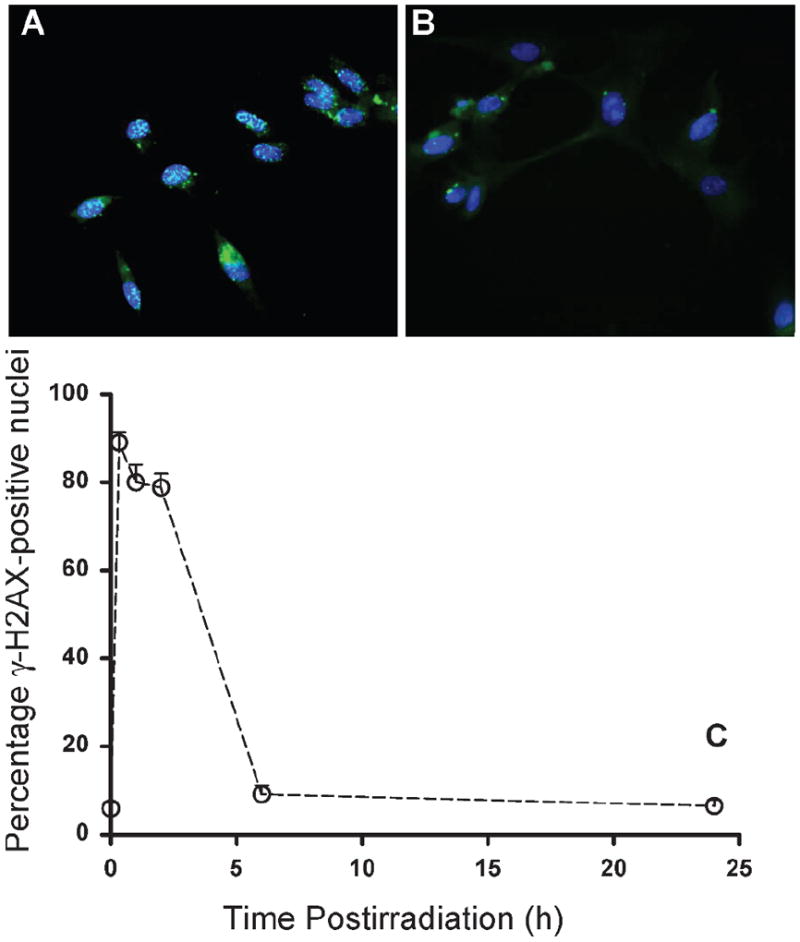
Effects of γ radiation on the yield of γ-H2AX-positive satellite cell as a function of time after 5 Gy γ irradiation (panel C). Panel A: 2 h after irradiation; panel B: 24 h after irradiation. Data are reported as means ± SE. γ-H2AX-positive foci at 20 min, 1 h and 2 h were significantly different from control values at P < 0.001.
Apoptosis of Irradiated Satellite Cells
To determine whether irradiated satellite cells undergo apoptosis, PARP cleavage and annexin V assays were performed. Both end points gave qualitatively similar data (Fig. 5), and over the selected time course, apoptosis was generally increased and was dependent on dose. At 48 h, apoptosis was elevated by ~1.3–1.7-fold over controls.
FIG. 5.
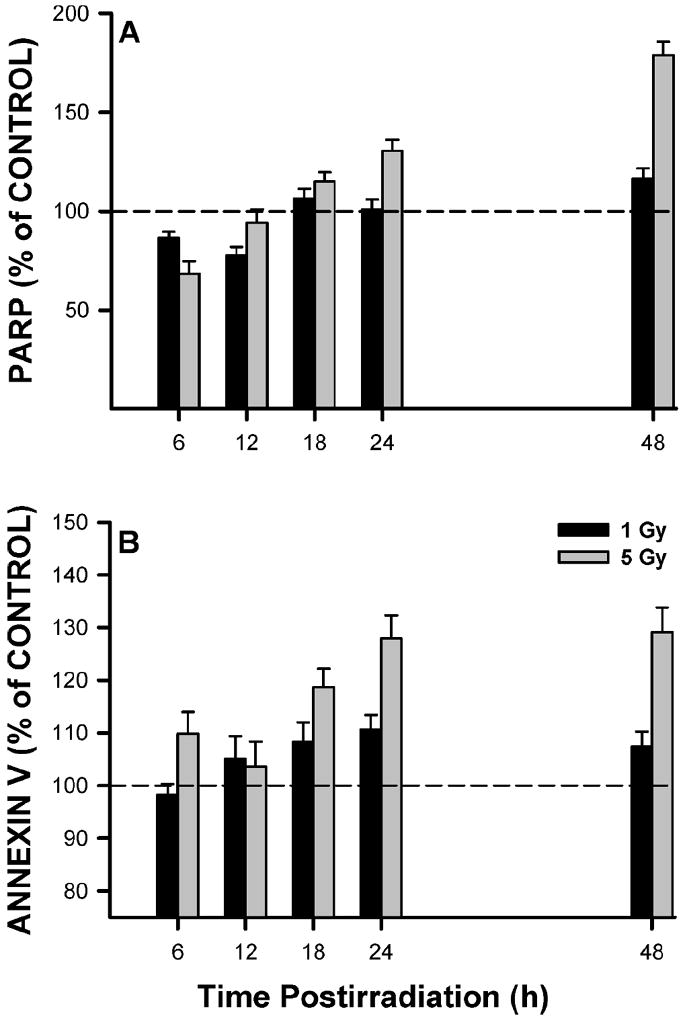
Satellite cells were irradiated and fixed at various times for FACS analysis of apoptosis by PARP cleavage (panel A) or annexin V binding (panel B). PARP and annexin V levels are expressed relative to the control levels (represented by dashed line) at the same times. Data are means of triplicate measurements ± SE.
Elevation of Oxidative Stress in Irradiated Satellite Cells
As part of our working hypothesis, we postulate that oxidative stress induced by γ radiation negatively affects the ability of satellite cells to proliferate or differentiate. As shown in Fig. 7A and B, we observed that ROS/RNS levels increase after irradiation as detected by elevations in both CM-H2DCFDA- and MitoSOX-derived fluorescent signals. There was a substantial decrease in CM-H2DCFDA levels at 6 h; however, this was followed by significant increases at each of the later times (Fig. 6A). MitoSOX levels gradually increased after irradiation such that they were elevated by ~80% at 72 h (Fig. 6B). The oxidative changes reported here are not likely due to marked differences in dye uptake and efflux across the cells, because redox-insensitive fluorescent dye analogs did not show any dose-dependent changes (data not shown).
FIG. 7.
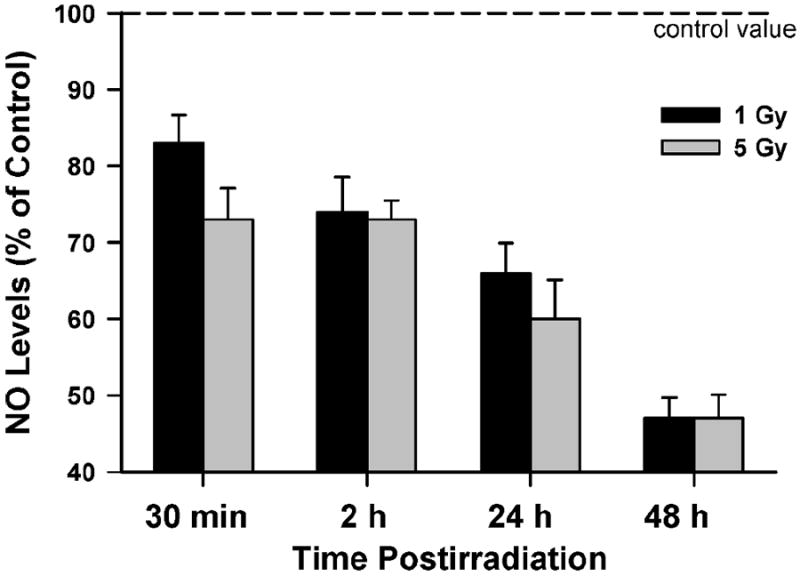
Effects of 1 and 5 Gy γ radiation on NO production by satellite cells. Each irradiation condition was run in duplicate; the height of each bar represents the mean NO level (of all cells run in a given experiment) expressed relative to the control (unirradiated) condition. Data are reported as means ± SE.
FIG. 6.
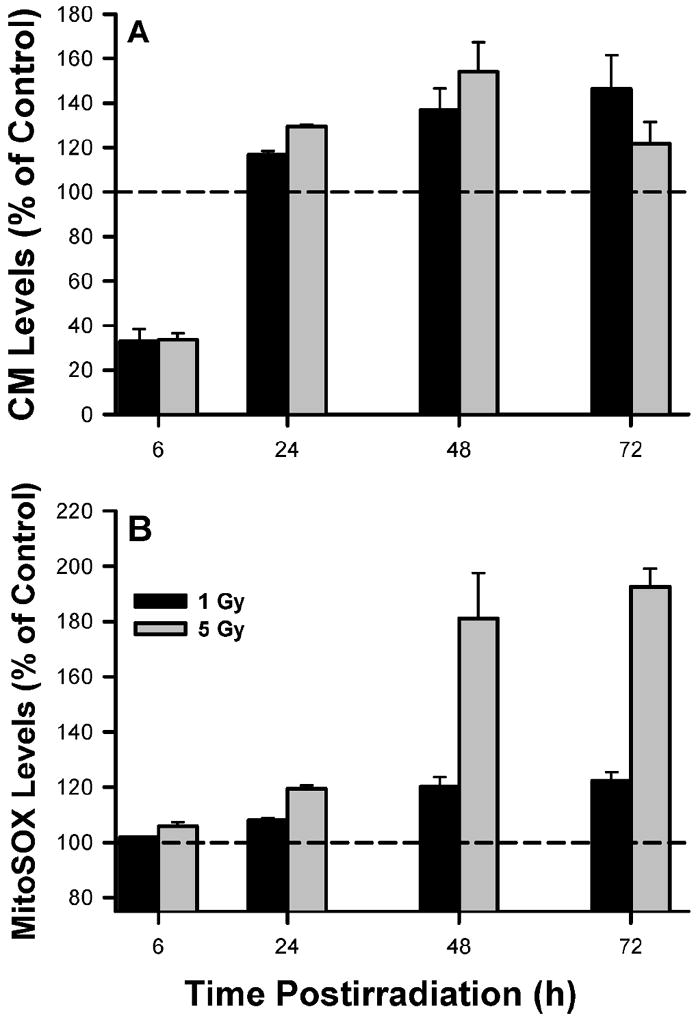
Oxidative stress in satellite cells. Absolute fluorescence values were normalized to unirradiated controls (represented by dashed line) to derive the relative fluorescent ROS levels. Data are means of triplicate measurements ± SE. For both the CM-H2DCFDA and MitoSOX data, there were main effects between groups and time that were significant at P ≤ 0.01.
γ Radiation Reduces NO Production in Satellite Cells
Allen and colleagues (22-24, 30-35) showed that NO plays a central role in promoting satellite cell proliferation. This led us to examine the effects of γ radiation on NO levels in satellite cells. Satellite cells were irradiated with 1 and 5 Gy. As shown in Fig. 7, both doses of radiation produced a rapid and progressive reduction in NO to 49 (1 Gy) and 47% (5 Gy) 48 h after irradiation.
Satellite Cell Proliferation can be Rescued Using a NO Donor
We also examined whether a NO donor (Sulindac) could rescue proliferation of irradiated satellite cells. Satellite cells were irradiated with 5 Gy, treated with Sulindac for 48 h, and then counted. As shown in Fig. 8, we observed a significant (P ≤ 0.02) dose–response effect, with 1 μM Sulindac producing ~15% increase in satellite cell counts.
FIG. 8.
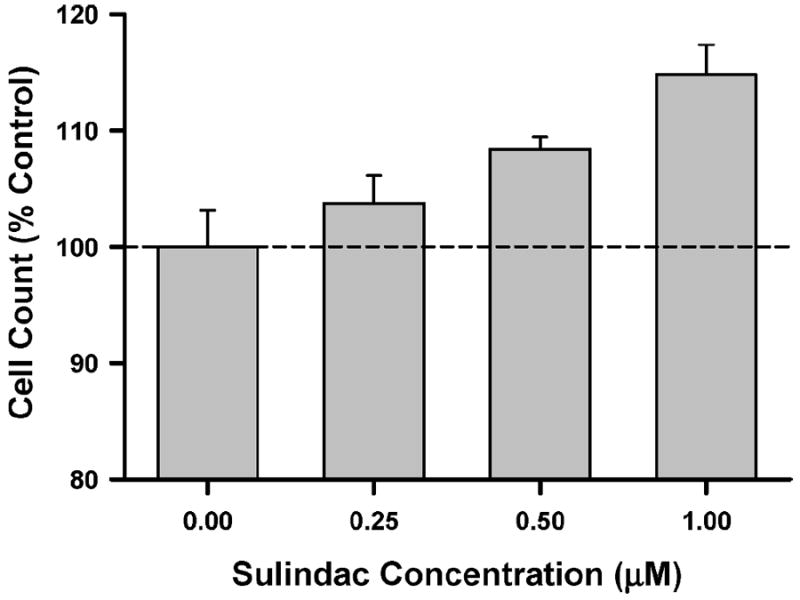
Effects of NO donor on cell counts in irradiated satellite cells. Satellite cells were exposed to 5 Gy and then treated with Sulindac for 48 h. Data were analyzed using a one-way ANOVA, resulting in an F ratio of 6.9 (P = 0.013). Dashed horizontal line is reference line for control condition. Data are means ± SE of triplicate measurements.
DISCUSSION
Skeletal muscle is thought to be highly radioresistant; however, this perspective deserves further consideration given that myogenic precursor cells (e.g., satellite cells) play a fundamental role in regulating the growth and health of skeletal muscle. Cells undergoing irradiation are susceptible not only to radiation-induced ionizations and resultant cellular damage but also to protracted free radical damage resulting from perturbations to redox homeostasis, proliferating cells are more susceptible to the harmful effects of radiation than quiescent cells, and few studies (if any) have examined the effects of clinically relevant doses of γ radiation on myogenic precursor cells.
Given these considerations, the findings of this study provide a unique series of baseline measurements that are important from a number of standpoints. First, to our knowledge, this is the only study to date to examine the effects of clinically relevant doses of γ radiation on key markers of satellite cell proliferation and viability. Second, we observed that clinically relevant doses of γ radiation produce significant elevations in oxidative stress, which may impair satellite cell proliferation. Finally, a very novel finding of this study is that γ radiation markedly reduces NO levels in satellite cells. This is an important observation because it has been reported that satellite cell proliferation is dependent on a NO-mediated pathway (23, 35). Each of these key observations may be critical to explain muscle wasting in patients subjected to clinical irradiation. While the causal role of radiation in precipitating clinical complications of irradiated muscles is uncertain at present, our studies provide an important backdrop for the points discussed below.
Effects of γ Radiation on Satellite Cell Proliferation and Viability
As noted above, very little is known about the effects of clinically relevant doses of γ radiation on satellite cell proliferation and differentiation. Thus a major focus of the current study was on issues related to proliferation. We observed that doses of 2 and 5 Gy reduced satellite cell proliferation by approximately 50 and 70%, respectively. The reduction in satellite cell proliferation was associated with several key phenomena: an elevation in apoptosis as seen in dose-dependent increases in PARP and annexin V and persistent cell cycle arrest at both the G1/S and G2/M checkpoints. As shown in Fig. 4, satellite cells demonstrate a rapid repair of DSBs that is virtually complete 6 h after 5 Gy γ irradiation. The rapid disappearance of focus-positive satellite cells suggests that they possess no intrinsic defect(s) in the repair of these cytotoxic lesions. Radiation also activated cell cycle checkpoints, because the fraction of S-phase cells declined as cells were delayed in progressing past the G1/S and G2/M checkpoints 48 h postirradiation (Fig. 4). By 48 h, apoptosis was high, likely reflecting the inability of cells to circumvent checkpoint controls.
Cell viability as measured using the XTT assay mirrored that of the cell counts, and we interpret these data to indicate that the metabolic capacity of the surviving cells was unchanged from that of control cells; this appears to be true across a broad range of doses of γ radiation (1–10 Gy). From a redox perspective, the XTT data are interesting because they suggest that the surviving cells and their mitochondria have the potential to contribute significantly to chronically elevated oxidative stress, thereby negatively affecting satellite cell proliferation and differentiation (see below).
γ Radiation Produces Early and Persistent Oxidative Stress in Satellite Cells
The cellular redox state controls a wide variety of key processes regulated by an intricate balance between pro- and antioxidant forces in the cell. Our findings are the first to characterize the changes in oxidative stress imposed on satellite cells by γ radiation. Specifically, we observed that γ radiation produced dose-dependent increases in ROS/RNS levels that persisted for several days postirradiation. These observations are consistent with the working hypothesis that γ radiation might inhibit satellite cell proliferation via elevated oxidative stress.
Although few studies have examined the effects of altered redox state on satellite cell proliferation and differentiation, Chakravarthy et al. (36), Csete et al. (37), and Lee et al. (38) demonstrated that elevated oxidative stress can negatively affect both proliferation and differentiation. Both Chakravarthy et al. (36) and Csete et al. (37) demonstrated the importance of redox state by manipulating the oxygenation of the tissue culture medium: cells cultured using 20% O2 demonstrated a significant reduction in proliferation and differentiation compared to cells cultured at 6% O2. A more direct approach was taken by Lee et al. (38), who manipulated the oxidative stress of satellite cells by creating glutathione peroxidase 1 (GPX1) null satellite cells and observed that proliferation was significantly reduced. The reduction in satellite cell proliferation induced via the GPX1 null condition was associated with significant elevations in the expression of key myogenic bHLH transcription factors (i.e., MyoD and myogenin) by about five- to ninefold (38). Furthermore, Lee et al. (38) observed that differentiation was dramatically reduced in the GPX1 null cells when exposed to high levels of O2, finding that only ~20% of the GPX1 null satellite cells were incorporated into myotubes as compared to ~80–90% of wild-type satellite cells. Collectively, these findings provide important data supporting our working hypothesis that oxidative stress induced by γ radiation can negatively affect satellite cell proliferation and differentiation.
Satellite Cell NO Levels are Reduced by γ Radiation
Radiation has the potential to reduce satellite cell proliferation by lowering NO levels via a coupled reaction whereby increases in the superoxide anion (O2•−) act to quench NO levels by reacting to form peroxynitrite (39, 40). To date, however, no studies have examined the effects of γ radiation on NO levels in satellite cells. We observed that γ radiation produced a progressive reduction in NO levels (see Fig. 7). Allen and colleagues provided extensive data supporting the concept that NO plays a central role in mediating satellite cell proliferation via a NO/MMP2/HGF/c-met pathway (22-24, 30-35). Specifically, they reported that satellite cell proliferation could be inhibited using a nitric oxide synthase (NOS) inhibitor, NG-nitro-l-argininemethylester (L-NAME), and increased using NO donors like sodium nitroprusside (SNP). More recent findings by Allen and his colleagues (41, 42) provide definitive evidence that MMP2 mediates the stretch-dependent activation of satellite cells in a nitric oxide-dependent manner. In contrasting the effects of 1 and 5 Gy on satellite cell proliferation and NO levels, one might challenge the importance of NO in regulating the response of satellite cells to radiation. We point out that there is no reason a priori to expect that the dose response for cell killing and/or proliferation would be coincident with radiation-induced changes in NO levels. While the reduction in satellite cell proliferation is due to the cell killing and cell cycle perturbation, the finding that radiation-induced reductions in NO levels occur at low doses (<5 Gy) suggests the existence of a unique radioresponsive pathway involving NO. The fact that NO levels do not track the drop in cell numbers (Fig. 2) suggests that the radiation-induced inhibition of NO may reach a threshold at lower doses. A preponderance of data suggest that cell killing does not show such a dose threshold. Furthermore, we have observed that a NO donor can rescue satellite cell proliferation at doses of γ radiation up to 5 Gy (Fig. 8) and that mechanical stretch of irradiated (5 Gy) satellite cells can completely rescue satellite cell proliferation (data not shown). The stretch activation of satellite cell proliferation is known to act via elevations in NO levels. Given this background, our collective data raise the intriguing possibility that the reduction in satellite cell proliferation may in part be mechanistically linked to a radiation-induced reduction in NO levels.
Summary
There are over 600 muscles in the human body, and skeletal muscles comprise approximately 40% of the body’s mass. Due to the ubiquitous nature of skeletal muscle, it is difficult to irradiate a clinically involved structure without also irradiating skeletal muscle. Thus it is somewhat surprising that the effects of radiation on satellite cells are not better understood. Our findings demonstrate that clinically relevant doses of γ radiation are capable of impairing satellite cell proliferation and inducing significant oxidative stress. Further work is required to understand mechanism(s) underlying the role of elevated oxidative stress and the accompanying reductions in NO levels, especially as they apply to those processes influencing the proliferation and differentiation of satellite cells.
Acknowledgments
This work was supported by grants from the California Institute for Regenerative Medicine no. RS-1-00413 (CLL and VJC) and a pilot project (VJC) supported through NIH Grant HD50837 to the National Skeletal Muscle Research Center at the University of California, San Diego, and by the National Space Biomedical Research Institute through NASA NCC-9-58 (BT, CLL). The authors would also like to thank Drs. Ron Allen and Frank Booth for their thoughts and input regarding the isolation of satellite cells.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- 6.Ashton LA, Bruce W, Goldberg J, Walsh W. Prevention of heterotopic bone formation in high risk patients post-total hip arthroplasty. J Orthop Surg (Hong Kong) 2000;8:53–57. doi: 10.1177/230949900000800210. [DOI] [PubMed] [Google Scholar]
- 7.Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006;65:1289–1299. doi: 10.1016/j.ijrobp.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am. 1981;63:201–208. [PubMed] [Google Scholar]
- 9.Lo TC, Healy WL, Covall DJ, Dotter WE, Pfeifer BA, Torgerson WR, Wasilewski SA. Heterotopic bone formation after hip surgery: prevention with single-dose postoperative hip irradiation. Radiology. 1988;168:851–854. doi: 10.1148/radiology.168.3.3136510. [DOI] [PubMed] [Google Scholar]
- 10.Parkinson JR, Evarts CM, Hubbard LF. Radiation therapy in the prevention of heterotopic ossification after total hip arthroplasty. Hip. 1982:211–227. [PubMed] [Google Scholar]
- 11.Pellegrini VD, Jr, Evarts CM. Radiation prophylaxis of heterotopic bone formation following total hip arthroplasty: current status. Semin Arthroplasty. 1992;3:156–166. [PubMed] [Google Scholar]
- 12.Roth A, Fuller J, Fahrmann M, Anders J, Sachse A, Sander K, Venbrocks R. Prophylaxis of heterotopic bone formation by radiotherapy – a comparison between pre- and postsurgical activity. Acta Chir Orthop Traumatol Cech. 2005;72:38–41. [PubMed] [Google Scholar]
- 13.Seegenschmiedt MH, Keilholz L, Martus P, Goldmann A, Wolfel R, Henning F, Sauer R. Prevention of heterotopic ossification about the hip: final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39:161–171. doi: 10.1016/s0360-3016(97)00285-x. [DOI] [PubMed] [Google Scholar]
- 14.Seegenschmiedt MH, Makoski HB, Micke O. Radiation prophylaxis for heterotopic ossification about the hip joint—a multicenter study. Int J Radiat Oncol Biol Phys. 2001;51:756–765. doi: 10.1016/s0360-3016(01)01640-6. [DOI] [PubMed] [Google Scholar]
- 15.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res Treat. 2005;94:1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 16.Keramopoulos A, Tsionou C, Minaretzis D, Michalas S, Aravantinos D. Arm morbidity following treatment of breast cancer with total axillary dissection: a multivariated approach. Oncology. 1993;50:445–449. doi: 10.1159/000227227. [DOI] [PubMed] [Google Scholar]
- 17.Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44:449–457. doi: 10.1080/02841860510029905. [DOI] [PubMed] [Google Scholar]
- 18.Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat. 2008;110:19–37. doi: 10.1007/s10549-007-9710-9. [DOI] [PubMed] [Google Scholar]
- 19.Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26:78–84. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 20.Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, Sugden E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat. 2007;106:19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 21.Sugden EM, Rezvani M, Harrison JM, Hughes LK. Shoulder movement after the treatment of early stage breast cancer. Clin Oncol (R Coll Radiol) 1998;10:173–181. doi: 10.1016/s0936-6555(98)80063-0. [DOI] [PubMed] [Google Scholar]
- 22.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi R, Wuollet AL, Tabata K, Nishimura S, Tabata S, Mizunoya W, Ikeuchi Y, Allen RE. A role for calciumcalmodulin in regulating nitric oxide production during skeletal muscle satellite cell activation. Am J Physiol Cell Physiol. 2009;297:922–929. doi: 10.1152/ajpcell.00471.2008. [DOI] [PubMed] [Google Scholar]
- 24.Tatsumi R, Yamada M, Katsuki Y, Okamoto S, Ishizaki J, Mizunoya W, Ikeuchi Y, Hattori A, Shimokawa H, Allen RE. Low-pH preparation of skeletal muscle satellite cells can be used to study activation in vitro. Int J Biochem Cell Biol. 2006;38:1678–1685. doi: 10.1016/j.biocel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Fuchtbauer EM, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn. 1992;193:34–39. doi: 10.1002/aja.1001930106. [DOI] [PubMed] [Google Scholar]
- 26.Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- 27.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yablonka-Reuveni Z, Paterson BM. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J Histochem Cytochem. 2001;49:455–462. doi: 10.1177/002215540104900405. [DOI] [PubMed] [Google Scholar]
- 29.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol. 1999;181:499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve. 2004;30:654–658. doi: 10.1002/mus.20114. [DOI] [PubMed] [Google Scholar]
- 33.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 34.Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. 2002;13:2909–2918. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell Mol Life Sci. 2001;58:1150–1158. doi: 10.1007/PL00000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Shin HS, Shireman PK, Vasilaki A, Van Remmen H, Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 41.Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol. 2008;40:2183–2191. doi: 10.1016/j.biocel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Yamada M, Tatsumi R, Kikuiri T, Okamoto S, Nonoshita S, Mizunoya W, Ikeuchi Y, Shimokawa H, Sunagawa K, Allen RE. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve. 2006;34:313–319. doi: 10.1002/mus.20601. [DOI] [PubMed] [Google Scholar]


