Abstract
Background
Apoptosis is induced by ethanol in human placental trophoblast cells, possibly disrupting placentation and contributing to intrauterine growth restriction in fetal alcohol spectrum disorder (FASD). Ethanol induces programmed cell death in several embryonic tissues by raising intracellular Ca2+. Therefore, the role of Ca2+ signaling in ethanol-induced apoptosis was examined using human first trimester cytotrophoblast cell lines, examining the hypothesis that apoptosis is dependent on intracellular Ca2+ signaling.
Methods
Using HTR-8/SVneo and SW.71 cytotrophoblast cell lines, real-time intracellular Ca2+ concentration was monitored by fluo-4 epifluorescence microscopy and apoptosis was assessed by flow cytometry of cells fluorescently labeled for DNA fragmentation (TUNEL) and annexin V binding.
Results
Intracellular Ca2+ concentrations increased synchronously in all cells within 10 s of exposure to 50 mM ethanol, but not at lower ethanol concentrations (10–25 mM) incapable of inducing apoptosis. Trophoblast cells treated with inhibitors of Ca2+ signaling (BAPTA-AM, U73122, xestospongin D, BAPTA, SKF-96365) produced no intracellular Ca2+ transients after exposure to 50 mM ethanol and were protected from cell death induced by ethanol.
Conclusions
Ethanol-induced apoptosis in human cytotrophoblast cells, identified by DNA fragmentation and externalized phosphatidylserine, was dependent upon Ca2+ signaling. Both intracellular Ca2+ mobilization and extracellular Ca2+ influx were required, as well as phosphatidylinositol signaling. Inhibition by SKF-96365 suggests that the capacitative Ca2+ entry mechanism that utilizes TRPC channels was activated by ethanol. Apoptosis occurs downsteam of Ca2+ signaling in trophoblasts, and may contribute to placental insufficiency and poor fetal growth associated with FASD.
Keywords: trophoblast, alcohol, apoptosis, calcium, intracellular signaling, intrauterine growth restriction, fetal alcohol spectrum disorder
INTRODUCTION
Ethanol, a powerful teratogen, causes apoptosis in neural regions of the developing embryo and is the third most recognizable cause of neurodevelopmental disability in the United States (Hannigan and Armant, 2000). Common characteristics of fetal alcohol spectrum disorder (FASD) include facial and cranial physical defects such as microcephaly, reduction in size of the palpebral fissures, thinning of the vermilion of the upper lip, and impaired behavioral and cognitive ability (Hannigan and Armant, 2000). Prenatal ethanol exposure also causes intrauterine growth restriction (IUGR), which is generally associated with reduced survival of the progenitor cytotrophoblast cell population, as well as syncytiotrophoblast, within the placenta (Axt et al., 1999; Barrio et al., 2004; Erel et al., 2001; Ishihara et al., 2002; Levy et al., 2002; Smith et al., 1997). The mechanism underlying the IUGR and reduced cytotrophoblast survival is unknown. While some apoptosis is important for the normal growth and remodeling of the placenta, exaggerated apoptosis within placental cell populations is strongly associated with adverse pregnancy outcomes including IUGR and pre-eclampsia (reviewed in (Sharp et al., 2010)). One explanation for the reduced placentation and subsequent IUGR in alcohol-exposed pregnancy could be increased apoptosis within placental cytotrophoblasts. Indeed, ethanol exposure using the human first trimester cytotrophoblast cell line, HTR-8/SV neo (Graham et al., 1993), produces substantial programmed cell death that proves to be apoptotic based on quantification of both DNA fragmentation (TUNEL) and phosphatidylserine externalization (Annexin V binding), as well as other criteria (Wolff et al., 2007). Moreover, the apoptosis displayed a linear dose response between 25 and 100 mM ethanol (Wolff et al., 2007). Apoptosis within human placental trophoblast cells could contribute to IUGR in FASD by disrupting placentation, as well as reduce nutrient transport and produce endothelial dysfunction (Sharp et al., 2010). The mechanism by which ethanol reduces trophoblast survival within the placenta has not been elucidated and would be of critical importance to understanding the etiology of FASD.
In several model systems including neural crest progenitors (Debelak-Kragtorp et al., 2003; Garic-Stankovic et al., 2005) and cerebellar granule neurons (Kouzoukas et al., 2013), ethanol induces programmed cell death by raising the concentration of free cytoplasmic Ca2+. The source of Ca2+ is attributed to the entry of extracellular Ca2+, it’s externalization from the endoplasmic reticulum, and its capacitative entry using Stim1 and Orais (Putney, 2009). In both models, the ethanol-induced apoptosis is specifically blocked by chelation of intracellular Ca2+ before ethanol exposure, and depletion of extracellular Ca2+ results in a partial block of ethanol-induced apoptosis (Garic-Stankovic et al., 2005). Additionally, phosphoinositide-specific phospholipase-C (PLC) is required for an ethanol-dependent Ca2+ transient leading to apoptosis (Garic-Stankovic et al., 2005), and for neural crest it is associated with increased IP3 levels. Several blockers of the transient, including the IP3 receptor antagonists (Xestospongin D, 2-APB) and inhibitors of PLC-mediated phosphoinositide production (U73122, ET-18-OCH3) appear to block the transmission of the apoptotic signal (Debelak-Kragtorp et al., 2003). This pathway also mediates ethanol-induced apoptosis within the early zebrafish embryo (Flentke et al., 2014b). In all these models, there is a linear relationship between cell death and either ethanol concentration or time of exposure (Debelak-Kragtorp et al., 2003; Flentke et al., 2014b; Kilburn et al., 2006; Pantazis et al., 1993). Taken together, these studies suggest that the intracellular Ca2+ transient and its downstream signaling represent a broadly conserved mechanism of ethanol's action.
Interestingly, in preimplantation embryos, similar ethanol concentrations induce a Ca2+ transient that also originates from the activity of PLC (Stachecki and Armant, 1996). But in sharp contrast to the previous models, ethanol does not trigger apoptosis or programmed cell death in the preimplantation mouse embryo. Instead, ethanol’s Ca2+ transient accelerates mouse embryonic development to the blastocyst stage (Stachecki and Armant, 1996). Thus, while ethanol initiates Ca2+ mobilization in multiple cell types, the consequences are cell type and stage specific (Kilburn et al., 2006; Smith et al., 2006).
These findings raise questions as to whether ethanol induces elevated levels of intracellular Ca2+ in human cytotrophoblast cells and whether Ca2+ signaling mediates apoptosis under those conditions. Here, we examine the effect of ethanol on intracellular Ca2+ concentration and it’s correlation to ethanol-induced apoptosis in HTR-8/SVneo cells, as well as another first trimester human cytotrophoblast cell line, SW.71, which was transformed by transfection with telomerase rather than SV40 T antigen (Straszewski-Chavez et al., 2009). Furthermore, we investigate the intracellular signaling pathway that mediates ethanol-induced apoptosis by depleting intracellular and extracellular Ca2+, using inhibitors of the IP3 signaling cascade and with an inhibitor of capacitative Ca2+ entry.
MATERIALS AND METHODS
Human Cytotrophoblast Cell Culture and Ethanol Exposure
HTR -8/SVneo and SW.71 cytotrophoblast cells were cultured in a 1:1 mixture of Dulbecco’s modified eagle’s medium and Ham’s F12 (DMEM/F12; Life Technologies, Grand Island, NY) containing 10% fetal bovine serum (Life Technologies). Culture medium was changed every two to three days and cells were passaged with trypsin – EDTA (Life Technologies). Cells were then cultured serum-free for an additional 18–24 h in media containing 5 mg/ml BSA. Ethanol (Mid-West Grain Company, Perkin, Il) was prepared in serum-free medium immediately before addition at 10, 25, 50 and 100mM concentrations. Cytotrophoblast cells were also treated in certain experiments 30 min prior to ethanol addition with 10 µM of U73122, U73343, xestospongin D, SKF-96365 (Millipore, Billerica, MA), 10 µM BAPTA-AM, or 1 mM BAPTA (Sigma, St. Louis, MO).
Intracellular Ca2+ Measurement
HTR-8/SVneo cytotrophoblast cells were grown in 96 well strip plates (2500 cells/well) to 50% confluence and cultured overnight in serum-free medium. Cells were loaded with 4 µM fluo-4-AM (Life Technologies) for 30 min at 37°C, and rinsed twice with modified BWW medium (Sigma). For certain experiments, inhibitors were added simultaneously during loading with fluo-4-AM. To monitor intracellular Ca2+ transients, cells were illuminated at 10 or 20 second intervals for fluorescence evaluation. Images were obtained using a Leica (Wetzlar, Germany) DM IRB epifluoresence microscope interfaced with a Hamamatsu Orca Digital camera (Hamamatsu City, Japan) and analyzed using Simple PCI imaging software system (Hamamatsu). Mean fluorescence intensity was evaluated over an entire field and intracellular Ca2+ concentration ([Ca2+]i) was calculated using the following formula:
where Kd (345 mM) is the dissociation constant of the Ca2+ indicator, F is the fluorescence intensity, Fmin is the relative background fluorescence, and Fmax is the maximum fluorescence intensity obtained after equilibrating the intracellular and extracellular Ca2+ with 5 nM ionomycin at the end of each experiment.
Cell Death Assays and Flow Cytometry
HTR-8/SVneo and SW.71 cytotrophoblast cells were treated for 1 h in T-25 cell culture flasks with vehicle (PBS) or ethanol, with and without inhibitor pretreatments. Cells were then rinsed twice with sterile PBS and detached using prewarmed (37°C) cell dissociation buffer (Sigma). The cell suspension was transferred to a 15 ml conical tube, centrifuged at 3000 RPM and washed three times with PBS.
Cell death based on DNA fragmentation was quantified using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method. Cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 10 min and the TUNEL assay was performed using a fluorescein-based cell death detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. Cells were then placed in 12 X 75 mm 5 ml polystyrene round-bottom tubes (500 µl PBS) at 1 million cells/ml, and cells with elevated fluorescence were counted using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ) flow cytometer and BD Cell Quest Pro flow cytometry analysis software.
Annexin V binding to the cell surface was measured to quantify apoptosis based on externalized phosphatidylserine. Using a BD Pharmigen FITC Annexin V Apoptosis Detection Kit, 100,000 cells suspended in 100 µl annexin V binding buffer (0.01 M HEPES, pH 7.4, 0.14 M NaCl, 0.25 mM CaCl2) were incubated with FITC Annexin V and propidium iodide for 15 min at 4°C. An additional 400 µl annexin binding buffer was added and cells were transferred to 5 ml polystyrene round-bottom tubes. At least 2000 cells at a final concentration of 200,000 cells/ml were analyzed by flow cytometry. Cells with low propidium iodide labeling and high FITC labeling were counted and analyzed using the flow cytometry software.
Statistics
Each experiment was repeated three times on separate days with different cell preparation. Data were analyzed using SPSS Version 21.0 (IBM Corp., 2012). For experiments in which time (0–120 min) was the variable, one-way analyses were conducted to examine TUNEL index for HTR-8/SVneo cells, and Annexln V binding index for HTR-8/SVneo and SW.71 cells. Tukey’s method was used for mean separations. Quadratic regression models were fitted to predict the time effects on TUNEL and Annexln V binding indices. For assessment of Ca2+ signaling inhibitors and ionomycin on ethanol-induced cell death, independent sample t-tests were used to detect the effects of ethanol on each treatment. One-way analyses of variance with Tukey’s post hoc tests were performed across the treatments. Differences were considered significant at p<0.05.
RESULTS
Ethanol Exposure Increases Apoptosis in Cytotrophoblast Cells
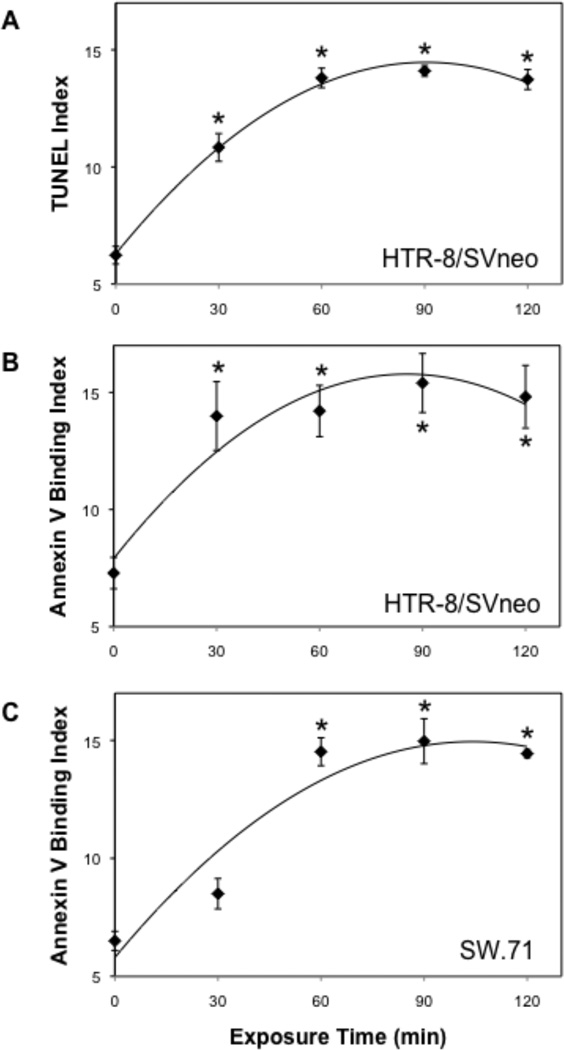
Human HTR-8/SVneo cytotrophoblast cells exposed to ethanol were previously examined in situ for cell death by TUNEL assay, indicating an increase compared to vehicle treatment that reached significance at 50 mM within 30 min (Wolff et al., 2007). Measuring TUNEL by flow cytometry, we confirmed that 50 mM ethanol significantly increased the population of cells that were positive for TUNEL and negative for propidium iodide uptake (Fig. 1A), suggesting that programmed cell death was taking place within 30 to 60 min of the original ethanol exposure. Indeed, externalization of phosphatidylserine was detected with similar kinetics by flow cytometric analysis of annexin V binding on the cell surface (Fig. 1B). This observation was confirmed in a second first trimester cytotrophoblast cell line, SW.71 (Fig. 1C). We conclude that 50 mM ethanol optimally induces apoptosis in human cytotrophoblast cells within 1 h.
Figure 1. Effect of ethanol on apoptosis in human cytotrophoblast cells.
Cytotrophoblasts were exposed to 50 mM EtOH and assessed for apoptosis using TUNEL and Annexin V-binding methods. Apoptosis was assessed in HTR-8/SVneo cells using both the TUNEL (A) and Annexin V (B) procedures. Annexin V binding was also assessed in SW.71 cytotrophoblast cells (C). Experiments were repeated three times and the averages are shown with error bars indicating the SEM. *, p < 0.05 compared to control (0 min).
Ethanol Exposure Increases Cytoplasmic Free Ca2+ in Cytotrophoblast Cells
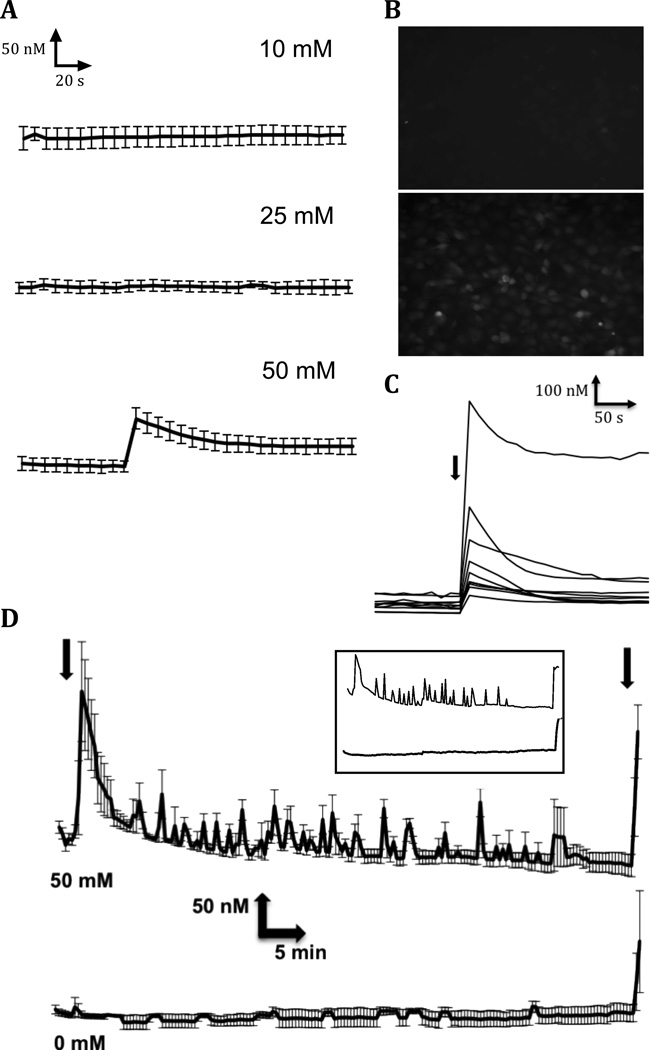
To determine if ethanol disrupts Ca2+ homeostasis in human cytotrophoblast cells as it does in other embryonic and neuronal cell types (De et al., 1999; Debelak-Kragtorp et al., 2003; Kowalczyk et al., 1996; Markovits et al., 1994; Simasko et al., 1999; Stachecki and Armant, 1996; Webb et al., 1996), intracellular Ca2+ concentration was monitored in real time after exposing HTR-8/SVneo cells to ethanol. Fluorescence imaging of nearly confluent cells pre-loaded with fluo-4-AM was monitored at 10 s intervals before and after addition of vehicle or ethanol at 10, 25 or 50 mM (Fig. 2A). Exposure to 50 mM ethanol, but not to lower concentrations of ethanol or vehicle, resulted in a significant elevation of cytoplasmic Ca2+ concentration within 10 s that subsided over the next 5 min (Fig. 2A-C). This result suggested a correlation with our finding that exposure of cytotrophoblast cells to ethanol significantly increased apoptosis at 50 mM, but not at lower alcohol concentrations, as shown in prior studies (Wolff et al., 2007). Averaging across the entire field of cells from three experiments, mean intracellular Ca2+ concentration initially increased from 143.5 nM (SE: 9.5 nM) to 206.9 nM (SE: 14.6 nM) after addition of 50 mM ethanol (Fig. 2A). There was great variation in the magnitude of the increase in Ca2+ level among individual cells, with differential concentrations ranging from 40 to 650 nM (Fig. 2C). However, the initial transient occurred synchronously across the field of cells (Fig. 2B). Because apoptosis does not occur until 30 to 60 min after exposure to 50 mM ethanol (Fig. 1), intracellular Ca2+ concentration was monitored for 1 h (Fig. 2D). Spontaneous transients continue to occur intermittently in ethanol treated cells (upper tracings), while no Ca2+ transients were observed over the same time period in vehicle-treated cells (lower tracings). We conclude that Ca2+ transients are induced repeatedly in cytotrophoblast cells during exposure to concentrations of ethanol capable of causing apoptosis.
Figure 2. Effects of ethanol on intracellular Ca2+ concentration in HTR-8/SVneo human cytotrophoblast cells.
A. Ethanol at the indicated concentrations was added to fluo-4-loaded cells after 90 s while the intracellular Ca2+ concentration was monitored in real time at 10-s intervals. Average Ca2+ concentrations were calculated from confluent fields for a total of 5 minutes. Experiments were repeated three times and the averages with error bars indicating the SEM are shown. B. Examples of fluo-4 fluorescence 20 s after adding 0 mM (upper panel) or 50 mM (lower panel) ethanol to a field of cytotrophoblast cells. C. Individual cells were monitored for 5 min after exposure (arrow) to 50 mM ethanol. Intracellular Ca2+ concentration was monitored to illustrate the variability of Ca2+ transients among cytotrophoblast cells. D. Initial Ca2+ transients and subsequent smaller transients were observed when intracellular Ca2+ concentration was monitored for 1 h after cells were exposed (first arrow) to 50 mM or 0 mM ethanol, as indicated. Averages and SEMs are shown for three experiments. After 1 h, cells were exposed to 5 nM ionomycin (second arrow) to demonstrate that the fluorescent dye was still responsive to the intracellular Ca2+ concentration. Insert shows examples of individual cell tracings for 50 mM (upper) or 0 mM (lower) ethanol treatments.
Regulation of Intracellular Ca2+ Levels by Ethanol
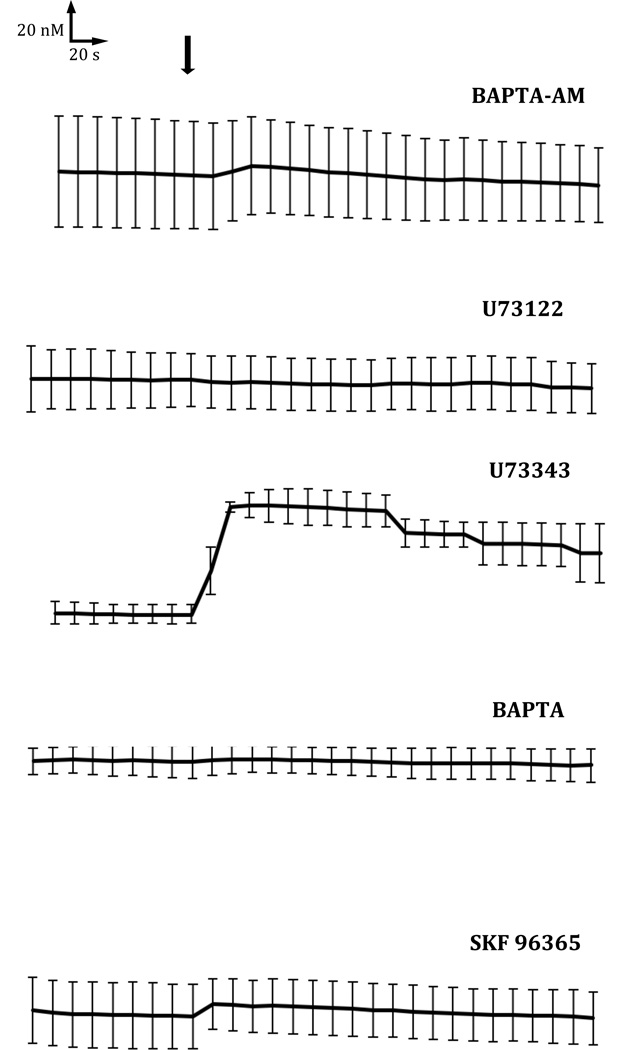
Intracellular Ca2+ signaling was monitored in cytotrophoblast cells preloaded with fluo-4-AM while various sources of cytoplasmic Ca2+ mobilization were inhibited pharmacologically. IP3 is produced by activation of phosphoinositide-specific PLC and the subsequent hydrolysis of phosphatidylinositol 4,5-bisphosphate (Rhee and Bae, 1997). To examine the role of PLC and IP3 signaling after ethanol treatment, cytotrophoblast cells were treated before exposure with 10 µM U73122, which inhibits PLC-mediated phosphoinositide production (Carvou et al., 2007; Zheng et al., 1995). SKF-96365, a compound that specifically inhibits Ca2+ entry through plasma membrane channels (Merritt et al., 1990; Singh et al., 2010), was also used in these experiments. Intracellular Ca2+ concentrations remained unchanged in cytotrophoblast cells exposed to 50 mM ethanol after they were first treated with the intracellular Ca2+ chelator BAPTA-AM, an extracellular Ca2+ chelator BAPTA, U-73122, or SKF-96365 (Fig. 3). When the inhibited samples were monitored for 1 h, no increase in Ca2+ was observed (not shown). An inactive analogue of U-73122, U73343, did not attenuate the Ca2 transient induced by ethanol. Equilibrating the intracellular and extracellular Ca2+ with 5 nM ionomycin at the end of each experiment produced a significant increase in Ca2+ regardless of inhibitor treatment (not shown).
Figure 3. Source of ethanol-induced Ca2+ mobilization.
HTR-8/SVneo cells were treated with BAPTA-AM, U73122, U73343, BAPTA or SKF-96365, as indicated, and simultaneously loaded with 5 mM fluo4-AM for 30 min at 37°C. Cell fluorescence was monitored at 10 s intervals with addition of 50 mM ethanol after 90 s (arrow). Ca2+ levels were monitored for a total of 5 min. Experiments were repeated three times and the averages with error bars indicating the SEM are shown.
Ethanol-Induced Apoptosis Requires Ca2 Signaling
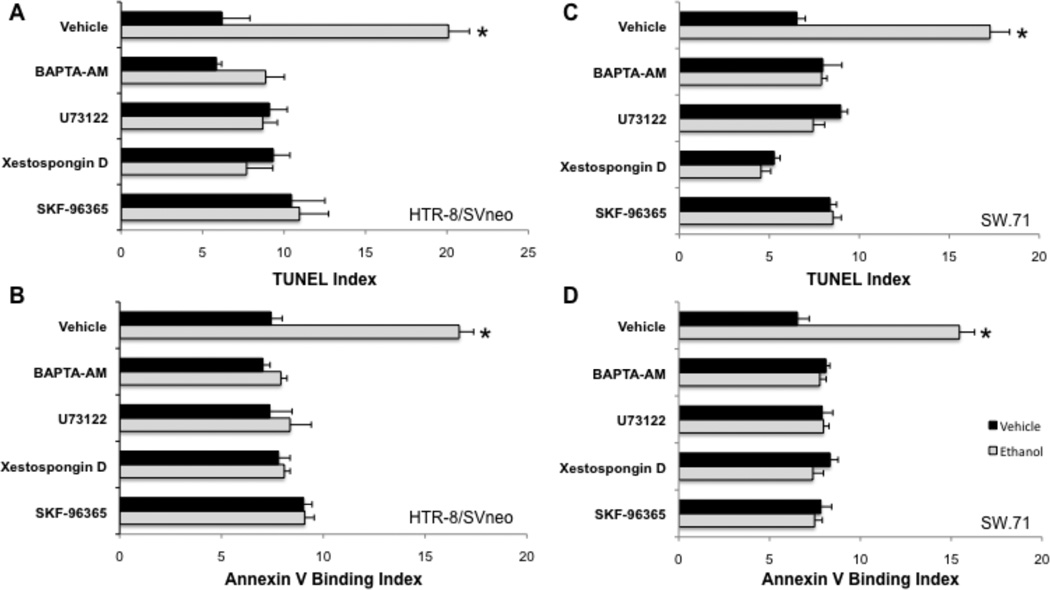
HTR-8/SV-neo and SW.71 cytotrophoblast cells pretreated with vehicle displayed an increase in apoptosis after exposure to 50 mM ethanol, as assessed by TUNEL and Annexin V binding (Fig. 4). Cell death, determined by TUNEL, increased significantly (p<0.05, n=6) 60 min after HTR-8/SV-neo cells were treated with 5 nM ionomycin (mean, 11.1; SE, 0.31), compared to control non-treated cells (mean, 2.97; SE, 0.68), suggesting that elevation of the Ca2+ concentration is sufficient to induce cell death. Pretreatment of the cells with the intracellular Ca2+ chelator BAPTA-AM prevented the apoptosis, confirming a requirement for intracellular Ca2+ signaling. The involvement of intracellular Ca2+ release via the IP3 pathway was demonstrated by the addition of inhibitors directed against phosphoinositide release and signaling. Both xestospongin D and U73122 pretreatments prevented apoptosis of cytotrophoblasts after exposure to ethanol (Fig. 4). SKF-96365 also blocked apoptosis (Fig. 4).
Figure 4. Requirements of Ca2+ signaling for ethanol-induced apoptosis.
HTR-8/SVneo (A-B) or SW.71 (C-D) cytotrophoblast cells were preincubated with vehicle or the indicated Ca2+ signaling inhibitors, as in Fig. 3, and then treated with vehicle or 50 mM ethanol. After 60 min, cells were assessed for apoptosis by the TUNEL (A, C) or Annexin V-binding (B, D). Experiments were repeated three times and the averages with error bars indicating the SEM are shown. *, p < 0.05 compared to vehicle treatment.
DISCUSSION
Normal human trophoblast functions, including implantation, hormone production and secretion, exchange between the maternal and fetal circulatory systems and maintenance of the maternal-fetal barrier, are essential for fetal survival, growth and development. Cytotoxic-induced trophoblast dysfunction can cause adverse pregnancy outcomes (McAleer and Tuan, 2004). Here, we have investigated the mechanism of cell death in ethanol-exposed human trophoblast cells in light of reports that ethanol exposure during gastrulation induces cell death in chick and mouse embryos (Debelak-Kragtorp et al., 2003; Kilburn et al., 2006), and that ethanol-induced apoptosis in chick embryos is dependent on intracellular Ca2+ and the IP3 signaling pathway (Debelak-Kragtorp et al., 2003; Garic-Stankovic et al., 2005). In the chick neural crest, ethanol mobilizes intracellular Ca2+ by activation of Gαi/o protein and subsequent interaction of Gβγ with PLCβ (Garic-Stankovic et al., 2005). Downstream of Ca2+, ethanol exposure suppresses β-catenin/Wnt signaling in neural crest cells that might induce cardiac, skeletal and abnormal neural development associated with the pathogenesis of FAS (Flentke et al., 2011). Furthermore, calmodulin kinase II has been identified as another critical mediator of ethanol-induced cell death (Garic et al., 2011) and it mediates the β-catenin loss (Flentke et al., 2014a).
In preimplantation mouse embryos, ethanol exposure accelerates development, rather than causing apoptosis, again through activation of the phospholipase C pathway and mobilization of intracellular Ca2+ (Stachecki and Armant, 1996). Increased apoptosis caused by alcohol exposure during later embryonic development could be a key factor contributing to the teratogenic effect of ethanol, particularly fetal growth restriction. Ethanol clearly induces apoptosis of human cytotrophoblast cells, based on increased numbers of pyknotic nuclei, TUNEL, annexin V binding, activation of caspase activity and absence of extracellular LDH activity associated with necrosis (Wolff et al., 2007). We now report that signaling downstream of intracellular Ca2+ is required for programmed cell death of human cytotrophoblast cells exposed to ethanol.
There is a strong correlation between the dose-dependency of apoptosis and production of intracellular Ca2+ transients in human cytotrophoblast cells. Significant increases were observed in both apoptosis and intracellular Ca2+ concentration when cells were exposed to 50 mM (229 mg/dL) or higher ethanol. While this value is pharmacologically high, similar blood alcohol levels occur in pregnant alcoholics and it is consistent with other cell culture studies (Kouzoukas et al., 2013). There was also a temporal correlation between apoptosis and intracellular Ca2+ signaling in cytotrophoblast cells. The maximal level of apoptosis occurred at 1 h during ethanol exposure and, concomitantly, intracellular Ca2+ oscillations persisted for approximately 1 h. Indeed, elevation of the intracellular Ca2+ concentration by treatment with ionomycin proved to be sufficient for induction of apoptosis 1 hour later in cytotrophoblast cells. The pharmacological signatures of intracellular Ca2+ signaling and apoptosis, determined with inhibitors of several upstream regulatory pathways, were similar. Chelation of intracellular Ca2+ with BAPTA-AM or extracellular Ca2+ with BAPTA inhibited both apoptosis and the production of intracellular Ca2+ transients by ethanol. Both were sensitive to inhibitors of PLC and TRPC channels, suggesting that the mechanism of cytoplasmic Ca2+ mobilization was store-operated Ca2+ entry mediated by TRPC channels. This mechanism, involving ethanol’s mobilization of Ca2+ stores, strongly parallels the mechanism underlying ethanol-mediated apoptosis for diverse neuronal lineages including neural crest, neuroectoderm, primary cerebellar granule neurons, and astrocytes (Garic-Stankovic et al., 2005; Hirata et al., 2006; Kilburn et al., 2006; Kouzoukas et al., 2013), and its extension here to a placental stem cell lineage suggests this represents a broader response of highly proliferative populations to ethanol exposure.
The evident increase in cell death and reduction in proliferation of the human cytotrophoblasts (Wolff et al., 2007) is consistent with previous studies conducted with animal models (Kilburn et al., 2006; Smith, 1997; Smith et al., 2006). In this investigation, concentrations of ethanol were used within the range of social and binge alcohol consumption. Hence, our findings support the hypothesis that maternal alcohol consumption during pregnancy and gestation could jeopardize placental function by inducing apoptosis. Our data suggest that the apoptotic signal is transduced through the IP3 pathway and further reinforced by the capacitative uptake of extracellular Ca2+.
Although these studies have detailed how ethanol induces apoptosis in human cytotrophoblast cells, there are limitations. While an immortalized first trimester human trophoblast cell line was used for these and previous studies of ethanol, there could be differences between the responses of cell lines and primary first trimester cytotrophoblast cells. All experiments were conducted in serum-free medium, which while not optimal for cell growth, does not predispose the cells to increased apoptosis. Additionally, these experiments cannot be replicated in vivo in viable pregnancies. Caution should be exercised in the interpretation of experiments using pharmacological inhibitors. U73122 is a widely used PLC inhibitor (Carvou et al., 2007; Zheng et al., 1995), but has also been reported to enhance PLC activity, alter phosphoinositide recycling, and can promote Ca2+ release (Mogami et al., 1997; Vickers, 1993). Although SKF-96365 can inhibit Ca2+ influx through TRPC channels at micromolar concentrations, it is also capable of blocking the activity of recombinant voltage-gated Ca2+ channels (Singh et al., 2010). Taken together, the consistent suppression by these agents of ethanol-medicated calcium release support a model in which the calcium transient originates from the activity of IP3 and PLC. However, it remains to be verified whether the observed requirements for both an active IP3 pathway and influx of extracellular Ca2+ indeed signify the involvement of a store-operated Ca2+ entry mechanism.
The findings of this study support the hypothesis (Wolff et al., 2007) that maternal alcohol consumption during pregnancy could jeopardize trophoblast accumulation in the developing placenta by inducing apoptosis. Furthermore, data suggest a mechanism in which the apoptotic signal is transduced through Ca2+ signaling that involves the IP3 pathway and capacitative influx of extracellular Ca2+. Prenatal exposure to alcohol can affect virtually all organ systems, triggering cell death, constriction of blood vessels, reduction of blood flow and oxygen transport to the placenta, and disruption of nerve cell development to the brain (Hannigan and Armant, 2000). Resultant impairments can cause learning and attention deficits, mental retardation, IUGR and individuals with smaller brains. Ethanol has been shown at the cellular level to reduce gene expression of aspartyl-asparaginyl β-hydroxylase (AAH), which is critical in regulation of cell motility and invasion of extravillous trophoblast cells (Gundogan et al., 2008; Jia et al., 1992). Pregnant rats exposed to chronic ethanol feeding demonstrate impaired placentation, significantly reduced levels of AAH, and IUGR (Gundogan et al., 2008). In this same experiment, toxic effects occurred in the placenta and fetus, including reduced thickness of the placenta, vascular dysfunction, impairment of oxygen delivery to the uteroplacental interface, reduced trophoblast motility and invasiveness, smaller pups and increased fetal demise (Gundogan et al., 2008). The resulting poor trophoblast survival and dysfunction could link prenatal alcohol exposure to the development of IUGR associated with FASD.
Acknowledgments
SUPPORT: This research was supported by NIH grant R37AA11085 to SMS, R21HD067629 to DRA and the Intramural Research Program of the NICHD, NIH.
REFERENCES
- Axt R, Kordina AC, Meyberg R, Reitnauer K, Mink D, Schmidt W. Immunohistochemical evaluation of apoptosis in placentae from normal and intrauterine growth-restricted pregnancies. Clin.Exp.Obstet.Gynecol. 1999;26:195–198. [PubMed] [Google Scholar]
- Barrio E, Calvo MT, Romo A, Alvarez R, Gutierrez JI, Naval J, Ferrandez LA. Intrauterine growth retardation: study of placental apoptosis. J.Pediatr.Endocrinol.Metab. 2004;17(Suppl 3):451–456. [PubMed] [Google Scholar]
- Carvou N, Norden AG, Unwin RJ, Cockcroft S. Signalling through phospholipase C interferes with clathrin-mediated endocytosis. Cell Signal. 2007;19:42–51. doi: 10.1016/j.cellsig.2006.05.023. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva NI, Sarkar DK. Effect of voltage-dependent calcium channel blockers on ethanol-induced beta-endorphin release from hypothalamic neurons in primary cultures. Alcohol Clin Exp Res. 1999;23:850–855. [PubMed] [Google Scholar]
- Debelak-Kragtorp KA, Armant DR, Smith SM. Ethanol-induced cephalic apoptosis requires phospholipase C-dependent intracellular calcium signaling. Alcohol Clin Exp Res. 2003;27:515–523. doi: 10.1097/01.ALC.0000056615.34253.A8. [DOI] [PubMed] [Google Scholar]
- Erel CT, Dane B, Calay Z, Kaleli S, Aydinli K. Apoptosis in the placenta of pregnancies complicated with IUGR. Int J Gynaecol Obstet. 2001;73:229–235. doi: 10.1016/s0020-7292(01)00373-3. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. Calcium-mediated repression of beta-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2011;91:591–602. doi: 10.1002/bdra.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Hernandez M, Smith SM. CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J Neurochem. 2014a;128:523–535. doi: 10.1111/jnc.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Klingler RH, Tanguay RL, Carvan MJ, 3rd, Smith SM. An Evolutionarily Conserved Mechanism of Calcium-Dependent Neurotoxicity in a Zebrafish Model of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2014b doi: 10.1111/acer.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic A, Flentke GR, Amberger E, Hernandez M, Smith SM. CaMKII activation is a novel effector of alcohol's neurotoxicity in neural crest stem/progenitor cells. J Neurochem. 2011;118:646–657. doi: 10.1111/j.1471-4159.2011.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez MR, Chiang PJ, Debelak-Kragtorp KA, Flentke GR, Armant DR, Smith SM. Ethanol triggers neural crest apoptosis through the selective activation of a pertussis toxin-sensitive G-protein and a phospholipase Cá-dependent Ca2+ transient. Alcohol Clin Exp Res. 2005;29:1237–1246. doi: 10.1097/01.alc.0000172460.05756.d9. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, Wands JR, de la Monte SM. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29:148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Armant DR. Alcohol in pregnancy and neonatal outcome. Seminars in Neonatology. 2000;5:243–254. doi: 10.1053/siny.2000.0027. [DOI] [PubMed] [Google Scholar]
- Hirata H, Machado LS, Okuno CS, Brasolin A, Lopes GS, Smaili SS. Apoptotic effect of ethanol is potentiated by caffeine-induced calcium release in rat astrocytes. Neurosci Lett. 2006;393:136–140. doi: 10.1016/j.neulet.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- Jia S, VanDusen WJ, Diehl RE, Kohl NE, Dixon RA, Elliston KO, Stern AM, Friedman PA. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J Biol Chem. 1992;267:14322–14327. [PubMed] [Google Scholar]
- Kilburn BA, Chiang PJ, Wang J, Flentke GR, Smith SM, Armant DR. Rapid Induction of Apoptosis in Gastrulating Mouse Embryos by Ethanol and Its Prevention by HB-EGF. Alcohol Clin Exp Res. 2006;30:127–134. doi: 10.1111/j.1530-0277.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzoukas DE, Li G, Takapoo M, Moninger T, Bhalla RC, Pantazis NJ. Intracellular calcium plays a critical role in the alcohol-mediated death of cerebellar granule neurons. J Neurochem. 2013;124:323–335. doi: 10.1111/jnc.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk CL, Stachecki JJ, Schultz JF, Leach RE, Armant DR. Effects of alcohols on murine preimplantation development: relationship to relative membrane disordering potency. Alcohol Clin Exp Res. 1996;20:566–571. doi: 10.1111/j.1530-0277.1996.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- Markovits A, Premecz G, Lorincz I, Bagi G, Nagy J, Farkas T, Foldes I. Ethanol-induced signal transducing mechanism associated with a transient antiviral state in human amniotic cells. J Stud Alcohol. 1994;55:495–502. doi: 10.15288/jsa.1994.55.495. [DOI] [PubMed] [Google Scholar]
- McAleer MF, Tuan RS. Cytotoxicant-induced trophoblast dysfunction and abnormal pregnancy outcomes: role of zinc and metallothionein. Birth Defects Res C Embryo Today. 2004;72:361–370. doi: 10.1002/bdrc.20024. [DOI] [PubMed] [Google Scholar]
- Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Lloyd Mills C, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324(Pt 2):645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis NJ, Dohrman DP, Goodlett CR, Cook RT, West JR. Vulnerability of cerebellar granule cells to alcohol-induced cell death diminishes with time in culture. Alcohol Clin Exp Res. 1993;17:1014–1021. doi: 10.1111/j.1530-0277.1993.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko SM, Boyadjieva N, De A, Sarkar DK. Effect of ethanol on calcium regulation in rat fetal hypothalamic cells in culture. Brain Res. 1999;824:89–96. doi: 10.1016/s0006-8993(99)01188-9. [DOI] [PubMed] [Google Scholar]
- Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010;160:1464–1475. doi: 10.1111/j.1476-5381.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- Smith SM. Alcohol-induced cell death in the embryo. Alcohol Health Res World. 1997;21:287–295. [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Debelak-Kragtorp KA, Miller MW. The Developing Brain: Lessons Learned from Alcohol and Nicotine Exposures. New York, NY: Oxford University Press; 2006. Neural crest and alcohol exposure; pp. 279–294. [Google Scholar]
- Stachecki JJ, Armant DR. Transient release of calcium from inositol 1,4,5-trisphosphate- specific stores regulates mouse preimplantation development. Development. 1996;122:2485–2496. doi: 10.1242/dev.122.8.2485. [DOI] [PubMed] [Google Scholar]
- Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers JD. U73122 affects the equilibria between the phosphoinositides as well as phospholipase C activity in unstimulated and thrombin-stimulated human and rabbit platelets. J Pharmacol Exp Ther. 1993;266:1156–1163. [PubMed] [Google Scholar]
- Webb B, Suarez SS, Heaton MB, Walker DW. Calcium homeostasis in cultured embryonic rat septohippocampal neurons is altered by ethanol and nerve growth factor before and during depolarization. Brain Res. 1996;729:176–189. [PubMed] [Google Scholar]
- Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR. Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod. 2007;77:53–60. doi: 10.1095/biolreprod.106.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Paik WY, Cesnjaj M, Balla T, Tomic M, Catt KJ, Stojilkovic SS. Effects of the phospholipase-C inhibitor, U73122, on signaling and secretion in pituitary gonadotrophs. Endocrinology. 1995;136:1079–1088. doi: 10.1210/endo.136.3.7867562. [DOI] [PubMed] [Google Scholar]