Abstract
Plant cells in tissues experience mechanical stress not only as a result of high turgor, but also through interaction with their neighbors. Cells can expand at different rates and in different directions from neighbors with which they share a cell wall. This in connection with specific tissue shapes and properties of the cell wall material can lead to intricate stress patterns throughout the tissue. Two cellular responses to mechanical stress are a microtubule cytoskeletal response that directs new wall synthesis so as to resist stress, and a hormone transporter response that regulates transport of the hormone auxin, a regulator of cell expansion. Shape changes in plant tissues affect the pattern of stresses in the tissues, and at the same time, via the cellular stress responses, the pattern of stresses controls cell growth, which in turn changes tissue shape, and stress pattern. This feedback loop controls plant morphogenesis, and explains several previously mysterious aspects of plant growth.
Introduction
There exists a group of questions in plant developmental biology that have been open and unanswered for many decades, in some cases, even for centuries. Among them: the mechanism by which leaves and flowers are arranged regularly around the stem [1, 2]; positioning of lateral organs along the root [3]; how plant cells choose their plane of division [1, 4]; whether cell expansion or cell division initiates organ formation [5, 6]; how plants organize their tissues so as to allow them to withstand the large and changing stresses of wind and gravity [7]. Recent experiments, combined with computational models, suggest that these apparently disparate phenomena all have a common basis, and can be explained by a common set of hypotheses.
The common basis is the response of individual cells to mechanical stress, and the interrelated cellular and supracellular feedbacks involved in mechanical stress response. This realization can be arrived at through work from many different laboratories, in what appear to be many different areas of plant biology: cytoskeletal organization [8-13], cell wall structure and biosynthesis [14, 15], cellular anisotropy in expansion [16], and patterns of hormone response [17, 18]. Studies in all of these areas are converging on an integrated view of plant tissue growth and differentiation, which involves feedback between mechanical stress, hormone flux, cell growth, cell wall biosynthesis, and cell division [19].
For this review we will begin where it began for our laboratories, in studies of phyllotaxis, the regular pattern of leaves and flowers around stems. The most common phyllotactic pattern is the spiral one, recognized since antiquity [20]. In this pattern, each successive primordium arises around 130 to 140 degrees from the previous one. This process leads after many repeats to the familiar patterns seen in sunflowers and pineapples and many other plant structures. The resemblance of this angle to the golden angle (137.5 degrees, in which a golden ratio of 1.618 is obtained when a circle is segmented) has attracted attention of scientists and formation of this pattern has been simulated and commented upon by botanists, physicists and mathematicians for almost 150 years [1, 17, 21-29].
Impact of Auxin on developmental control of the shoot apical meristem
Auxin (indole-3 acetic acid) a plant hormone is known to play crucial role in regulating several aspects of plant development such as cell division, cell growth, plant tropisms, shoot architecture, and lateral organ formation [30-33]. It has been known since the 1930s that an elevated local concentration of auxin is causal in the initiation of a new leaf or flower at the shoot apex [34], and thus, that the question of the pattern of organs around a stem resolves to the question of how auxin concentration changes at the shoot apex. Auxin, uniquely (so far) among plant hormones, has a specific transport system [35-37]: it is acid-trapped in plant cells, and is allowed out by a plasma membrane (PM) auxin efflux carrier whose distribution in plant cells can be asymmetric – thereby allowing auxin to depart from cells directionally. This facilitates complex and dynamic patterns of auxin flow through plant tissues and leads to the local concentration peaks that initiate organs at the shoot apex [38]. To understand auxin flow in the shoot apex when new leaves or flowers are forming, immunolocalization and live imaging of fluorescent reporter fusions for the efflux carrier have been done [31, 39], and have revealed that the net flow of auxin in shoot tips is up the auxin gradient such that any cell directs its auxin toward neighboring cells that have a higher auxin concentration (the energy for this transport is indirect, coming from the pH difference between cytoplasm and extracellular spaces, which is generated at the expense of ATP by proton ATPases [40]). Modeling a sheet of cells (representing the epidermis of a shoot apical meristem) in which auxin is transported up the auxin gradient demonstrates that this property is sufficient to generate and maintain a spiral phyllotactic pattern of auxin peaks [27, 28, 41]. The dynamics of auxin concentration change in a shoot apex, as visualized by live imaging of auxin concentration reporters and of the auxin efflux carrier [31], matches closely the predictions made by one of the models [27] indicating a possible solution to the longstanding problem of the development of the phyllotactic pattern, but also raising a mechanistic question: how does a cell in the shoot apex “know” the auxin concentration of its neighbors, so that it can direct its auxin efflux carrier to its PM adjacent to the neighbors with more auxin?
Wall mechanics in pattern formation at the shoot apex
One of the earliest known effects of auxin in a plant cell is their ability to promote elongation in excised stem and hypocotyl segments [42, 43]. The actual mechanism behind this effect of auxin is up for debate, however several findings suggest that auxin promotes secretion of protons into the apoplast resulting in a decrease in apoplastic pH, which promotes the cleavage of load bearing bonds in the cell wall resulting in cell elongation [44]. Heyn showed auxin caused alteration of mechanical properties of plant tissues by measurements of deflection under imposed load [42]. More recently direct measurement of tissue rigidity at the shoot apex with atomic force microscopy shows that auxin application causes a reduction in the observed elastic modulus prior to organ outgrowth [33, 45, 46]. This effect of wall loosening, possibly combined with an increase in the turgor pressure of the cell causes more rapid cellular expansion with increasing concentrations of auxin [33, 47, 48]. One possibility, therefore, is that a cell responds to the auxin concentration of its neighbors by sensing which of its walls is being highly stressed by the expansion of the neighbor (as adjacent plant cells share a wall, and do not slide with respect to one another). There are additional reasons to consider a physical rather than a chemical signal, among them experiments showing that new primordia of leaves or flowers can be induced not only by auxin, but also by expansin, which weakens cell walls as does auxin, but without the hormonal effects [49], and that phyllotactic pattern is influenced by additional treatments that change the elastic modulus of the cell wall [45, 50]. If cells direct auxin toward neighbors that are rapidly expanding, any treatment that changes local expansion rates or local cell wall strength should affect the subcellular localization of the auxin efflux carrier. That this is the case has been shown by controlling the subcellular location of the efflux carrier protein via stress changes in shoot meristems caused by local cell ablations [17]. Thus, one effect of mechanical stress on at least shoot apex cells is that it causes preferential localization of an auxin efflux carrier to the PM adjacent to the most stressed or strained walls (Figure 1).
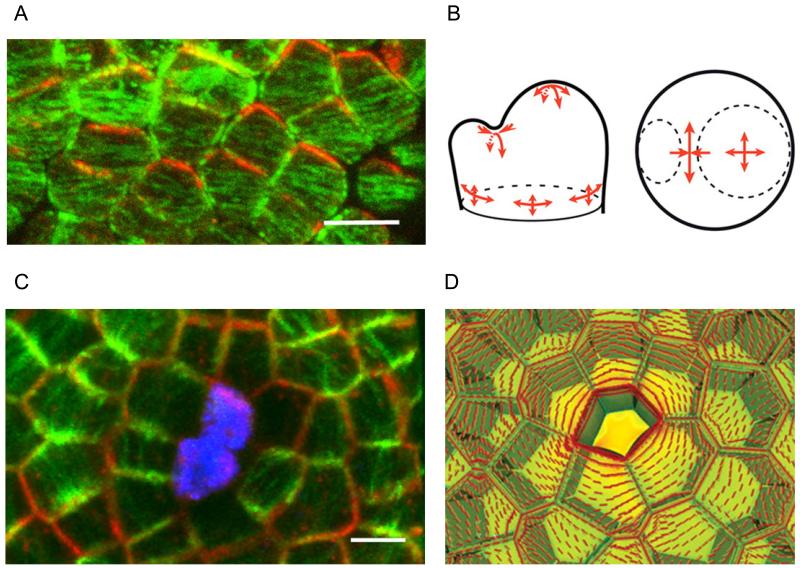
Figure 1. PIN1 polarity and MT orientations are co-aligned with mechanical stress patterns in boundary regions and around ablated cells in the SAM.
A. Double PIN1–microtubule immunosignal in the boundary domain: PIN1 (red) and microtubule (green) patterns are correlated. Scale bar = 5 μm (From Figure 1B in Heisler et al., 2010 [16]).
B. Stress pattern in different regions of SAM. Note that stress is strongly anisotropic (red arrows) in boundary region between primordium and SAM apex (From Figure 3B in Hamant et al., 2008 [7] Reprinted with permission from AAAS).
C. Co-alignment of PIN1-GFP (red) and microtubules (green) after ablation. Ablated cells are stained with propidium iodide (blue). Scale bar = 5 μm (From Figure 2C in Heisler et al., 2010 [16]).
D. Stress pattern around ablated site of SAM. Note that a circumferential stress pattern (red lines) is formed around the ablated site (From Figure 4A in Hamant et al., 2008 [7] Reprinted with permission from AAAS).
Mechanical forces regulate microtubule array organization
Another aspect of plant cells has long been known to respond to mechanical stress – the cytoskeleton. Unlike animal cells, plant cells lack microtubule-organizing centers or centrosomes. Advances have been made in understanding how centrosome-aided arrays are generated [51]. However our comprehension of non-centrosome based microtubule (MT) array organization is scant. Plant interphase cells typically have a cortical MT array, with MTs running in nearly parallel courses around the cell, just under the PM. Green suggested that MTs interact by means of inter-MT shear to maximize their overlap, while remaining attached to the PM (possibly by bridge-like connections) and this would result in a ring-like MT organization along the minimal circumference of the cell [11]. However MT arrays change both with cell type and developmental stage: a typical cylindrical root epidermal cells exhibits random MT array organization immediately after cell division and shifts to a more stereotypical transverse array, which at a later developmental stage becomes longitudinal as cell growth starts to slow [52, 53]. In addition to these developmental cues the MTs also reorient in response to externally applied forces [54-56]. Live imaging of MT arrays in the shoot apex also shows that different cells have different array behaviors. Cells near the tip of the shoot apical meristem continually reorient their MT arrays, such that the arrays in these cells appear to be in random orientations, and in no particular relation to the arrays of their neighbors. On the sides of the meristem, and in the saddle-shaped boundaries between floral primordia and the meristem, the MTs have preferred orientations, the orientations are generally fixed and not dynamic, and adjacent cells have their MT arrays in the same direction [8]. The shoot tip therefore has independent cellular organization of the cytoskeleton, while cells away from the tip have a supracellular organization displaying arrays coordinated across multiple cells (Figure 2). Experiments in which the apex is compressed by a tiny vise, or in which patterns of cells are ablated so as to change local stress patterns (by releasing the turgor of ablated cells) indicate that the MT arrays in cells with a strong preferential direction of stress are responding to physical stress, such that the MTs align parallel to the maximal principal direction of anisotropic stresses, and remain fixed in this orientation (despite continued treadmilling of the MTs). When stress is isotropic, that is, the same or similar in all directions, the MT arrays have no preferential direction, and change their direction every few hours [8]. The cause of the supracellular organization thus appears to be anisotropic stress in the tissue. Computational models of tissue stresses in the shoot apex, either spring or finite element method models, predict isotropic stresses in the tip and anisotropic stresses on the periphery and in the boundaries between primordia and the meristem, matching the direction of the experimentally observed supracellular MT arrays [8]. More recently, mechanical forces arising as a result of local growth variations in cells were also proposed to drive growth in the shoot apical meristem [57]. The ability of the MTs to respond to changes in mechanical stresses is proposed to be required for maintenance of this growth heterogeneity. Consistent with this scenario microtubule severing mutant katanin that lacked mechanical response had homogeneous growth locally in the shoot apical meristem [57]. That KATANIN mutants are required for mechanoresponse, and recent live cell imaging studies on SPIRAL2 protein, suggest that KATANIN-mediated MT severing is reduced at MT crossovers due to the presence of SPIRAL2 [58]. If an increase in tensile force on MTs could trigger disassociation of SPIRAL2 from MT crossover sites, it would promote KATANIN-mediated severing of MTs not aligned along the maximal stress direction, perhaps providing a mechanism for stress regulation of MT alignment.
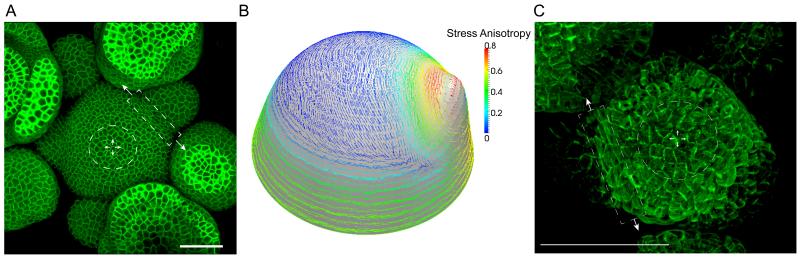
Figure 2. Mechanical stress dependent feedback loops regulate microtubule organization at the shoot apical meristem.
A. Maximum intensity projection of an Arabidopsis shoot apical meristem having a dome shaped central domain which gives rise to floral primordia along the periphery. Meristem dome has isotropic distribution of stresses represented by a circle, whereas the boundary domain cells have anisotropic stresses (boxed enclosure).
B. Shell element model showing stress pattern in different regions of SAM. Note that stress is strongly anisotropic in boundary region between primordium and SAM apex.
C. Microtubule organization in the shoot apical meristem showing random organization of microtubules in the center of the dome, and cells at the boundary domain having microtubules parallel along the long axis of the cell. Scale bars 50 μm
Plant cells, at least in the shoot apical meristem, thus respond in two different ways to mechanical stress, first the auxin efflux carrier concentrates adjacent to walls whose tensional stress or strain is highest, and cortical MTs organize parallel to the maximal principal direction of anisotropic stress of the cell (and therefore of its walls, which bear the stress). These two responses are separate since depolymerization of MTs does not appear to change the subcellular location of the efflux carrier protein, and changes in auxin concentration do not clearly alter the MT array, but the two responses are correlated, as they both respond to the mechanical state of each cell in the tissue [17].
Microtubules guide cellulose microfibril synthesis
Cortical MT orientation correlates with an additional aspect of plant cells, their direction of cellulose microfibril (CMF) deposition into the cell wall. CMFs are considered the major stress-bearing component of the cell wall, in fact, the elastic modulus of these fibrils is around half of that of steel [59, 60]. Prior to the identification of microtubules Green observed that treatment of algal cells with colchicine, a mitotic spindle disrupting drug, lead to isotropic swelling of cylindrical cells. The treatment resulted in the randomization of cell wall polymers suggesting that colchicine targeted the disruption of components organizing the CMFs [61]. Following this MTs were discovered in grazing transmission electron microscope sections of plant cells, and the micrographs also showed that the CMFs in the walls above the cortical microtubules were parallel to the cytoskeletal elements [62]. Subsequent work has demonstrated that the cellulose synthase complexes (CSCs), which sit in the PM, ride along the cortical MTs, thus accounting for the early observation [9]. The implication of this is that plant cells, at least in the shoot apical meristem where MTs align to stress, lay down CMFs parallel to the principal direction of anisotropic cellular stress. This would tend to reinforce the cells against the stress, causing a reduction in stress perception by the cells over time due to reinforcement of the cell wall.
Supracellular feedbacks imposed by mechanical stress
Given that physical stress in a plant tissue leads to a directional reorientation of the MT cytoskeleton [8], this in turn could lead to deposition of CMFs in the cell wall thereby decreasing the stress per unit area along the direction of maximal stress. Consider now the behavior of an expanding cell in a field of plant cells – first, the direction of expansion. It has long been known [63] that plant cells expand anisotropically, and perpendicular to the fibers in the cell wall, as well as perpendicular to the helical MT array, as if the array were a spring being extended [64, 65]. The strain (growth direction) of the cell in this case is perpendicular to the previous maximal direction of anisotropic stress, which, via the MT response to stress, is the direction of the recently deposited CMFs. This strain changes the stress pattern in adjacent cells (as the common wall is extending), which should in turn change (or reinforce) their MT array, the subsequent wall reinforcement, and then the subsequent expansion direction of the neighboring cells. As this feedback would be in constant effect in the entire plant tissue, it should be possible to predict the direction of expansion of each cell in a plant tissue, given information on the initial conditions of anisotropy of the walls in the tissue. In this way, once the dynamics and strength of the links between stress, cytoskeleton, and cell wall synthesis are understood, plant morphogenesis, which depends upon cell expansion and divisions, should become predictable and eventually controllable.
There is an important piece of information missing, though, in an attempt to predict the dynamics of morphogenesis through supracellular feedbacks on stress, anisotropic expansion, and wall synthesis and deposition – the rate of expansion of each cell. This brings us back to auxin. At the same time that meristem cells are responding to stress by aligning MTs, reinforcing cell walls, and dividing with new walls placed according to supracellular stress patterns, the auxin efflux carrier is transporting auxin toward cells that are expanding more rapidly than their neighbors, thus sending auxin up the auxin gradient, and increasing the auxin concentration of cells already high in auxin [17]. This positive feedback on cell expansion is countered, though, by wall reinforcement and cell division, which alter the supracellular stress pattern, thereby changing the direction of auxin flow. There are two major feedback circuits at work, stress via MTs to cell wall changes, and stress via the auxin efflux carrier to auxin flow and concentration changes both these circuits interact because each serves to change the stress pattern.
Can mechanical stresses influence spatial domains of cellular identity in the shoot apical meristem?
Can these feedbacks, and supracellular organization, really explain the activities of the shoot apical meristem? Consider one aspect of the meristem that in the past has been noted, but not understood: the behavior of the cells located between a developing floral primordium, and the meristem, the so-called boundary region [66]. The cells in the boundary region have a number of unusual characteristics. First, they are elongated across the saddle region between the meristem and the flower primordium, rather than generally isotropic, as are other cells in the shoot meristem. Second, they divide in an unexpected plane. It has long been noted that elongated cells tend to divide so that the new wall is of minimal area, and so that the daughter cells are about equal in size [4]. Boundary region cells divide the long way, with a new wall of maximal area [66]. Third, boundary region cells have their MTs fixed and parallel to the long direction of the cells, across the saddle between floral primordium and meristem [8]. All of these aspects can be explained by the supracellular stress hypothesis because stress is highly anisotropic in regions with such shapes [8, 67] (given that the epidermal cells of the meristem are under tension [68]), and parallel to the plane dividing meristem from developing flower. This orients the MTs, which in turn dictate the plane of cell division, which leads to the elongated shapes of the cells (whose strain is perpendicular to the stress, as expected if the cell walls are anisotropic, and resist expansion the long way, parallel to the MTs and therefore to the reinforcing CMFs). Boundary region cells also have a fixed pattern of auxin efflux carrier localization in their PMs, such that PIN1 protein is aligned with the long axis of the boundary and local MT bundles (Figure 1) [17, 31]. That these cells maintain their MT and efflux carrier patterns over time indicates that the supracellular stress is never fully resisted by new cell walls and by wall reinforcement – the growth of the tissue as a whole overcomes the negative feedbacks on stress at the single cell level.
It is also the case that boundary region cells express different genes than other meristematic regions, such as CUC2 and LAS, and along with the center of the meristem, STM [69, 70]. Whether this is due to auxin concentration changes that occur as a result of the changing stress pattern in the meristem, which cause auxin depletion in the boundary region as primordia form [31], or due to responses to stress through the MT system, is still unanswered – but either is an open possibility.
Mechanical cues orient cell division planes
Another aspect of plant cell behavior that can be understood in light of the supracellular stress theory is the control of cell division. Cell division affects morphogenesis both by changing the array of cells in a tissue, and by reinforcing the tissue against stress by inserting a new anticlinal wall, which would reduce stress. Cell division plane in plants, is predicted by the MT cytoskeleton: while interphase plant cells have helical arrays of MTs, these arrays condense (as far as is known, without a change in orientation) to form preprophase bands before cell division [71].
Early concepts of cell division planes however were proposed based on the geometry of the cell [4, 72, 73]. More recently alternative theories based on physical forces have been proposed. Once such observation reported by Vesecky and Lintilhac was that compression of callus tissue by means of a small vise, led by the subsequent division of the callus cells to files of cells whose new walls were perpendicular to the compression [56]. If we assume that callus cells respond to stress as do shoot meristem cells, this is the behavior expected based on the stress hypothesis where compression of the callus in one direction would cause tension in a perpendicular direction, leading to alignment of MTs parallel to the vise jaws. Collapse of these MT arrays to form a preprophase band, parallel to the previous direction of the helical array (and therefore parallel to the maximal stress direction at an earlier time), would mark the position of the new cell wall that will form after mitosis. The plane of each new cell wall should therefore be as predictable as the direction of CMF deposition since both are parallel to the earlier main principal direction of anisotropic stress in a cell’s walls, as applied by the neighboring cells. However, it should be noted that contradictory observations of new walls parallel to the plane of compression were also reported [74], potentially indicating additional levels of control [75].
Extending the physical stress hypothesis across scales
One large and still largely open question regarding the supracellular stress hypothesis is its applicability outside of the shoot apical meristem, where it was developed, and predominantly tested. As pointed out above, the patterns of cell division in callus (which is a root-like tissue, [76]) can be predicted after applied stress, suggesting that the MT system is present there. Recent work in our laboratories indicates that the MT stress system is functional in the epidermal cells of cotyledons (Sampathkumar at al., unpublished). MT patterns in these cells represent both cellular and supracellular stresses – the cells, which have a jigsaw puzzle piece shape [77], are expected to have high stress at indentations (due to the shape of the cells and their turgor pressure), and in these areas the MT cytoskeleton is aligned [77, 78],. A map of elastic modulus generated using atomic force microscopy further shows fiber like cell wall reinforcement resembling CMFs parallel to the predicted maximal stress directions, and to the MTs. The rest of the cell has a changing pattern of MTs, with little alignment, as in the isotropically stressed cells at the tip of a shoot apex. This can be changed to an aligned pattern, however, by imposition of directional stresses, such as when the cotyledon is cut with a razor, which directionally relieves the tension that the epidermis normally experiences. Early work on changes in MT pattern in roots shows that MT alignment is affected by physical forces [54], and this can be interpreted in light of the later live-imaging studies to represent realignment in response to stress (rather than strain or some other cellular feature). More recently applications of compressive forces were shown to change MT pattern in older leaf tissues of Arabidopsis [79]. Thus it seems possible that all plant cells have the MT stress response, and that cell wall and cell division may be predictable from stress patterns and cellular anisotropy in many or all tissues.
Whether the auxin mechanical stress system exists in other cells is a more open question. The auxin efflux carriers of Arabidopsis are the best studied, there are 8 members of the PINFORMED (PIN) gene family that codes for them, and additional efflux carrier-like genes [80]. The gene that codes for the efflux carrier that acts in floral phyllotaxis is PIN1 [38]. PIN3 is the one that appears to redirect auxin in response to gravity, thereby allowing gravitropic responses [32]. PIN3 redistribution in response to changes in the direction of the gravity vector is directed by sedimentation within specialized cells of starch-filled plastids called statoliths. Whether mechanical stress or some other cause is involved (such as creating a ligand-receptor interaction when the plastid reaches the endoplasmic reticulum or plasma membrane or triggering an ER-mediated response) is not known [81, 82]. The positions of various other PIN proteins such as PIN1 and PIN2 in roots has been studied in depth, and a computational model has been built based on PIN distinct localization domains in various zones in the root to explain the auxin flux and maintenance of an auxin maximum at the root quiescent center [80, 83]. Recent studies using cellulose synthesis mutants and inhibitors revealed that the normal polarized localization of PINs in root cells requires a cellulose cell wall matrix, suggesting that mechanical stress could also be involved in control of PIN polarity in root [84]. However, unlike the L1 layer of the SAM, where the stress pattern can be determined based on shape, the mechanical stress pattern in root cells (especially in inner layers) has not been modeled, thus we do not yet know if the supracellular control of chemical signaling via auxin is correlated with MT control in cells of this tissue.
Putative role of additional molecular elements in mechanosensing and perception
Beside MTs and PIN proteins, various other plant responses to mechanical perturbations have been documented. These mechanosensing behaviors can range from the whole plant/organ level, to the level of cellular gene transcription, to sub-cellular organelles [85, 86]. It is well known that apart from the MT cytoskeleton, actin filaments (AFs) also reorganize to physical perturbations [13]. AFs dynamically interact with the MT cytoskeleton in plants [87]. Furthermore AFs, unlike MTs, are necessary for polar localization of PIN1 [88]. Pharmacological and genetic disruption of AFs also affect global distribution of CSCs at the PM [89-91], apparently by influencing the rate of delivery of the CSCs and the lifetime of these complexes on the PM [91], however, and not by directing CSC movement.
Mechanosensing at the whole plant/organ level has been known for centuries [92]. Some plants have evolved specialized organs to carry out spectacular mechanical (though not developmental) responses, examples of which include the fast movement of the trap (less than 1 second) in the carnivorous plant Venus’ Flytrap triggered by insects [93], the mechanically induced leaflet closure in the sensitive plant Mimosa pudica [92], and the touch-sensitive tendril coiling in vines and climbing plants [94]. Examples of developmental mechanosensing at the organ level can also be found. Plant root growth demonstrates obstacle avoidance behavior which can be against gravity [92], and which appears to be a general behavior in different species [95]. Bending of an Arabidopsis root can induce lateral root formation, an auxin-mediated developmental process [96]. In above-ground tissue, it has been shown that touching can inhibit inflorescence elongation of Arabidopsis [85]. In woody plants, mechanical stress can induce local changes in cell wall and growth activity, forming a local strengthened wood structure called reaction wood which helps the plant hold its branches in a fixed position [97, 98]. Classical models postulate that differential auxin distribution around the stem is required for formation of the reaction wood [98], however, more recent direct experimental studies do not support differential auxin distribution [97]. At a cellular level, it has been shown that plant TCH genes (which encode different types of proteins such as calmodulins and xyloglucan endotransglucosylase/hydrolases) can rapidly respond to mechanical stimulation [99, 100]. A microarray-based genome wide search for mechanically responsive genes revealed that over 2.5% of genes in the Arabidopsis genome are rapidly upregulated by touching [100], which is mediated via the jasmonic acid signaling pathway [101]. Mechanical stress-activated gene expression is not unique to Arabidopsis plant, for example, in the woody plant poplar, ZFP2 gene expression is induced in a linear manner by bending of the stem [102]. An array of subcellular responses have been associated with mechanical stress. It is widely considered that plants respond to transient mechanical stresses by action of stress-activation PM channels that change internal ion concentrations, such as calcium [103], and different families of mechanical stress activated ion channels have been identified in plants [104-107]. However, ion concentration change does not give a directional signal in response to anisotropic stress, but is rather a non-directional response to any membrane strain. This stress-sensing mechanism is not likely, therefore, to be the one that regulates auxin flow in the shoot apex. Various subcellular compartments demonstrate different movements induced by micro-indentation, with nuclei [108], ER [13] and peroxisomes [13] moving toward the touching site, and chloroplasts moving away [109]. How these mechanosensing responses at different levels are coordinated, and if they relate to the mechanosensing relevant to developmental pattern formation, is unknown.
Conclusion and future directions
Thus, there is progress – an overall view of plant development and growth as being controlled by specific feedbacks between cellular and tissue stresses, that act by changing hormone transport, cell wall biosynthesis, and perhaps other cellular properties (Figure 3). But there is also challenge – finding the sensors for stress in cells and cell walls, and developing the computational models that are critical for developing and testing hypotheses of the physical control of development. Studies on understanding how mechanical signals are perceived and transduced into gene expression changes would lead to a more complete picture of the molecular mechanisms underlying this aspect of development. This would require better comprehension of how cells, tissues and organs are able to differentiate between a wide range of stimuli such as physical, chemical and environmental cues. Advances in techniques that provide direct measurements of mechanical properties coupled with high resolution live cell imaging strategies is necessary to provide better insight of how physical control morphogenesis and development. Another important step in understanding organogenesis is development of quantitative 3D image analysis and modeling techniques [123, 124]. Both progress and challenges combine to define a new field of plant growth, and of developmental biology of plants and of animals.
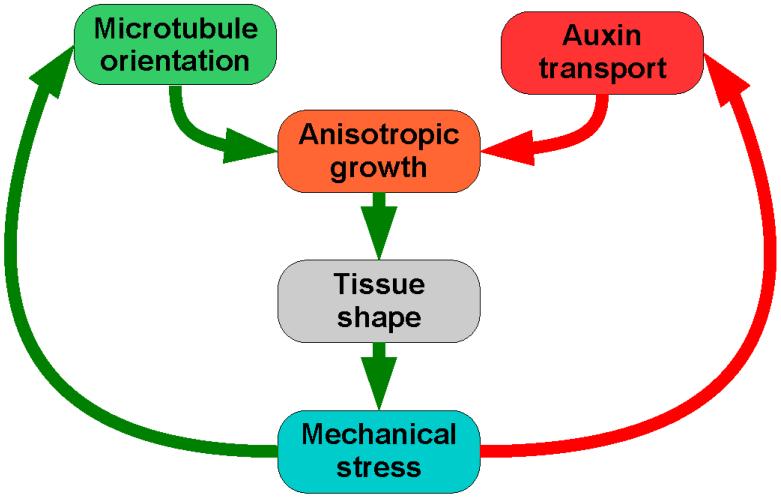
Figure 3. Feedbacks in tissue morphogenesis.
Stress controls microtubule orientation, which in turn controls cellulose deposition, and therefore cell wall anisotropy. At the same time, and separately, stress controls auxin transport direction, and as auxin regulates cell expansion, this also feeds into anisotropic growth. Such growth creates tissue shape, and the combination of turgor pressure and tissue shape creates the mechanical stress tensor field. This field then feeds back to regulate subsequent microtubule orientation and hormone movement. An analogy is Einstein’s theory of gravitation: as Wheeler (1980) [118] summarized it, “Space tells matter how to move and matter tells space how to curve.” In the case of plant development, mechanical stresses tell cells how to grow, and cell growth creates mechanical stress – and morphology.
BOX 1. Interdisciplinary approach to understand role of mechanical forces in biology.
Stress is a quantity that is not easily experimentally measurable on microscopic level. Only a tissue level observations of deformation after micro-incisions can help us differentiate between tensile and compressive stresses, but reliable measurement of stress levels on a cellular level is not easily done. For this reason cellular models of tissues are invaluable in deciphering the connection between mechanics of a cell and tissue, hormonal transport and biochemical regulation of cellular processes. In such models the information about stress and strain can be easily obtained. However the correctness of readout can depend on the appropriate choice of loading forces, boundary conditions and material properties. These parameters have to be assumed or measured. There is increasing number of measurements concerning material properties of different plant tissues [46, 110, 111]. They usually produce results in terms of apparent elasticity modulus and possibly a Poisson ratio. One has, however, to realize that these parameters describe a linear response of a material (Hooke’s law) which might not be an appropriate model of a plant cell wall, as such a complicated composite bio-material, is likely to exhibit nonlinear relations between stress (force per unit area) and strain (displacement). Such measurements should also take into account possible anisotropy of a tissue in its plastic and viscoelastic behavior. Therefore, correct measurement and modeling of a tissue or cell is a both challenging, and crucial.
Cellular models of living tissues have, in addition to the considerations above, to cope with updates of cell geometry and topology due to cell proliferation, and to dynamic and local changes in material properties during growth and morphogenesis. This also presents a modeling challenge.
BOX 2. Role of mechanical forces in morphogenesis in other systems.
Mechanosensing also plays a role in pattern formation in animal systems. As in plants, mechanosensing can influence organisms at different levels, ranging from the organ level, to cellular gene expression, to the subcellular level. One of the earliest proposed “rules” for mechanosensing at the organ level in animals is Wolff’s Law [112], which states that human and animal bone will adapt to the direction of its mechanical stress. However, more recent morphological studies tend to reject the validity of this rule [113]. It has also been proposed that mechanical stress regulates animal embryo development [114]. More clear and conclusive examples of mechanosensing at the organ level include the hearing and touch functions of animals, which are dependent on neural responses to mechanical forces [114]. At cellular level, mechanical stress may regulate serum response factor-dependent gene transcription in migrating Drosophila border cells [115], Twist gene expression in Drosophila embryo development [116], and cell growth and division patterns in cultured dog kidney cells [117]. At the subcellular level, mechanical stress can regulate the activity of stretch-sensitive channels [114], polarization of both actin and MT cytoskeletons [118, 119], and stabilization of the nucleus via increasing lamin-A level [120]. However, there is one striking difference between mechanical stress sensing in plants and animals. In plant cells, due to the existence of a cell wall, turgor pressure is the main cause of cell wall/PM mechanical stress, with the pressure magnitude in the range of 105 – 106 Pa. [121]. In animal cells, the actin cytoskeleton is the main factor generating forces against the PM, causing a membrane tension many orders of magnitude lower [122]. Given this huge difference in cellular forces, the mechanical sensing machinery in plants and animals could well be different.
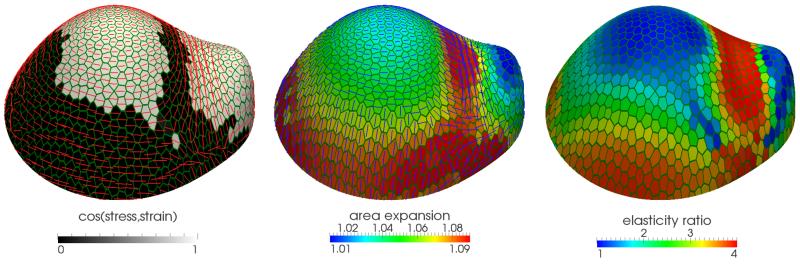
Figure BOX1.
Figure (modified from Bozorg et al. 2014 [125]) shows an example of a stress feedback model of a meristem with anisotropic cells. In the first image, white and black indicate regions where, because of the stress pattern and anisotropy, maximal stress and strain are parallel. They are perpendicular in the black area. This plays out into realistic levels and positions of expansion and stiffness as shown in the subsequent images.
Acknowledgements
Our research on mechanical forces in plants is supported by the Department of Energy Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, of the US Department of Energy [DE-FG02-88ER13873] to E.M.M, the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation (through Grant GBMF3406) to EMM and Swedish Research Council for P.K.
References
- 1.Hofmeister W. Handbuch der physiologischen Botanik. Vol. 1. Engelmann; Leipzig: 1868. Allgemeine Morphologie der Gewächse; pp. 405–664. [Google Scholar]
- 2.Turing AM. The chemical basis of morphogenesis. 1953. Bull Math Biol. 1990;52:153–197. doi: 10.1007/BF02459572. discussion 119-152. [DOI] [PubMed] [Google Scholar]
- 3.Laskowski M, Grieneisen VA, Hofhuis H, Colette A, Hogeweg P, Marée AF, Scheres B. Root system architecture from coupling cell shape to auxin transport. PLoS biology. 2008;6:e307. doi: 10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Errera L. Über zellformen und siefenblasen. Bot Centralbl. 1888;34:395–398. [Google Scholar]
- 5.Haber AH. Nonessentiality of Concurrent Cell Divisions for Degree of Polarization of Leaf Growth. I. Studies with Radiation-Induced Mitotic Inhibition. American Journal of Botany. 1962;49:583–589. [Google Scholar]
- 6.Kaplan DR, Hagemann W. The Relationship of Cell and Organism in Vascular Plants. BioScience. 1991;41:693–703. [Google Scholar]
- 7.Moulia B, Coutand C, Lenne C. Posture control and skeletal mechanical acclimation in terrestrial plants: implications for mechanical modeling of plant architecture. American Journal of Botany. 2006;93:1477–1489. doi: 10.3732/ajb.93.10.1477. [DOI] [PubMed] [Google Scholar]
- 8.Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. Developmental patterning by mechanical signals in Arabidopsis. Science. 2008;322:1650–1655. doi: 10.1126/science.1165594. [DOI] [PubMed] [Google Scholar]
- 9.Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto K, Williamson RE, Wasteneys GO. New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol. 2000;124:1493–1506. doi: 10.1104/pp.124.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green PB. Organogenesis-A Biophysical View. Annual Review of Plant Physiology. 1980;31:51–82. [Google Scholar]
- 12.Lloyd CW, Clayton L, Dawson PJ, Doonan JH, Hulme JS, Roberts IN, Wells B. The cytoskeleton underlying side walls and cross walls in plants: molecules and macromolecular assemblies. J Cell Sci Suppl. 1985;2:143–155. doi: 10.1242/jcs.1985.supplement_2.8. [DOI] [PubMed] [Google Scholar]
- 13.Hardham AR, Takemoto D, White RG. Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC plant biology. 2008;8:63. doi: 10.1186/1471-2229-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Phys. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 16.Baskin TI. Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol. 2005;21:203–222. doi: 10.1146/annurev.cellbio.20.082503.103053. [DOI] [PubMed] [Google Scholar]
- 17.Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jonsson H, Traas J, Meyerowitz EM. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010;8:e1000516. doi: 10.1371/journal.pbio.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama N, Smith RS, Mandel T, Robinson S, Kimura S, Boudaoud A, Kuhlemeier C. Mechanical regulation of auxin-mediated growth. Current biology: CB. 2012;22:1468–1476. doi: 10.1016/j.cub.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Robinson S, Burian A, Couturier E, Landrein B, Louveaux M, Neumann ED, Peaucelle A, Weber A, Nakayama N. Mechanical control of morphogenesis at the shoot apex. Journal of experimental botany. 2013;64:4729–4744. doi: 10.1093/jxb/ert199. [DOI] [PubMed] [Google Scholar]
- 20.Theophrastus . Enquiry into plants. Harvard University Press; Cambridge, MA, USA: 1948. [Google Scholar]
- 21.Mitchison GJ. Phyllotaxis and the fibonacci series. Science. 1977;196:270–275. doi: 10.1126/science.196.4287.270. [DOI] [PubMed] [Google Scholar]
- 22.Douady S, Couder Y. Phyllotaxis as a Dynamical Self Organizing Process Part I: The Spiral Modes Resulting from Time-Periodic Iterations. J Theor Biol. 1996;178:255–273. [Google Scholar]
- 23.Douady S, Couder Y. Phyllotaxis as a Dynamical Self Organizing Process Part II: The Spontaneous Formation of a Periodicity and the Coexistence of Spiral and Whorled Patterns. J Theor Biol. 1996;178:275–294. [Google Scholar]
- 24.Douady S, Couder Y. Phyllotaxis as a Dynamical Self Organizing Process Part III: The Simulation of the Transient Regimes of Ontogeny. J Theor Biol. 1996;178:295–312. [Google Scholar]
- 25.Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1952;237:37–72. [Google Scholar]
- 26.Newell AC, Shipman PD, Sun ZY. Phyllotaxis: Cooperation and competition between mechanical and biochemical processes. J Theor Biol. 2008;251:421–439. doi: 10.1016/j.jtbi.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci U S A. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RS, Guyomarc’h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model of phyllotaxis. Proc Natl Acad Sci U S A. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C. Integration of transport-based models for phyllotaxis and midvein formation. Genes & development. 2009;23:373–384. doi: 10.1101/gad.497009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himanen K, Boucheron E, Vanneste S, Engler JD, Inze D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current biology: CB. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 32.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 33.Braybrook SA, Peaucelle A. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS One. 2013;8:e57813. doi: 10.1371/journal.pone.0057813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snow M, Snow R. Auxin and leaf formation. New Phytologist. 1937;36:1–18. [Google Scholar]
- 35.Goldsmith MHM. The Polar Transport of Auxin. Annual Review of Plant Physiology. 1977;28:439–478. [Google Scholar]
- 36.Mitchison GJ. The Dynamics of Auxin Transport. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1980;209:489–511. [Google Scholar]
- 37.Rubery PH, Sheldrake AR. Carrier-mediated auxin transport. Planta. 1974;118:101–121. doi: 10.1007/BF00388387. [DOI] [PubMed] [Google Scholar]
- 38.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. The Plant cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:1627–1632. doi: 10.1073/pnas.0510130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones AM. Botany: Auxin transport: Down and out and up again. Science. 1998;282:2201–2202. doi: 10.1126/science.282.5397.2201. [DOI] [PubMed] [Google Scholar]
- 41.Kramer EM. Computer models of auxin transport: a review and commentary. Journal of experimental botany. 2008;59:45–53. doi: 10.1093/jxb/erm060. [DOI] [PubMed] [Google Scholar]
- 42.Heyn ANJ. The physiology of cell elongation. Bot. Rev. 1940;6:515–574. [Google Scholar]
- 43.Cleland R. Auxin and Cell Elongation. In: Davies P, editor. Plant Hormones and their Role in Plant Growth and Development. Springer; Netherlands: 1987. pp. 132–148. [Google Scholar]
- 44.Rayle DL, Cleland RE. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Hofte H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Current biology: CB. 2011;21:1720–1726. doi: 10.1016/j.cub.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 46.Milani P, Gholamirad M, Traas J, Arneodo A, Boudaoud A, Argoul F, Hamant O. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 2011;67:1116–1123. doi: 10.1111/j.1365-313X.2011.04649.x. [DOI] [PubMed] [Google Scholar]
- 47.Cleland R. Cell Wall Extension. Annual Review of Plant Physiology. 1971;22:197–222. [Google Scholar]
- 48.Kierzkowski D, Nakayama N, Routier-Kierzkowska AL, Weber A, Bayer E, Schorderet M, Reinhardt D, Kuhlemeier C, Smith RS. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science. 2012;335:1096–1099. doi: 10.1126/science.1213100. [DOI] [PubMed] [Google Scholar]
- 49.Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C. Induction of Leaf Primordia by the Cell Wall Protein Expansin. Science. 1997;276:1415–1418. [Google Scholar]
- 50.Peaucelle A, Louvet R, Johansen JN, Hofte H, Laufs P, Pelloux J, Mouille G. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Current biology: CB. 2008;18:1943–1948. doi: 10.1016/j.cub.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 51.Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 52.Baskin TI, Meekes HT, Liang BM, Sharp RE. Regulation of growth anisotropy in well-watered and water-stressed maize roots. II. Role Of cortical microtubules and cellulose microfibrils. Plant Physiol. 1999;119:681–692. doi: 10.1104/pp.119.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traas J, Derksen J. Microtubules and cellulose microfibrils in plant cells. Simultaneous demonstration in dry cleave preparations. European journal of cell biology. 1989;48:159–164. [Google Scholar]
- 54.Hush JM, Overall RL. Electrical and mechanical fields orient cortical microtubules in higher plant tissues. Cell Biology International Reports. 1991;15:551–560. [Google Scholar]
- 55.Landrein B, Hamant O. How mechanical stress controls microtubule behavior and morphogenesis in plants: history, experiments and revisited theories. Plant J. 2013;75:324–338. doi: 10.1111/tpj.12188. [DOI] [PubMed] [Google Scholar]
- 56.Lintilhac P, Vesecky T. Stress-induced alignment of division plane in plant tissues grown in vitro. Nature. 1984;307:363–364. [Google Scholar]
- 57.Uyttewaal M, Burian A, Alim K, Landrein B, Borowska-Wykret D, Dedieu A, Peaucelle A, Ludynia M, Traas J, Boudaoud A, et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell. 2012;149:439–451. doi: 10.1016/j.cell.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 58.Wightman R, Chomicki G, Kumar M, Carr P, Turner SR. SPIRAL2 Determines Plant Microtubule Organization by Modulating Microtubule Severing. Current biology: CB. 2013;23:1902–1907. doi: 10.1016/j.cub.2013.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng QZ, Wang SQ, Harper DP. Effects of process and source on elastic modulus of single cellulose fibrils evaluated by atomic force microscopy. Compos Part a-Appl S. 2009;40:583–588. [Google Scholar]
- 60.Mann J, Roldan-Gonzalez L. X-ray measurements of the elastic modulus of cellulose crystals. Polymer. 1962;3:549–553. [Google Scholar]
- 61.Green PB. Mechanism for Plant Cellular Morphogenesis. Science. 1962;138:1404–1405. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]
- 62.Ledbetter MC, Porter KR. A “MICROTUBULE” IN PLANT CELL FINE STRUCTURE. The Journal of Cell Biology. 1963;19:239–250. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castle ES. Membrane tension and orientation of structure in the plant cell wall. Journal of Cellular and Comparative Physiology. 1937;10:113–121. [Google Scholar]
- 64.Wasteneys G, Williamson R. Microtubule orientation in developing internodal cells of Nitella: a quantitative analysis. European journal of cell biology. 1987;43:14–22. [Google Scholar]
- 65.Wasteneys G, Williamson R. Reassembly of microtubules in Nitella tasmanica: quantitative analysis of assembly and orientation. European journal of cell biology. 1989;50:76–83. [Google Scholar]
- 66.Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development. 2004;131:4225–4237. doi: 10.1242/dev.01261. [DOI] [PubMed] [Google Scholar]
- 67.Kutschera U, Niklas KJ. The epidermal-growth-control theory of stem elongation: An old and a new perspective. Journal of Plant Physiology. 2007;164:1395–1409. doi: 10.1016/j.jplph.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Green PB. Transductions to Generate Plant Form and Pattern: An Essay on Cause and Effect. Annals of Botany. 1996;78:269–281. doi: 10.1006/anbo.1996.0121. [DOI] [PubMed] [Google Scholar]
- 69.Aida M, Ishida T, Tasaka M. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development. 1999;126:1563–1570. doi: 10.1242/dev.126.8.1563. [DOI] [PubMed] [Google Scholar]
- 70.Takada S, Hibara K, Ishida T, Tasaka M. The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development. 2001;128:1127–1135. doi: 10.1242/dev.128.7.1127. [DOI] [PubMed] [Google Scholar]
- 71.Wasteneys GO. Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]
- 72.Hofmeister W. Zusätze und Berichtigungen zu den 1851 veröffentlichten Untersuchungen der Entwicklung höherer Krytogamen. Jahrb. Wiss. Bot. 1863;3:259–293. [Google Scholar]
- 73.Sachs J. Über die Anordnung der Zellen in jüngsten Pflanzentheilen. Arb. Bot. Inst. Würzburg. Arb. Bot. Inst. Würzburg. 1878;2:46–104. [Google Scholar]
- 74.Lynch TM, Lintilhac PM. Mechanical signals in plant development: a new method for single cell studies. Dev Biol. 1997;181:246–256. doi: 10.1006/dbio.1996.8462. [DOI] [PubMed] [Google Scholar]
- 75.Louveaux M, Hamant O. The mechanics behind cell division. Current Opinion in Plant Biology. 2013;16:774–779. doi: 10.1016/j.pbi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Sugimoto K, Jiao Y, Meyerowitz EM. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Developmental Cell. 2010;18:463–471. doi: 10.1016/j.devcel.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24:551–575. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 79.Jacques E, Verbelen JP, Vissenberg K. Mechanical stress in Arabidopsis leaves orients microtubules in a ‘continuous’ supracellular pattern. BMC Plant Biol. 2013;13:163. doi: 10.1186/1471-2229-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dettmer J, Friml J. Cell polarity in plants: when two do the same, it is not the same. Current opinion in cell biology. 2011;23:686–696. doi: 10.1016/j.ceb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 81.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 82.Leitz G, Kang BH, Schoenwaelder MEA, Staehelin LA. Statolith Sedimentation Kinetics and Force Transduction to the Cortical Endoplasmic Reticulum in Gravity-Sensing Arabidopsis Columella Cells. Plant Cell. 2009;21:843–860. doi: 10.1105/tpc.108.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grieneisen VA, Xu J, Maree AFM, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 84.Feraru E, Feraru MI, Kleine-Vehn J, Martiniere A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J. PIN polarity maintenance by the cell wall in Arabidopsis. Current biology: CB. 2011;21:338–343. doi: 10.1016/j.cub.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 85.Braam J. In touch: plant responses to mechanical stimuli. New Phytologist. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 86.Hamant O. Widespread mechanosensing controls the structure behind the architecture in plants. Current Opinion in Plant Biology. 2013;16:654–660. doi: 10.1016/j.pbi.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Sampathkumar A, Lindeboom JJ, Debolt S, Gutierrez R, Ehrhardt DW, Ketelaar T, Persson S. Live cell imaging reveals structural associations between the actin and microtubule cytoskeleton in Arabidopsis. The Plant cell. 2011;23:2302–2313. doi: 10.1105/tpc.111.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 89.Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, Gonneau M, Hofte H, Vernhettes S. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. The Plant cell. 2009;21:1141–1154. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol. 2009;11:797–806. doi: 10.1038/ncb1886. [DOI] [PubMed] [Google Scholar]
- 91.Sampathkumar A, Gutierrez R, McFarlane HE, Bringmann M, Lindeboom J, Emons AM, Samuels L, Ketelaar T, Ehrhardt DW, Persson S. Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 2013;162:675–688. doi: 10.1104/pp.113.215277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Darwin C. The power of movements in plants. William Clowes and Sons, Ltd.; London: 1880. [Google Scholar]
- 93.Darwin C. Insectivorous plants. John Murray; London: 1893. [Google Scholar]
- 94.Darwin C. The movements and habits of climbing plants. John Murray; London: 1906. [Google Scholar]
- 95.Massa GD, Gilroy S. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J. 2003;33:435–445. doi: 10.1046/j.1365-313x.2003.01637.x. [DOI] [PubMed] [Google Scholar]
- 96.Richter GL, Monshausen GB, Krol A, Gilroy S. Mechanical Stimuli Modulate Lateral Root Organogenesis. Plant Physiol. 2009;151:1855–1866. doi: 10.1104/pp.109.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hellgren JM, Olofsson K, Sundberg B. Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine. Plant Physiol. 2004;135:212–220. doi: 10.1104/pp.104.038927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Timell T. Compression wood in gymnosperms. Vol. 2. Springer-Verlag; Heidelberg: 1986. pp. 983–1262. [Google Scholar]
- 99.Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 100.Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. The New phytologist. 2005;165:429–444. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 101.Chehab EW, Yao C, Henderson Z, Kim S, Braam J. Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Current biology: CB. 2012;22:701–706. doi: 10.1016/j.cub.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 102.Coutand C, Martin L, Leblanc-Fournier N, Decourteix M, Julien JL, Moulia B. Strain mechanosensing quantitatively controls diameter growth and PtaZFP2 gene expression in poplar. Plant Physiol. 2009;151:223–232. doi: 10.1104/pp.109.138164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. The Plant cell. 2009;21:2341–2356. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci U S A. 2012;109:19015–19020. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci U S A. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furuichi T, Iida H, Sokabe M, Tatsumi H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant signaling & behavior. 2012;7:1022–1026. doi: 10.4161/psb.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Current biology: CB. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 108.Gus-Mayer S, Naton B, Hahlbrock K, Schmelzer E. Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc Natl Acad Sci U S A. 1998;95:8398–8403. doi: 10.1073/pnas.95.14.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato Y, Kadota A, Wada M. Mechanically induced avoidance response of chloroplasts in fern protonemal cells. Plant Physiol. 1999;121:37–44. doi: 10.1104/pp.121.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandes AN, Chen X, Scotchford CA, Walker J, Wells DM, Roberts CJ, Everitt NM. Mechanical properties of epidermal cells of whole living roots of <i>Arabidopsis thaliana</i>: An atomic force microscopy study. Physical Review E. 2012;85:021916. doi: 10.1103/PhysRevE.85.021916. [DOI] [PubMed] [Google Scholar]
- 111.Wu J-Z, Lin Y, Zhang X-L, Pang D-W, Zhao J. IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. Journal of experimental botany. 2008;59:2529–2543. doi: 10.1093/jxb/ern119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolff J. Das Gesetz der Transformation der Knochen. A. Hirschwald; Berlin: 1892. [Google Scholar]
- 113.Morimoto N, Ponce de Leon MS, Zollikofer CP. Exploring femoral diaphyseal shape variation in wild and captive chimpanzees by means of morphometric mapping: a test of Wolff’s law. Anatomical record. 2011;294:589–609. doi: 10.1002/ar.21346. [DOI] [PubMed] [Google Scholar]
- 114.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Somogyi K, Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 116.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Current biology: CB. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 117.Puliafito A, Hufnagel L, Neveu P, Streichan S, Sigal A, Fygenson DK, Shraiman BI. Collective and single cell behavior in epithelial contact inhibition. Proc Natl Acad Sci U S A. 2012;109:739–744. doi: 10.1073/pnas.1007809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Asnacios A, Hamant O. The mechanics behind cell polarity. Trends in cell biology. 2012;22:584–591. doi: 10.1016/j.tcb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Celik E, Abdulreda MH, Maiguel D, Li J, Moy VT. Rearrangement of microtubule network under biochemical and mechanical stimulations. Methods. 2013;60:195–201. doi: 10.1016/j.ymeth.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L, Hukin D, Pritchard J, Thomas C. Comparison of plant cell turgor pressure measurement by pressure probe and micromanipulation. Biotechnology letters. 2006;28:1147–1150. doi: 10.1007/s10529-006-9075-x. [DOI] [PubMed] [Google Scholar]
- 122.Petersen NO, McConnaughey WB, Elson EL. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proceedings of the National Academy of Sciences. 1982;79:5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fernandez R, Das P, Mirabet V, Moscardi E, Traas J, Verdeil J-L, Malandain G.g., Godin C. Imaging plant growth in 4D: robust tissue reconstruction and lineaging at cell resolution. Nature Methods. 2010;7:547–553. doi: 10.1038/nmeth.1472. [DOI] [PubMed] [Google Scholar]
- 124.Schiessl K, Kausika S, Southam P, Bush M, Sablowski R. JAGGED Controls Growth Anisotropy and Coordination between Cell Size and Cell Cycle during Plant Organogenesis. Current biology: CB. 2012;22:1739–1746. doi: 10.1016/j.cub.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bozorg B, Krupinski P, Jönsson H. Stress and Strain Provide Positional and Directional Cues in Development. PLoS Comput Biol. 2014;10:e1003410. doi: 10.1371/journal.pcbi.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]