Abstract
Background
Studies were carried on the decolorization of the textile dye reactive blue 19 (RB 19) by a novel isolate of Coprinus plicatilis (C. plicatilis) fungi. We describe an in vitro optimization process for decolorization and its behavior under different conditions of carbon and nitrogen sources, pH, temperature and substrate concentration.
Results
The optimal conditions for decolorization were obtained in media containing intermediate concentrations of ammonium oxalate and glucose (10 g/L) as nitrogen and carbon sources, respectively, at 26°C and pH = 5.5. Maximum decolorization efficiency against RB 19 achieved in this study was around 99%. Ultra-violet and visible (UV-vis) spectrophotometric analyses, before and after decolorization, suggest that decolorization was due to biodegradation.
Conclusions
This effect was associated with laccase enzyme displaying good tolerance to a wide range of pH values, salt concentrations and temperatures, suggesting a potential role for this organism in the remediation of real dye containing effluents. In conclusion, laccase activity in C. plicatilis was firstly described in this study.
Keywords: Reactive blue 19, White rot fungi, Decolorization, Metabolite, UV-vis
Introduction
Treatment of synthetic dyes in wastewater is a matter of great concern. Several physical and chemical methods have been employed for the removal of dyes [1]. However, these procedures have not been widely used due to high cost, formation of hazardous by products and intensive energy requirement [2]. Worldwide over 10,000 different dyes and pigments are used in dyeing and printing industries. The total world colorant production is estimated to be 8.00.000 tons per year and at least 10% of the used dyestuff enters the environment through wastes [3,4]. Wastewater from textile industries constitutes a threat to the environment in many parts of the world. Although some of the dyes are not themselves toxic, after release into the aquatic environment their degradation products are often carcinogenic [5,6]. The existing technologies for decolorization of textile dyeing effluents like adsorption, precipitation, membrane filtration, chemical degradation and photochemical degradation are relatively expensive and commercially unattractive [7,8]. Coagulation-flocculation has major operational problems as it generates large amounts of sludge. Adsorption technique is also more expensive as it involves the use of powdered activated carbon as adsorbent and disposal of spent adsorbent [9] is still a problem. Microbial decolorization is a potential and an effective alternative for the decolorization of wastewater. Microbial decolorization methods of textile dye containing effluents have been reviewed and reported. White-rot fungi such as Phanerochaete chrysosporium[10,11], Trametes versicolor[12,13], Bjerkandera adusta[14], Pycnoporus cinnabarinus[15] and Phanerochaete sordida[16] have been shown to decolorize textile dyes or coloured effluents [17].
In recent years, the utilization of biodegradative abilities of some white rot fungi seems to be promising. They do not require preconditioning to particular pollutants and owing to their extracellular non-specific free radical-based enzymatic system they can degrade to nondetectable levels or even completely eliminate a variety of xenobiotics including synthetic dyes. Many white rot fungi (Phanerochaete chrysosporium, Pleurotus ostreatus, Bjerkandera adusta, Trametes versicolor, etc.) have been intensively studied in connection with their ligninolytic enzyme production and their decolorization ability [18-25]. This capability is due to extracellular non-specific and non-stereoselective enzyme systems composed of laccases (EC 1.10.3.2), lignin peroxidases (EC 1.11.10.14) and manganese peroxidases (EC 1.11.1.13) [26]. Laccase based decolorization treatments are potentially advantageous to bioremediation Technologies since the enzyme is produced in larger amounts mainly by numerous fungi [27,28]. Laccase belongs to a family of multi-copper oxidases that are widespread in nature. Laccases are related to oxidation of a range of aromatic, toxic and environmentally problematic substrates [29] and particular interest with industrial applications. The potential applications are in the textile industry [30], detoxification of pollutants and industrial effluents [31], pulp and paper industry [32], food and pharmaceutical industries [33] biosensor and biofuel applications [34].
The aim of the present work was to characterize the biodegradation of the textile dye Remazol reactive blue 19, by the action of soluble extracts from the white rot fungus C. plicatilis. We describe an in vitro optimization process for decolorization and its behavior under different conditions of carbon and nitrogen sources, pH, temperature and substrate concentration.
Materials and methods
Dyes and chemicals
Textile Remazol dye: reactive blue 19 (RB 19) was supplied by Dystar (Kocaeli, Turkey). 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was obtained from Sigma Chemical Company (St. Louis, MO, USA). All chemicals used were of the highest purity available and of analytical grade.
Culture conditions
Mycelial suspension of Coprinus plicatilis isolated in our university fungus research Laboratory. The white rot fungi Coprinus plicatilis (C. plicatilis) were maintained on 2% (w/v) malt agar slants at 4°C and were then activated at 26°C for 3 days. The mycelium were harvested with a sterile 0.9% NaCl solution and were then inoculated into 100 mL of 2% malt extract broth (pH = 4.5) in 250 mL Erlenmeyer flasks at 26°C and 175 rpm for 4 days. Pellets were inoculated into the medium consisting of 10 g/L glucose, 1.0 g/L of NH4H2PO4, 0.05 g/L of MgSO4.7H2O, 0.01 g/L of CaCl2, 0.025 g/L of yeast extract. Cultivation was carried out in an orbital shaker incubator, at 26°C, 175 rpm [35]. At the beginning of the fourth day of incubation, dye solution was added to the flasks, aseptically, at desired concentrations. Aliquots were assayed for laccase activity. Experiments were performed in 250 mL Erlenmeyer flasks containing 50 mL of liquid medium. For biomass calculation, mycelia were filtered through previously dried and tared Whatman No. 1 filter papers, washed with distilled water and dried at 50°C to constant weight.
Spectrophotometric analysis
Aliquots of 1–2 mL volume of clear dye solution were taken from each reaction flask at regular time intervals and measured immediately using a UV-vis recording double beam spectrophotometer (Shimadzu UV-1601). Decolorization was determined spectrophotometrically by monitoring the absorbance at the wavelength maximum for this dye, and by the reduction of the major peak area in the visible region for dye. The percentage of in culture decolorization was calculated as;
Decolorization percentage, R (%)
| (1) |
Biodegradation Rate = (C0-C)/Incubation day
C0: Initial concentration of dye, C: Last concentration of dye
Optimization of carbon and nitrogen content for enzyme production
Six different carbon substrates (glucose, sucrose, starch, maltose, fructose and glycerol) and nitrogen sources (sodium nitrate, urea, ammonium tartrate, ammonium carbonate, ammonium oxalate and peptone) were used to substitute the original carbon and nitrogen sources. The assessed concentrations were 5, 10 and 15 g/L. All inoculated media were incubated for 15 days at 26°C in the dark and the supernatant or eluted extracts collected for further analysis.
Effect of pH, temperature and copper supplementation on enzyme production
Cultures optimized for carbon and nitrogen content were supplemented with copper sulfate (0.1-5 mM) and incubated for 15 days at 26°C. Supernatants or soluble extracts were then submitted to decolorization. Additionally, destaining activity was measured in samples derived from cultures grown at 26, 30 and 35°C for 15 days and at pH values from 5.5 to 7.0 at 26°C for 15 days.
Enzyme assays
Laccase (Lac) (EC 1.10.3.2) production was assessed by a measurement of the enzymatic oxidation of 2, 22-azinobis-(3 ethylbenzothiazoline- 6-sulphonic acid) (ABTS) at 420 nm (ϵ = 3.6 × 104 cm-1 M-1) [36]. The reaction mixture contained 300 μL of extracellular fluid, 300 μL of 1 μM ABTS and 0.1 M Na Acetate buffer (pH = 4.5, 400 μL). One unit of enzyme activity is defined as the amount of enzyme that oxidizes 1 μmol ABTS in one minute.
Effect of pH, temperature and salt concentration on decolorization
Soluble extracts derived from 15-day-old solid or liquid cultures, carried out under optimized conditions, were tested for decolorization. Decolorization of RB 19 (50 mg/L) was carried out at pH values from 2 to 9 (intervals of 0.5 units), adjusted by using 50 mM citrate-phosphate buffer. The temperatures assessed were 20, 30, 40, 50, 60 and 70°C. For testing susceptibility to salt, different NaCl concentrations (0.05, 0.1, 0.2, 0.4 and 0.6 M) were used in the assay.
Statistical analysis
Standard deviations of the results of triplicate samples from the flask studies were calculated using the Microsoft Excel Spreadsheet Program.
Results
Screening using reactive dyes
C. plicatilis cultures were initially exposed to RB 19 textile dye. In culture decolorization occurred only with RB 19. The total flattening of the UV-vis spectra of the samples indicates that decolorization was accompanied by biodegradation since the smoothening of absorbance peaks at 600 and at 280 nm is consistent with the reduction of azo linkages and the loss of aromatic rings (Figure 1).
Figure 1.
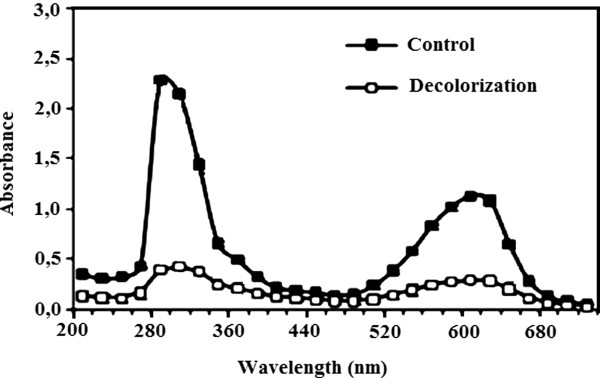
UV-vis spectrum of a solution of RB19 before and after incubation with C. plicatilis cultures.
Effect of dye concentration and water content on fungal growth
When C. plicatilis was cultured in malt extract medium containing 50 mg/L RB 19, the percentage decolorization increased over time, reaching a peak of 99% decolorization after 15 days of incubation (Figure 2). The decolorization was not directly associated with fungal growth since over the period of 5–10 days the biomass level fell while the percentage decolorization was still increasing rapidly. In culture decolorization tests were then done with increasing concentrations of RB 19. Although these concentrations delayed fungal growth slightly, L. crinitus was able to develop in RB 19 concentrations as high as 200 or even 250 mg/L, giving percentage in culture decolorizations of 93.7% and 77.4%, respectively, after 15 days (Figure 3).
Figure 2.
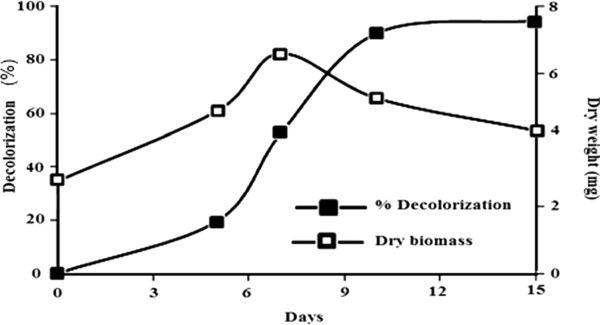
Decolorization of RB19 and growth of C. plicatilis in liquid minimal medium containing 50 mg/L dye. The absorbance of the culture supernatant was determined at 585 nm.
Figure 3.
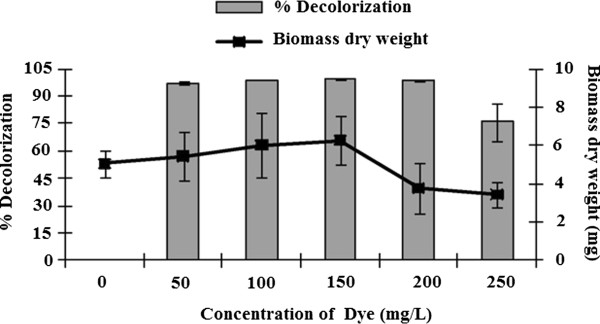
Effect of dye concentration on fungal growth and in culture decolorization. Absorbance at 585 nm was assessed after 15 days of culture.
In order to determine whether the fungal products responsible for dye decolorization were secreted or mycelium-associated, supernatants and mycelia from dead liquid cultures and eluted extracts from dead solid cultures were subjected to decolorization. Decolorization was highest in supernatants and eluted extracts, with relatively low decolorization being detected in mycelia (results not shown). Decolorization eluted from solid cultures was very similar to that obtained in the supernatants obtained from liquid cultures. Also, an RB 19 concentration of 50 mg/L was selected. At this concentration the dye was totally consumed and slightly higher decolorizations were obtained in the culture extracts.
Effect of carbon and nitrogen content on decolorization
In the case of white rot fungi, it has been reported that the production of lignin modifying enzymes (LMEs) and decolorization varies greatly, according to the type and concentration of carbon and nitrogen sources in culture media. These effects were therefore investigated. C. plicatilis was grown on solid minimal media that contained glucose, fructose, maltose, starch, sucrose or glycerol as the sole carbon source. For each substrate, three concentrations (5, 10 and 15 g/L) were tested. After 15 days of incubation, decolorization was performed using extracts from the solid medium. Drastic differences in the decolorization were observed. C. plicatilis produced the highest levels of decolorization when grown in 10 g/L glucose; 5 g/L maltose or 10 g/L fructose, with the decolorization ranging from 63.7% to 84.8%. Higher concentrations of these carbon sources did not lead to higher decolorization. With some carbon sources the yield of decolorization was very low. For example, with 15 g/L glycerol and 10 g/L starch decolorization of only 5-15% were produced (Figure 4A).
Figure 4.
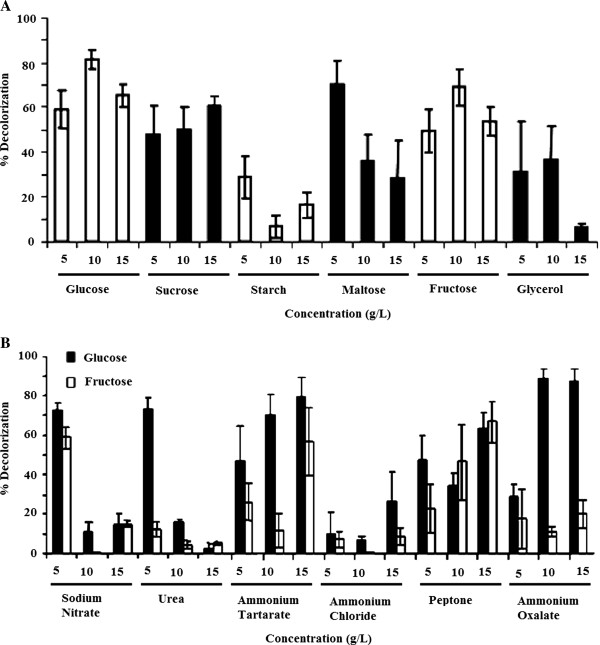
Effect of carbon and nitrogen on decolorization. A; Effect of carbon type and concentration on decolorization in solid medium. B; Effect of nitrogen source and concentraation on decolorization in solid media containing fructose (10 g/L) and glucose (10 g/L) as carbon source.
When nitrogen sources were assessed, using fructose (10 g/L) and glucose (10 g/L) as carbon sources (maltose was omitted due to its high cost), soluble extracts derived from cultures containing 5 g/L sodium nitrate, 15 g/L ammonium tartrate and 10 g/L ammonium oxalate performed the highest decolorization (Figure 4B). Again, some substrates promoted the decolorization and some of them, such as urea and ammonium chloride, were inhibitory. A significant observation is that the effect of a nitrogen source can depend on the accompanying carbon substrate. Thus, soluble extracts derived from cultures containing 5 g/L urea led to a 10% decolorization of this dye when combined with 5 g/L fructose in the culture medium, but when combined with 5 g/L glucose, this same concentration of urea gave a 5-fold higher decolorization. A similar effect was observed for 10 and 15 g/L ammonium oxalate with fructose and glucose.
Effect of pH, temperature and copper amount of cultures on decolorization of dye
Cultures were done in solid medium with optimized carbon and nitrogen contents (10 g/L glucose and 10 g/L ammonium oxalate) in five different initial pH values (at 26°C) and three different temperatures (with initial pH = 5.5). Other cultures were performed (at 26°C and initial pH = 5.5) in the presence of three different copper sulfate concentrations. Extracts from 4 day cultures were tested for decolorization. Decolorization of dye was better in extracts derived from cultures incubated at 26°C and with initial pH values ranging from 5.5 to 6.5 (Table 1). Extracts derived from copper supplemented cultures did not display an improvement in decolorization and at copper sulfate concentrations above 1 mM the decolorization was relatively low (Table 1).
Table 1.
Effect of pH, temperature and copper concentration on decolorization (initial dye concentration; 50 mg/L)
|
T°C % Decolorization |
26 95.1 ± 0.6 |
30 21.4 ± 2.3 |
35 0.04 ± 0.04 |
|
|
Cu
2+
(mM) % Decolorization |
0 79.7 ± 2.2 |
0.1 84.3 ± 3.6 |
1.0 51.2 ± 1.3 |
2.0 49.6 ± 1.1 |
| pH % Decolorization | 5.5 99.2 ± 0.6 | 6 81.3 ± 1.2 | 6.5 68.7 ± 2.4 | 7 11.4 ± 1.7 |
Laccase activities
Figure 5 shows variations of laccase activities during decolorization within the ten days period for this organism. In an attempt to determine the possible role of ligninolytic enzymes on dye decolorization, laccase enzyme activity was monitored during the decolorization of this dye. In dye containing liquid cultures, laccase specific activity of this fungi was observed to have decreased by increasing dye concentration. These results emphasized the role of laccase in RB 19 decolorization. At higher concentrations levels than 50 mg/L RB 19, considered enzyme activities were diminished sharply, which is likely due to the toxicity of synthetic dyes as mentioned before (Figure 5).
Figure 5.
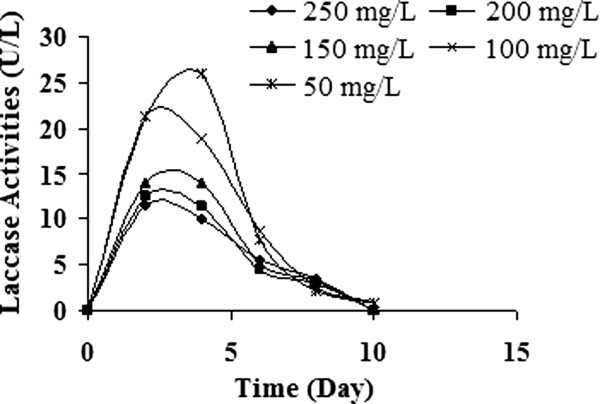
Alteration of laccase enzyme activities.
Discussions
Several species and strains have been assessed for biodegradation of different pollutants such as crude oil [37], pentachlorophenol [38], DDT [39], trinitrotoluene [40] and some textile dyes [41,42]. Here we report laccase activity in C. plicatilis, a relatively unexplored Coprinus species, and its participation in the decolorization of the textile dye reactive blue 19. Reactive blue 19 (RB 19) is a vinyl sulfone azo dye. Laccase activity in C. plicatilis was firstly described. Copper supplementation can stimulate laccase synthesis since the enzyme uses copper as cofactor, but the ion can also inhibit the growth of the organism [43]. Previous attempts to degrade it have focused on photo-catalytic and chemo-oxidative processes [44-46]. Although these processes may degrade the dye partially or even totally, they have several drawbacks such as the generation of by-products, including chemical sludge, and high investment and operating costs [1].
It used to be generally accepted that carbon and nitrogen limitation favored the production of lignolytic enzymes in white rot fungi [47]. However, more recent results are somewhat contradictory. For example, in L. edodes, Buswell et al. [48] obtained 5-fold higher laccase levels under high nitrogen conditions than in low-nitrogen cultures, while Hatvani and Mécs [49], working on the biodegradation of dyes by Lentinus sp. grown in solid media, found that faster decolorization occurred at very low NH4Cl, peptone and malt extract concentrations. On the other hand, the lignolytic activity of L. edodes grown in liquid culture was stimulated by high N concentrations [50]. In the present work, the decolorization was affected by the type and concentration of the nitrogen source, but different trends occurred for different sources: higher concentrations of ammonium salts resulted in higher decolorization, while for sodium nitrate and urea higher production levels were obtained at the lower concentrations. Moreover, the final result varied drastically with the type of carbohydrate present in the culture medium. For example, the high decolorization obtained in cultures containing 5 g/L urea was almost lost when glucose was replaced with fructose as the main carbon source. A similar effect was observed for ammonium oxalate.
Conclusions
C. plicatilis cultures were able to decolorize and biodegrade the textile dye reactive blue 19. The decolorization was highly influenced by medium composition and culture conditions, being higher in media containing intermediate concentrations of ammonium oxalate and glucose. Decolorization of dye was associated with laccase displaying good tolerance to a wide range of pH values and temperatures, suggesting a potential role for this organism and enzyme in the remediation of real dye containing effluents. In conclusion, laccase activity in C. plicatilis was firstly described in this study.
Competing interests
The authors have declared no conflict of interest.
Authors’ contribution
HAA: Designed experiments and drafted the manuscript. MCT: carried out the experiments, analyzed data. AAU: carried out the experiments. All authors have read and approved the final manuscript.
Contributor Information
Hatice A Akdogan, Email: hardag@pau.edu.tr.
Merve C Topuz, Email: mervecanpolat88@gmail.com.
Asiye A Urhan, Email: chemist_ardag@hotmail.com.
Acknowledgements
The author also would like to thank Shimadzu Co. for their advice.
References
- Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluents: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol. 2001;77:247–255. doi: 10.1016/S0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Hai FI, Yamamoto K, Fukushii K. Hybrid treatment systems for dye. Crit Rev Environ Sci Technol. 2007;37:315–377. doi: 10.1080/10643380601174723. [DOI] [Google Scholar]
- Palmieri G, Cennamo G, Sannia G. Remazol brilliant R decolourisation by the fungus pleurotus ostreatus and its oxidative enzymatic system. Enzyme Microb Technol. 2005;36:17–27. doi: 10.1016/j.enzmictec.2004.03.026. [DOI] [Google Scholar]
- Levin L, Papinutti L, Forchiassin F. Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresour Technol. 2004;94:169–176. doi: 10.1016/j.biortech.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Banat IM, Nigam P, Singh D, Marchant R. Microbial decolorization of textiledye- containing effluents: a review. Bioresour Technol. 1996;58:217–227. doi: 10.1016/S0960-8524(96)00113-7. [DOI] [Google Scholar]
- Shyam SD, Satyshari D, Bhattacharyya BC. Dye decolorization in a column bioreactor using wood-degrading fungus Phanerochaete chrysosporium. Indian Chem Eng A. 1995;37:176–180. [Google Scholar]
- Sarasa J, Roche MP, Ormad MP, Gimeno E, Puig A, Ovelleiro JL. Treatment of a wastewater resulting from dyes manufacturing with ozone and chemical coagulation. Water Res. 1998;32:2721–2727. doi: 10.1016/S0043-1354(98)00030-X. [DOI] [Google Scholar]
- Konsowa AH. Decolorization ofwastewater containing direct dye by ozonation in a batch bubble column reactor. Desalination. 2003;158:233–240. doi: 10.1016/S0011-9164(03)00458-2. [DOI] [Google Scholar]
- Blanquez P, Casas N, Font X, Gabarrell X, Sarra M, Caminal G, Vicent T. Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res. 2004;38:2166–2172. doi: 10.1016/j.watres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Kirby N, McMullan G, Marchant R. Decolorization of an artificial textile effluent by Phanerochaete chrysosporium. Biotechnol Lett. 1995;17:761–764. doi: 10.1007/BF00130365. [DOI] [Google Scholar]
- Swamy J, Ramsay J. The evaluation of white rot fungi in the decolorization of textile dyes. Enzyme Micro Technol. 1999;24:130–137. doi: 10.1016/S0141-0229(98)00105-7. [DOI] [Google Scholar]
- Nilsson I, Moller A, Mattiasson B, Trubindamayugi MS, Welander U. Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enzyme Microb Technol. 2006;38:94–100. doi: 10.1016/j.enzmictec.2005.04.020. [DOI] [Google Scholar]
- Mehna A, Pratima B, Pramod KB. Studies on decolorization of effluent from a small pulp mill utilizing agri-residues with Trametes versicolor. Enzyme Microb Technol. 1994;22:18–22. [Google Scholar]
- Heinfling A, Martinez MJ, Martinez AT, Bergbauer M, Szewzyk U. Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl Environ Microbiol. 1998;64:2788–2793. doi: 10.1128/aem.64.8.2788-2793.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliephake K, Lonergan GT. Laccase variation during dye decolorization in a 200-L packed-bed bioreactor. Biotechnol Lett. 1996;8:881–886. [Google Scholar]
- Koichi H, Yoshio W, Kazunori N. Decolorization of azo dye by the white rot Basidimycete Phanerochaete sordida by itsmanganese peroxidase. J Biosci Bioeng. 2003;95:455–459. doi: 10.1016/s1389-1723(03)80044-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan SV, Murthy DVS. Statistical optimization for decolorization of textile dyes using Trametes versicolor. J Hazard Mater. 2009;165:909–914. doi: 10.1016/j.jhazmat.2008.10.072. [DOI] [PubMed] [Google Scholar]
- Borchert M, Libra JA. Decolorization of reactive dyes by the white rot fungus Trametes versicolor in sequencing batch reactors. Biotech Bioeng. 2001;75:313–321. doi: 10.1002/bit.10026. [DOI] [PubMed] [Google Scholar]
- Conneely A, Smyth WF, McMullan G. Study of the white-rot fungal degradation of selected phthalocyanine dyes by capillary electrophoresis and liquid chromatography. Anal Chim Acta. 2002;451:259–270. doi: 10.1016/S0003-2670(01)01415-5. [DOI] [Google Scholar]
- Jarosz-Wilkołazka A, Rdest-Kochman’ska J, Malarczyk E, Wardas W, Leonowicz A. Fungi and their ability to decolorize azo and anthraquinonic dyes. Enzyme Microbiol Technol. 2002;30:566–572. doi: 10.1016/S0141-0229(02)00022-4. [DOI] [Google Scholar]
- Martins MAM, Lima N, Silvestre AJD, Queiroz MJ. Comparative studies of fungal degradation of single or mixed bioaccessible reactive azo dyes. Chemosphere. 2003;52:967–973. doi: 10.1016/S0045-6535(03)00286-8. [DOI] [PubMed] [Google Scholar]
- Moldes D, Couto SR, Cameselle C, Sanroma’n MA. Study of the degradation of dyes by MnP of Phanerochaete chrysosporium produced in a fixed-bed bioreactor. Chemosphere. 2003;51:295–303. doi: 10.1016/S0045-6535(02)00406-X. [DOI] [PubMed] [Google Scholar]
- Moreira MT, Palma C, Mielgo I, Feijoo G, Lema JM. In vitro degradation of a polymeric dye (Poly R-478) by manganese peroxidase. Biotech Bioeng. 2001;75:362–368. doi: 10.1002/bit.10052. [DOI] [PubMed] [Google Scholar]
- Rodrı’gues E, Pickard A, Vazquez-Duhalt R. Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol. 1999;38:27–32. doi: 10.1007/PL00006767. [DOI] [PubMed] [Google Scholar]
- Andleeb S, Atiq N, Ali MI, Rehman F, Hameed A, Ahmad S. Biodegradation of anthraquinone dye by Aspergillus niger sa1 in self designed fluidized bed bioreactor. Iran J Environ Health Sci Eng. 2010;7:371–376. [Google Scholar]
- Heinzkill M, Bech L, Halkier T, Schneider P, Anke T. Characterization of laccases and peroxidases from wood rotting fungi (Family Coprinaceae) Appl Environ Microbiol. 1998;64:1601–1606. doi: 10.1128/aem.64.5.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Georg MG. Decolourization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol. 2000;66:3357–3362. doi: 10.1128/AEM.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FJ, Hong YZ, Xiao YZ, Xu YH, Fan W. High production of laccase B from Trametes sp. in Pichia pastoris. World J Microbiol Biotechnol. 2007;23:741–745. doi: 10.1007/s11274-006-9286-2. [DOI] [Google Scholar]
- Canas AI, Camarero S. Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly process. Biotechnol Adv. 2010;28(6):694–705. doi: 10.1016/j.biotechadv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases – occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Trovaslet-Leroy M, Jolivalt C, Froment M, Brasme B, Lefebvre B, Daveloose D, Nachon F, Masson P. Application of laccase-mediator system (LMS) for the degradation of organophosphorus compounds. Chem Biol Interact. 2010;187:393–396. doi: 10.1016/j.cbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Widsten P, Kandelbauer A. Laccase applications in the forest products industry: A review. Enzym Microb Technol. 2008;42:293–307. doi: 10.1016/j.enzmictec.2007.12.003. [DOI] [Google Scholar]
- Desai SS, Nitvanand C. Microbial laccases and their applications: a review. Asian J Biotechnol. 2011;3:98–124. doi: 10.3923/ajbkr.2011.98.124. [DOI] [Google Scholar]
- Odaci D, Timur S, Pazarlioglu N, Montereali MR, Vastarella W, Pilloton R, Telefoncu A. Determination of phenolic acids using Trametes versicolor laccase. Talanta. 2011;71:312–317. doi: 10.1016/j.talanta.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Ardag Akdogan H, Kasikara Pazarlioglu N. Fluorene Biodegradation by P.ostreatus-I: Biodegradation by Free Cells. Process Biochem. 2011;46:834–839. doi: 10.1016/j.procbio.2010.12.011. [DOI] [Google Scholar]
- Eggert C, Temp U, Dean JF, Eriksson KE. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- Adenipekun CO, Fasidi IO. Bioremediation of oil-polluted soil by Lentinus subnudus, a Nigerian white-rot fungus. Afr J Biotechnol. 2005;4:796–798. [Google Scholar]
- Machado KMG, Matheus DR, Monteiro RTR, Bononi VLR. Biodegradation of pentachlorophenol by tropical basidiomycetes in soils contaminated with industrial residues. World J Microbiol Biotechnol. 2005;21:297–301. doi: 10.1007/s11274-004-3693-z. [DOI] [Google Scholar]
- Van Chung T, Khue DN, Minh DB, Cheng F. Use of fungal humus for 1,1,1- trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) polluted soil treatment. Chem Asian J. 2009;21:5967–5972. [Google Scholar]
- Samson J, Langlois E, Lei J, Piche Y, Chenevert R. Chenevert, Removal of 2,4,6-trinitrotoluene and 2,4-dinitrotoluene by fungi (Ceratocystis coerulescens, Lentinus lepideus and Trichoderma harzianum) Biotechnol Lett. 1998;20:355–358. doi: 10.1023/A:1005371112287. [DOI] [Google Scholar]
- Minussi RC, de Moraes SG, Pastore GM, Duran N. Biodecolorization screening of synthetic dyes by four white-rot fungi in a solid medium: possible role of siderophores. Lett Appl Microbiol. 2001;33:21–25. doi: 10.1046/j.1472-765X.2001.00943.x. [DOI] [PubMed] [Google Scholar]
- Gomes E, Aguiar AP, Carvalho CC, Bonfa MRB, da Silva R, Boscolo M. Ligninases production by basidiomycetes strains on lignocellulosis agricultural residues and their application in the decolorization of synthetic dyes. Braz J Microbiol. 2009;40:31–39. doi: 10.1590/S1517-83822009000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutova VV, Revin VV, Myakushina YA. The effect of copper ions on the production of laccase by the fungus Lentinus (Panus) tigrinus. Appl Biochem Microbiol. 2008;44:619–623. doi: 10.1134/S0003683808060100. [DOI] [PubMed] [Google Scholar]
- Kurbus T, Le-Marechal AM, Voncina DB. Comparison of H2O2/UV, H2O2/O3 and H2O2/Fe2+ processes for the decolorisation of vinylsulphone reactive dyes. Dyes Pigm. 2003;58:245–252. doi: 10.1016/S0143-7208(03)00085-8. [DOI] [Google Scholar]
- Voncina DB, Le-Marechal AM. Reactive dye decolorization using combined ultrasound/H2O2. Dyes Pigm. 2003;59:173–179. doi: 10.1016/S0143-7208(03)00101-3. [DOI] [Google Scholar]
- Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J Colloid Interface Sci. 2006;295:159–164. doi: 10.1016/j.jcis.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Kirk K, Farrell RL. Enzymatic “combustion”: the microbial degradation of lignin. Ann Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Buswell JA, Cai Y, Chang ST. Effect of nutrient nitrogen and manganese on manganese peroxidase and lactase production by Lentinula (Lentinus) edodes. FEMS Microbiol Lett. 1995;128:81–88. doi: 10.1111/j.1574-6968.1995.tb07504.x. [DOI] [Google Scholar]
- Hatvani N, Mécs I. Effect of the nutrient composition on dye decolorisation and extracellular enzyme production by Lentinus edodes on solid medium. Enzyme Microbiol Technol. 2002;30:381–386. doi: 10.1016/S0141-0229(01)00512-9. [DOI] [Google Scholar]
- Leatham GF, Kirk TK. Regulation of ligninolytic activity by nutrient Nin white rot basidiomycetes. FEMS Microbiol Lett. 1983;7:16–65. [Google Scholar]


