Abstract
Age-related hearing loss (AHL), also known as presbycusis, is a universal feature of mammalian aging and is the most frequently occurring sensory disorder in the elderly population. AHL is characterized by a decline of auditory function and loss of hair cells and spiral ganglion neurons in the cochlea of the inner ear. It has been postulated that AHL occurs gradually as a result of the cumulative effect with aging of exposure to noise, diet, oxidative damage, and mitochondrial DNA mutations. However, the molecular mechanisms of AHL remain unclear and no preventative or therapeutic interventions have been developed. A growing body of evidence suggests increased oxidative damage with aging to macromolecules such as DNA, proteins, and lipids may play a causal role in aging and age-related diseases. Caloric restriction (CR) extends the lifespan of most mammalian species, delays the onset of multiple age-related diseases, and attenuates both the degree of oxidative damage and the associated decline in physiological function. Here, we review studies on CR’s ability to prevent cochlear pathology and AHL in laboratory animals and discuss potential molecular mechanisms of CR’s actions.
Keywords: Aging, Age-related hearing loss, Presbycusis, Caloric restriction, Cochlea, Oxidative stress, Mitochondria, Apoptosis
INTRODUCTION
Age-related hearing loss (AHL), known as presbycusis, is a universal feature of mammalian aging and is the most frequently occurring sensory disorder in the elderly [1]. In the US, people over 65 years of age numbered 36.3 million in 2004, representing 12.4% of the US population, and this elderly population is expected to grow to 20% of the US population by 2030 (Administration on Aging. Statistics on the Aging Population: http://www.aoa.gov/prof/Statistics/statistics.asp). In Japan, people over 65 years of age numbered 25.6 million in 2007 (20.0% of the population) (Statistic Bureau, Ministry of Internal Affairs and Communications of Japan: http://www.stat.go.jp/english/data/jinsui/2.htm) and this elderly population is expected to grow to be 35.9 million (39.0% of the population) in 2050 (National Institute of Population and Social Security Research: http://www.ipss.go.jp/index-e.html). AHL affects nearly half the population between 48 and 92 years of age in Wisconsin [2], and 23% of people between 65 and 75 years of age and 40% of people over 75 years of age in the US [3, 4]. Because of the high prevalence of AHL and because the overall number of people with it is rapidly growing as lifespan increases in industrialized nations, AHL is a major social and health problem.
AHL is characterized by the progressive deterioration of auditory function with aging [1, 4, 5] and is thought to occur as a result of the cumulative effect of exposure to noise, diet, oxidative damage, and mitochondrial DNA (mtDNA) mutations. In humans, there are several age-associated pathological changes to the inner ear as hearing loss progresses. These changes include degeneration of the sensory hair cells, spiral ganglion (SG) cells, and stria vascularis cells [1, 5]. None of these cochlear long-lived cell types regenerates in mammals, and extensive cell loss leads to permanent hearing impairment. Schuknecht and co-workers [6] defined sensory, neural, and strial types of AHL based on correlations between cochlear pathology and audiogram; approximately 75% of cases of AHL represent a pure sensory, neural, or strial type, or a mixture of two or more types, and in the remaining 25% of cases the cochlear pathology does not correlate with the audiogram. Therefore, the major cause of AHL is the loss of hair cells, SG neurons, and stria vascularis cells in the cochlea of the inner ear. However, despite intensive studies in the AHL field, the molecular mechanisms of AHL remain unclear and no preventative or therapeutic interventions have been developed in humans.
Caloric restriction (CR) extends the lifespan of most mammalian species and is the only intervention shown to slow the rate of aging in mammals [7-12]. Maximum lifespan is thought to be increased by reducing the rate of aging, while the average lifespan can be increased by improving environmental conditions. Indeed, the average lifespan of humans has dramatically increased as a result of improved diet and environmental health conditions, whereas the maximum lifespan has remained largely unchanged. CR extends the lifespan in species as diverse as birds, protozoans, water fleas, spiders, guppies, and rodents [7-10]. In laboratory rodents, CR delays the onset of age-related diseases such as lymphomas, breast cancer, prostate cancer, nephropathy, cataracts, diabetes, hypertension, hyperlipidemia, and autoimmune diseases [7-10]. CR also reduces degeneration of neurons in animal models of Parkinson’s disease [13]. In monkeys, CR results in signs of improved health including reduced body fat, higher insulin sensitivity, increase in HDL and reduction in VLDL levels [14]. At the molecular level, CR results in a significant reduction in the levels of oxidative protein damage in the brains, hearts, and livers of aging mice [15]. Although CR improves most of the physiological parameters in strains and species evaluated, it should be noted that CR may also have adverse effects in a certain condition. For example, it has been shown that testicular atrophy was increased in both Brown Norway and Fischer 344 rats under CR [16].
Previous studies have shown that CR delays the onset of AHL and reduces cochlear pathology in mice [17-20] and rats [3]. The mechanisms postulated to be responsible for the beneficial effects of CR on aging and AHL include reduced oxidative damage and reduced levels of mtDNA mutations [7-12, 21-25]. Herein, we review the evidence that CR delays the onset of AHL in laboratory animals. We focus on the different CR regimens employed, and discuss potential molecular mechanisms by which CR slows the rate of cochlear aging and prevents AHL.
STUDIES IN MICE
Two different dietary feeding regimens of CR have been widely used because of their reproducible abilities to retard the rates of aging in rodents [26]. In an “every day feeding (ED)” regimen, the animals receive food daily, but are limited to a specified amount which is usually 30-50% less than the ad libitum consumption by the control group. In an “every other day feeding (EOD)” regimen, the animals are deprived of food for a full day, every other day, and are fed ad libitum on the intervening days. As for housing regimens, the use of individual housing for all animals in CR studies has been widely used since it allows for the food intake of each animal to be controlled with great accuracy [27]. To investigate the effects of CR on AHL and to monitor the progression of AHL, electrophysiological tests of hearing function, auditory brainstem response (ABR), auditory nerve isoelectric (ANI), middle latency response (MLR), or distortion product otoacoustic emission (DPOAE) tests have been used in laboratory animals [3, 17-20, 28-31].
The AU/Ss (AU) strain is characterized as a normal-hearing mouse, but develops gradual hearing loss in the second half of its life, while the AKR strain is characterized as a short-lived mouse strain (mean lifespan = 323 days) (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Yuan2) which develops early onset of hearing loss by 1-2 months of age [18]. In a study of 18 month old AU mice, CR animals were fed standard lab chow ad libitum EOD (only on Monday, Wednesday, and Friday), while control mice were fed ad libitum daily for a 17 month period. All mice were housed with 1-3 per cage and ANI analysis was used to monitor the progression of AHL [18]. At 18 months of age, both control and CR mice displayed significant threshold elevations at the 2, 4, 8, 32, and 64 kHz frequencies; however, the mean thresholds of CR mice were significantly lower than those of controls at all the frequencies measured, indicating that CR delays the onset of AHL in the AU strain. CR mice weighed 14% less than controls and CR increased the lifespan of the AU mice by 44%. In a study of the AKR strain [18], the same dietary feeding and housing regimens as described above were followed starting from 2 months of age until 4 months of age. At 4 months of age, both control and CR mice displayed large threshold elevations at the 2, 4, 32, and 64 kHz frequencies, and there were no differences in hearing threshold between control and CR animals at all the frequencies measured, indicating that CR does not delay the onset of AHL in this strain. This may be due to the fact that the onset of AHL in this strain occurs at a very young age (1-2 months of age) which may be too early for CR to be beneficial. CR mice weighed 22% less than controls, but CR was not found to increase the lifespan of the AKR mice. However, this study was done with very small sample sizes (N = 6). Consequently, this study had low statistical power and statistically significant lifespan improvements may have gone undetected.
The CBA/J (CBA) mouse strain is long-lived (mean lifespan = 679 days) (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Yuan2) and has normal hearing for the first half of its life, but gradually develops AHL thereafter [19, 32]. Sweet and co-workers [19] performed two interesting CR studies using CBA mice: CR for an early 8 month period (from 2 until 10 months of age) for the Study 1 group; the Study 2 group was subjected to 17 months of CR (from 10 until 27 months of age). The same feeding and housing methods as described for the AU and AKR strain studies were employed. The ABR analysis was used to monitor the progression of AHL. Both control and CR mice from the Study 1 group displayed large and similar ABR threshold elevations at all frequencies measured (4, 8, 16, 32, and 64 kHz); these results indicate that CR until midlife (from 2 to 10 months of age) can not prevent AHL in this strain. This may be due to the fact that CR was stopped too early in the life of the CBA mice to be beneficial. As expected, CR did not increase the lifespan of the Study 1 group mice. CR mice from the Study 2 group also displayed ABR threshold elevations; however, the thresholds were less severely elevated at frequencies below 64 kHz and the mean thresholds of CR mice were significantly lower than those of controls at 8, 16, and 32, kHz frequencies, indicating that middle-age onset CR can delay the onset of AHL in this strain. These results suggest that CR may only reduce AHL if the restriction spans the period when hearing function decline occurs. Interestingly, the CR after midlife regimen did not increase the lifespan of the Study 2 group mice. As with the AKR strain study, the CBA strain study was done with small sample sizes (N = 15) and statistically significant lifespan improvements may have gone undetected.
C57BL/6 (B6) mice are very long-lived (mean lifespan = 901 days) (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Yuan2) and have been used extensively to study aging [7] and as a model of AHL [17, 28, 32]. It is also well known that B6 mice respond to CR with a robust extension of lifespan [7]. This strain displays the classic pattern of AHL, including loss of sensory hair cells and SG neurons by 12-15 months of age [17, 28]. We have shown previously that CR can completely prevent the onset of AHL in the B6 strain [17]. In this study, B6 mice were calorie restricted to 75% of the control intake starting at two months of age and housed individually for a 13 month period. CR mice were fed agar-based semi-purified powdered diets EOD (only on Monday, Wednesday, and Friday), receiving 63 kcal/week of the diet, while control mice were also fed the diet EOD and received 84 kcal/week of the diet [27]. The control mice were not fed ad libitum daily to avoid large individual variations among the caloric intake of the control group and obesity in the control mice. ABR analysis was used to monitor the progression of AHL. At 15 months of age, the mean ABR thresholds of CR mice were significantly lower than those of age-matched controls at the 4, 8, 16 kHz frequencies, and not significantly different from those of 4 month old mice at all the frequencies measured. Thus, CR prevents the onset of AHL that would otherwise occur in B6 mice by 15 months of age. CR mice weighed 25% less than the controls and displayed only minor cochlear degeneration; in contrast, age-matched control mice displayed severe cochlear cell loss.
Willott and co-workers [20] performed a large scale CR study using 4 different mouse strains: B6, DBA/2J (DBA), WB/ReJ (WB), and BALB/cByJ (BALB). In their study of B6 mice calorie restricted to 70% of the control intake, all animals were housed 4 mice per cage for a 22 month period (from 1 until 23 months of age) and ABR analysis was used to monitor the progression of AHL. The animals were fed the CR or control measured amount of food daily and total amounts of all diet components except starch and corn oil were the same. CR significantly reduced SG neuron loss compared to controls; however, no ABR thresholds were obtained even with 100 dB SPL at 4, 8, 16, 24, and 32 kHz frequencies in both the control and CR mice. In contrast to the results of our studies in 15 month old B6 mice, these results indicate that all the mice displayed severe to complete hearing loss, and that CR did not prevent the AHL of the 23 month old B6 mice. Different strains of mice have a different age of onset for AHL. The DBA strain displays hearing loss by 3 months of age (mean lifespan = 701 days) [20, 32], while WB strain and BALB strains (mean lifespan = 664 days) strains develop severe hearing loss between 16 and 23 months of age (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Yuan2) [20, 33]. Willott and co-workers employed the same diet regimens to study DBA, WB, and BALB strains [20]. Again, CR failed to delay the onset of AHL in these 3 strains when tested at 23 months of age; no ABR thresholds were obtained even with 100 dB SPL at 4, 8, 16, 24, and 32 kHz frequencies in both the control and CR mice of the DBA strain group, while both the control and CR mice of the WB and BALB strain groups displayed large ABR threshold elevations at all frequencies measured (4, 8, 16, 32, and 64 kHz). This may be due to the fact that the time points of the hearing tests in these strains were too late for CR to be beneficial since all strains develop severe to complete hearing loss by 3-23 months of age. Different methods of housing (4 mice/cage) may also account for the different effects of CR on the onset of AHL in these strains. Summaries of dietary regimens used in the mouse studies are given in Table 1. CR increased the lifespan of the WB strain, while most of the DBA and BALB mice died before the age of 23 months. CR did not increase the lifespan of the B6 strain. Again, statistically significant differences may have gone undetected because the sample sizes were small (N = 2-12).
Table 1.
List of CR Regimens in Mouse Studies
| Category | Mouse | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AU | AKR | CBA | CBA | B6 | B6 | DBA | WB | BALB | |
| Age at Test (month) | 18 | 4 | 10 | 27 | 15 | 23 | 23 | 23 | 23 |
| Number/Diet Group | 4 | 6 | 10 | 7-9 | 6 | 4 | 4 | 6-8 | 2-5 |
| CR% | NA | NA | NA | NA | 25 | 30 | 30 | 30 | 30 |
| Number/Cage | 1-3 | 1-3 | 1-3 | 1-3 | 1 | 4 | 4 | 4 | 4 |
| Feeding | EOD | EOD | EOD | EOD | EOD | ED | ED | ED | ED |
| Feeding Period (months) | 17 | 2 | 8 | 17 | 13 | 22 | 22 | 22 | 22 |
| Onset of CR (month) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Control Diet (kcal/week) | NA | NA | NA | NA | 84 | NA | NA | NA | NA |
| CR Diet (kcal/week) | NA | NA | NA | NA | 63 | NA | NA | NA | NA |
| Age of AHL Onset (month) | 18 | 1-2 | 18 | 18 | 12-15 | 12-15 | 3 | 16-23 | 16-23 |
| Hearing Test | ANI | ANI | ANI | ABR | ABR | ABR | ABR | ABR | ABR |
| Frequency tested (kHz) | 2-64 | 2-64 | 4-64 | 4-64 | 4-16 | 4-32 | 4-32 | 4-32 | 4-32 |
| CR Prevention of AHL | Yes | No | No | Yes | Yes | No | No | No | No |
| Reference | [18] | [18] | [19] | [19] | [17] | [20] | [20] | [20] | [20] |
STUDIES IN RATS
The Fischer 344 strain of rats displays severe hearing loss by 20 months of age [3]. In a study of CR in male Fischer rats, the animals were calorie restricted to 70% of the control intake beginning at one month of age, then housed individually for 24-25 months after which the progression of AHL was measured by ABR [3]. CR rats were fed 11.2 g of a standard rodent diet daily, while control rats were fed ad libitum. Both control and CR rats displayed large ABR threshold elevations at 3, 6, 9, 12, and 18 kHz frequencies, but the mean thresholds of CR rats were significantly lower than those of controls at all the frequencies measured. CR animals also had significantly reduced hair cell loss compared to controls. Thus, CR delays the onset of AHL in rats, as has been shown in the AU, CBA and B6 strains of mice. Summaries of dietary regimens used in the rat study are given in Table 2.
Table 2.
List of CR Regimens in Rat and Monkey Studies
| Category | Rat | Monkey | |
|---|---|---|---|
| Fischer | Rhesus (UW) | Rhesus (NIA) | |
| Age at Test | 26-27 months | 11-23 years | 19-20 years |
| Number/Diet Group | 3-5 | 33-35 | 24-26 |
| CR% | 30 | 30 | 30 |
| Number/Cage | 1 | 1 | 1 |
| Feeding | ED | ED | ED |
| Feeding Period | 24-25 months | 3-9 years | 12-13 years |
| Onset of CR | 1 month | 8-14 years | 11-14 years |
| Control Diet (kcal/week) | NA | NA | NA |
| CR Diet (kcal/week) | NA | NA | NA |
| Age of AHL Onset | 20 months | 25-31 years | 25-31 years |
| Hearing Test | ABR | ABR, MLR | ABR, MLR, DPOAE |
| Frequency tested (kHz) | 3-12 | Click (100-3000) | Click (100-3000) |
| CR Prevention of AHL | Yes | Yes | No |
| Reference | [3] | [29, 30, 35] | [31, 34] |
STUDIES IN MONKEYS
Rhesus monkeys are long-lived animals with a maximum lifespan of 40 years [34] and display decreased auditory function by 25-31 years of age [29, 34]. There are two large studies ongoing at the University of Wisconsin-Madison (UW) and the National Institute on Aging (NIA) designed to test the hypothesis that CR retards the rates of aging and delays the onset of AHL in a primate species [30, 31, 34, 35]. In the UW study, 33 rhesus monkeys were on CR diets (20 males and 13 females) and 35 controls were fed ad libitum (21 males and 14 females) daily; CR was maintained at 30% less than the calories consumed by the ad libitum group [30, 35]. At the time of the auditory tests, the ages of the monkeys were between 11 and 23 years and the monkeys had been in the dietary study 3-9 years. The animals were housed individually to minimize aggressive encounters and to control food access. All animals were daily fed pelleted, semi-purified diets which contain 15% lactalbumin, 10% corn oil and 65% carbohydrate in the form of sucrose and corn starch. ABR and MLR analyses were used to monitor the progression of AHL. A previous study has shown that aged rhesus monkeys display smaller peak amplitudes of ABR waves compared to young controls. In the UW study, the peak IV amplitude of ABR waves of CR female monkeys was significantly larger compared to control females. In addition, the binaural peak IV amplitude of ABR waves decreased significantly faster with age for control monkeys compared to CRs. The mean ABR threshold (click stimuli) of CR male monkeys was lower than that of control males, but the difference was not statistically significant. Together, these early results indicate that some components of auditory function in the aged monkeys were maintained by CR.
In the NIA study, 26 rhesus monkeys were on CR diets and 24 controls were fed ad libitum to investigate the effects of CR on the onset of AHL [31]. CR was maintained at 30% less the calories consumed by the ad libitum group. At the time of the tests, the mean ages of the CR and control monkeys were 20.4 years and 18.7 years respectively, and monkeys had been in the CR study 12-13 years at the time of testing. Dietary feeding and housing regimens similar to those of the UWM study were employed to study CR in monkeys at the NIA. ABR, MLR, and DPOAE analyses were used to monitor the progression of AHL. No significant effects of CR were found on any parameters examined. At the time of the hearing tests, the age range of the control monkeys was 13-32 years, while that of the CR monkeys was 13-36 years. Therefore, the lack of effect of CR may be due to the fact that some of the monkeys were still relatively young at the time of the tests and had not reached the age (25 years) at which AHL begins to be clearly evident with hearing tests, and where CR is most likely to have an effect on auditory function. It would be interesting to evaluate frequency-specific hearing loss, specifically high frequency-specific hearing loss in the control and CR monkeys when they all reach 25-31 years of age, since elevation of high-frequency ABR thresholds is the first sign of AHL [1]. Summaries of dietary regimens used in the monkey studies are given in Table 2.
POTENTIAL MECHANISMS FOR COCHLEAR PROTECTION AND THE DELAY OF AGE-RELATED HEARING LOSS BY CR
The molecular mechanisms by which CR slows the rate of cochlear aging and prevents AHL remain unclear and the specific molecular pathways are unknown. However, there is a growing body of evidence suggesting potential mechanisms for cochlear cell protection and the delay of AHL by CR. The free radical theory of aging postulates that aging is the result of increased or accumulated oxidative damage caused by reactive oxygen species (ROS) [36, 37]. It is now widely accepted that mitochondria are a major source of ROS and a major site of ROS-induced oxidative damage, and that ROS production increases with age [36, 38]. The theory is supported by the observations that over-expressing the mitochondrial antioxidant gene MnSOD [39] or the mitochondrial iron regulator protein frataxin [40] significantly increases longevity in Drosophila, while over-expressing a mitochondrially-targeted catalase gene results in reduced age-related pathology and moderately increased lifespan in mice [41]. Oxidative damage caused by ROS is also postulated to play a role in AHL [4, 21, 22]. Numerous studies have reported that ROS are generated in the cochlea exposed to noise of an intensity that leads to hearing loss [42, 43]. Age-related cochlear hair cell loss is enhanced in mice lacking the antioxidant enzyme Sod1 [44], while mice lacking the antioxidant enzyme Gpx1 or Sod1 show enhanced susceptibility to noise-induced hearing loss [45, 46]. Furthermore, levels of oxidative protein damage increase with age in the cochlea of CBA mice [47]. CR is thought to result in reduced levels of ROS [7-9]. Indeed, many studies have shown that CR reduces oxidative damage to DNA, proteins, and lipids in mammals [7-9, 36]. In mice, most of the age-related increase in oxidative DNA damage occurs in postmitotic tissues such as brain and heart, and most of the attenuation of the damage by CR also occurs in the postmitotic tissues [7-9]. In agreement with this hypothesis, CR reduces levels of mtDNA deletions and delays the onset of AHL in rats [3]. Enhancing antioxidant levels also promotes hair cell survival [48, 49]. Moreover, enhancing antioxidant defenses by supplementation with antioxidants including vitamin E, vitamin C, and melatonin significantly reduces AHL in rats [3]. Collectively, the mechanisms of cochlear cell protection by CR may involve decreased production of ROS with resulting reduction in oxidative damage to nuclear and/or mitochondrial DNA, proteins, or lipids in the aged cochlea (Fig. 1).
Fig. (1).
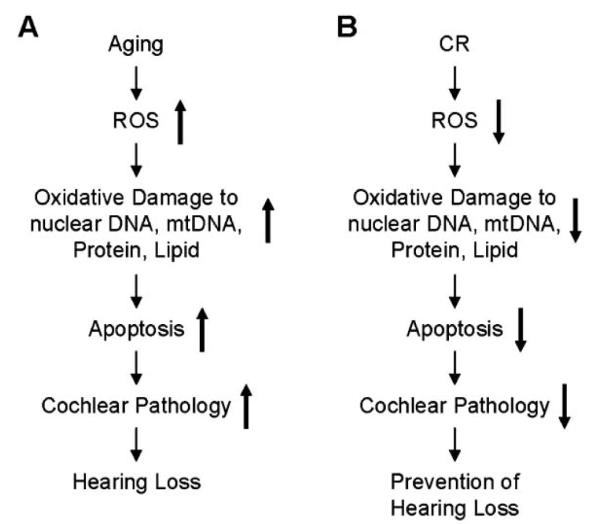
Potential molecular mechanisms by which: (A) aging leads to cochlear cell loss and the onset of AHL and (B) CR prevents cochlear pathology and AHL.
Mitochondria have been postulated to play an important role in mammalian aging [23, 24, 50]. The mitochondrial theory of aging postulates that ROS generated inside mitochondria damage key mitochondrial components such as mtDNA, membranes, and respiratory chain proteins [51]. Such damage accumulates with age, leads to mitochondrial dysfunction and eventually to tissue dysfunction. Mitochondria are the main source of cellular energy, generating most cellular ATP. The mammalian mitochondrial genome consists of 37 genes, encoding 13 proteins of the electron transport oxidative phosphorylation system [24, 52]. Because mtDNA plays essential roles in energy metabolism and cellular apoptosis, mtDNA mutations have been hypothesized to contribute to mammalian aging. Indeed, previous studies have shown that mtDNA point mutations accumulate with aging in humans [53], and that accumulation of mtDNA mutations leads to premature aging in mice [23], indicating a causal role of mtDNA mutations in mammalian aging. It has been postulated that mtDNA mutations may contribute to AHL [23, 24, 54, 55]. In support of this hypothesis, hearing loss is a common symptom in patients harboring inherited mtDNA mutations [56]. Several mutations in the Polg gene (mitochondrial DNA polymerase gamma) have been identified as a cause of human disorders such as Alpers syndrome and deafness [57, 58]. Specific mtDNA point mutations also contribute to mitochondrial disorders in humans such as MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes) and MERRF (myoclonic epilepsy and ragged red fibres), the symptoms of which include hearing loss [54, 55, 59]. Furthermore, more than 100 different deletions of mtDNA have been associated with mitochondrial disorders, and of these, some mtDNA deletions cause Kearns–Sayre syndrome which involves hearing loss [55]. In mice, accumulation of mtDNA mutations leads to the onset of AHL [23, 25]. CR is postulated to protect mtDNA by reducing production of ROS. In support of this hypothesis, CR reduces levels of mtDNA deletions in the skeletal muscle [60] and liver [61] of aged rats. CR also results in reduced levels of mtDNA deletions in the auditory nerve and stria vascularis of the cochlea [3]. Taken together, these studies indicate that the mechanisms of cochlear cell protection by CR may involve reduced levels of mtDNA damage in the aged cochlea (Fig. 1).
ROS-induced oxidative damage is thought to promote programmed cell death or apoptosis [62]. Apoptosis can occur through two major pathways: the intrinsic pathway, also known as the mitochondrial pathway, is initiated when the outer mitochondrial membrane loses its integrity, while the extrinsic pathway is initiated through ligand binding to cell surface receptors [63, 64]. In mammals, mitochondria play a major role in apoptosis that is regulated by Bcl-2 family members. Of the Bcl-2 family members, the proapoptotic proteins Bak and Bax have been proposed to play a central role in promoting mitochondrial-mediated apoptosis. These Bcl-2 proteins promote permeabilization of the outer mitochondrial membrane, leading to cytochrome c release in the cytosol. Growing evidence suggests that an apoptosis program contributes to aging and age-related degenerative diseases [23-25, 62, 65]. In the animal model of Parkinson’s disease, CR lowers symptom severity and levels of apoptosis in neurons [13]. CR also reduces levels of caspase-3 and caspase-9 in the brain of aged rats, suggesting that CR is neuroprotective [66]. We have shown previously that CR prevents AHL in mice, reduces the levels of apoptosis, and reduces the levels of the mitochondrial apoptosis activator Bak in the aged cochlea [17]. Thus, the mechanisms of cochlear cell protection by CR may involve reduced levels of apoptosis (Fig. 1).
CONCLUSIONS
An expanding body of evidence suggests that oxidative damage contributes to the development of Parkinson’s disease, Alzheimer’s disease, and other age-associated neurodegenerative diseases including AHL [4, 7-10, 13]. As discussed earlier, organs such as brain and cochlea which consist of postmitotic cells are particularly susceptible to oxidative damage since most of the aged neurons and any sensory cells are thought not to regenerate in mammals, and extensive cell loss leads to permanent tissue dysfunction. Therefore, we speculate that oxidative damage caused by ROS may play a causal role in AHL leading to cochlear cell loss via apoptosis, and CR may reduce the oxidative damage to nuclear DNA, mtDNA, proteins, and lipids in the cochlea which thereby reduces apoptosis and delays the onset of AHL (Fig. 1).
REFERENCES
- [1].Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- [2].Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The epidemiology of hearing loss study. Am J Epidemiol. 1998;148:879–86. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- [3].Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110:727–38. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- [4].Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res. 2007;226:185–93. doi: 10.1016/j.heares.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [5].Jennings CR, Jones NS. Presbyacusis. J Laryngol Otol. 2001;115:171–8. doi: 10.1258/0022215011906984. [DOI] [PubMed] [Google Scholar]
- [6].Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- [7].Weindruch R, Walford RL. The retardation of aging and diseases by dietary restriction. Charles C Thomas; Springfield: 1988. [Google Scholar]
- [8].Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel deaconess medical center. Caloric intake and aging. N Engl J Med. 1997;337:986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- [11].Fontán-Lozano A, López-Lluch G, Delgado-García JM, Navas P, Carrión AM. Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol Neurobiol. 2008;38:167–77. doi: 10.1007/s12035-008-8040-1. [DOI] [PubMed] [Google Scholar]
- [12].Guarente L. Mitochondria-a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–6. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–9. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- [14].Rezzi S, Martin FP, Shanmuganayagam D, Colman RJ, Nicholson JK, Weindruch R. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp Gerontol. 2009;44:356–62. doi: 10.1016/j.exger.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–33. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- [16].Lipman RD, Dallal GE, Bronson RT. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. J Gerontol A Biol Sci Med Sci. 1999;54:B478–91. doi: 10.1093/gerona/54.11.b478. [DOI] [PubMed] [Google Scholar]
- [17].Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007;28:1613–22. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- [18].Henry KR. Effects of dietary restriction on presbyacusis in the mouse. Audiology. 1986;25:329–37. doi: 10.3109/00206098609078397. [DOI] [PubMed] [Google Scholar]
- [19].Sweet RJ, Price JM, Henry KR. Dietary restriction and presbyacusis: periods of restriction and auditory threshold losses in the CBA/J mouse. Audiology. 1998;27:305–12. doi: 10.3109/00206098809081601. [DOI] [PubMed] [Google Scholar]
- [20].Willott JF, Erway LC, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res. 1995;88:143–55. doi: 10.1016/0378-5955(95)00107-f. [DOI] [PubMed] [Google Scholar]
- [21].Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–8. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- [22].Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–97. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- [23].Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- [24].Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, et al. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol Aging. 2008;29:1080–92. doi: 10.1016/j.neurobiolaging.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechansims. J Neurochem. 2003;84:417–31. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- [27].Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–65. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- [28].Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–8. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Torre P, III, Fowler CG. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta) Hear Res. 2000;142:131–40. doi: 10.1016/s0378-5955(00)00025-3. [DOI] [PubMed] [Google Scholar]
- [30].Fowler CG, Torre P, III, Kemnitz JW. Effects of caloric restriction and aging on the auditory function of rhesus monkeys (Macaca mulatta): the University of Wisconsin study. Hear Res. 2002;169:24–35. doi: 10.1016/s0378-5955(02)00335-0. [DOI] [PubMed] [Google Scholar]
- [31].Torre P, III, Mattison JA, Fowler CG, Lane MA, Roth GS, Ingram DK. Assessment of auditory function in rhesus monkeys (Macaca mulatta): effects of age and calorie restriction. Neurobiol Aging. 2004;25:945–54. doi: 10.1016/j.neurobiolaging.2003.09.006. [DOI] [PubMed] [Google Scholar]
- [32].Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–32. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- [34].Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–6. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- [35].Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–49. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- [36].Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [37].Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [38].Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–36. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- [39].Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–72. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Runko AP, Griswold AJ, Min KT. Overexpression of frataxin in the mitochondria increases resistance to oxidative stress and extends lifespan in Drosophila. FEBS Lett. 2008;582:715–9. doi: 10.1016/j.febslet.2008.01.046. [DOI] [PubMed] [Google Scholar]
- [41].Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- [42].Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–8. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- [43].Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–36. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- [44].McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- [45].Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–54. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 2004;50:2012–8. doi: 10.1373/clinchem.2004.037788. [DOI] [PubMed] [Google Scholar]
- [47].Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–12. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol. 2001;6:117–23. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- [49].Kawamoto K, Sha S-H, Minoda R, Izumikawa M, Kuriyama H, Schacht J, et al. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–81. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- [50].Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [52].Wallace DC, Shoffner JM, Trounce I, Brown MD, Ballinger SW, Corral-Debrinski M, et al. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochim Biophys Acta. 1995;1271:141–51. doi: 10.1016/0925-4439(95)00021-u. [DOI] [PubMed] [Google Scholar]
- [53].Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–9. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- [54].Kokotas H, Petersen MB, Willems PJ. Mitochondrial deafness. Clin Genet. 2007;71:379–91. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- [55].Pickles JO. Mutation in mitochondrial DNA as a cause of presbyacusis. Audiol Neurootol. 2004;9:23–33. doi: 10.1159/000074184. [DOI] [PubMed] [Google Scholar]
- [56].Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, Turnbull DM, et al. The spectrum of hearing loss due to mitochondrial DNA defects. Brain. 2000;123(Pt 1):82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- [57].Mancuso M, Filosto M, Bellan M, Liguori R, Montagna P, Baruzzi A, et al. POLG mutations causing ophthalmoplegia, sensorimotor polyneuropathy, ataxia, and deafness. Neurology. 2004;62:316–8. doi: 10.1212/wnl.62.2.316. [DOI] [PubMed] [Google Scholar]
- [58].Nguyen KV, Østergaard E, Ravn SH, Balslev T, Danielsen ER, Vardag A, et al. POLG mutations in Alpers syndrome. Neurology. 2005;65:1493–5. doi: 10.1212/01.wnl.0000182814.55361.70. [DOI] [PubMed] [Google Scholar]
- [59].Fischel-Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24:303–13. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- [60].Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–81. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- [61].Cassano P, Lezza AM, Leeuwenburgh C, Cantatore P, Gadaleta MN. Measurement of the 4,834-bp mitochondrial DNA deletion level in aging rat liver and brain subjected or not to caloric restriction diet. Ann NY Acad Sci. 2004;1019:269–73. doi: 10.1196/annals.1297.045. [DOI] [PubMed] [Google Scholar]
- [62].Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–95. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [63].Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- [64].Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [66].Shelke RR, Leeuwenburgh C. Lifelong caloric restriction increases expression of apoptosis repressor with a caspase recruitment domain (ARC) in the brain. FASEB J. 2003;17:494–6. doi: 10.1096/fj.02-0803fje. [DOI] [PubMed] [Google Scholar]


