Abstract
Increased tissue permeability is commonly associated with hypoxia of many origins. Since hypoxia-inducible factor (HIF) represents a predominant hypoxia signaling mechanism, we compared hypoxia-elicited changes in tissue barrier function in mice conditionally lacking intestinal epithelial hypoxia-inducible factor-1α (hif1a). Somewhat surprisingly, these studies revealed that mutant hif1a mice were protected from hypoxia-induced increases in intestinal permeability in vivo. Guided by microarray analysis of tisses derived from these mutant hif1a mice, we identified HIF-1-dependent repression of vasodilator-stimulated phosphoprotein (VASP), a molecule with established importance in control of cytoskeletal dynamics, including barrier function. Studies at the mRNA and protein level confirmed hypoxia-elicited repression of VASP in murine tissue, cultured epithelia and endothelia, as well as in human saphenous vein ex vivo. Targeted repression of VASP by siRNA recapitulated our findings with hypoxia, and directed over-expression of VASP abolished hypoxia-induced barrier dysfunction. Studies in the cloned human VASP promoter revealed hypoxia-dependent transcriptional repression, and functional studies by chromatin immunoprecipitation (ChIP) and site directed mutagenesis revealed hypoxia-dependent binding of HIF-1α to the human VASP promoter. These studies identify HIF-1-dependent repression of VASP as a control point for hypoxia-regulated barrier dysfunction.
Keywords: Vasodilator Stimulated Phosphoprotein; Hypoxia; Barrier Function, Actin
Introduction
Limited oxygen availability (hypoxia) is coincident with a variety of inflammatory, cardiovascular and infectious conditions. Among other influences, hypoxia has been shown to increase tissue permeability. Central to an intact barrier is the cytoskeleton, wherein dynamic reorganization of actin filaments control both passive and active movement of solutes across cell monolayers (1). It is currently thought that hypoxia influences barrier properties though formation of stress fibers, and active reorganization of the cytoskeleton in hypoxia (2-4). For example, in alveolar epithelial cells exposure to hypoxia results in decreased actin organization associated with a decreased zonula occludens-1 (ZO-1) protein levels (5). Our own work has demonstrated that as a major actin-binding protein vasodilator stimulated phosphoprotein (VASP) co-localizes with ZO-1 at tight junctions, and plays a key role in establishing and maintaining barrier function (1, 6).
VASP is composed of three major domains: an EVH1 domain, an EVH2 domain, and a central proline-rich region.(7) As VASP contains both actin-binding and actin monomernucelating capabilities, this molecule is of particular interest for the reorganization of cytoskeleton The actin-binding and cross-linking capabilities localize to the COOH-terminal EVH2 domain (8). Loss of VASP function function results in loose associations between epithelia and failure to re-seal barrier following insult (9). In endothelial cells, VASP functions in membrane ruffling, aggregation and tethering of actin filaments during the formation of endothelial cell–substrate and cell-cell contacts. Moreover, VASP expression is increased in endothelial cells during angiogenesis and at most phases involving cell shape change. (10) These previous investigations objectively demonstrate the importance of VASP in development, organization and mobility of the cytoskeleton.
In the present study, we examined basic elements of tissue permeability in hypoxia. As directed by microarray results from conditional hif1a−/− mice, identified VASP as a crucial molecule in the regulation of tissue permeability during hypoxia. Molecular analysis revealed a novel HIF-1α-dependent repression mechanism. Our data show, for the first time, a regulatory pathway for VASP expression involving HIF-1α-regulated tissue permeability during hypoxia.
Materials and Methods
Hif1a−/− mice and exposure to hypoxia
For the production of conditional colon mutants lacking hif1a allele, Fabpl4x at-132/Cre mice were bred as described previously (11). Genotyping of mice was carried out at 8–12 weeks of age as described previously (12, 13). After genotyping, littermate controls and conditional hif1a mutant mice were subjected to environments of normoxia (21% O2) or normobaric hypoxia (8% O2, 92% N2) for 8h. At the beginning of the experiment mice were gavaged with 0.6 mg/g body weight of FITC-dextran (mol wt 4kDa, at a concentration of 80 mg/ml; Sigma-Aldrich) to examine intestinal permeability as described previously (14). Mice were sacrificed and cardiac puncture was performed for serum analysis of FITC-dextran concentration. Organs were harvested for protein and mRNA analysis. All procedures involving animals were performed according to NIH guidelines for use of live animals and were approved by the Institutional Animal Care and Use Committee at Brigham and Women’s Hospital and the University of Colorado Health Sciences Center.
Endothelial Macromolecule Paracellular Permeability Assay
Using a modification of methods previously described (15), HMEC-1 were seeded on polystyrene permeable inserts (0.4-μm pore, 6.5-mm diam; Costar Corp.) and studied 5–7 d after seeding (2–3 d after confluency). Inserts were exposed to normobaric hypoxia (pO2=20 torr) for up to 48h. After this inserts were placed in Hanks buffered saline (HBSS+)-containing wells (1.0 ml), and HBSS+ was added to inserts apically (100 μl). At the start of the assay (t = 0), FITC-labeled dextran 70 kD (concentration 3.5 μM) was added to fluid within the insert. The size of FITC-dextran, 70 kDa, approximates that of human albumin, both of which have been used in similar endothelial paracellular permeability models (16). Fluid from opposing well (reservoir) was sampled (50 μl) over 60 min (t = 20, 40, and 60 min) after exposure to hypoxia for 24h and 48h. Fluorescence intensity of each sample was measured (excitation, 485 nm; emission, 530 nm; Cambrex FLX 800) and FITC-dextran concentrations were determined from standard curves generated by serial dilution of FITC-dextran. Paracellular flux was calculated by linear regression of sample fluorescence (15)
Transcriptional Analysis
Semiquantitative analysis was performed employing real-time PCR RT-PCR (iCycler; Bio-Rad Laboratories Inc.) to examine VASP expression levels in HMEC-1 and T84 cells after confluent cells were exposed to 4, 8, 24 and 48h hypoxia. Primer sets contained 10pM each of the sense primer 5′- GAA AAC CCC CAA GGA TGA AT -3′ and the antisense primer 5′- GGA AGT GGT CAC CGA AGA AG -3′. The primer set was amplified using increasing numbers of cycles of 94°C for 1 min, 60°C for 2 min, 72°C for 4 min, and a final extension of 72°C for 7 min. Samples were controlled for ß-actin using following primers: sense 5′-GGT GGC TTT TAG GAT GGC AAG-3′, antisense 5′-ACT GGA ACG GTG AAG GTG ACA G-3′, 162 bp). After approval by the institutional review board, saphenous vein material was obtained from patients undergoing coronary artery bypass grafting (CABG). Equal portions of dissected vein tissue were subjected to normoxia or hypoxia (pO2 20 torr) for 8h. After hypoxic exposure, RNA was isolated from the tissues and real-time PCR was performed as described above.
Mouse analysis for mRNA levels was performed also employing real-time PCR using sense primer 5- TGG GCT ACA GGC TTG TCA CT -3 and 5- GAG AAG AAA AGC CAC ACA GGT T -3 antisense primer. Mouse ß-actin expression evaluated with: sense 5′-CTC TCC CTC ACG CCA TCC TG-3′, antisense 5′- TCA CGC ACG ATT TCC CTC TCA G-3′.
Human and mouse protein analysis
Cell culture and mouse tissue samples were normalized for protein levels before applying them in non-reducing conditions to SDS containing polyacrylamide gels. Antibodies used for Western blotting included mouse polyclonal anti–VASP (BD Transduction Laboratories) for human VASP analysis and rabbit polyclonal anti-VASP (Alexis Biochemicals) for mouse VASP analysis. Actin was stained using rabbit anti-Actin (Sigma-Aldrich). Blots were washed, and species-matched peroxidase-conjugated secondary antibody was added. Labeled bands from washed blots were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech). Blots were scanned and densitometry analysis performed using NIH Image J Software.
Immunofluorescent staining
HMEC-1 were grown to confluence on acid washed 12 mm glass coverslips. Monolayers were washed once in phosphate buffered saline and fixed for 10 minutes at room temperature in 1% paraformaldehyde in cacodylate buffer (0.1M sodium cacodylate; pH 7.4, 0.72% sucrose). The monolayers were permeabilized for 10 minutes in PBS containing 0.2% Triton X-100 and 3% BSA. After washing twice with PBS, the cells were stained for 1 hour with a monoclonal anti-VASP (12.5 μg/ml, BD Transduction Labs, Lexington, KY). After washing, the monolayers were incubated with goat anti-mouse Oregon Green (1μg/ml, Molecular Probes, Eugene, OR). Nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI, 10μg/ml, Molecular Probes, Eugene, OR). Coverslips were mounted in polyvinylalcohol and viewed at 400x on a fluorescence microscope (Nikon, Melville, NY). Image pixel densities were calculated using Image J (National Institutes of Health, Bethesda, MD).
VASP pGL3- reporter assay
The VASP 5′ flanking region of the human VASP gene was cloned by PCR and ligated into a pGL3 firefly luciferase reporter vector and sequenced with basic pGL3 vector primer GL2. As a control for hypoxia, cells were transfected with a pGL3-based HRE plasmid containing four tandem HIF-1 enhancer sequences from the 3′-region of the erythropoietin gene (17). HMEC-1 and HeLa cells were transfected using standard methods with lipofectamine 2000 for 5h. After transfection, media was changed and cells were subjected to hypoxia or normoxia for 24 h. Luciferase activity was assessed (Turner Designs) using a luciferase assay kit (Promega). All firefly luciferase activity was normalized with respect to the constitutively expressed protein. In a subset of experiments, HIF-1–binding site mutations were introduced in VASP pGL3 reporter plasmids to evulate the functional importance of the HIF-1 α binding. Mutations were performed using the Quickchange in vitro site-directed mutagenesis system (Stratagene). The original sequence in position 22-25 containing the HIF-1 binding site CGTG was mutated to GCCA and the pGL3 vector amplified with Top Ten fast growing bacteria. After extraction from bacteria the pGL3 vector containing the mutation was sequenced again to confirm mutation at the specified site. Again, HeLa cells were transfected with the original VASP pGL3 reporter and the mutated reporter and firefly luciferase assay performed after exposure of cells to 24h of normobaric hypoxia.
Chromatin Immunoprecipitation (ChIP) Assay
HMEC and T84 cells were fixed with 1% paraformaldehyde. Chromatin derived from isolated nuclei was sheared by using a F550 microtip cell sonicator (Fisher Scientific). After centrifugation, supernatants containing sheared chromatin were incubated with an anti-HIF-1α antibody (Novus Biologics, Littleton, CO) or control IgG (OE). Protein A Sepharose was then added, the incubation overnight, and immune complexes subsequently eluted. Complexes were next treated with RNase and proteinase K and were extracted with phenol/chloroform and then with chloroform. DNA was precipitated, washed, dried, resuspended in water, and analyzed by PCR (33 cycles). The primers used in this analysis spanned 165 bp around the putative HIF-1-binding site within the VASP promoter (sense, 5′-CCA CTT CCT CCT CAC CTT CC -3′, and antisense, 5′- TTC TCC CTG AAA TCC CAT CTT -3′).
Results
Analysis of epithelial barrier in mice conditionally lacking intestinal epithelial hif1a
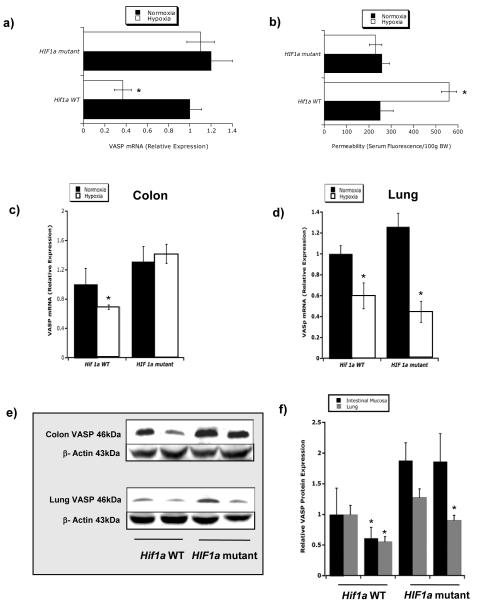
We have previously reported the generation and characterization of conditional hif1a mutant mice (13). These previous studies revealed a protective role for intestinal epithelial HIF-1α in a murine model of colitis. Since epithelial cells are the primary determinant of mucosal barrier function, we compared intestinal permeability to 4kDa FITC dextran in WT and conditional hif1a mutants in response to hypoxia (8hr at 8% O2; 92% N2), a condition we have previously shown to significantly increase intestinal permeability. As shown in Figure 1a, while wild-type animals showed a predictable increase in permeability to fluorescent tracer (p<0.025), conditional hif1a mutant animals were unexpectedly protected from increased intestinal permeability (p = not significant), thereby implicating HIF-1α in increased intestinal permeability during hypoxia.
Figure 1.
Role of epithelial HIF-1α in intestinal permeability a) Serum fluorescence in mice exposed to hypoxia. Quantitation of serum FITC-dextran (per 100g weight) in serum fluorescence of wild-type mice (WT) and conditional hif 1α-null mice exposed to normobaric hypoxia (n = 4 per group). Mice were gavaged with 0.6mg/g 4kDa Fitc-dextran before exposure to hypoxia. b) Epithelial VASP expression pattern in conditional hif1a mutant tissue. Microarray analysis of murine epithelial VASP transcript in conditional hif1a −/− mutant and littermate control animals. Data are expressed as relative VASP mRNA±SD (* indicates p<0.01), where transcript levels in control animals were normalized to 1 and based on internal microarray standards. Results are derived from four animals in each condition. c) VASP mRNA in mice exposed to hypoxia. Analysis in WT and conditional hif1a −/− mice exposed to normoxia and normobaric hypoxia (8% O2, 92% N2 for 8h). Lack of VASP mRNA decrease in conditional hif1a−/− mice in colonic mucosa compared to WT mice. d) VASP mRNA levels in lung e.) Western Blot analysis of VASP protein in colonic scrapings and lung tissue of WT and conditional hif1a −/− mice exposed to normoxia (Nx) and normobaric hypoxia (Hx). Densitometry evaluation of relative VASP expression levels normalized to actin levels in colonic scrapings compared to lung tissue in WT and conditional hif1a−/− mice.
To search for hif1a-dependent genes involved in epithelial barrier regulation, we profiled conditional hif1a mutant animals (n=5) and littermate controls (n=5) subjected to hypoxia (4hr at 8% O2; 92% N2) by microarray analysis. This analysis revealed that while the vast majority of genes (>96%) were unchanged between the animal populations, one barrier-related gene was significantly different between these animal populations. Specifically, these studies revealed that vasodilator-stimulated phosphoprotein (VASP) was significantly repressed by hypoxia in wild-type animals, but not changed in conditional hif1a mutant animals (Figure 1b). We and others have previously identified VASP as an actin-binding protein important in the dynamic regulation of barrier function (18-20), and therefore, we hypothesized that VASP repression in hypoxia represents a HIF-regulated pathway important in barrier regulation. Initially, we verified our microarray findings in conditional hif1a mutant animals. As show in Figure 1c, VASP mRNA levels were significantly reduced in colonic epithelia of hif1a WT (p<0.025), but not in hif1a mutant animals after subjection to hypoxia (p = not significant). Importantly, this difference was reflected only in the Fabpl4x at-132/Cre conditionally deleted tissue (colon, see Figure 1c), and other organs demonstrated a wild-type phenotype with regard to VASP repression in hypoxia, including the lung (Figure 1d), liver and kidney(data not shown).
We next compared VASP protein expression between conditional hif1a mutant animals and littermate controls. As shown in Figure 1e, western blot analysis was utilized to examine VASP expression, and revealed that protein expression paralleled changes in mRNA, wherein wild-type animals expressed significantly less VASP than hif1a mutant littermates, thereby implicating a hif1a-dependent repression of VASP expression. Densitometry verified these results (Figure 1f), reflecting the hypoxia-dependent downregulation of VASP (p<0.05) and the unchanged VASP expression profile in conditional hif1a mutant tissues (p = not significant).
Hypoxia-dependent repression of VASP in human tissue and in human cell lines
We next extended our findings in the mouse to examine whether hypoxia similarly regulates human VASP expression. First, we addressed whether hypoxia influenced VASP expression in human saphenous vein ex vivo. As shown in Figure 2a, explants of human saphenous vein exposed to hypoxia (pO2 20 torr for 8h) resulted in a 56±11% decrease in VASP mRNA expression relative to actin controls (p<0.025). Second, we utilized two individual barrier cell types, namely human microvascular endothelia (HMEC-1) and human colonic epithelia (T84 cells) to examine VASP expression in hypoxia. As shown in Figure 2b, exposure of T84 and HMEC-1 to a time course of hypoxia (0 – 48h) revealed a sustained and significant decrease in VASP mRNA for both cell lines examined (p<0.025 by ANOVA).
Figure 2.
Influence of hypoxia on human VASP expression and function. a) Human saphenous vein was exposed to normoxia (pO2 147 torr, 8h) or hypoxia (pO2 20 torr, 8h) and VASP mRNA was evaluated by real-time PCR (n=6). b) VASP expression in cell culture in hypoxia. VASP mRNA expression levels were evaluated by real-time PCR in HMEC-1 and T84 cells exposed to normoxia or hypoxia for indicated periods of time (n=4). In panels c and d, HMEC-1 were grown to confluence on glass coverslips, exposed to a 24 h period of normoxia (panel c) or hypoxia (panel d) and VASP was localized by immunofluorescence (green) with DAPI counterstain (blue). Pictures were taken at 400x. e) Western blot analysis of VASP expression in hypoxia. VASP western blot analysis in HMEC and T84 cells exposed to normobaric hypoxia d.) Paracellular Flux across HMEC in hypoxia. Passive Flux evaluated with 70 kD FITC-dextran of HMEC-1 plated on polystyrene transwell inserts exposed to normobaric hypoxia (1% O2, 99% N2) for 24h and 48h, as indicated. Data are expressed as mean±SD (n=8).
Localization of VASP by immunofluorescence in HMEC-1 revealed a prominent loss of VASP (63±11% decrease in pixel density, n = 14, p<0.025) in hypoxia relative to normoxia (Figure 2c and 2d). A confirmatory decrease in VASP protein expression by western blot was evident in both HMEC-1 and in T84 cells (Figure 2c), suggesting that similar to our findings in murine models in vivo (Figure 1), hypoxia represses VASP protein and mRNA expression in human cells and tissues.
Impact of VASP expression on barrier function
We next sought to determine whether a functional cause and effect relationship exists between hypoxia and VASP repression. First, we determined whether conditions which repress VASP protein (i.e. hypoxia, see Figure 2d and 2e) influence permeability in human cells in vitro. For these purposes, we exposed HMEC-1 to hypoxia (0 -48h) and examined paracellular permeability to FITC-labelled dextran. As shown in Figure 2f, increasing time points of hypoxia increased paracellular permeability in a time-dependent manner (p<0.01 by ANOVA).
We next induced repression of VASP through use of siRNA and examined changes in permeability (i.e. independent of hypoxia). As shown in Figure 3a, loading of HMEC-1 with VASP siRNA, but not a non-specific control siRNA, decreased VASP mRNA and protein by nearly 80%. Examination of permeability in these cells (Figure 3b) revealed a 68±8% increased in paracellular permeability (p<0.025 compared to control monolayers).
Figure 3.
Influence of VASP repression and over-expression on permeability. a) HMEC-1 were grown on polystyrene permeable transwells, loaded with VASP siRNA and examined for protein (upper bands) and mRNA (lower bands). b) passive flux evaluated with 70 kD-FITC Dextran across the endothelial monolayers on day 2 post transfection. c) HMEC were transfected with empty plasmid or VASP CMV plasmid and exposed to normoxia or normobaric hypoxia (24h). Shown is a representative western blot for VASP and actin loading control. d) Analysis of paracellular permeability in HMEC-1 over-expressing VASP. Transfected HMEC-1 were plated on membrane permeable supports, exposed to normoxia or hypoxia and examined for permeability to 70kDa FITC dextran. Results are presented as the mean±st. dev. percent change in permeability relative to normoxia control, where * indicates significant increase in permeability (p<0.025 compared to control) and # indicates significant decrease in permeability (p<0.05 compared to control), where n=8 HMEC-1 monolayers per condition.
Third, we determined whether forced over-expression of VASP might protect against hypoxia-elicited increases in permeability. To do this, we transfected HMEC-1 with VASP cDNA on a heterologous viral promoter (CMV). Such transient transfections resulted in a nearly 6-fold increase in VASP protein expression over mock transfected controls (Figure 3c), with no significant changes associated with hypoxia. Using these cells, we examined the influence of hypoxia on paracellular permeability to 70kDa FITC-dextran. As shown in Figure 3d, forced over-expression of VASP in HMEC-1 resulted in a 22±5% decrease in baseline permeability compared to mock transfected controls (p<0.05). Moreover, when exposed to hypoxia, cells over-expressing VASP were protected from hypoxia-induced changes in permeability (Figure 3d). Indeed, while hypoxia increased permeability of control transfected cells by 40±6% (p<0.025, Figure 3d), cells transfected with VASP and exposed to hypoxia showed 34±6% decrease in permeability (p<0.05). Taken together, these findings suggest that VASP is a limiting factor for endothelial permeability during episodes of hypoxia.
Cloning of the VASP promoter and role of HIF in repression during hypoxia
We next examined mechanisms of VASP repression by hypoxia. In view of the likelihood of a transcription-mediated repression of VASP during hypoxia, attention was directed at the 5′-region of the VASP gene for potential hypoxia regulated transcription factor sequences. Available public databases (21) and analysis of full-length cDNA [Genbank NM_003370] identified the transcription start site of VASP at position –260 relative to the first codon. Based on these reports, we cloned a genomic fragment extending from positions -975 to +238 (Figure 4a) into pGL3 luciferase reporter vector and examined hypoxia-dependent activity (24 h hypoxia) following transient transfection in HMEC-1 and in HeLa cells. Consistent with mRNA and protein analysis (Figure 3), VASP promoter analysis revealed transcriptional repression in response to hypoxia. As shown in Figure 4b, transient transfection in HMEC-1 and HeLa cell resulted in a 62±5% and 64±10% loss of activity in hypoxia (p<0.025 for both), respectively, thus implicating transcriptional repression of the VASP promoter by hypoxia.
Figure 4.
VASP promoter analysis and HIF-1α binding analysis. a) Map of cloned VASP construct utilized here. Relative positions of each clone are annotated, as well as the transcription start site (TSS). b) HMEC-1 or HeLa cells, as indicated, were transfected with human VASP pGL3 reporter vector and exposed to normoxia or normobaric hypoxia (1% O2, 99% N2) for 24 hours post transfection (n=4). Results are presented as relative luciferase activity±sem, where * indicates p<0.025). c) ChIP assay analysis performed with HIF-1α antibody in HMEC-1 and T84 exposed to 24h normobaric hypoxia. d) Site-directed mutagenesis of HIF-1α binding site located at position - 522 to – 519 of pGL3 reporter construct shows attenuated VASP downregulation during hypoxia (n=4). Results are presented as relative luciferase activity±sem, where * indicates p<0.025). Also shown is a positive control plasmid encoding the HRE from the erythropoietin gene (EPO HRE).
Analysis of our cloned region of VASP revealed the existence of a potential binding site for HIF-1α (core sequence 5′-CGTG-3′) at position –522 to –519 relative to the transcription start site and a HIF-1 ancillary sequence (22) located 12 base pairs in the 3′ direction. Based on these findings, and recent studies indicating that HIF can function as a transcriptional repressor (23-25), we determined whether HIF-1α would bind the VASP promoter. For these purposes, we utilized ChIP analysis to examine binding of HIF-1α to the VASP promoter spanning the putative HIF-1α binding site in intact cells. As shown in Figure 4c, this analysis in two separate cell lines (HMEC-1 and T84) revealed a prominent band of 165 bp in nuclei derived from hypoxic, but not normoxic cells. No bands were evident in the beads only or H20 controls and pre-immunoprecipitation samples revealed equivalent DNA input, thereby indicating specific binding of HIF-1α to the proximal VASP promoter.
To determine the functional implications of the HIF-1α binding site in the VASP promoter, site-directed mutagenesis was employed. Here, we targeted the central HIFα binding site in cloned promoter (see Figure 4a), and as shown in Figure 4d, a three nucleotide mutation (ΔHIF consensus motif 5−- CACGTGG -3′ mutated to 5′- CAATCGG -3′ within HIF-1 site) resulted in a complete loss of repression. Indeed, hypoxia-dependent promoter activity was repressed by 53±6% in the wild-type construct (p<0.01 compared to normoxia) and induced by 26±7% in the ΔHIF construct (p = not significant compared to normoxia). As a HIF reference control, cells were transfected with a plasmid expressing the HRE from the 3′ region of the EPO (termed HRE), and revealed a 46±6 fold increase in activity with exposure to hypoxia (p<0.01 compared to normoxia). Taken together, these DNA binding and mutagenesis studies strongly suggest that the central HIFα site is critical for hypoxia-dependent repression of VASP.
Discussion
Dynamic organization of the cytoskeleton is critical to barrier maintenance and function (26). Guided by results from microarray analysis, we identified HIF-regulated expression of VASP in vivo as a regulatory mechanism of tissue permeability during hypoxia. VASP is a key actin-binding protein with demonstrated importance in junctional reorganization. Extensions of these studies in vitro and with epithelial conditional hif1a −/− mice revealed that HIF-dependent repression of VASP contributes to increased tissue permeability observed in various hypoxic models.
At the tissue and cellular level, HIF mediates the expression of an array of genes pivotal to survival in low oxygen states (27, 28). Consensus HIF binding sequences termed hypoxia responsive enhancers / elements (HRE) have been identified in a growing number of hypoxia responsive genes (28), some of which directly impact epithelial barrier function. In this regard, initial studies from our laboratory revealed that barrier function in cell lines derived from different tissues may be influenced by hypoxia to different degrees, with cell lines derived from colonic tissue being the most resistant (29). These studies indicated the differential expression of barrier protective genes in intestinal tissue and candidate genes for this effect were consecutively identified in ITF (29), CD73 (30), adenosine receptors (31) and MDR-1 (32). These studies, and others, have implicated HIF-1α as an adaptive barrier mechanism for both epithelial and endothelial cells. Notable, while increased permeability is widely observed in tissues subjected to hypoxic stress, less is know about direct mechanisms for such permeability changes. Several lines of evidence implicate HIF-dependent VASP repression as a component to increased tissue permeability during hypoxia. First, epithelial conditional hif1a−/− mice are generally resistant to increased permeability induced by hypoxia. Second, hypoxia-induced VASP repression was demonstrable in murine tissue, human tissue and various human cell lines. Third, molecular manipulation of VASP expression (e.g. siRNA and heterologous plasmid expression) independent of hypoxia resulted in a predictable change in barrier function. Fourth, cloning of the VASP promoter identified HIF-1α binding and function at the transcriptional level. Thus, it is likely that HIF-dependent VASP regulation contributes, at least in part, to changes in tissue permeability associated with hypoxia.
VASP plays a central role in mediating actin dynamics within endothelial and epithelial cells and is involved in stress fiber formation (10). In endothelial and epithelial formation of stress fibers or destruction of cytoskeletal structures is associated with an increase in permeability (5, 33, 34). In addition, passive cell retraction as a result of cytoskeleton rearrangement and attenuation of cell-cell and cell-substrate junctions also plays a key role in mediating cellular contractile response and changes of paracellular permeability (35, 36). This rearrangement transposes its force on cell cell junctions through indirect attachment of actin fibers with tight and adherens junctions. Previous investigations have focused on demonstrating influence of hypoxia on cytoskeletal arrangement and development, but focused primarily on the influence of this in the central nervous system (37-39). Chemically induced hypoxia through ATP-depletion results in disruption of the actin cytoskeleton in renal epithelial cells. Ankyrin and spectrin are dissociated from the actin cytoskeleton in low ATP states causing intracellular redistribution.(40-42) A redistribution of actin in cells exposed to chemical hypoxia persist longer than 3 hours and is associated with increased paracellular flux.(43) In addition, ischemia is a cause for cells to loose their polarity, to open tight junctions and as a result increase paracellular permeability.(44) ZO-1 plays a critical role in tight junction complex assembly. Modification of ZO-1 and other tight junctional protein expression leads to increased paracellular permeability.(5, 45, 46) This shows that the regulation of the cytoskeletal proteins during hypoxia can have a direct influence on barrier properties of endothelial and epithelial cells and as such are consistent with a decreased VASP expression (5).
Taken together, our results demonstrate a fundamental control process for VASP as a central mechanism of barrier regulation. Efforts to better understand a possible beneficial role of VASP in barrier function could result in therapeutic modalities for a variety of disease processes that are associated with impaired barrier function.
References
- 1.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in protein kinase A-induced changes in endothelial junctional permeability. Faseb J. 2002 doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- 2.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L749–760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- 3.Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1173–1182. doi: 10.1152/ajplung.00309.2005. [DOI] [PubMed] [Google Scholar]
- 4.Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem. 2002;277:42596–42602. doi: 10.1074/jbc.M205863200. [DOI] [PubMed] [Google Scholar]
- 5.Bouvry D, Planes C, Malbert-Colas L, Escabasse V, Clerici C. Hypoxia-induced Cytoskeleton Disruption in Alveolar Epithelial Cells. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2005-0478OC. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol. 2002;282:C1235–1245. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 7.Haffner C, Jarchau T, Reinhard M, Hoppe J, Lohmann SM, Walter U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. Embo J. 1995;14:19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- 9.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 10.Price CJ, Brindle NP. Vasodilator-stimulated phosphoprotein is involved in stress-fiber and membrane ruffle formation in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:2051–2056. doi: 10.1161/01.atv.20.9.2051. [DOI] [PubMed] [Google Scholar]
- 11.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- 12.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-Adenosine Monophosphate Promotes Endothelial Barrier Function via CD73-mediated Conversion to Adenosine and Endothelial A2B Receptor Activation. J. Exp. Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno-Yagyu Y, Hashida R, Mineo C, Ikegami S, Ohkuma S, Takano T. Effect of PGI2 on transcellular transport of fluorescein dextran through an arterial endothelial monolayer. Biochem Pharmacol. 1987;36:3809–3813. doi: 10.1016/0006-2952(87)90442-4. [DOI] [PubMed] [Google Scholar]
- 17.Sheta EA, Trout H, Gildea JJ, Harding MA, Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene. 2001;20:7624–7634. doi: 10.1038/sj.onc.1204972. [DOI] [PubMed] [Google Scholar]
- 18.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. Faseb J. 2002;16:583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol. 2002;282:C1235–1245. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 20.Sporbert A, Mertsch K, Smolenski A, Haseloff RF, Schonfelder G, Paul M, Ruth P, Walter U, Blasig IE. Phosphorylation of vasodilator-stimulated phosphoprotein: a consequence of nitric oxide- and cGMP-mediated signal transduction in brain capillary endothelial cells and astrocytes. Brain Res Mol Brain Res. 1999;67:258–266. doi: 10.1016/s0169-328x(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita R, Suzuki Y, Wakaguri H, Tsuitani K, Nakai K, Sugano S. DBTSS: DataBase of Human Transcription Start Sites, progress report 2007. Large-scale collection and characterization of promoters of human and mouse genes 5′-end SAGE for the analysis of transcriptional start sites. Nucleic Acids Res. 2006;34:D86–89. doi: 10.1093/nar/gkj129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura H, Weisz A, Ogura T, Hitomi Y, Kurashima Y, Hashimoto K, D’Acquisto F, Makuuchi M, Esumi H. Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J Biol Chem. 2001;276:2292–2298. doi: 10.1074/jbc.M008398200. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibla JC, Khoury J, Kong T, Robinson A, Colgan SP. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2006;291:C282–289. doi: 10.1152/ajpcell.00564.2005. Epub 2006 Mar 2029. [DOI] [PubMed] [Google Scholar]
- 25.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2004;16:16. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 26.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe PJ, O’Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J. Exp. Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 29.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford KM, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Ex. Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 (HIF-1) mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 32.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 33.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128:96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- 34.Wu NZ, Baldwin AL. Transient venular permeability increase and endothelial gap formation induced by histamine. Am J Physiol. 1992;262:H1238–1247. doi: 10.1152/ajpheart.1992.262.4.H1238. [DOI] [PubMed] [Google Scholar]
- 35.Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22:309–315. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- 36.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu RJ, Bennett V. In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s) J Biol Chem. 1991;266:18200–18205. [PubMed] [Google Scholar]
- 38.Lee A, Morrow JS, Fowler VM. Caspase remodeling of the spectrin membrane skeleton during lens development and aging. J Biol Chem. 2001;276:20735–20742. doi: 10.1074/jbc.M009723200. [DOI] [PubMed] [Google Scholar]
- 39.Roberts-Lewis JM, Siman R. Spectrin proteolysis in the hippocampus: a biochemical marker for neuronal injury and neuroprotection. Ann N Y Acad Sci. 1993;679:78–86. doi: 10.1111/j.1749-6632.1993.tb18290.x. [DOI] [PubMed] [Google Scholar]
- 40.Doctor RB, Bennett V, Mandel LJ. Degradation of spectrin and ankyrin in the ischemic rat kidney. Am J Physiol. 1993;264:C1003–1013. doi: 10.1152/ajpcell.1993.264.4.C1003. [DOI] [PubMed] [Google Scholar]
- 41.Molitoris BA, Dahl R, Hosford M. Cellular ATP depletion induces disruption of the spectrin cytoskeletal network. Am J Physiol. 1996;271:F790–798. doi: 10.1152/ajprenal.1996.271.4.F790. [DOI] [PubMed] [Google Scholar]
- 42.Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]
- 43.Kwon O, Phillips CL, Molitoris BA. Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am J Physiol Renal Physiol. 2002;282:F1012–1019. doi: 10.1152/ajprenal.00294.2001. [DOI] [PubMed] [Google Scholar]
- 44.Molitoris BA. Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton. Am J Physiol. 1991;260:F769–778. doi: 10.1152/ajprenal.1991.260.6.F769. [DOI] [PubMed] [Google Scholar]
- 45.Cavanaugh KJ, Jr., Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–591. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]