Abstract
Visual acuity and motion perception are degraded during head movements unless the eyes counter-rotate so as to stabilize the line of sight and the retinal image. The vestibulo-ocular reflex (VOR) is assumed to produce this ocular counter-rotation. Consistent with this assumption, oscillopsia is a common complaint of patients with bilateral vestibular weakness.
Shanidze et al. (2010b) described compensatory eye movements in normal guinea pigs that appear to anticipate self-generated head movements. These responses effectively stabilize gaze and occur independently of the vestibular system. These new findings suggest that the VOR stabilizes gaze during passive perturbations of the head in space, but anticipatory responses may supplement or even supplant the VOR during actively generated head movements. This report reviews these findings, potential neurophysiological mechanisms, and their potential application to human clinical treatment of patients with vestibular disease.
Keywords: vestibulo-ocular reflex (VOR), efference copy, cervico-ocular reflex (COR), vestibular nuclei, oscillopsia, guinea pigs
Introduction
The most primitive eye movements were initiated by the semicircular canals in fish (the vestibulo-ocular reflex or VOR) so as to stabilize retinal images with respect to the environment (reviewed by Walls1). In its most basic form, the VOR is a 3-neuron reflex arc linking the labyrinths to the extraocular muscles of the eyes2. Because of its relatively large bandwidth and short latency, the VOR is ideally suited to compensate for unexpected perturbations of the head in space, and it is this aspect of VOR function that is most often tested in the laboratory or clinic using passive whole body rotations of animal or human subjects. In contrast to this passive stimulus, in more natural environments animals move voluntarily and, more specifically, move their heads relative to their bodies or their bodies in space. For example, primates typically shift their direction of gaze by making a saccade to an eccentric spatial location and then rotating their heads in the same direction3. Since the desired gaze direction is often achieved with the saccade (unless the target is very eccentric, >20 degrees), the eyes must counter-rotate during the head turn in order to stabilize the retinal image and maintain the newly acquired direction of gaze. Although some of the experimental findings are controversial, it is probable that the VOR plays a significant role in the ocular counter rotation that stabilizes the retinal image during coordinated eye-head gaze shifts4–6.
Unlike the primate, the guinea pig’s retina is relatively homogeneous and consists primarily of rods7,8. Thus, guinea pigs have poor visual acuity9 and the term “gaze direction” is ambiguous for this animal since their vision is effectively panoramic (guinea pigs do have a horizontally aligned visual streak that they use to detect the horizon8). Despite their relatively poor acuity, retinal image stability would enhance vision and, more importantly, enable these afoveate animals to distinguish the motion of objects in their environment from retinal image motion produced by self-motion1,10. Thus, guinea pigs do exhibit a VOR in response to passive whole body rotation in the dark and in the light11,12. However, when tested in the laboratory, in the dark, with passive rotations, the guinea pig’s VOR compensates for only about 50% of the animal’s passively induced head movement12. During more natural, self-generated head movements, guinea pigs generate significantly larger compensatory eye movements, which compensate on average for 81% of head rotational speed and, in some instances, achieve 100% compensation13. A description of this remarkable behavior is the focus of this report.
Methods
Experimental and surgical procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan’s University Committee on Use and Care of Animals.
Data were obtained from five guinea pigs (4 pigmented, 1 albino), some of which were used in the study of passive responses described in a previous report12. The methods were identical to those of that report, where their descriptions can be found in greater detail.
For this study, the trunks of the guinea pigs were restrained within the experimental apparatus. However, the animals’ heads were free to move and there were frequent bursts of self-generated head movements. Although some of these movements appeared to be irritative, i.e., rapid shaking of the head, most were exploratory movements during which the animal smelled, chewed or oriented toward some feature of the experimental environment. All analyses of active movements included in this report occurred in the absence of any passive rotational stimulus and all occurred in the dark, unless described otherwise.
During the experiment, fully awake animals were restrained and placed on a servo-controlled turntable (Neurokinetics, Inc, Pittsburgh, PA). The restraint consisted of a polycarbonate box, which had an adjustable width that could be comfortably adjusted to fit the animal’s trunk. The animal’s body position was fixed relative to the turntable via the restraint box, but its head was able to move freely. Eye and head movements were recorded using the electromagnetic search coil technique12,14. A Primelec search coil system (D. Florin, Ostring, Sw; model CS681) generated three orthogonal electromagnetic fields about the guinea pig. The Primalec field coils were stationary relative to the world. With this configuration, measured eye and head movement signals were eye-in-space and head-in-space relative to the earth earth-fixed coordinate frame established by the field coils.
Eye position and head position data were each sampled at 1,000 Hz by a dedicated data acquisition system (CED Power 1401, Cambridge Electronic Design, Cambridge, UK). Data were analyzed offline using custom software written in the Spike 2 (Cambridge Electronic Design) and MATLAB (The MathWorks, Inc, Natick, MA) environments. A smoothing filter was applied to all acquired position channels and eye and head velocity were computed by differentiating the position data.
Two broad strategies were employed to analyze active head movements. First, segments of active head movements (>5 msec in duration) were selected by a software algorithm using two criteria: that no passive stimulus was present (intervals of active head movements were not predictable and were interspersed with passive body rotations) and head speed exceeded 5 deg/sec. Each segment was analyzed to compute the gain of the compensatory response by regression of eye-in-head against head-in-space velocity. The latency of the compensatory eye response was determined by cross correlating these variables over the same data segments. The latency was assumed to be the lag associated with the maximum correlation coefficient. Alternatively, some data segments were selected by one of the authors during which discrete head and eye movements occurred and which approximated the speeds and accelerations of the passive whole body transient perturbations analyzed in a previous report12. Each of these segments was analyzed for gain and latency using the same approach described above for the automated analysis. For those segments, brief intervals containing rapid eye movements were excluded from the analysis. About 150 minutes total of experimental time in the intact animals was included, from which 31 minutes were selected as being long enough with sufficient head movement activity to warrant analysis.
Results
Figure 1A shows a typical example of a guinea pig abruptly rotated from 0 to 90 degrees/sec in ~100 msec. The eye-in-head trace is inverted to clearly show the delayed response of the animal’s VOR during this trial (~10 msec). The lowermost trace shows that eye velocity relative to the world is reduced by the VOR to about 30% of the head speed. Figure 1B shows the linear relationship of eye-in-head to head-in-space speed during the initial portion of the response; the regression line has a slope of −0.71 and is highly significant (R2 >0.99). This compensatory response is comparable to the passive VOR responses we reported in a prior study12.
Figure 1.
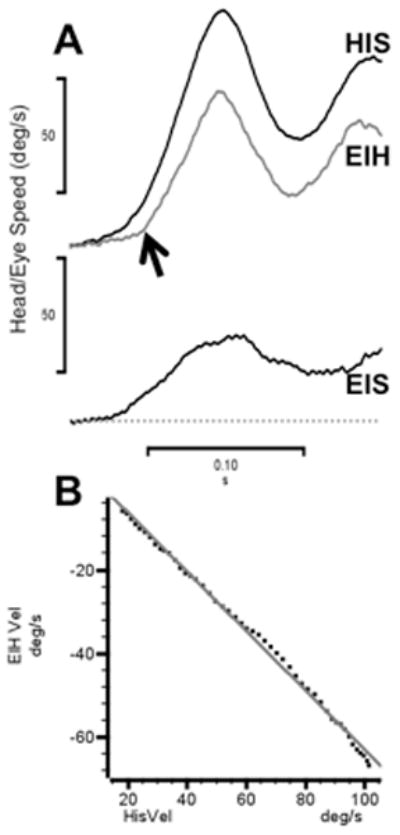
During passive rotation the guinea pig’s VOR is delayed in time and fails to compensate fully for head velocity. A. Head-in-space velocity (black trace, HIS) and eye-in-head velocity (gray trace, EIH) evoked by an abrupt passive whole body rotation. Lowermost trace is head-in-space velocity (EIS). B. Linear regression of EIH and HIS (data from panel A) in the time interval following the quick phase eye movement. The best-fit regression is EIH = 8.1±0.43–0.71±0.006 * HIS (R2 ≥ 0.99).
When the same animal generates active head turns, the compensatory eye movement is larger and produces significantly more retinal stability than is seen in that animal during passive whole body rotations. Figure 2 shows two typical examples of this behavior. In Figure 2A the animal reorients itself and makes a rapid (~150 deg/sec) head turn to the right. In near synchrony with the head movement, the eye counter-rotates and then executes an anti-compensatory quick phase eye movement in the same direction as the head movement. The quick phase is followed by additional ocular counter-rotation that compensates for the on-going head turn. The lowermost trace shows that the eye-in-space speed is essentially zero throughout the rapid head turn except during the quick phase anti-compensatory eye movement. This observation is confirmed quantitatively by the linear regression data illustrated in Figure 2B. During the head turn that follows the quick phase, the ocular counter-rotation compensated for ~85% of the head speed (regression slope = −0.85). Furthermore, the latency of this response as computed by waveform cross correlation was −1.0 msec. A longer sequence of active head turns made by the same animal is illustrated in Figure 2C. In this example, the eye-in-head trace (gray) is inverted to illustrate how accurately the compensatory response mirrors the head-in space velocity (black) in amplitude and in temporal synchrony. Figure 2D shows the linear regression of eye speed relative to head speed; for this example the ocular counter-rotation is 100% compensatory (slope = −1.0) and the latency is 0.0 msec.
Figure 2.
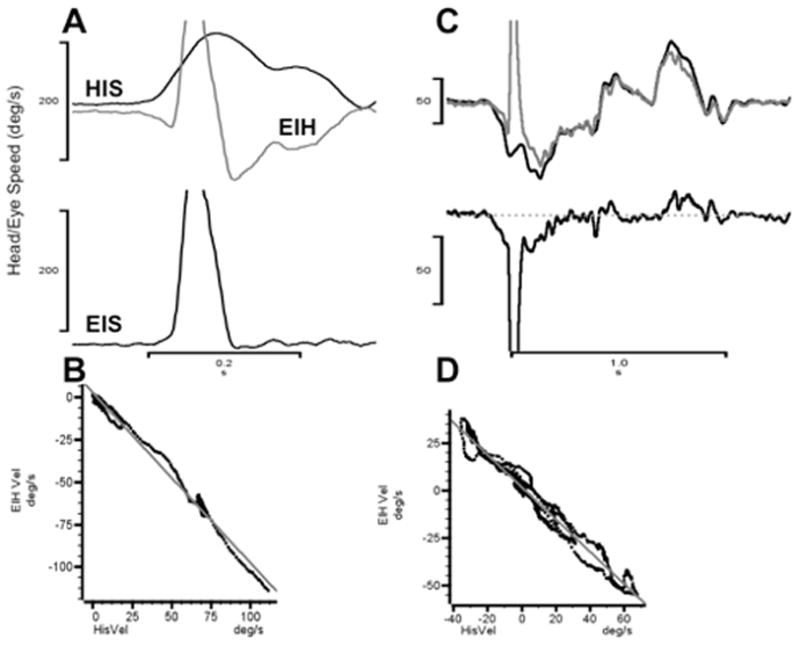
Anticipatory responses during self-generated head movements. A. Brief rightward head turn. Traces labeled as in Figure 1. B. Linear regression of EIH and HIS (data from panel A) following the quick phase eye movement. The best-fit regression is EIH = 1.8±0.15–0.85±0.005 * HIS (R2 ≥ 0.99). C. Self-generated sequence of head movements. In this panel, the EIH trace (gray) is inverted to show how closely it matches HIS velocity temporally and in amplitude. D. Linear regression of EIH and HIS (data from panel C) following the quick phase eye movement. The best-fit regression is EIH = 2.6±0.35–1.00±0.007 * HIS (R2 ≥ 0.99).
Figure 3A shows the distribution of latencies for 74 segments of anticipatory responses to self-generated head movements in 3 animals13. To compute response latency, cross correlations of eye-in-head and head-in-space velocities were performed. The mean anticipatory latency was 0.1±2.5 msec (standard deviation). To ensure that segment lengths were not confounding the results, a regression of segment length to lag was performed and no relationship was found (R2 = 0.029). Additionally, the 74 segments were broken up into 25 msec long intervals, analyzed for latency and binned. The procedure confirmed the results shown in Figure 3 (mean −0.2±0.27 msec, n = 47,827). Most of the computed latencies were less than zero, verifying the anticipatory nature of the responses illustrated in Figure 2.
Figure 3.
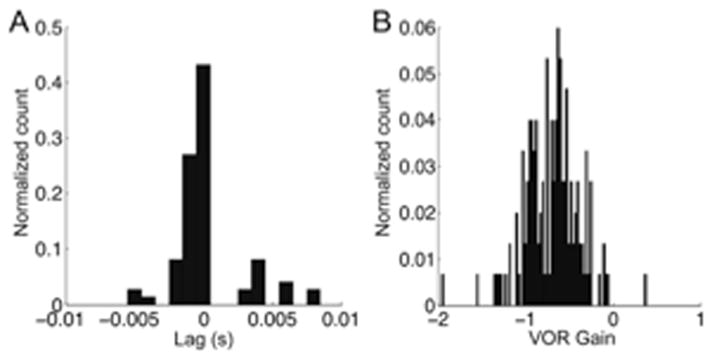
A. Distribution of eye movement latencies (lags) associated with self-generated head movements. B. Distribution of regression slopes (compensatory gain) of eye versus head velocity associated with self-generated head movements. The “normalized count” is the count of items in each bin divided by the total number of counts. From Shanidze et al. 13.
We analyzed the 74 data segments further to establish the relationship of head and eye velocity. Eye and head velocity were recorded for multiple segments of active head movement that occurred in the absence of passive rotation. Linear regressions of eye-in-head versus head-in-space velocity were done for consecutive points of each active head movement segment. Segments for which no valid cross correlation value could be found were excluded from the analysis. Figure 3B shows the resultant distribution of slope values. Statistically, the slopes were normally distributed with a mean of −0.80, i.e., the anticipatory movements compensated on average for 80% of head velocity. To determine if there was a relationship between response gain and latency, the gain values were re-plotted subject to certain conditions. The data set was divided into two distributions one with only gain values associated with lags less than 2 msec (n = 61) and another with values associated with lags greater than or equal to 2 msec. The mean of the smaller lag subset was slightly higher (−0.81± 0.23) than that of the other subset (mean = −0.74 ± 0.19), but the difference was not statistically significant.
Discussion
In a previous report, we showed that anticipatory responses similar to those illustrated in Figure 2 occurred in animals with complete bilateral peripheral vestibular lesions13. Those results and the near-zero or negative latency of the anticipatory responses (Figure 3A) argue for an extra vestibular origin for these remarkable gaze stabilizing eye movements. The two most likely extra vestibular sources for the observed responses are proprioceptive inputs from the neck and/or an efference copy signal related to the voluntary head movements15. Studies in non-human primates have demonstrated the presence of signals, encoded by the firing rates of vestibular neurons, which were related to head rotation relative to the trunk5,16–18. Many of these same vestibular neurons also receive monosynaptic vestibular inputs. Furthermore, Cullen and her colleagues have shown that presumptive proprioceptive inputs related to head on body rotations reach secondary vestibular neurons in the VOR pathway where they are apparently masked in the intact monkey19–21. This finding and the short latency of the anticipatory responses argue against, but do not exclude, a proprioceptive origin for the responses observed in guinea pigs. For example, our latency measurement is relative to the onset of head movement, but the EMG activity of neck muscles is likely to precede our estimate of the initiation of the head movement; thus a response, initiated by proprioceptive inflow, might be seen to have zero latency when measured behaviorally.
Efference copy signals related to voluntary head movements could also produce anticipatory responses. Signals linked to voluntary head/body movements have been described in the intact monkey’s VOR pathway3,4,21,22. Dichgans et al.23 reported accurate compensatory responses in labyrinthectomized monkeys during active head movements and those responses persisted after surgical lesions that interrupted neck proprioception. Dichgans’ experiments showed that efference copy signals could produce compensatory responses in animals with vestibular lesions, and that these responses could be pre-programmed23. Our results, which illustrate similar anticipatory responses in intact guinea pigs, are a significant extension of these previous studies. One can no longer assume that the VOR is the only, or even the principle, mechanism by which retinal image stability is achieved during voluntary head movements in an animal with an intact vestibular system – regardless of what is ultimately shown to be the source of the anticipatory response.
These findings could have important clinical implications if anticipatory responses that compensate for voluntary head movements can be demonstrated in humans. For example, patients with bilateral vestibular weakness typically experience oscillopsia (perceived motion of the environment) as a result of retinal instability during movement24–26, suggesting that human anticipatory responses, if present, incompletely compensate for head movements. In contrast, the data presented in this report imply that oscillopsia should be minimal in guinea pigs given the robustness of their anticipatory responses, even in animals with complete bilateral vestibular lesions.
Diagnostic vestibular tests rely on passive rotation of patients whose heads are supported relative to the body. Anticipatory responses in humans cannot be detected using this test protocol; instead, patients must be instructed to rotate their heads voluntarily. In a study of labyrinthine deficit patients (who had no response bilaterally to caloric irrigation), patients produced larger compensatory responses during active eye-head tracking than during passive rotations; behavior consistent with an extra vestibular mechanism such as an anticipatory response27. This study was later extended and confirmed by Waterston et al.28. These reports suggest that human patients with bilateral vestibular disease can produce eye movements that are at least partially compensatory for self-generated head movements. However, to the best of our knowledge, the origin of these responses has not been systematically studied in normal subjects or in patients, and it is not known if their origin is the cervico-ocular reflex29,30 or efference copy-driven eye movements that anticipate planned head movements. Regardless of their origin(s), these compensatory responses should be subjected to further study to determine why they appear to be less than fully compensatory and how they can be enhanced in labyrinthine deficient patients.
Figure 4.
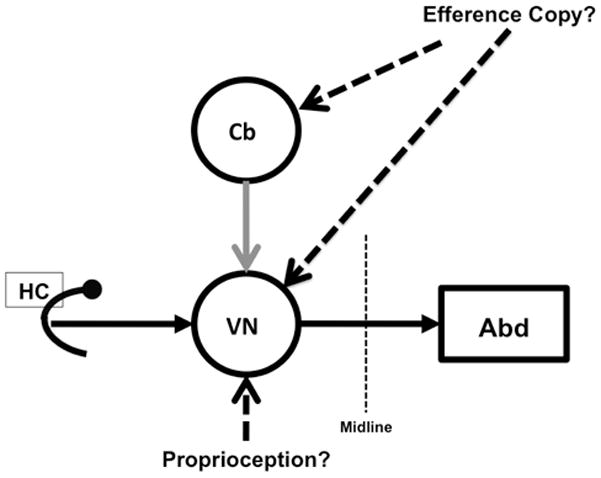
Vestibular inflow and proprioceptive inputs from the neck and/or efference copy signals, which encode head on trunk movement interact within central VOR pathways to produce anticipatory responses. In this model, the proprioceptive inputs are presumed to act directly on vestibular neurons. The efference copy signal reach vestibular neurons via the cerebellum and/or directly (dashed lines).
Acknowledgments
We want to acknowledge the contributions of Kevin Lim, Ph.D. for his programming expertise; Dwayne Valliencourt designed and built the specialized animal restraints used for this study and Chris Ellinger kept our electronics running. This research was supported by the following National Institutes of Health grants: P30 NDC005188-07, R21- DC008607-01, and T32 DC000011 30.
References
- 1.Walls GL. The evolutionary history of eye movements. Vision Research. 1962;2:69–80. [Google Scholar]
- 2.Lorente de No R. Vestibulo-ocular reflex arc. Archives of Neurological Psychiatry. 1933;30:245–291. [Google Scholar]
- 3.Bizzi E, Kalil RE, Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971:173. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- 4.McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. Journal of neurophysiology. 1999;82:416. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- 5.Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. Journal of Neurophysiology. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- 6.Roy JE, Cullen KE. Brain stem pursuit pathways: dissociating visual, vestibular, and proprioceptive inputs during combined eye-head gaze tracking. Journal of neurophysiology. 2003;90:271–290. doi: 10.1152/jn.01074.2002. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury BP. Retinotopic organization of the guinea pig’s visual cortex. Brain Res. 1978;144:19–29. doi: 10.1016/0006-8993(78)90432-8. [DOI] [PubMed] [Google Scholar]
- 8.Hughes A. The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Crescitelli F, editor. The visual sytem in vertebrates. Springer; Berlin, Heidelberg, New York: 1977. pp. 613–756. [Google Scholar]
- 9.Buttery RG, Hinrichsen CF, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Res. 1991;31:169–187. doi: 10.1016/0042-6989(91)90110-q. [DOI] [PubMed] [Google Scholar]
- 10.Land MF. Motion and vision: why animals move their eyes. J Comp Physiol A. 1999;185:341–352. doi: 10.1007/s003590050393. [DOI] [PubMed] [Google Scholar]
- 11.Escudero M, de Waele C, Vibert N, Berthoz A, Vidal PP. Saccadic eye movements and the horizontal vestibulo-ocular and vestibulo-collic reflexes in the intact guinea-pig. Experimental Brain Research. 1993;97:254–262. doi: 10.1007/BF00228694. [DOI] [PubMed] [Google Scholar]
- 12.Shanidze N, Kim AH, Raphael Y, King WM. Eye-head coordination in the guinea pig I. Responses to passive whole-body rotations. Exp Brain Res. 2010;205:395–404. doi: 10.1007/s00221-010-2374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanidze N, Kim AH, Loewenstein S, Raphael Y, King WM. Eye-head coordination in the guinea pig II. Responses to self-generated (voluntary) head movements. Exp Brain Res. 2010;205:445–454. doi: 10.1007/s00221-010-2375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Transactions in Biomedical Engineering. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 15.Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nucleus neurons. Experimental Brain Research. 2000;135:511. doi: 10.1007/s002210000542. [DOI] [PubMed] [Google Scholar]
- 16.Gdowski GT, Boyle R, McCrea RA. Sensory processing in the vestibular nuclei during active head movements. Archives italiennes de biologie. 2000;138:15. [PubMed] [Google Scholar]
- 17.Gdowski GT, Belton T, McCrea RA. The neurophysiological substrate for the cervico-ocular reflex in the squirrel monkey. Experimental Brain Research. 2001;140:253–264. doi: 10.1007/s002210100776. [DOI] [PubMed] [Google Scholar]
- 18.Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen KE, Brooks JX, Sadeghi SG. How Actions Alter Sensory Processing: reafference in the vestibular system. Annals of the New York Academy of Sciences. 2009;1164:29–36. doi: 10.1111/j.1749-6632.2009.03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res. 2011;210:377–388. doi: 10.1007/s00221-011-2555-9. [DOI] [PubMed] [Google Scholar]
- 21.Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. Journal of Neuroscience. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- 23.Dichgans J, Bizzi E, Morasso P, Tagliasco V. Mechanisms underlying recovery of eye-head coordination following bilateral labyrinthectomy in monkeys. Experimental Brain Research. 1973;18:548–562. doi: 10.1007/BF00234137. [DOI] [PubMed] [Google Scholar]
- 24.Bronstein AM. Vision and vertigo: some visual aspects of vestibular disorders. J Neurol. 2004;251:381–387. doi: 10.1007/s00415-004-0410-7. [DOI] [PubMed] [Google Scholar]
- 25.Leigh RJ. Oscillopsia: impaired vision during motion in the absence of the vestibulo-ocular reflex. J Neurol Neurosurg Psychiatry. 1998;65:808. doi: 10.1136/jnnp.65.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinne T, Bronstein AM, Rudge P, Gresty MA, Luxon LM. Bilateral loss of vestibular function: clinical findings in 53 patients. journal of neurology. 1998;245:314–321. doi: 10.1007/s004150050225. [DOI] [PubMed] [Google Scholar]
- 27.Leigh RJ, Sharpe JA, Ranalli PJ, Thurston SE, Hamid MA. Comparison of smooth pursuit and combined eye-head tracking in human subjects with deficient labyrinthine function. Exp Brain Res. 1987;66:458–464. doi: 10.1007/BF00270678. [DOI] [PubMed] [Google Scholar]
- 28.Waterston JA, Barnes GR, Grealy MA, Luxon LM. Coordination of eye and head movements during smooth pursuit in patients with vestibular failure. J Neurol Neurosurg Psychiatry. 1992;55:1125–1131. doi: 10.1136/jnnp.55.12.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai T, Zee DS. Eye-head coordination in labyrinthine-defective human beings. Brain Res. 1978;144:123–141. doi: 10.1016/0006-8993(78)90439-0. [DOI] [PubMed] [Google Scholar]
- 30.Chambers BR, Mai M, Barber HO. Bilateral vestibular loss, oscillopsia, and the cervico-ocular reflex. Otolaryngol Head Neck Surg. 1985;93:403–407. doi: 10.1177/019459988509300322. [DOI] [PubMed] [Google Scholar]


