Abstract
A secondary prevention trial in older people with amyloid accumulation at high risk for Alzheimer’s disease dementia should provide insights into whether anti-amyloid therapy can delay cognitive decline.
Alzheimer’s disease (AD) afflicts more than 13% of individuals over the age of 65, and remains one of the most feared consequences of aging. Converging biomarker and imaging data from both genetic at-risk and age at-risk cohorts suggest that the pathophysiological process of AD begins more than a decade prior to the stage of dementia (1-3). Despite compelling genetic and biomarker evidence, the role of amyloid accumulation in the pathogenesis of AD that occurs in late life remains controversial. Disappointing results from clinical trials testing amyloid-modifying therapies in individuals with AD dementia suggest that these interventions may be starting too late in the disease process to alter the clinical course (4). Like other fields such as cancer, HIV/AIDS, diabetes, and heart disease, the best opportunity to modify the course of AD will be prior to widespread damage and clinical symptoms. Thus, a new era of AD “secondary prevention” trials is beginning, aimed at preventing the progression of clinical symptoms in individuals in whom the pathophysiological process of AD has already begun. The majority of these secondary prevention trials are being conducted in genetic at-risk cohorts. The A4 trial (Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease; www.adcs.org/Studies/A4.aspx) is the first prevention trial in clinically normal older individuals identified as “at-risk” for progression to AD dementia on the basis of evidence of brain amyloid accumulation on PET imaging.
The concept of preclinical AD has rapidly evolved with recent advances in biomarker and imaging techniques that can detect evidence of AD pathology in the brains of healthy individuals without evidence of cognitive impairment (1). Autopsy, PET amyloid imaging and cerebrospinal (CSF) fluid studies have shown that about one third of clinically normal older individuals harbor evidence of amyloid plaque accumulation. Amyloid accumulation in clinically normal individuals is often associated with AD-like abnormalities on functional and structural imaging and increases in tau protein in the CSF, a component of neurofibrillary tangles in the brain, another pathological hallmark of AD. These amyloid-positive yet clinically normal older individuals also demonstrate subtle decreases in performance on challenging memory tests and are at increased risk for cognitive decline over 3-5 years. Additional research is needed to elucidate the individual risk of cognitive decline associated with markers of amyloid accumulation, and most importantly, to determine if anti-amyloid treatment, if administered early enough, can slow cognitive decline.
The A4 study is a three-year, placebo-controlled, randomized clinical trial conducted in 1000 older individuals with evidence of amyloid accumulation on screening PET scans, that will test whether anti-amyloid treatment can slow the rate of cognitive decline on a composite measure of sensitive neuropsychological tests. The amyloid-modifying drug used in the first A4 trial is solanezumab, a humanized monoclonal antibody targeting the mid-peptide domain of amyloid-β (made by Eli Lilly and Company). We anticipate that the A4 trial design will serve as a platform for future secondary prevention trials with other anti-amyloid agents, such as β-secretase inhibitors, and, ultimately, for combinations of therapeutic agents targeting more than one mechanistic target in preclinical AD.
A guiding hypothesis of the A4 program is that the impact of anti-amyloid therapy may be greatest prior to clinically evident mild cognitive impairment (MCI) or dementia, as there is often evidence of substantial neurodegeneration even at the MCI stage. The first step in the screening process is cognitive assessment to identify individuals who are clinically normal but at highest risk for imminent cognitive decline. Individuals who score higher than one standard deviation above the age- and education-normative values will not be eligible, as they are less likely to harbor amyloid accumulation and to decline towards MCI or symptomatic AD over the three-year period of the trial. Individuals who score in the clearly impaired range (<1.5 S.D. below age and education norms) on screening tests will not be eligible, as they may already be manifesting MCI. Eligible individuals must have a Clinical Dementia Rating (CDR) total score of zero with no evidence of dementia or impaired daily life function. Individuals who report subtle subjective cognitive concerns or self-awareness of memory changes will be eligible for the A4 study, as long as these subjective changes do not interfere with their daily function (as reported by the study partner) to merit a CDR of 0.5 (MCI or questionable dementia). Recent studies suggest that these “worried well” older individuals, who report recent memory changes despite normal cognitive test performance, may indeed be more likely to harbor amyloid accumulation and be at increased risk of cognitive decline.
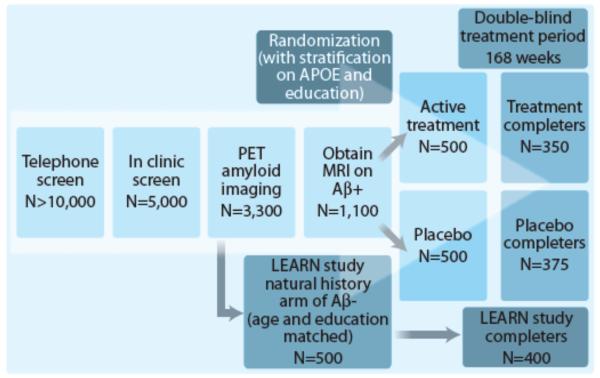
More than 5000 healthy individuals, 65-85 years of age, will be screened to identify ~1000 participants eligible for randomization into two treatment arms (solanezumab n=500; placebo n=500; Fig. 1). The A4 trial has the goal of improving diversity by requiring that one out of every five individuals screened come from an under-represented minority group. Eligible participants will undergo PET imaging for amyloid deposition with 18-F-Florbetapir. To qualify for randomization into the active treatment or placebo arms, participants must show evidence of elevated amyloid accumulation on both a visual read and a quantitative measurement (Standardized Uptake Value Ratio; SUVr) of the florbetapir PET scan. Based on previous data, we anticipate that ~30% of individuals over age 65 will demonstrate evidence of amyloid accumulation that is sufficient to qualify for enrollment.
Figure 1. Screening and Randomization Algorithm for the A4 Study.
The A4 trial will enroll clinically normal older individuals with amyloid accumulation, who are at increased risk for cognitive decline due to AD. Five thousand clinically normal older individuals will be screened to identify those with increased amyloid accumulation on PET imaging; those individuals will be randomized into the anti-amyloid treatment arm with solanezumab or placebo arm. Individuals who do not show evidence of elevated amyloid accumulation may be eligible to participate in the LEARN study, a companion natural history observation arm that will run parallel to the A4 treatment arm with identical cognitive assessments. A4 and LEARN study participants will be followed for 168 week treatment and observation periods.
An important aspect of the A4 study is how to educate participants and inform them about their screening amyloid PET results. After carefully weighing the pros and cons of disclosing this information to participants, we decided that it was critically important to study the impact of the disclosure process. If the A4 trial is successful, clinically normal older individuals might undergo such screening in clinical settings in the future. Furthermore, as indicated by surveys and focus groups, potential participants want to understand the significance of their amyloid PET results in order to make a fully informed decision about their participation in the A4 trial (5). Importantly, the A4 study educational materials clearly explain that although the limited longitudinal studies published to date suggest that elevated amyloid is associated with an increased risk of cognitive decline and progression to MCI and AD dementia, there is insufficient information to predict a given individual’s risk of cognitive decline; indeed, some individuals with elevated amyloid accumulation may not develop any cognitive symptoms within their lifetime. We have incorporated psychological assessments into the screening and disclosure process to ensure that individuals enrolling in the A4 study are ready to receive information about their amyloid status and to monitor them carefully for any adverse reaction. Finally, we will measure how disclosure impacts neuropsychological test performance, the perception of cognitive symptoms, quality of life and perceived risk of AD dementia in participants with and without evidence of amyloid accumulation.
The primary outcome for the A4 trial, the ADCS-PACC (Alzheimer’s Disease Cooperative Study-Preclinical Alzheimer’s Cognitive Composite), is a composite of well-validated neuropsychological tests that were selected specifically because of their sensitivity in tracking the earliest evidence of decline from “normal” to subtly abnormal cognitive performance, in contrast to outcome measures typically used in AD dementia trials. ADCS-PACC includes four tests: a picture-word list learning test (6), a paragraph recall test, a timed executive function test, and a global cognitive measure. We performed power estimates with combinations of similar cognitive measures using longitudinal data from the ADNI (AD Neuroimaging Initiative), AIBL (Australian Imaging Biomarker and Lifestyle), and ADCS-PI (ADCS-Prevention Instrument) studies. Specifically, we investigated rates of decline in: 1) older individuals with and without evidence of amyloid accumulation according to PET and CSF markers, 2) one or two APOE ε4 alleles compared to non-carriers, and 3) those who progressed to MCI compared to those who remained cognitively stable. Based on these estimates, A4 is powered to detect a treatment effect of ~30% slowing of the rate of cognitive decline. This effect size would have a meaningful impact on the clinical course and public health cost of AD, as delaying dementia by just five years is estimated to decrease federal expenditures by more than 50% (http://www.alz.org/documents_custom/trajectory.pdf).
The A4 study also incorporates a number of innovative secondary and exploratory outcome measures, including cognitive tests administered on an iPad. This computerized assessment is based on the CogState platform using several card-playing measures of reaction time and visual recognition memory that have shown promise in detecting decline in the preclinical and prodromal stages of AD. We also incorporated two new memory measures known to depend on hippocampal function: the Face Name Associative Memory Exam (7) and the Behavioral Pattern Separation-Object test (8). The A4 study also emphasizes participant reported outcomes, as an advantage of earlier intervention is that less impaired individuals can provide accurate assessments of their own cognitive function in daily-life activities and subjective memory concerns. Although it is unlikely that we will observe large effects on functional outcomes in a population that is clinically normal at baseline, over the initial three and a half year trial, these measures may provide crucial evidence that very early intervention can impact longer term clinical indicators.
Although cognition is the primary outcome, it is important to acquire biomarker and imaging outcomes that may one day provide reliable “theragnostic” markers to predict and track therapeutic responses. Volumetric and functional connectivity magnetic resonance imaging will be acquired four times during the course of the A4 trial. Repeat florbetapir PET amyloid imaging will be acquired at the end of the trial to assess effects on the accumulation of fibrillar amyloid. CSF measures of amyloid-β1-42, tau, and phospho-tau will be acquired in a subset of participants. Tau-PET imaging will be acquired in a subset of A4 participants using a new ligand that binds to the hyperphosphorylated helical filament form of tau found in neurofibrillary tangles. Importantly, the A4 study will investigate whether there is a “critical window” for anti-amyloid therapy based on the degree of neurodegeneration at baseline.
A broader goal of the A4 study is to acquire additional information on the risk of cognitive decline associated with amyloid accumulation. Thus, another 500 individuals will be recruited, who “screen-fail” for the A4 study because they do not demonstrate elevated amyloid on PET imaging. They will participate in the Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) natural history observational arm of the A4 study, which will run in parallel to the treatment arm with identical cognitive assessments performed every six months, and imaging and biomarker outcomes collected at the end of the study. The LEARN cohort will serve as an essential comparison group to the placebo arm of the A4 study to quantify amyloid-related cognitive decline, and will also provide data on non-amyloid factors associated with cognitive decline.
The A4 trial will be conducted through the ADCS utilizing 60 sites in the United States, Canada and Australia. The A4 study is funded by a public-private partnership, including the National Institute on Aging, Eli Lilly and Company, the Alzheimer’s Association and several philanthropic organizations. Clinical and imaging data from the screening cohort will be made publicly available early in the course of the study, and the full dataset, including data from the A4 treatment arms and the LEARN cohort, will be shared after completion of the primary analyses at the end of the trial.
The A4 study complements ongoing secondary prevention trials in rare genetic risk cohorts who carry a deterministic genetic mutation, including the Dominantly Inherited Alzheimer Network (DIAN) (2), which is enrolling younger individuals from families with causative mutations in amyloid precursor protein (APP), presenilin-1 (PSEN1) and presenilin-2 (PSEN2), and the Alzheimer Prevention Initiative (API) (3), which is enrolling individuals from a large kindred with a PSEN1 (E280A) mutation in Medellin, Colombia, as well as families with other PSEN1 mutations in the United States. There are also prevention trials planned for late-onset genetic risk groups including APOE ε4 homozygotes and individuals carrying the TOMM40 risk allele. The Collaboration for Alzheimer Prevention (CAP) consortium was formed to facilitate interactions among these secondary prevention trials and to harmonize the collection of clinical, biomarker, and imaging outcomes to maximize our ability to translate findings from trials with genetic at-risk individuals to trials in older individuals at risk due to biomarker status. The US Food and Drug Administration (FDA) and European Medicines Authorities (EMA) participated in open discussions with CAP membership about the challenges of designing very early stage AD trials and have released draft guidance (9) that has enabled A4 and other early intervention studies to proceed through an innovative regulatory pathway.
The success of these trials does not require that amyloid is the cause of AD, merely that amyloid is one critical factor that can be targeted prior to widespread, irreversible neurodegeneration. An analogous situation might be the development of cholesterol lowering medications for cardiovascular disease. Statins were developed with public-private partnerships between the NIH and pharmaceutical companies, beginning with early trials in patients with familial hypercholesterolemia and followed by large secondary prevention trials in individuals with a history of minor myocardial infarction. Although cholesterol is clearly only one factor involved in the pathogenesis of ischemic heart disease, lowering cholesterol with statin treatment has decreased cardiac morbidity by ~25%, with a 14% reduction in all-cause mortality (10). Given the age-linked prevalence of AD and the increasing life expectancy in the developed world, a similar magnitude of reduction in AD-related morbidity would have enormous economic and social impact.
Acknowledgements
We thank the Alzheimer’s Disease Cooperative Study and the many individuals who have contributed to the A4 Study design.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012;367:795. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JB, Kosik KS, Tariot PN, Lopera F. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Jack CR, Jr., Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimers Dement. 2013;9:356. doi: 10.1016/j.jalz.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49:2776. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368:1169. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 10.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. The Cochrane database of systematic reviews. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]