Abstract
Functional imaging studies frequently report that the hippocampus is engaged by successful episodic memory retrieval. However, considering that concurrent encoding of the background environment occurs during retrieval and influences medial temporal lobe activity, it is plausible that hippocampal encoding functions are reduced with increased attentional engagement during effortful retrieval. Expanding upon evidence that retrieval efforts suppress activity in hippocampal regions implicated in encoding, this study examines the influence of retrieval effort on encoding performance and the interactive effects of encoding and retrieval on hippocampal and neocortical activity. Functional magnetic resonance imaging was conducted while subjects performed a word recognition task with incidental picture encoding. Both lower memory strength and increased search duration were associated with encoding failure and reduced hippocampal and default network activity. Activity in the anterior hippocampus tracked encoding, which was more strongly deactivated when incidental encoding was unsuccessful. These findings highlight potential contributions from background encoding processes to hippocampal activations during neuroimaging studies of episodic memory retrieval.
Keywords: fMRI, hippocampus, episodic memory, encoding, retrieval
INTRODUCTION
Episodic memory is frequently investigated as a system comprising two distinct yet complementary functions. The first involves encoding the features of an experience into memory, a process mediated largely by the brain’s medial temporal lobe. The second is the subsequent retrieval of a memory from storage, via interactions between medial temporal regions and neocortical association areas to reactivate representations of the episodic features (for review of the medial temporal lobe in episodic encoding and retrieval, see Squire and Zola-Morgan, 1991; Schacter and Wagner, 1999; Squire et al., 2004). However, encoding processes cannot be cleanly dissociated from retrieval, and a deeper understanding of their functional interactions is critical to elucidating the mechanisms of episodic memory. For instance, the intent to retrieve a memory (Buckner et al., 2001) or retrieval success (Huijbers et al., 2009) can influence further encoding of both task-relevant and task-irrelevant information, and memory decisions can bias subsequent operations towards encoding or retrieval (Duncan et al., 2012). Psychological models of memory propose that encoding and retrieval can operate concurrently and may facilitate or compete with one another (Glover, 1989; Tulving and Thomson, 1973; Storm et al., 2006). While evidence suggests medial temporal lobe subregions play distinct roles that span encoding and retrieval, it remains unclear how the interaction between encoding and retrieval is mediated by such subregions, which are integrated and mostly thought to subserve both functions.
The role of the hippocampus in encoding episodic memories has been established by evidence from patients with hippocampal damage (Scoville and Milner, 1957), animal electrophysiology and lesion studies (Zola-Morgan et al., 1994; Suzuki and Eichenbaum, 2000; Wood et al., 2000; Wirth et al., 2003; Leutgeb et al., 2005), and supported by human local field potential (Fernandez et al., 1999) and functional neuroimaging studies (Brewer et al., 1998; Wagner et al., 1998), demonstrating postlesion anterograde and temporally graded retrograde amnesia as well as increased neuronal and metabolic activity during successful encoding. Although the medial temporal lobe has also been strongly implicated in memory retrieval, there is conflicting evidence over the circumstances under which the hippocampus is involved in retrieval. While some human neuroimaging studies fail to report hippocampal responses during retrieval, others indicate that the hippocampus signals successful recollection or familiarity (Schacter et al., 1996; Gabrieli et al., 1997; Eldridge et al., 2000; Stark and Squire, 2000; Wais et al., 2010b) or the retrieval of strong memories or contextual details (Cansino et al., 2002; Ross and Slotnick, 2008; Wais, 2011; Yu et al., 2011). Thus, while functional imaging has failed, as yet, to delineate the bases for hippocampal involvement in retrieval, a general consensus over hippocampal involvement in encoding exists. As such, it is important to consider possible contributions from encoding processes to hippocampal responses observed during retrieval tasks that would otherwise be attributed directly to retrieval functions. Activity in the medial temporal lobe has been shown to vary with shifts in attentional focus that fluctuate with dynamic retrieval demands (Nee and Jonides, 2008). Thus, any component of retrieval, including (1) reactivation that facilitates memory recovery or (2) cognitive control operations directing search efforts, could interfere with ongoing encoding functions, an interaction that would be evidenced by a diminished response in hippocampal regions that mediate encoding.
Indeed, there is evidence that encoding processes remain online during memory retrieval and are tracked by medial temporal lobe regions that support episodic memory. Incidental encoding of novel stimuli occurs during recognition tasks and is associated with encoding-dependent modulation of frontal (Buckner et al., 2001) and hippocampal (Stark and Okado, 2003) activity. For instance, Stark and Okado (2003) report that hippocampal responses during a scene recognition task were greater to subsequently remembered than forgotten nontarget foils, an effect also observed during an intentional scene encoding task. Additional evidence demonstrates that even task-irrelevant information of the background environment can be encoded during memory retrieval and suggests a competitive interaction between encoding and retrieval mechanisms (Huijbers et al., 2009). In their study, Huijbers et al. (2009) report that successful, relative to failed, word recognition, impaired incidental encoding of simultaneously presented scenes, and activity in areas of the medial temporal lobe and visual cortex associated with encoding success was reduced during encoded hit as compared to encoded miss trials. Together, these observations suggest that encoding of both task-relevant and task-irrelevant information remains active during retrieval, may be mediated by retrieval task demands and, in conjunction with retrieval processes, interactively regulates activity in regions of the medial temporal lobe.
Prior functional magnetic resonance imaging (fMRI) studies provide evidence for an increased hippocampal blood oxygen level dependent (BOLD) signal by retrieval success (for review see Eichenbaum et al., 2007), as well as increasing hippocampal activity with increasing recognition confidence, consistent with nonlinear recollection (Daselaar et al., 2006) or linear familiarity signals (Kirwan et al., 2009). However, memory strength is correlated with attention and cognitive control functions that support retrieval efforts. Recent findings suggest that the hippocampal response to retrieval success or strength can be driven in part by attentional demands of the retrieval task, such as engaging in retrieval mode or directed memory search (Israel et al., 2010; Reas et al., 2011). These studies identified a negative BOLD signal change in anterior hippocampus associated with retrieval attempt that is amplified by both response time and difficulty of retrieving the memory. Anterior hippocampal activity during retrieval is correlated with activity in regions of the default network (Israel et al., 2010; Huijbers et al., 2011; Reas et al., 2011), which has been shown to deactivate in response to increasing attentional demands across a variety of cognitive states (Raichle et al., 2001; McKiernan et al., 2003; Buckner et al., 2008). Considering the previously discussed evidence that episodic memory encoding is heavily dependent on the hippocampus and that brain processes underlying concomitant encoding functions may contribute to activations during retrieval, hippocampal deactivation during retrieval has been linked to a reduction in background encoding processes, which may be modulated by attentional engagement during the retrieval attempt (Reas et al., 2011).
The present study directly tested whether the magnitude of deactivation in the anterior hippocampus during retrieval, previously associated with memory search efforts, is related with incidental encoding of task-irrelevant information. During event-related fMRI subjects performed a recognition test on previously studied words with concurrent picture presentation, and incidental encoding was measured by a subsequent picture recognition test. On the basis of evidence for a dissociation of memory functions along the long axis of the hippocampus (Lepage et al., 1998; Prince et al., 2005; Daselaar et al., 2006), region of interest analyses of encoding-retrieval interactions were performed in anterior, middle, and posterior hippocampus. Whole brain analysis was conducted to identify regions outside the hippocampus sensitive to retrieval search demands and correlated with hippocampal activity. Words were studied as paired associates in order to trigger greater search for associated episodic features in response to word cues as well as to increase dependence of the retrieval response on the hippocampus, which may selectively support memories with multiple attributes (Wixted and Squire, 2011). Study repetitions were varied to manipulate memory strength, and hence the degree of search required for retrieval. Given the correlation between memory strength and retrieval search demands, behavioral or neural effects of study repetitions could be explained either by higher memory strength or attenuated search levels driven by greater study. While we acknowledge the longstanding semantic ambiguity that exists for the term “search,” it will henceforth be used to refer broadly to the attentional operations under an individual’s control that guide the evaluation of stored information during a directed retrieval attempt (Atkison and Shiffrin, 1968).
Competing hypotheses were tested to investigate the behavioral interactions between encoding and retrieval as well as the neural correlates of such interactions. Activity in the anterior hippocampus was expected to be regulated by incidental encoding, resulting in greater activation during trials with subsequently remembered than forgotten pictures. Alternative hypotheses were constructed regarding the interaction between encoding performance and memory strength or success. First, based on the interpretation that the previously reported hippocampal deactivation during effortful retrieval is associated with suppressed encoding functions (Reas et al., 2011), we proposed that encoding would be impaired during word recognition trials for lower strength memories. Such lower strength memories are assumed to elicit more demanding search efforts, relative to trials for higher strength memories. If memory search was found to reduce encoding, hippocampal activity was predicted to be modulated by preretrieval search duration, approximated by response times, and correlated with activity in regions previously shown to be sensitive to retrieval search efforts including medial prefrontal, superior temporal, and medial and lateral parietal cortex. Second, if retrieval success rather than the attentional demands of the retrieval task interfere with encoding (Huijbers et al., 2009), then encoding should be worse during word recognition hit than miss trials. This might be expected if the neural resources for encoding and retrieval directly overlap and are depleted by concurrent retrieval, or if a recovered memory captures internal attention and diverts it away from the external environment.
METHODS
Participants
Twenty right-handed, English-speaking volunteers with normal or corrected vision participated in this study. All subjects were recruited from the University of California, San Diego (UCSD) community and surrounding areas and gave informed written consent in accordance with criteria of the UCSD Institutional Review Board. Three participants were excluded from further analysis due to excessive motion, word recognition accuracy more than three standard deviations below the mean, or below chance picture recognition accuracy. Data from the remaining 17 participants (seven male, mean age ± standard deviation = 26.2 ± 4.0 years) are reported.
Stimuli
Stimuli for the word recognition task included 240 English nouns (Reas et al., 2011), divided into sets of 80 low-study targets, 80 high-study targets, and 80 novel foils. 360 color pictures of everyday objects (Bakker et al., 2008) were used in the incidental picture encoding task, including 240 target images, and 120 novel foils.
Experimental Paradigm
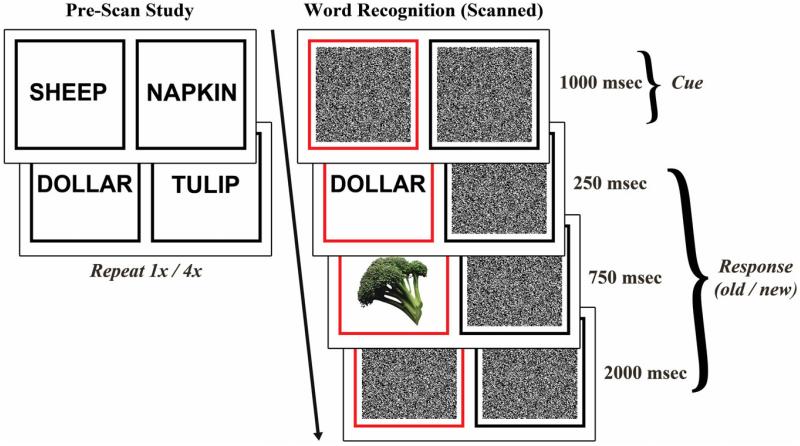
During a prescan associative memorization task, participants were presented word pairs and instructed to remember each word pair association. Half of the paired associates were presented once and half were presented four times and are thus referred to as low-study and high-study, respectively. Words were pseudorandomly combined into 80 pairs and presented in 200 trials over the course of four 200-second study runs. Each pair was displayed for three seconds, followed by a fixation cross for 1 s (Fig. 1, left).
FIGURE 1.
Behavioral protocol. Left: Prior to scanning, subjects studied word pair associates. Low-study pairs were presented once and high-study pairs were presented four times. Right: During event-related fMRI, subjects performed a word recognition task with incidental picture encoding. A previously studied or novel word was presented, immediately followed by a picture. Subjects were instructed to respond “old” or “new” to the word and attend to both the word and picture. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
After a delay of ~20 min, event-related fMRI data were acquired while participants performed a word recognition task with concurrent incidental picture encoding. In each trial a black box and a red box were presented for 1000 msec, the red box serving as a cue for the stimulus location, which was the same as the location at study. A previously studied or novel word appeared in the red box for 250 msec, followed by a picture for 750 msec and visual masking noise for 2000 msec (Fig. 1, right). Participants were instructed to respond “old” or “new” to indicate whether the presented word was previously studied or novel by responding with their right hand using two buttons of a response box. They were given no explicit instructions to remember the pictures, but were told to respond as quickly and accurately as possible while attending to both the word and picture in each trial. Trials were jittered with 0.5–10 s of fixation baseline, calculated to optimize the study design for modeling the hemodynamic response to trials (Dale and Buckner, 1997; Dale, 1999). Eighty low-study, 80 high-study, and 80 novel words were pseudorandomly distributed across five 388 s runs. Word assignment to the three conditions was randomized and counterbalanced across participants. Each picture was randomly paired with a word, and pairing between pictures and word-condition (low-study, high-study, novel) was counterbalanced across participants.
After scanning, participants completed a brief distractor task of serial 7s subtraction to minimize recency effects before proceeding to further testing. A surprise, self-paced picture recognition test was then administered using the 240 target pictures that had been previously presented during the word recognition test along with 120 novel foils. Participants were instructed to respond “old” or “new” to indicate whether the picture was previously shown or novel. Lastly, a self-paced cued recall test was administered to assess associative memory for each word pair. One word from each pair was presented and participants verbally reported the word’s pair.
fMRI Data Acquisition
Imaging was performed using a 3.0 T General Electric scanner at the UCSD Keck Center for Functional MRI. Field maps were acquired to measure and correct for static field inhomogeneities (Smith et al., 2004). Functional data were acquired using a gradient-echo, echo-planar, T2*-weighted pulse sequence (time repetition = 2.5 s, one shot per repetition, echo time = 30, flip angle = 90°, bandwidth = 31.25 MHz, field of view = 220 mm, matrix = 64 × 64). Each functional volume contained 40 slices (in-plane resolution = 3.4 × 3.4 mm, slice thickness = 4 mm) oriented perpendicular to the long axis of the hippocampus. The first five volumes were discarded to allow for signal equilibration. A high resolution T1-weighted anatomical scan (1 × 1 × 1 mm) was acquired using an inversion recovery prepared spoiled gradient recalled sequence providing high gray-white contrast for anatomical delineation. An additional T1-weighted structural scan was acquired in the same plane and of the same voxel size as the functional scans to confirm alignment between the functional and high-resolution anatomical images.
fMRI Data Processing
Functional data were corrected for spatial distortions using field maps (Smith et al., 2004), and data from each of five runs were reconstructed using the AFNI suite of programs (Cox, 1996). Slices were temporally aligned and coregistered using a three-dimensional image alignment algorithm. Nonbrain voxels were removed using a threshold mask of the functional data. Functional runs were smoothed with a 4 mm full-width half-maximum Gaussian blur, corrected for motion and concatenated. Anatomical images and the functional data were normalized to Talairach space (Talairach and Tornoux, 1988) after manually defining standard landmarks on the anatomical images.
The region of interest large deformation diffeomorphic metric mapping (ROI-LDDMM) alignment technique was applied to improve alignment of the medial temporal lobe between subjects (Miller et al., 2005). For each subject, previously described landmarks were used to define medial temporal lobe subregions, including the hippocampus (Chera et al., 2009), perirhinal and entorhinal cortices (Insausti et al., 1998) and parahippocampal cortex (Stark and Okado, 2003), on Talairach transformed images. These anatomical regions of interest for each subject were normalized using ROI-LDDMM to a modified model of a previously created template segmentation (Kirwan et al., 2007). Functional imaging data underwent the same ROI-LDDMM transformation as was applied to the anatomical data.
Whole Brain Analysis
To examine effects of study repetitions and incidental encoding during retrieval, word recognition trials were sorted according to word condition (low-study, high-study, novel) and picture encoding (subsequently remembered or forgotten, henceforth referred to as “encoded” and “not-encoded”), yielding six trial classes for multiple regression analysis: low-study encoded, low-study not-encoded, high-study encoded, high-study not-encoded, novel encoded, and novel not-encoded. In a separate regression analysis to examine effects of retrieval success and encoding, trials were sorted according to word recognition success (hits, misses, correct rejections, and false alarms) and picture encoding. Multiple regression analysis was performed by generating a general linear model with regressors for each of the task conditions along with six motion regressors obtained from the registration process. Signal deconvolution with TENT basis functions (Cox, 1996) was used to estimate the hemodynamic response for each condition for the 15 s following the stimulus onset. T-tests (P < 0.01, two-tailed and corrected for multiple comparisons) were performed on parameter estimates from the 5–10 s period of each condition, selected based on reported peak of impulse response curves from a prior study using a similar task (Reas et al., 2011).
Amplitude-modulated regression was conducted to identify regions in which the magnitude of the BOLD response correlated with response time. A general linear model was constructed with six regressors for each condition, sorted according to word type and encoding (low-study words, encoded pictures; low-study words, not-encoded pictures; high-study words, encoded pictures; high-study words, not-encoded pictures; novel words, encoded pictures; novel words, not-encoded pictures), six regressors for each condition weighted by response times, and six motion regressors. The magnitude of BOLD signal modulation by response time across all trials was estimated from the response-time-weighted regressors. Group-level t-tests were performed on the resulting correlation maps (P < 0.01, corrected for multiple comparisons).
Functional connectivity analysis was performed using the anatomically defined bilateral anterior hippocampus as a seed region (http://afni.nimh.nih.gov/sscc/gangc/SimCorrAna.html). Each subject’s whole brain connectivity map was tested for an interaction of the correlation with study-level (low-study vs. high-study) and encoding (encoded vs. not-encoded), and group-level t-tests (P < 0.01, corrected for multiple comparisons) were performed on the resulting interaction maps.
For all whole-brain analyses, correction for multiple comparisons was computed using a Monte Carlo simulation on a whole-brain functional volume in AFNI (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to determine the minimum cluster size necessary to achieve a family-wise error rate of P < 0.05 with a voxel-wise threshold of P < 0.01. Significant clusters, including at least six contiguous voxels, were displayed on a statistical map overlaid onto an across-subject averaged structural image.
Hippocampal ROI Analysis
A structural mask was drawn on the across-subject averaged T1-weighted anatomical image to divide the hippocampus into left and right anterior (y = −7 to −18), middle (y = −19 to −26) and posterior (y = −27 to −38) regions of interest. The hemodynamic response function in each hippocampal subregion was extracted and averaged across subjects to examine the signal time-course in an impulse-response plot, and beta-values from 5 to 10 s after stimulus onset were submitted to repeated-measures analysis of variance (ANOVA).
RESULTS
Behavioral Performance
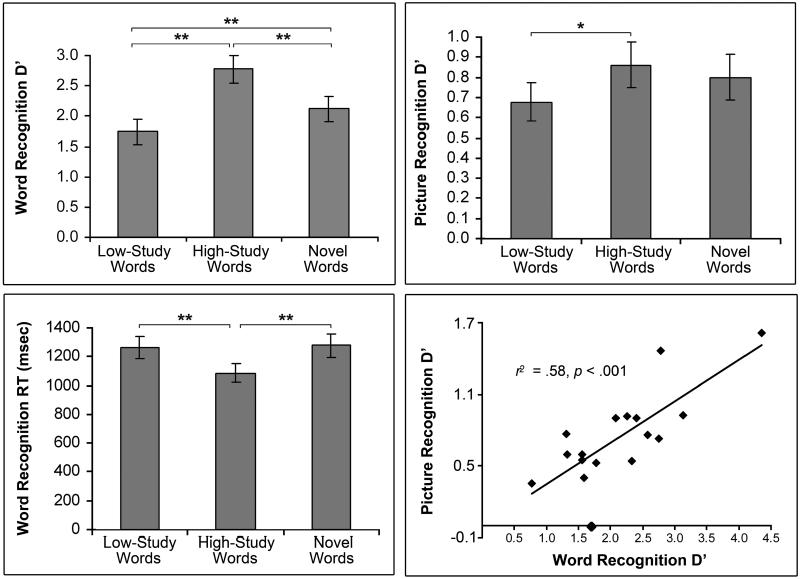
In the word recognition task, subjects responded “old” to 64 (±4)% (mean ± standard error) of low-study words, 90 (±2)% of high-study words and correctly rejected 88 (±3)% of novel words (Fig. 2, top left). D′ values, computed as z(hit rate) – z(false alarm rate), were higher for high-study than low-study words (2.8 ± 0.2 vs. 1.7 6 0.2; t(16) = 10.24, P < 0.001). Response times differed by word condition (F(2,32) = 17.33, P < 0.001) and were faster for high-study (1086 ± 65 msec) than low-study (1264 ± 77; P < 0.001) and novel (1276 ± 82 msec; P < 0.001) trials (Fig. 2, bottom left). Postscan cued recall was more accurate for high-study than low-study word pairs (78 ± 5 vs. 37 ± 6%; t(16) = 9.48, P < 0.001), confirming better associative memory for more highly studied pairs.
FIGURE 2.
Behavioral performance. Mean (± standard error) word recognition accuracy (top left; low-study vs. high-study d′: 1.7 ± 0.2 vs. 2.8 6 0.2; t(16) = 10.24, P < 0.001), word recognition response times (bottom left) and picture recognition d′ values (top right) for low-study, high-study and novel word trials. Recognition d′ scores are plotted for old words (x-axis) and for pictures presented during old-word trials (y-axis) for each subject (bottom right). * P < 0.01, ** P < 0.001
D′ scores were calculated for the postscan picture recognition task to assess incidental picture encoding during the scanned word recognition task. D′ values were significantly above chance (.78 ± 0.10; t(16) = 7.76, P < 0.001), indicating that subjects successfully encoded the pictures despite no explicit memorization instructions. Picture encoding differed by word condition (F(2,32) = 4.17, P < 0.05), with better subsequent memory for pictures paired with high-study than low-study words (0.86 ± 0.10 vs. 0.68 ± 0.10; P < .01; Fig. 2, top right). Encoding did not differ between pictures paired with novel words and those paired with either of the old-word conditions (Ps > 0.05) or between hit and miss trials (P = 0.22). To test the effect of study-level on incidental encoding with retrieval success held constant, the analysis was performed on hit trials only, comparing the low-study versus high-study conditions. In addition, to test effects of retrieval success within a strength condition, low-study hits were compared to low-study misses. Picture encoding d′ scores were better for high-study than low-study hits (0.86 ± 0.11 vs. 0.65 ± 0.11; t(16) = 2.74, P < 0.05), but did not differ between low-study hits and misses (P = 0.71), indicating an effect of study-level, but not retrieval success, on encoding. High-study misses were too infrequent to allow analysis of this effect within the high-study condition on all subjects. However, a separate ANOVA on a subset of 14 subjects confirmed a main effect of study-level on incidental encoding (high-study: 0.85 ± 0.12, low-study: 0.58 ± 0.09; F(1,13) = 8.23, P < 0.05), but no effect of retrieval success (P = 0.45) nor interaction between study-level and retrieval success on encoding (P = 0.54). Response times were longer for trials associated with unsuccessful than successful picture encoding (1240 ± 72 vs. 1157 ± 71 msec; t(16) = 4.58, P < 0.001). Response times remained longer for not-encoded than encoded trials when comparing low-study trials alone (1293 ± 77 vs. 1210 ± 81 msec; t(16) = 2.71, P < 0.05) and a trend for this effect was observed for the high-study condition (P = 0.09), suggesting an effect of response time on encoding success beyond the effect of study-level.
To investigate whether subjects with superior word recognition would also demonstrate better incidental encoding or conversely, might more effectively inhibit task-irrelevant information and thus show diminished encoding, the Pearson’s correlation between word and picture recognition d′ scores were computed. Word and picture recognition d′ scores were positively correlated, such that subjects with better word recognition also demonstrated better incidental encoding (r = 0.75, P < 0.001). Although encoding did not significantly differ between word recognition hit and miss trials, if successful encoding were more frequent during successful word recognition, this correlation could have been related to the higher frequency of word hits in higher performing subjects. Therefore, to control for word recognition hit rates, picture recognition d′ scores were computed separately for word recognition hit and miss trials and hit and miss d′ scores were subsequently averaged. After correction, word recognition and picture encoding remained positively correlated across subjects (r = 0.76, P < 0.001, Fig. 2, bottom right).
Hippocampal Responses to Encoding and Retrieval
To confirm consistency with prior reports of hippocampal activity associated with memory strength, retrieval success or encoding success, general linear tests contrasted (1) high-study > low-study trials, (2) word hits > misses, (3) word hits > correct rejections, and (4) encoded > not-encoded pictures. Greater BOLD responses (P < 0.01) were observed for hits than misses throughout bilateral hippocampus, for hits than correct rejections throughout left and middle right hippocampus, and for encoded than not-encoded pictures in bilateral anterior hippocampus. At a reduced threshold (P < 0.05, corrected for multiple comparisons), activity throughout the body of the right hippocampus was greater for high-study than low-study trials.
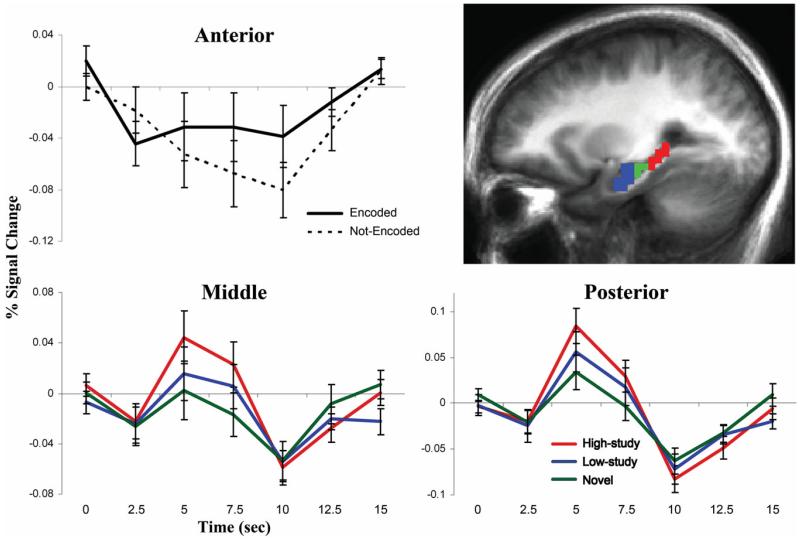
BOLD responses in the anatomically defined anterior, middle, and posterior hippocampus were analyzed to examine how concurrent picture encoding and memory strength or search might interact to influence hippocampal activity. Repeated measures ANOVA was performed with factors of region (anterior, middle, and posterior), hemisphere, word condition (low-study, high-study, and novel) and incidental picture encoding (encoded and not-encoded). A main effect of region (F(2,32) = 8.56, P < 0.01) reflected less activity in anterior than middle (P < 0.05) and posterior (P < 0.01) subregions, and an effect of word condition (F(2,32) = 3.50, P < 0.05) revealed that high-study words elicited greater activation than novel words across all regions (P < 0.01). An interaction between region and encoding (F(2,32) = 4.41, P < 0.05) was also observed. Bilateral anterior hippocampus demonstrated greater activation during trials with successful than unsuccessful picture encoding (F(1,16) = 5.74, P < 0.05; Fig. 3, top), and a trend for an encoding by word condition interaction (P = 0.05). Impulse response curves revealed a task-negative BOLD signal change, such that anterior hippocampus was more strongly deactivated during not-encoded than encoded trials. In contrast, responses in middle and posterior bilateral hippocampus were not associated with subsequent picture memory (Ps > 0.10), but showed main effects of word condition (middle: F(2,32) = 4.13, P < 0.05; posterior: F(2,32) = 3.63, P < 0.05), reflecting greater task-positive activation to high-study than novel words (Ps < 0.01; Fig. 3, bottom).
FIGURE 3.
Hippocampal BOLD responses. Anterior (blue), middle (green) and posterior (red) hippocampal ROIs are overlaid on a sagittal cross-section of the mean anatomical image of all subjects (top right). Impulse-response plots display the time-course of the percent signal change (± standard error) in anatomically-defined bilateral anterior, middle and posterior hippocampus. Anterior hippocampus was more deactivated during word recognition trials when pictures were not-encoded than encoded (P < 0.05, top left). Middle and posterior hippocampus, which were not influenced by incidental encoding during retrieval (Ps > 0.10), were more activated during high-study than novel word recognition trials (P < 0.01, bottom). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Behavioral results indicated that incidental picture encoding success was influenced by study-level, but not by recognition success. However, to test whether recognition success and encoding might interactively regulate hippocampal activity, a second ANOVA was performed on old word recognition trials with factors of region, hemisphere, recognition success (hit, miss), and incidental picture encoding. One subject was excluded from this analysis due to word recognition performance at ceiling. Across all regions, hippocampal activity was greater for hit than miss trials (F(1,14) = 7.50, P < 0.05) and recognition success interacted with region F(2.28) = 5.63, P < 0.01). Anterior (F(1,14) = 6.02, P < 0.05) and middle (F(1,14) = 14.16, P < 0.01) hippocampus demonstrated greater activity during hit than miss trials, and posterior hippocampus showed a trend for this effect (P = 0.09). Notably, recognition success did not interact with encoding (P = 0.52), consistent with the behavioral findings that study-level, and not recognition success, interacted with incidental concurrent encoding.
While the between-condition contrasts suggested a general hippocampal sensitivity to memory strength and success, further subregion analyses revealed a spatially selective sensitivity to incidental encoding behaviorally associated with study-level. To investigate encoding-related activity during opposing memory strength or retrieval success conditions, BOLD responses were compared between low-study trials with successful picture encoding and high-study trials with unsuccessful encoding, as well as between word miss trials with successful encoding and word hit trials with unsuccessful encoding. No hippocampal activations were observed for either of these contrasts, suggesting possible spatial overlap of hippocampus-mediated encoding and retrieval functions.
Hippocampal and Default Network Deactivation by Search
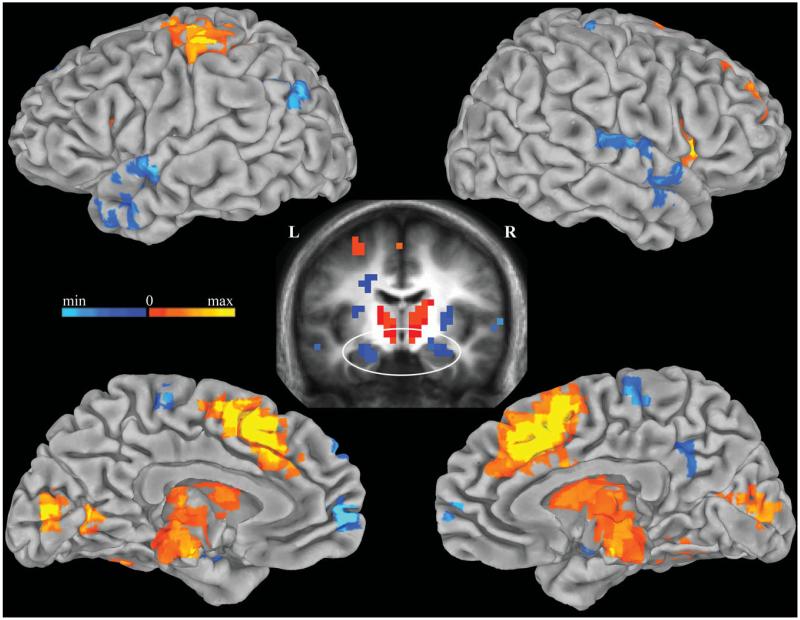
Although hippocampal activity differed between word conditions, time spent at study is correlated with both increased memory strength and reduced search requirements at retrieval; this inherent correlation renders it difficult to dissociate effects of strength and search based on study-level alone. Since response times are known to positively correlate with degree of memory search (Sternberg, 1966), trial-by-trial BOLD signal modulation by search was measured by correlating BOLD responses with response times across all word recognition trials. Bilateral anterior hippocampus, medial prefrontal cortex, posterior cingulate, superior temporal, and left inferior parietal cortex were negatively correlated with response times (P < 0.01), reflecting greater deactivation with longer response times (Fig. 4). To confirm that this correlation was not influenced by differences in response times between low-study and high-study trials, the analysis was performed separately on these conditions. Response times remained negatively correlated with activity in these regions for the high-study condition (P < 0.01), and for the low-study condition at a reduced threshold (P < 0.05). Neither study-level nor encoding success interacted with the reaction time correlation in these regions. Thus, hippocampal and default network activations were more strongly negative for trials requiring longer preresponse search times, and this dependence upon search duration was robust against differences in memory strength and encoding success.
FIGURE 4.
Correlation between BOLD signal and response times. Negative correlations between BOLD signal and response times are displayed in cool colors and positive correlations are shown in warm colors (P < 0.01). Significant clusters are overlaid on the pial surface of the Talaraich and Tournoux N27 average brain. A coronal cross-section (y = −10) of the mean anatomical image of all subjects (middle) displays clusters in bilateral anterior hippocampus negatively correlated with response times. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Connectivity with the Anterior Hippocampus
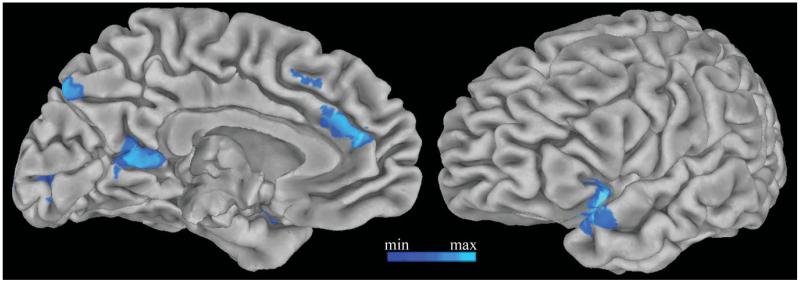
Activity in the anterior hippocampus and default network demonstrated a similar negative correlation with search duration, suggesting possible interactions between these regions that may vary according to attentional fluctuations across retrieval trials. Using a seed region of the structurally-defined bilateral anterior hippocampus, functional connectivity analysis was conducted to identify hippocampal network activity. To examine how hippocampal connectivity is modulated by study-level and encoding, whole-brain correlation maps were contrasted between low-study and high-study trials, and between encoded and not-encoded trials. Left dorsolateral prefrontal cortex, cingulate gyrus, and intraparietal sulcus were more strongly correlated with the hippocampus during the low-study condition (P < 0.01). Hippocampal connectivity with the medial prefrontal cortex, posterior cingulate, and left superior temporal cortex was stronger when pictures were not encoded than encoded (P < 0.01, Fig. 5). Together, these findings show that hippocampus and default network regions simultaneously deactivate with increasing search duration and functionally interact in a manner regulated by trial-by-trial encoding processes.
FIGURE 5.
Functional connectivity with the anterior hippocampus. Regions showing more strongly correlated activity with the bilateral anterior hippocampus during trials when pictures were not-encoded than encoded (P < 0.01) are displayed on the pial surface of the Talaraich and Tournoux N27 average brain. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Hippocampal Deactivation Tracks Encoding Performance
Prior findings suggest that regional deactivation of the hippocampus during effortful episodic memory retrieval may reflect suppression of encoding functions by memory search (Reas et al., 2011). The present study examined BOLD responses in anatomically defined hippocampal regions of interest during a combined intentional retrieval with incidental encoding task to determine whether retrieval effort (1) influences concurrent encoding and (2) modulates hippocampus-dependent encoding activity.
Consistent with previous reports, regions of the hippocampus exhibited reductions in activity and the degree of reduction was associated with recall performance. The anterior hippocampal deactivation observed during this recognition task has been previously documented during cued recall of visual (Israel et al., 2010) and verbal (Reas et al., 2011) paired associates. This consistently observed, retrieval-related reduction in hippocampal activity from baseline warrants further discussion. Although retrieval-related differences in hippocampal activity levels are frequently attributed directly to retrieval success, this interpretation fails to account for the finding of highest hippocampal activity before task onset. Any number of mental processes may be active during the less restricted task-free or lower-load inter-trial intervals, including mind wandering, reflection upon a previous trial or anticipation of the next. Though specific cognitive processes comprising the periods between retrieval trials are difficult to fully characterize, the prestimulus baseline would not be expected to elicit greater mnemonic retrieval than the retrieval task itself. It is therefore challenging to interpret the reduced BOLD response during recognition as a neural signature of memory retrieval without considering other factors.
The present study demonstrates that during retrieval the anterior hippocampus is also modulated by encoding of task-irrelevant information, exhibiting a larger negative deflection from baseline on word recognition trials during which background pictures were not encoded, relative to trials when pictures were successfully encoded. Simple subtraction of absolute activity level showed greater hippocampal activity for subsequently remembered versus forgotten task-irrelevant stimuli, extending prior evidence for involvement of the hippocampus and adjacent medial temporal lobe in incidental encoding of task-relevant novel (Stark and Okado, 2003) and task-irrelevant background stimuli (Huijbers et al., 2009). Taken together, the results support the hypothesis that baseline elevated hippocampal activity is associated with encoding processes, including those that encode the ongoing stream of task-irrelevant information, that are reduced during effortful retrieval.
Memory Search is Associated with Reduced Encoding and Deactivation of Hippocampus and Default Network
Hippocampal suppression during episodic memory retrieval has been attributed to retrieval effort and attentional engagement during the recall task, as it is present regardless of retrieval success and maximally deactivated for the attempted recall of weaker memories (Reas et al., 2011). Several lines of evidence from the current study support the proposal that more effortful retrieval attempts inhibit background encoding processes subserved by the anterior hippocampus, resulting in deactivation associated with both elevated search and impaired encoding function.
First, encoding performance depended upon the study-level of the memory to be retrieved, rather than retrieval success. Encoding was impaired for low-study recognition trials, relative to high-study trials, the former which are presumed to represent weaker memories and thus demand greater search effort at retrieval. These assumptions are supported by lower recognition and cued recall accuracy and longer response times for low-study than high-study words. Furthermore, responses were faster for trials with successful than unsuccessful encoding, indicating that encoding success was related with reduced search time. Notably, encoding was not influenced by recognition success, and the difference between the low-study and high-study conditions remained while holding retrieval success constant. Thus, the current findings suggest that although recognition success does not influence encoding, the attempted retrieval of lower strength memories disrupts incidental encoding of concurrent, task-irrelevant stimuli. These results differ from those of a prior study where successful recognition was associated with reduced incidental encoding accuracy and encoding-related medial temporal lobe activity (Huijbers et al., 2009). Variation in experimental design may partially account for these differences. For example, D′ scores for both the recognition and encoding tasks were substantially lower in the Huijbers et al. (2009) study than for the present low-study condition, raising the possibility that the recognition task used in the prior study was more attentionally demanding. Degree of search engagement might further be influenced by whether a stimulus is perceived as weakly familiar versus novel, even if both were previously encountered during study. It is possible that weakly familiar items might engage more search, and thus greater interference with concurrent encoding, than those items erroneously perceived as novel and rejected outright. Additionally, both retrieval search and success could interact competitively with encoding; though the present study did not identify an effect of retrieval success on incidental encoding, this does not exclude the possibility that retrieval success might also regulate encoding but that its effect here was below detection threshold. While the preceding interpretation supposes that fluctuations in retrieval-dependent attentional processes influence encoding, the reverse relationship, that encoding functions affect retrieval, is also possible. For example, interference from distracting stimuli has been shown to diminish recall performance and reduce the hippocampal response to retrieval (Wais et al., 2010a). In the present study, attentional capture by the external environment might monopolize attention resources to facilitate encoding while impairing retrieval, reducing retrieval accuracy when pictures are concurrently encoded. However, given that this effect was not observed, and that levels of distraction were balanced across trials, this scenario seems less likely.
Second, activity in the anterior hippocampus was simultaneously modulated by incidental encoding success and memory search, as approximated by retrieval response times (Sternberg, 1966). Longer search duration was associated with greater deactivation in bilateral anterior hippocampus and regions of the default network. Although trial-by-trial variability in response times cannot be cleanly dissociated from differences in memory strength, this finding suggests a direct relationship between suppression of these regions and the temporal duration of the retrieval effort. Other studies have presented similar evidence for a dependence of medial temporal lobe responses on attentional fluctuations during retrieval, in that retrieval demands that vary according to the present attentional focus correspondingly regulate medial temporal lobe responses to retrieval (Nee and Jonides, 2008).
Given prior evidence that the default network deactivates with focused, goal-directed tasks (Raichle et al., 2001; McKiernan et al., 2003), a correlated suppression of hippocampal and default network activity suggests that the encoding-related response in the anterior hippocampus might be mediated by the attentional demands of the retrieval task. Functional connectivity analysis confirmed that activity between the anterior hippocampus and default network regions was more strongly correlated when incidental encoding failed. The hippocampus has been found to correlate with the default network during episodic memory retrieval but not intentional encoding, such that the hippocampus activates during both successful encoding and retrieval, but the default network deactivates during successful encoding and unsuccessful retrieval (Vannini et al., 2010; Huijbers et al., 2011). However, in accordance with other reports of hippocampus-default network correlations when learning and retrieval occur simultaneously (Zeithamova et al., 2012), in the present study the hippocampus effectively tracked incidental encoding even while remaining coupled with regions of the default network. The present findings suggest that, beyond encoding or retrieval success, the degree of engagement in an intentional task may be an additional factor underlying functional correlations between the hippocampus and default network.
Concurrent Hippocampal Encoding and Retrieval Responses
Notably, the anterior to posterior extent of the hippocampus was more active during high-study than low-study trials as well as during hit relative to miss or correct rejection trials, consistent with a large body of literature illustrating that the hippocampus supports recognition success or retrieval of strong memories (for review see Eichenbaum et al., 2007). The detection of a concomitant encoding response does not necessarily invalidate the interpretation of such retrieval-related activations as veritable signatures of retrieval, as the hippocampus may subserve the balance between new learning and recovering old memories. For instance, reactivation of an old association can facilitate new learning (Zeithamova et al., 2012), while new learning can in turn diminish an original memory and the corresponding posterior hippocampal retrieval response (Kuhl et al., 2010). The present study extends support for a complex interplay between ongoing hippocampus-mediated encoding and retrieval processes, which may depend upon the successful recovery of old or the formation of new memories, the strength of the memory trace or the attentional processes supporting memory operations.
The dissociable anterior, middle, and posterior hippocampal responses further suggests that the structure supports both encoding and retrieval, but that these functions may be subserved by distinct subregions. BOLD responses in anterior regions were modulated by encoding while those in posterior regions were predominantly modulated by retrieval. This finding is consistent with prior human neuroimaging studies reporting an antero-posterior functional gradient that dissociates encoding and retrieval functions (Lepage et al., 1998; but see Schacter and Wagner, 1999; Prince et al., 2005), novelty versus recollection or memory for prior experiences (Daselaar et al., 2006; Poppenk et al., 2010), or content-specificity (Liang et al., 2012). In addition to hippocampal subfield specialization documented in animals (Kesner et al., 2004; Daumas et al., 2005; Leutgeb et al., 2007) and humans (Eldridge et al., 2005; Chen et al., 2011; Lacy et al., 2011), anterior versus posterior specialization is in agreement with distinct anatomical projections (Aggleton, 2012) and cytoarchitectonic and gene expression profiles (Fanselow and Dong, 2010) along the longitudinal axis of the hippocampus. The anterior overlap of encoding- and retrieval-related activations in the present study underscores the possibility that the hippocampus may actively continue to process or filter information from the external environment even when task demands do not require conscious encoding, and thus highlights the importance of considering contributions of underlying encoding-related activity when interpreting responses during retrieval.
CONCLUSIONS
The present study identified an anterior hippocampal BOLD signal sensitive to incidental encoding that overlapped with responses to retrieval success, strength and memory search. Reduced memory strength and extended search efforts were associated with impaired encoding performance and anterior hippocampus and default network deactivation, demonstrating that encoding-related processing in the hippocampus may be regulated by attentional engagement during retrieval. Together, these results suggest that encoding of task-irrelevant information remains active during intentional memory retrieval, influences activity in the anterior hippocampus and depends upon memory search. Such findings may explain why hippocampal activity is reduced from baseline during retrieval and most strongly deactivated during unsuccessful retrieval, highlighting potentially significant contributions of encoding functions to hippocampal responses during episodic memory retrieval.
Acknowledgments
Grant sponsor: National Institute of Neurological Disorders and Stroke
Grant sponsor: The General Electric Medical Foundation and the University of California, San Diego; Grant numbers: NINDS K02, NS067427.
REFERENCES
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Atkison RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The Psychology of Learning and Motivation: Advances in Research and Theory. Academic Press; New York: 1968. [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18:523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera BS, Amdur RJ, Patel P, Mendenhall WM. A radiation oncologist’s guide to contouring the hippocampus. Am J Clin Oncol. 2009;32:20–22. doi: 10.1097/COC.0b013e318178e4e8. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Frances B, Lassalle JM. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem. 2005;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Sadanand A, Davachi L. Memory’s penumbra: Episodic memory decisions induce lingering mnemonic biases. Science. 2012;337:485–487. doi: 10.1126/science.1221936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M, Van Roost D, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Glover JA. The testing phenomenon—Not gone but nearly forgotten. J Edu Psychol. 1989;81:392–399. [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. When learning and remembering compete: A functional MRI study. PLoS Biol. 2009;7:e11. doi: 10.1371/journal.pbio.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6:e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Israel SL, Seibert TM, Black ML, Brewer JB. Going their separate ways: Dissociation of hippocampal and dorsolateral prefrontal activation during episodic retrieval and post-retrieval processing. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2009.21198. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Shrager Y, Squire LR. Medial temporal lobe activity can distinguish between old and new stimuli independently of overt behavioral choice. Proc Natl Acad Sci U S A. 2009;106:14617–14621. doi: 10.1073/pnas.0907624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309(5734):619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cereb Cortex. 2013;23:80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci U S A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci U S A. 2008;105:14228–14233. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FI, Moscovitch M. Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci. 2010;30:4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas ET, Gimbel SI, Hales JB, Brewer JB. Search-related suppression of hippocampus and default network activity during associative memory retrieval. Front Hum Neurosci. 2011;5:112. doi: 10.3389/fnhum.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci. 2008;20:432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: Evidence from positron emission tomography. Proc Natl Acad Sci U S A. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Storm BC, Bjork EL, Bjork RA, Nestojko JF. Is retrieval success a necessary condition for retrieval-induced forgetting? Psychon Bull Rev. 2006;13:1023–1027. doi: 10.3758/bf03213919. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tornoux P. Georg Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging; p. p122. [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352–373. [Google Scholar]
- Vannini P, O’Brien J, O’Keefe K, Pihlajamaki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2011;21:22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wais PE. Hippocampal signals for strong memory when associative memory is available and when it is not. Hippocampus. 2011;21:9–21. doi: 10.1002/hipo.20716. [DOI] [PubMed] [Google Scholar]
- Wais PE, Rubens MT, Boccanfuso J, Gazzaley A. Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. J Neurosci. 2010a;30:8541–8550. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. J Cogn Neurosci. 2010b;22:109–123. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yu SS, Johnson JD, Rugg MD. Hippocampal activity during recognition memory co-varies with the accuracy and confidence of source memory judgments. Hippocampus. 2012;22:1429–1437. doi: 10.1002/hipo.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]