Abstract
Rationale
Endothelial dysfunction inflicted by inflammation is found in a host of cardiovascular pathologies. One hallmark event in this process is the aggregation and adhesion of leukocyte to the vessel wall mediated by the up-regulation of adhesion molecules (CAM) in endothelial cells at the transcriptional level. The epigenetic modulator(s) of CAM transactivation and its underlying pathophysiological relevance remain poorly defined.
Objective
Our goal was to determine the involvement of Brg1 and Brm in CAM transactivation and its relevance in the pathogenesis of atherosclerosis.
Methods and Results
In the present study, we report that pro-inflammatory stimuli augmented the expression of Brg1 and Brm in vitro in cultured endothelial cells and in vivo in arteries isolated from rodents. Over-expression of Brg1 and Brm promoted whereas knockdown of Brg1 and Brm abrogated transactivation of adhesion molecules and leukocyte adhesion induced by inflammatory signals. Brg1 and Brm interacted with and were recruited to the CAM promoters by NF-κB/p65. Conversely, depletion of Brg1 and Brm disrupted the kinetics of p65 binding on CAM promoters and crippled CAM activation. Silencing of Brg1 and Brm also altered key epigenetic changes associated with CAM transactivation. Of intrigue, 17β-estradiol antagonized both the expression and activity of Brg1/Brm. Most importantly, endothelial-targeted elimination of Brg1/Brm conferred atheroprotective effects to Apoe−/− mice on a Western diet.
Conclusion
Therefore, our data suggest that Brg1 and Brm integrate various pro-inflammatory cues into CAM transactivation and endothelial malfunction and as such may serve as potential therapeutic targets in treating inflammation related cardiovascular diseases.
Keywords: Endothelial dysfunction, inflammation, atherosclerosis, gene regulation, adhesion molecules
INTRODUCTION
Endothelial dysfunction is a prominent feature and in many cases serves as a direct cause for cardiovascular disease (CVD), owing largely to the fact that vascular endothelium provides a physical and functional sanctuary to the wholesomeness of the cardiovascular system.1, 2 One of the early events shared among different CVD is active recruitment of leukocytes to the vascular wall.3 Circulating leukocytes normally are unable to interact with the vascular endothelium due to insufficient surface contact. Under pathological circumstances, induction of adhesion molecules (CAM) primarily at the transcriptional level in endothelial cells allows the leukocytes to adhere and aggregate at focal points and perpetuates the maladaptive response.4
Mounting evidence has correlated inflammation with impaired endothelial functionality and ensuing cardiovascular mishaps.5 A host of inflammatory factors act either singularly or synergistically to amend the vascular endothelial transcriptome as means of advancing their pathogenic agenda. Specifically, increased CAM expression by pro-inflammatory stimuli has been observed in a number of cardiovascular pathologies, which include atherosclerosis, hypoxia-induced pulmonary hypertension, and acute lung injury.6–8 The nuclear factor NF-κB has emerged as a central coordinator key to the transactivation of CAM genes in response to inflammatory cues.9 The co-factors involved in this process that fine-tune NF-κB dependent CAM activation and their pathophysiological relevance in CVD, however, are not well understood.
Cardiovascular episodes are intimately associated with epigenetic alterations on promoters that ultimately lead to the switch of the vascular transcriptome and consequently development of CVD. Brahma related gene 1 (Brg1) and Brahma (Brm) are core components of the chromatin remodeling complex that utilizes ATP hydrolysis to alter the positioning of nucleosomes and in so doing dictates the outcome of transcription events taking place within the vasculature.10 Smale and colleagues have previously reported that Brg1 and Brm are involved in LPS-induced inflammatory response in macrophages.11 Recent investigations have implicated Brg1 and Brm in both organogenesis and disease development in the cardiovascular system.12–14 Here we report that augmented expression Brg1/Brm was observed in inflammation-challenged endothelial cells in vitro and in vivo. Brg1 and Brm, functioning as co-factors for NF-κB, help orchestrate key epigenetic events that result in CAM transactivation. Silencing of Brg1/Brm in vivo contributes to alleviated phenotypes in an animal model of atherosclerosis. Therefore, our data provide clear evidence implicating Brg1/Brm as a common link in inflammation induced endothelial dysfunction.
METHODS
An expanded Methods section can be found in the online data supplement. Briefly, human umbilical vein endothelial cell (HUVEC/EAhy926), human primary aortic cells (HAEC), human embryonic kidney cell (HEK293), and the Brg1/Brm-negative adenoma cell (SW-13) were maintained according to the vendors’ recommendations. Promoter activity was measured by transfection reporter assays. Expression of mRNA and protein was measured by real-time quantitative PCR and Western blotting, respectively. Interaction between endothelial cells and leukocytes was assessed by leukocyte adhesion assay in vitro and immunostaining in vivo. Knockdown of endogenous proteins was mediated by either small interfering RNA (siRNA) or short hairpin RNA (shRNA) carried by lentiviral particles. Protein binding to DNA was assayed by DNA affinity pull-down assay and chromatin immunoprecipitation (ChIP) assay. Apoe−/− mice were fed a high-fat diet for 6–8 weeks to induce atherosclerosis. All animal experiments have been performed following guidelines by the intramural Committee on Ethic Conduct of Animal Studies.
RESULTS
Pro-inflammatory stimuli activate the expression of Brg1 and Brm in endothelial cells
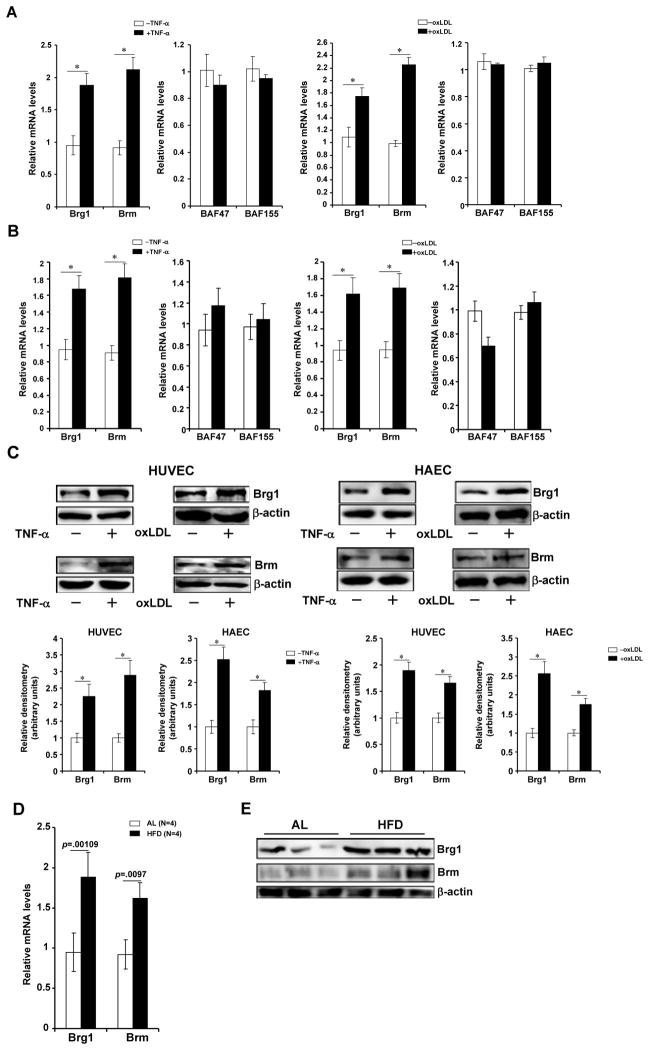
In order to assess the relevance of Brg1/Brm in the activation of adhesion molecules in endothelial cells, we first measured the expression of Brg1 and Brm in response to pro-inflammatory stimuli. Treatment of human umbilical endothelial cells (HUVEC/EAhyp26) with TNF-α, oxLDL, or LPS all led to significant induction of Brg1 and Brm levels (Fig. 1A, 1C, Online Figure IA, IB). Similarly, inflammatory treatments induced Brg1/Brm expression in human primary endothelial cells (Figs.1B, 1C, Online Figure IA, IB). In contrast, levels of BAF47 and BAF155, another two components of the mammalian SWI/SNF complex, were not significantly altered (Figs.1A, 1B, Online Figure IA). Interestingly, 17β-estradiol (E2), known to curb the inflammatory response in the vascular endothelium,15 tampered the up-regulation of Brg1/Brm expression (Online Figure IC). More importantly, Brg1 and Brm levels were elevated in arteries in vivo in rodents challenged with inflammatory stress (Figs.1D, 1E, Online Figure ID). Collectively, these data illustrate that expression levels of Brg1 and Brm might be correlated with intracellular inflammatory state.
Figure 1. Pro-inflammatory stimuli activate the expression of Brg1 and Brm in endothelial cells.
(A–C) Human umbilical vein endothelial cells (A) and primary aortic endothelial cells (B) were treated with TNF-α (10ng/ml) for 3 hours or oxLDL (100μg/ml) for 24 hours. mRNA (A, B) and protein (C) levels of Brg1 and Brm were probed with qPCR and Western respectively. Protein quantifications were performed with Image Pro based on three independent experiments. (D, E) Apoe−/− mice were fed a Western (HFD) or a control (AL) diet for 8 weeks. Aortic arteries were isolated and Brg1/Brm expression was probed by qPCR (D) and Western (E). Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
Brg1 and Brm are involved in the transcriptional activation of adhesion molecules by pro-inflammatory stimuli
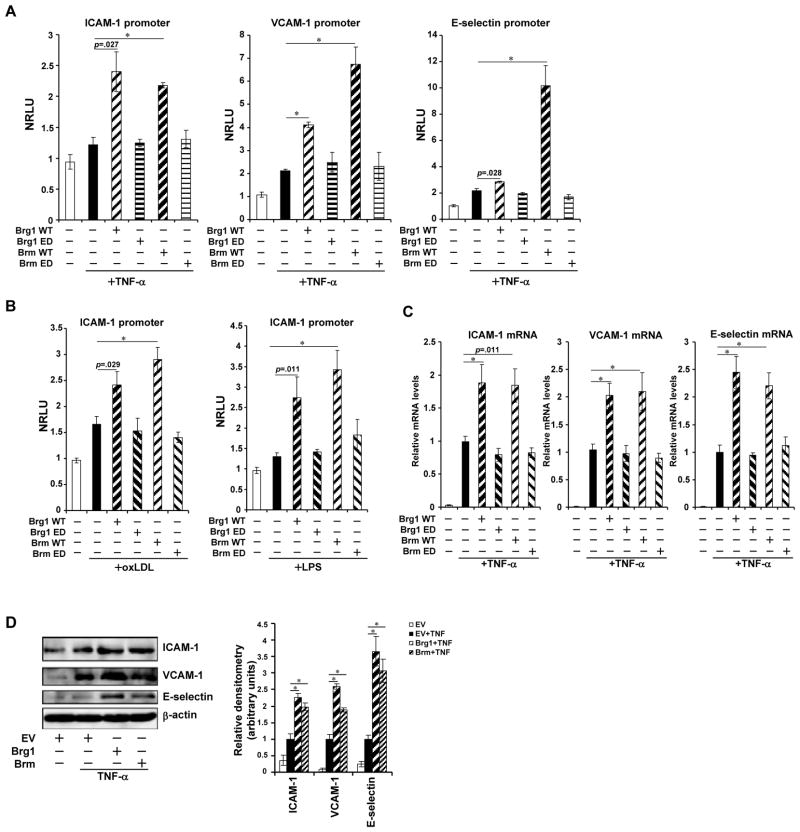
Having observed that the expression of Brg1 and Brm was up-regulated by pro-inflammatory stimuli in endothelial cells, we next evaluated the role of Brg1 and Brm in inflammation-induced CAM expression. By themselves, neither Brg1 nor Brm impacted promoter activities of adhesion molecules; they, however, potently augmented promoter activities of adhesion molecules in the presence of an individual pro-inflammatory signal (Online Figure IIA). The catalytic moiety of Brg1/Brm was clearly required for their function as enzyme deficient forms of Brg1 and Brm failed to activate TNF-α dependent transcription of CAM genes (Fig. 2A). Co-expression of Brg1 and Brm resulted in additional activation of CAM promoters (Online Figure IIB). Similar results were obtained in HUVECs treated with oxLDL or LPS (Fig. 2B), suggesting that Brg1/Brm may be a common link in inflammation induced transactivation of CAMs. In further support of this notion, over-expression of Brg1 and Brm in HUVECs enhanced activation of endogenous CAM expression in the presence of pro-inflammatory stimuli both at the mRNA level (Figs.2C, Online Figure IIC) and the protein level (Figs.2D, Online Figure IID). To further confirm the correlation between Brg1/Brm and CAM transactivation, we employed SW-13 cells in which both Brg1 and Brm are lacking. Ectopic expression of Brg1 and Brm significantly enhanced CAM induction by TNF-α (Online Figure IIE–IIG).
Figure 2. Brg1 and Brm enhance transcriptional activation of adhesion molecules by pro-inflammatory stimuli.
(A) Promoter luciferase fusion constructs for ICAM-1, VCAM-1 and E-selectin genes were transfected into HUVECs with either wild type (WT) or enzyme deficient (ED) Brg1 or Brm as indicated followed by treatment with TNF-α. Luciferase activities were expressed as normalized relative luciferase unit (NRLU). (B) ICAM-1 promoter construct was transfected into HUVECs with either WT or ED Brg1 or Brm followed by treatment with oxLDL or LPS as indicated. Luciferase activities were expressed as NRLU. (C, D) Brg1 or Brm expression constructs were transfected into HUVECs followed treatment with TNF-α. CAM levels were assessed by qPCR (C) and Western (D). Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
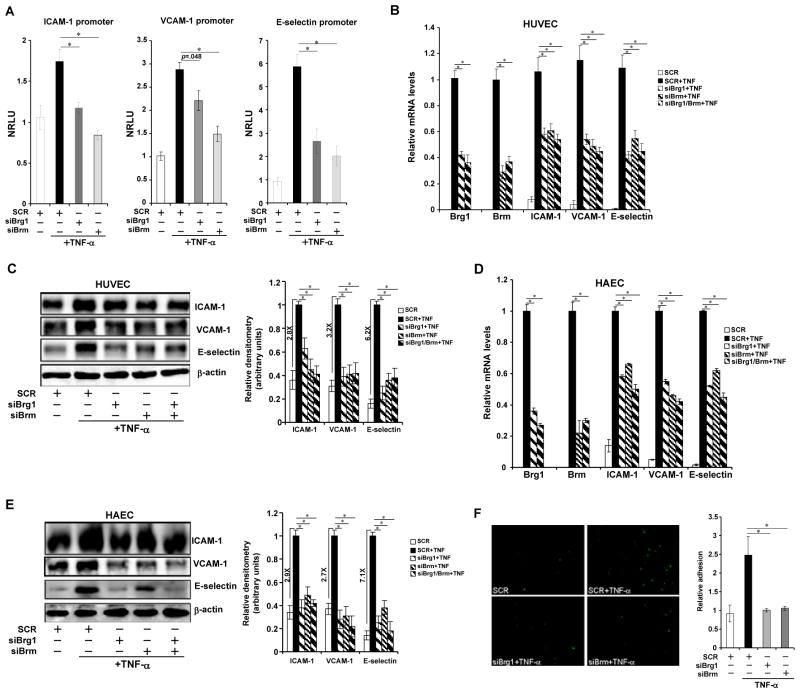
In contrast, knockdown of Brg1/Brm by either small interfering RNA (siRNA, Fig. 3A; Online Figure IIIA for validation) or short hairpin RNA16 (shRNA, Online Figure IIIB) attenuated promoter activities of all three CAM genes. In keeping with reduced CAM transcription, depletion of Brg1/Brm abrogated mRNA and protein levels of CAMs in HUVECs (Figs.3B, 3C, Online Figure IIIC) and HAECs (Figs.3D, 3E, Online Figure IIID) treated with TNF-α. Of note, co-depletion of Brg1 and Brm did not achieve additional suppression, indicating that proteins other than Brg1/Brm may become a rate-limiting factor when Brg1/Brm levels are sufficiently low. As a result, adhesion of leukocytes was severely crippled in the absence of Brg1/Brm (Fig. 3F). Consistently, knockdown of Brg1/Brm also abolished activation of CAM message levels by oxLDL (Online Figure IIIE, IIIF) or LPS (Online Figure IIIG, IIIH). Taken together, these data outline a scenario wherein Brg1 and Brm are intimately involved in the transactivation of CAMs by pro-inflammatory stimuli.
Figure 3. Depletion of Brg1 or Brm attenuates transcriptional activation of adhesion molecules by pro-inflammatory stimuli.
(A) Promoter luciferase fusion constructs for ICAM-1, VCAM-1 and E-selectin genes were transfected into HUVECs with either siRNA targeting Brg1 or Brm or scrambled siRNA (SCR) as indicated followed by treatment with TNF-α. Luciferase activities were expressed as NRLU. *, p<.05 (B–E) HUVECs were transfected with siBrg1, siBrm, or SCR followed by treatment with TNF-α. mRNA (B) and protein (C) levels of CAMs were assessed by qPCR and Western. Similar experiments were performed in HAECs (D, E). *, p<.05 (F) HUVECs were transfected with siBrg1, siBrm, or SCR followed by treatment with TNF-α. Leukocyte adhesion assay was performed as described under Methods. Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
Brg1 and Brm interact with and are recruited to the promoters by p65 and stabilize p65
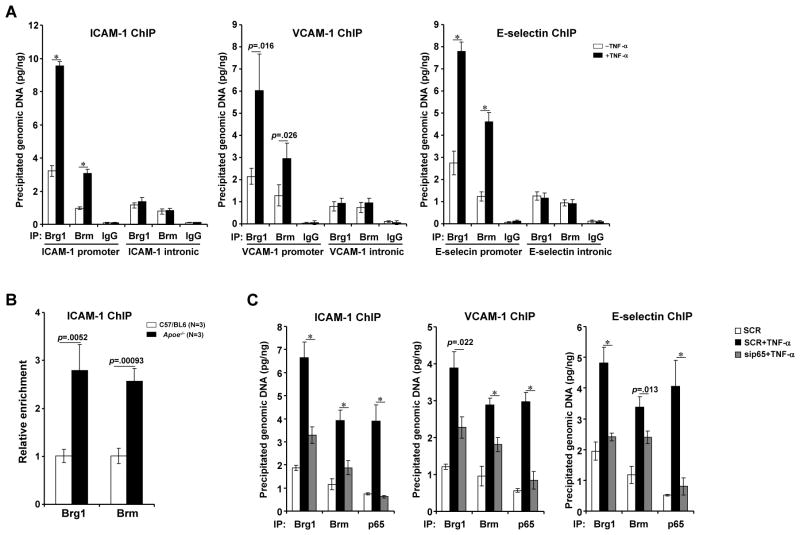
All three promoter constructs used in this study (Fig. 2A) harbor a conserved NF-κB site, alluding to the possibility that Brg1 and Brm may interact with and be recruited to the CAM promoters by NF-κB. Indeed, co-immunoprecipitations confirmed that Brg1/Brm formed a complex with p65 (Online Figure IVA, IVB). Inflammatory stimuli augmented occupancies of Brg1 and Brm to regions where NF-κB/p65 binds on all three CAM promoters as evidenced by ChIP assays (Figs.4A, Online Figure IVC). In contrast, there was less binding of Brg1/Brm on the intronic regions of CAM genes and the binding was not responsive to inflammatory signals (Fig. 4A and data not shown). Importantly, ex vivo ChIP assay revealed that there was more binding of Brg1 and Brm on the ICAM-1 promoter in arteries isolated from mice that developed atherosclerosis (Fig. 4B). Of note, Consistent with its ability to curtail the expression of Brg1/Brm, 17β-estradiol also disrupted the binding of Brg1 and Brm to the CAM promoters (Online Figure IVD).
Fig. 4. Brg1 and Brm interact with and are recruited to the promoters by p65.
(A) HUVECs were treated with TNF-α for 3 hours. ChIP assays were performed with anti-Brg1, anti-Brm, or IgG. Precipitated DNA was amplified by primers spanning the promoter or intronic region of indicated CAM gene. (B) C57/BL6 or Apoe−/− mice were fed a HFD diet for 8 weeks. Aortic arteries were isolated and ChIP assays were performed with indicated antibodies. N=3 for each group (C) HUVECs were transfected with sip65 or SCR followed by treatment with TNF-α for 3 hours. ChIP assays were performed with indicated antibodies. Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
To further probe the requirement of p65 for the recruitment of Brg1/Brm, DNA affinity pull-down assays were performed with nuclear protein extracted from HUVECs and HAECs. TNF-α treatment led to an increase in the binding of Brg1/Brm to a biotin-labeled ICAM-1 probe, which was abolished by cold oligos containing the p65 consensus sequence (Online Figure IVE). Similar observations were made in 293 cells transfected with exogenous Brg1 and Brm (Online Figure IVF). Finally, ablation of p65 by siRNA significantly hampered the recruitment of Brg1/Brm to the CAM promoter as demonstrated by ChIP assays (Fig. 4C) and blunt the enhancement of CAM transactivation (Online Figure IVG, IVH).
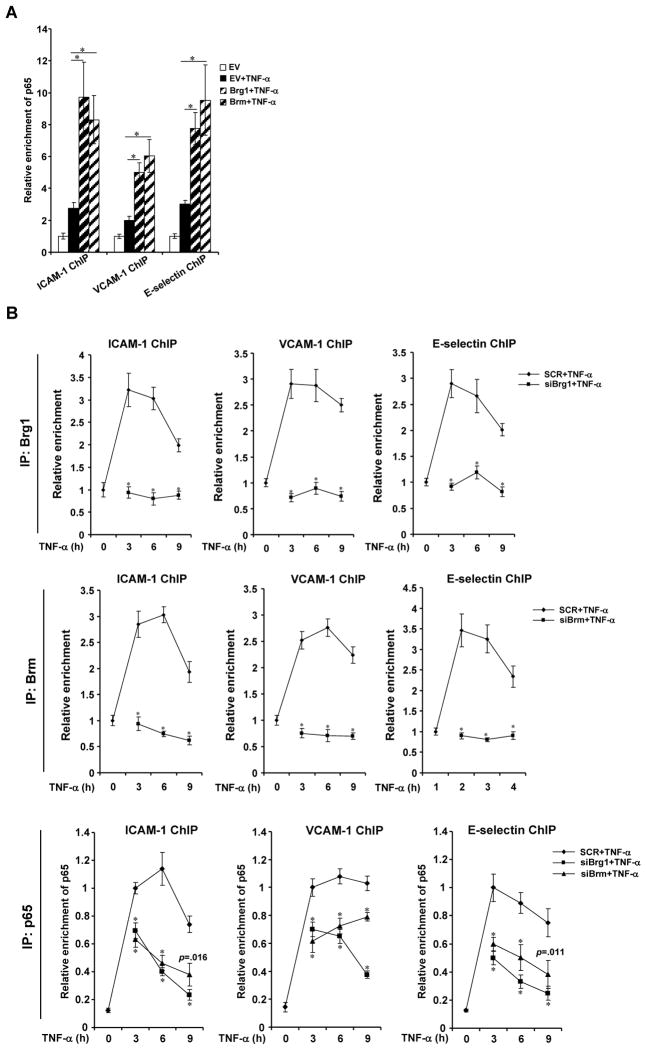
We next tackled the question whether Brg1 and Brm could affect the binding of p65 on CAM promoters in response to inflammatory signals. Ectopic expression of Brg1 or Brm in Brg1/Brm-negative SW13 cells significantly enhanced the occupancy of p65 on the CAM promoters as demonstrated by both ChIP analysis (Fig. 5A) and DNA-affinity pull-down assay (Online Figure VA, VB), indicating that Brg1 and Brm may facilitate the binding of p65. Meanwhile, treatment of endothelial cells with TNF-α led to a strong and sustained recruitment of p65 on all three CAM promoters up to 9 hours post exposure. Knockdown of Brg1 or Brm markedly accelerated the clearance of p65 from the CAM promoters in ChIP assays (Fig. 5B, Online Figure VC, VD). In aggregate, this line of data support a dynamic complex formed by Brg1, Brm, and p65 on the CAM promoters in response to inflammatory cues.
Figure 5. Brg1 and Brm stabilize p65 binding to the CAM promoters.
(A) SW-13 cells were transfected FLAG-Brg1 or FLAG-Brm followed by treatment with TNF-α. ChIP assays were performed with anti-p65. (B) HUVECs were transfected with siBrg1, siBrm, or SCR followed by treatment with TNF-α. Cells were harvested at indicated time points and ChIP assays were performed with anti-p65. Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
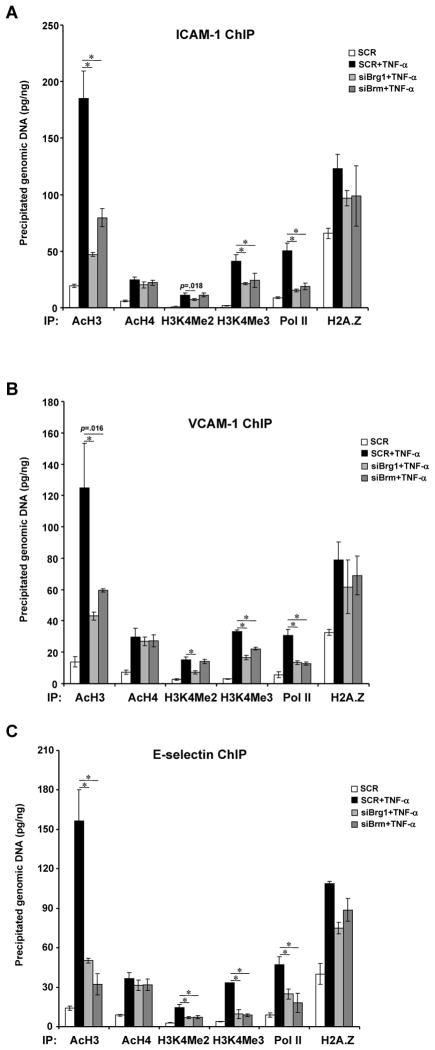
Brg1 and Brm coordinate key epigenetic alterations in response to pro-inflammatory stimuli
Since Brg1 and Brm constitute a key component of the epigenetic machinery, we examined how depletion of Brg1 and Brm would impact the chromatin structure of the CAM genes. Specific targeting siRNA for Brg1 or Brm significantly reduced their association with the CAM chromatin in response to TNF-α (Online Figure VIA). Consequently, active histone markers including acetylated histone H3 and methylated histone H3 lysine 4 were erased (Figs.6A–6C). This was accompanied by impaired recruitment of the basic transcriptional machinery as evidenced by inefficient binding of RNA Polymerase II. Curiously, levels of the histone variant H2A.Z that is commonly associated with active transcription was only modestly affected. In addition, elimination of Brg1 and Brm also abrogated active histone modifications and disrupted Pol II binding on the CAM chromatin when endothelial cells were treated with oxLDL (Online Figure VIB, VIC) or LPS (Online Figure VID). In all, these data strongly suggest that Brg1 and Brm contribute to CAM transactivation by maintaining a transcriptionally friendly chromatin environment for the accession of RNA polymerase.
Figure 6. Brg1 and Brm integrate key epigenetic events taking place on the CAM promoter in response to inflammation.
HUVECs were transfected with siBrg1, siBrm, or SCR followed by treatment with TNF-α. ChIP assays were performed with indicated antibodies. Data are shown as mean +SD of triplicates and are representative of one experiment out of three performed.
Brg1 and Brm deficiency averts detrimental effects exerted by pro-inflammatory stimuli in vivo
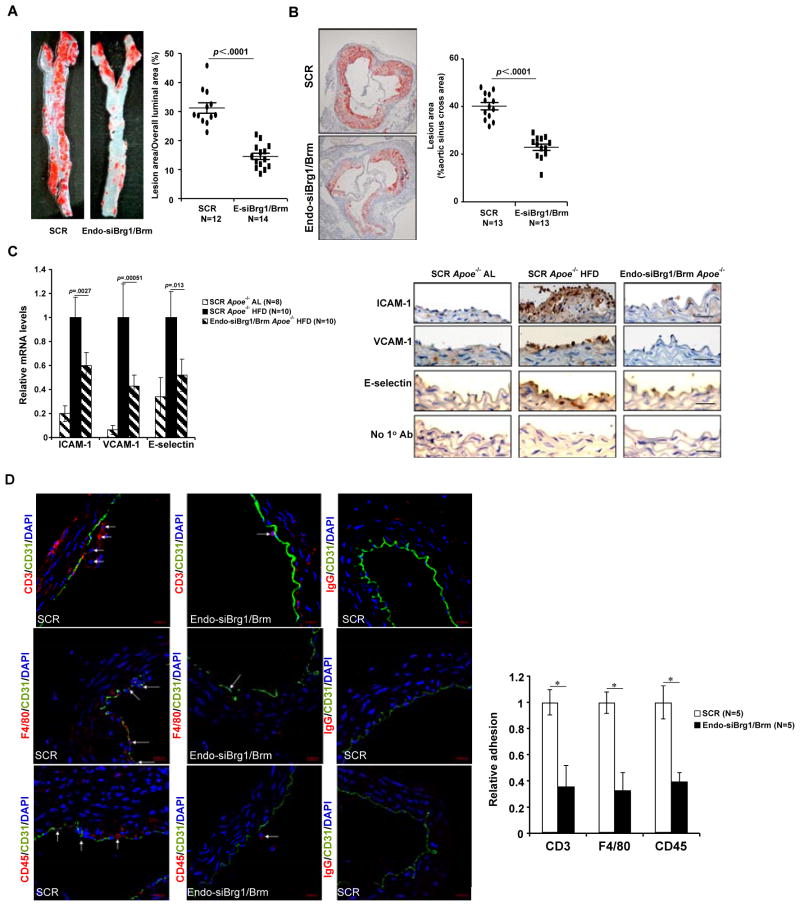
Finally, we probed the in vivo relevance of Brg1- and Brm-mediated CAM transactivation in atherosclerosis. To this end, we employed an endothelial-specific targeting system to knock down Brg1/Brm in vivo. Indeed, infection of in vitro cultured endothelial cells but not embryonic kidney cells resulted in suppression of Brg1/Brm expression (Online Figure VIIA). Moreover, immunostaining also revealed marked down-regulation of Brg1/Brm expression in the vascular endothelium (Online Figure VIIB), but not in circulating leukocytes (Online Figure VIIC). With endothelial-specific silencing of Brg1/Brm, there was a significant reduction in atherosclerotic lesion in mice (Figs.7A, 7B). Concomitantly, expression of ICAM1, VCAM1, and E-selectin was normalized in the aortic arteries of atherosclerotic mice (Fig. 7C). Consequently, adhesion of CD3+ lymphocytes, CD45+ activated leukocytes, and F4/80+ macrophages were reduced (Fig. 7D). In aggregate, these data clearly support the notion that silencing of Brg1 and Brm stalled development of atherosclerosis by normalizing endothelial function in vivo.
Figure 7. Brg1 and Brm depletion alleviates atherosclerotic lesion in Apoe−/− mice.
Apoe−/− mice were injected endothelial-specific lentivirus targeting Brg1/Brm (Endo-siBrg1/Brm) or scrambled sequence (SCR) and fed a Western (HFD) diet for 8 weeks to induce atherosclerosis as described under Methods. N=12–14 mice for each group. (A) Representative images of thoracic aorta isolated from mice injected with SCR or siBrg1/Brm and stained with oil red O. (B) Representative images of aortic sinus stained with oil red O. (C) Expression levels of CAM genes in aortic arteries were assessed by qPCR (N=8–10 mice for each group) and immunohistochemistry (N=5 mice for each group; representative images are shown). Scale bar, 20μM (D) Representative images of immunofluorescence staining of aortic arteries with indicated antibodies. Scale bar, 20μm. Arrows, immune cells adhered to the endothelium. Quantifications were performed using Image Pro and data are expressed as relative adhesion.
DISCUSSION
Inflammation triggered, endothelium dependent recruitment of leukocyte to the vessel wall and consequently the establishment of a pro-inflammatory milieu within the vasculature represents a hallmark event in the pathogenesis of a range of CVD.17 Key to this process is the transactivation of adhesion molecules mediated by NF-κB in response to inflammatory stimuli. Here we report that epigenetic factors Brg1 and Brm contribute to inflammation induced CAM transactivation and are critically involved in the pathogenesis of atherosclerosis.
By shaping up the chromatin structure, Brg1 and Brm play a versatile role in transcriptional regulation. Our data indicate that Brg1 and Brm represent an integral part of the pro-inflammatory program initiated by divergent injurious signals. Brg1 and Brm seem to be crucial in orchestrating key histone modifications indicative of an active transcription state that correlates with CAM up-regulation (Figure 6 and Figure S6B). It has been well documented that Brg1 and Brm are required for the recruitment and/or stabilization of several enzymes that catalyze the active histone marks including MLL1 (H3K4 methylation), CBP (histone H3 and H4 acetylation), and p300 (histone H3 and H4 acetylation).18–21 These modifications combined likely set up the stage for the recruitment of the basic transcriptional machinery. In fact, two recent papers highlight the importance of Brg1/Brm in stress induced gene expression. Naito et al provide evidence that ischemic stress harnesses Brg1 to induce the expression in TNF–α and MCP-1 in renal epithelial cells.22 Meanwhile, He et al point to the indispensability of Brg1 in HPV18 E6/E7 gene expression in HeLa cells.23 Of particular interest, while Brg1 is responsible for RNA Pol II recruitment to the TNF-α and MCP-1 promoters in response to ischemia without altering histone status, acetylation of histones H3 and H4 is clearly dependent on Brg1 for E6/E7 transactivation, which is more in line with our data presented here. Relying on extracellular and intracellular cues, Brg1 and Brm can be engaged in both active and repressive chromatin modification by switch binding partners.24–26 Paradoxically, Fish et al have reported that Brg1 helps restore eNOS expression during hypoxia/reoxygenation cycle by preventing the eviction of acetylated histones H3 and H4 on the eNOS promoter in endothelial cells27, indicating the Brg1 is beneficial in maintaining the vascular tone under stress conditions. These discrepancies reflect the gene-, cell-, and/or signal-specific nature of transcriptional regulation mediated by Brg1/Brm and need to be clarified by further research.
One of the intriguing issues regarding regulation of human pathobiology by Brg1 and Brm is their functional redundancy, i.e., how well one can compensate for the loss of the other both in embryogenesis and adult life. Brg1 and Brm clearly assume opposing roles in osteoblast differentiation.28 On the other hand, whereas Brm null mice are viable with slight overweight owing to increased cell proliferation, whole-body or tissue-specific Brg1 deletion causes lethality in mice, arguing for the irreplaceable role of Brg1 and as such lending support to the notion that Brm is non-essential in organogenesis especially in the cardiovascular system.13, 29–33 There has been, however, few definitive report dissecting the role of Brg1 and Brm in post-embryonic life. In hepatocyte, Brm, but not Brg1, is required for hypoxia induced transactivation of the erythropoietin gene and for the activation of Cyp7a1 gene in cholesterol metabolism. 34, 35 Hang et al demonstrate, by using a tetracycline inducible system that switches off Brg1 expression in the heart in adult mice, that Brg1 depletion greatly improves, but not completely abolishes, pressure overload induced cardiac hypertrophy, leaving open the question whether Brm could be responsible for the residual cardiomyocyte oversize observed in these mice.13 In the present study, we have observed that Brg1 and Brm play differential roles in regulating CAM transcription in response to different stimuli. For instance, knock-down of Brg1 does not appear to complete abrogate VCAM promoter activity/mRNA induced by TNF to basal levels (Fig. 3A, 3B); on the other hand, Brg1 siRNA dramatically down-regulates VCAM mRNA levels induced by LPS to below basal levels (Online Figure IIIG). This clearly reflects the complicated interplay between Brg1/Brm, specific gene/chromatin structure, and the upstream signal. Several key factors may contribute to the target specificity and hence biological relevance for Brg1 and Brm. Structurally, Brg1 differs from Brm in that Brg1 possesses a unique N-terminal domain that confers to Brg1 the ability to interact with and be recruited by zinc finger-type transcription factors such as KLF and GATA.36 Alternatively, tissue-specific expression/distribution of Brg1 and Brm may also play a role in their dedicated functionality. Compared with Brg1 that is preferentially expressed in epithelial cells, Brm expression is more enriched in smooth muscle and endothelial cells in healthy humans.37 It is noteworthy that under pathological conditions, the expression patterns of Brg1 and Brm could be significantly altered (Figure 1 and Online Figure I). We demonstrate here that there were subtle differences in CAM transactivation by Brg1 and Brm. Overall, however, there was little difference in the dependency of leukocyte adhesion on Brg1 or Brm in cultured endothelial cells (Figures 3F and data not shown), consistent with previous findings that Brg1 and Brm are equally important in maintaining the contractile phenotype of smooth muscle cells in vitro.38 Recently, it has been demonstrated that SMC-specific knockout of Brg1 on a Brm-null background led to increased neonatal death and an aggravated phenotype in surviving mice, strongly supporting a Brg1-independent role for Brm in the vasculature in vivo.32 Since we targeted both Brg1 and Brm in our animal models, it remains to be determined whether they contribute differentially to inflammation inflicted endothelial injury in vivo. High-throughput ChIP-seq profiling of Brg1/Brm binding in endothelial cells under different stress cues (LPS vs TNF-α) will enable a thorough analyses of the differential contribution of Brg1/Brm to gene expression.
Our data also revealed some subtle differences between Brg1 and Brm in terms of CAM transactivation by TNF–α For instance, Brm induced VCAM-1 and E-selectin promoter activities better than Brg1 in the presence of TNF–α (Figure 2A). As shown in Figures 1C and 1E, endogenous Brm levels appear to be lower than endogenous Brg1 levels, possibly rendering the cells more sensitive to the introduction of exogenous Brm than to Brg1 in terms of promoter activity. On the other hand, it is noteworthy that we used partial deletions of the CAM promoter constructs (ICAM-1, −393/+42, VCAM-1, −519/+20, and E-selectin, −188/+25) with the NF-κB binding motif included, which may not give the whole picture wherein Brg1/Brm regulate endogenous gene transcription. Indeed, endogenous VCAM-1 and E-selectin mRNA (Figure 2C) and protein (Figure 2D) levels respond to Brg1 over-expression and Brm over-expression equally well. The only way to compare the post-embryonic functionalities of Brg1 and Brm in the endothelium would be to generate an inducible (e.g., dox or estrogen) Cre line similar to the one used by Hung et al.13
To our best knowledge, the results as summarized in this manuscript represent heretofore the first direct evidence that links Brg1/Brm dependent CAM transactivation in endothelial cells to the pathogenesis of atherosclerosis (Figure 7) in mice. Brg1 and Brm have been shown to exert cell-specific effects in the cardiovascular system.11, 13, 32 Therefore, non-selective depletion of Brg1/Brm in vivo might have engendered equivocal data interpretation. For instance, atherosclerosis is widely believed pathology of inflammation. It is conceivable that, macrophage-specific Brg1/Brm, rather than endothelial-specific Brg1/Brm, contribute to atherogenesis since Brg1 and Brm are essential for NF-κB/p65 dependent production of IL-1, IL-6, and MCP-1.11, 22 Our endothelial-specific Brg1/Brm targeting system have largely avoided these potential ambiguities. Still, a recent investigation by Griffin and colleagues identified the Wnt/β-catenin pathway as a downstream target for Brg1.39 Because aberrant activation of Wntβ–catenin signaling has been correlated with atherosclerosis,40, 41 decelerated atherogenesis in mice without Brg1/Brm could stem from suppression of β-catenin activity.
In conclusion, our data suggest that Brg1/Brm serve as the common link that connects inflammation induced endothelial injury to the pathogenesis of atherosclerosis and HPH by coordinating epigenetic regulation of NF-κB dependent CAM transactivation. In light of the observation that Brg1 inactivation in the heart protects against pathological cardiac hypertrophy in mice,13 our findings reinforce the notion that (inadvertent) re-activation of certain genes (e.g., Brg1) required for embryonic development may pose great health risk in adult life. Therefore, targeting Brg1/Brm may offer new hope in the prevention and/or intervention of cardiovascular disorders.
Supplementary Material
Novelty and Significance.
What Is Known?
Endothelial injury inflicted by pro-inflammatory stimuli contributes to the genesis of cardiovascular disease (CVD).
Up-regulation of adhesion molecules (CAMs) by pro-inflammatory stimuli accompanies endothelial injury.
Brahma related gene 1 (Brg1) is essential for vascular development.
What New Information Does This Article Contribute?
Brg1 and Brm are activated by pro-inflammatory stimuli in endothelial cells in vitro and in vivo.
Brg1 and Brm promote CAM transactivation by influencing NF-κB/p65 kinetics and chromatin structure.
Endothelial specific targeting of Brg1/Brm attenuates atherogenesis in mice.
Pro-inflammatory stimuli-induced endothelial injury underlies the pathogenesis of several cardiovascular diseases. Transcriptional activation of adhesion molecules facilitates the interaction between the endothelium and circulating leukocytes contributing to the amplification and perpetuation of chronic inflammation. Here we report that several pro-inflammatory stimuli elevate the levels of Brg1 and Brm, core components of the mammalian chromatin remodeling complex, in cultured endothelial cells in vitro and in mice. Over-expression Brg1/Brm enhances, while depletion of Brg1/Brm attenuates, CAM transactivation. Brg1 and Brm interact with NF-κB/p65 and fine-tune the binding of p65 to target promoters. Brg1/Brm helps establish a “transcription friendly” chromatin structure for the recruitment of the basic transcription machinery. Endothelial specific targeting of Brg1/Brm in mice decreases atherogenesis. Thus, pharmaceutical inhibition of Brg1/Brm in the vascular endothelium represents a novel strategy for CVD treatment.
Acknowledgments
SOURCES OF FUNDING
This work was supported, in part, by grants from the National Basic Science Project of China (2012CB517503, 2012CB518201), the Program for New Century Excellent Talents in University of China (NCET-11-0991), National Natural Science Foundation of China (30971426/H2101, 81070120), Natural Science Foundation of Jiangsu Province (BK2012043), Ministry of Education (212059), and a Scientist Development Grant from American Heart Association (to J. Z.). YX is a Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Nonstandard Abbreviations and Acronyms
- Brg1
Brahma related gene 1
- Brm
Brahma
- ED
enzyme deficient/dead
- HAEC
human aortic artery endothelial cell
- HPEC
human pulmonary artery endothelial cell
- HUVEC
human umbilical vein endothelial cell
- ICAM
inter-cellular adhesion molecule
- NF-κB
nuclear factor kappa B
- WT
wild type
- VCAM
vascular cell adhesion molecule
Footnotes
DISCLOSURES
None
References
- 1.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 2.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 3.He P. Leucocyte/endothelium interactions and microvessel permeability: Coupled or uncoupled? Cardiovasc Res. 2010;87:281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 5.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation. 2010;122:1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of e-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 7.Liu SF, Ye X, Malik AB. Inhibition of nf-kappab activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 8.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297:L238–250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ. Inhibition of nuclear factor-kappab-mediated adhesion molecule expression in human endothelial cells. Circ Res. 1998;82:314–320. doi: 10.1161/01.res.82.3.314. [DOI] [PubMed] [Google Scholar]
- 10.Han P, Hang CT, Yang J, Chang CP. Chromatin remodeling in cardiovascular development and physiology. Circ Res. 2011;108:378–396. doi: 10.1161/CIRCRESAHA.110.224287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of swi/snf and mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguin P, Holloway AK, Mori AD, Wylie JN, Munson C, Zhu Y, Zhou YQ, Yeh RF, Henkelman RM, Harvey RP, Metzger D, Chambon P, Stainier DY, Pollard KS, Scott IC, Bruneau BG. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun. 2011;2:187. doi: 10.1038/ncomms1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, Chang CP. Chromatin regulation by brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme brg1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500. doi: 10.1242/dev.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo : Possible mechanisms for gender differences in atherosclerosis. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 16.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering rnas in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 17.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong KW, Kim K, Situ AJ, Ulmer TS, An W, Stallcup MR. Recognition of enhancer element-specific histone methylation by tip60 in transcriptional activation. Nat Struct Mol Biol. 2011;18:1358–1365. doi: 10.1038/nsmb.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paakinaho V, Makkonen H, Jaaskelainen T, Palvimo JJ. Glucocorticoid receptor activates poised fkbp51 locus through long-distance interactions. Mol Endocrinol. 2010;24:511–525. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirilly D, Wong JJ, Lim EK, Wang Y, Zhang H, Wang C, Liao Q, Wang H, Liou YC, Yu F. Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in drosophila. Neuron. 2011;72:86–100. doi: 10.1016/j.neuron.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito M, Zager RA, Bomsztyk K. Brg1 increases transcription of proinflammatory genes in renal ischemia. J Am Soc Nephrol. 2009;20:1787–1796. doi: 10.1681/ASN.2009010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H, Luo Y. Brg1 regulates the transcription of human papillomavirus type 18 e6 and e7 genes. Cell Cycle. 2012:11. doi: 10.4161/cc.11.3.19115. [DOI] [PubMed] [Google Scholar]
- 24.Bultman SJ, Gebuhr TC, Pan H, Svoboda P, Schultz RM, Magnuson T. Maternal brg1 regulates zygotic genome activation in the mouse. Genes Dev. 2006;20:1744–1754. doi: 10.1101/gad.1435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human swi/snf-associated prmt5 methylates histone h3 arginine 8 and negatively regulates expression of st7 and nm23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Chambers KJ, Faller DV, Wang S. Reprogramming of the swi/snf complex for co-activation or co-repression in prohibitin-mediated estrogen receptor regulation. Oncogene. 2007;26:7153–7157. doi: 10.1038/sj.onc.1210509. [DOI] [PubMed] [Google Scholar]
- 27.Fish JE, Yan MS, Matouk CC, St Bernard R, Ho JJ, Jr, Gavryushova A, Srivastava D, Marsden PA. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J Biol Chem. 2010;285:810–826. doi: 10.1074/jbc.M109.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flowers S, Nagl NG, Jr, Beck GR, Jr, Moran E. Antagonistic roles for brm and brg1 swi/snf complexes in differentiation. J Biol Chem. 2009;284:10067–10075. doi: 10.1074/jbc.M808782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial brg1 represses adamts1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (snf2alpha) EMBO J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou YJ, Chen M, Cusack NA, Kimmel LH, Magnuson KS, Boyd JG, Lin W, Roberts JL, Lengi A, Buckley RH, Geahlen RL, Candotti F, Gadina M, Changelian PS, O’Shea JJ. Unexpected effects of ferm domain mutations on catalytic activity of jak3: Structural implication for janus kinases. Mol Cell. 2001;8:959–969. doi: 10.1016/s1097-2765(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Chen M, Kim JR, Zhou J, Jones RE, Tune JD, Kassab GS, Metzger D, Ahlfeld S, Conway SJ, Herring BP. Swi/snf complexes containing brahma or brahma-related gene 1 play distinct roles in smooth muscle development. Mol Cell Biol. 2011;31:2618–2631. doi: 10.1128/MCB.01338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starmer J, Magnuson T. A new model for random x chromosome inactivation. Development. 2009;136:1–10. doi: 10.1242/dev.025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Zhang R, Beischlag TV, Muchardt C, Yaniv M, Hankinson O. Roles of brahma and brahma/swi2-related gene 1 in hypoxic induction of the erythropoietin gene. J Biol Chem. 2004;279:46733–46741. doi: 10.1074/jbc.M409002200. [DOI] [PubMed] [Google Scholar]
- 35.Miao J, Fang S, Lee J, Comstock C, Knudsen KE, Kemper JK. Functional specificities of brm and brg-1 swi/snf atpases in the feedback regulation of hepatic bile acid biosynthesis. Mol Cell Biol. 2009;29:6170–6181. doi: 10.1128/MCB.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadam S, Emerson BM. Transcriptional specificity of human swi/snf brg1 and brm chromatin remodeling complexes. Mol Cell. 2003;11:377–389. doi: 10.1016/s1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 37.Reisman DN, Sciarrotta J, Bouldin TW, Weissman BE, Funkhouser WK. The expression of the swi/snf atpase subunits brg1 and brm in normal human tissues. Appl Immunohistochem Mol Morphol. 2005;13:66–74. doi: 10.1097/00129039-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Zhang M, Fang H, El-Mounayri O, Rodenberg JM, Imbalzano AN, Herring BP. The swi/snf chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2009;29:921–928. doi: 10.1161/ATVBAHA.109.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T. The chromatin-remodeling enzyme brg1 modulates vascular wnt signaling at two levels. Proc Natl Acad Sci U S A. 2011;108:2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/beta-catenin signaling induces vsmc proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 41.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and t-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011;31:1625–1633. doi: 10.1161/ATVBAHA.111.227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.