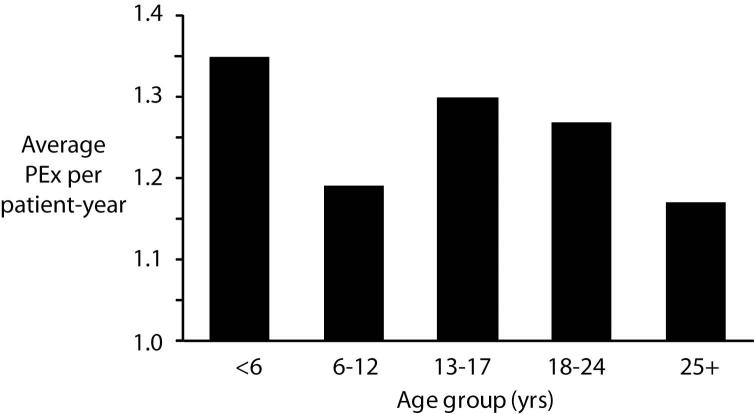SUMMARY
Rationale
Patients with cystic fibrosis experience frequent pulmonary exacerbations (PExs). Clinicians manage these episodes of worsening signs and symptoms in a variety of ways.
Objectives
To characterize the antibiotic management and associated change in lung function following PExs.
Methods
We used 2003-5 data from the Epidemiologic Study of Cystic Fibrosis to examine antibiotic treatment and the immediate and long term lung function change associated with clinician reported PExs.
Results
45,374 PExs were reported in 13,194 unique patients. Most PExs (73%) were treated with oral antibiotics, while 39% were treated IV and 24% were treated with inhaled antibiotics. The likelihood of non-IV vs. IV antibiotic treatment was associated with the patient’s age, stage of lung disease, and magnitude of lung function drop prior to the PEx. Following treatment, the average improvement in the FEV1 was 3.4±12.2 % predicted with a greater (5.1±12.7 % predicted) improvement following IV antibiotic treatment than with non-IV treatment (2.0±11.6 % predicted). When the best FEV1 from the year before was compared with 180 days following the PEx there was an average fall of 3.8±10.5 % predicted with little difference observed between antibiotic treatment routes. Patients with only one exacerbation during the 3-year study had a similar loss of lung function to patients with no reported exacerbations.
Conclusion
Clinicians treat the majority of PExs with oral antibiotics, particularly in younger, healthier patients. Pulmonary function improves with antibiotic therapy, however PExs are associated with lung function deterioration over time.
Keywords: cystic fibrosis, pulmonary exacerbation, antibiotics, epidemiology
INTRODUCTION
Lung disease in patients with cystic fibrosis (CF) is characterized by episodic increases in respiratory symptoms such as cough and sputum production, systemic symptoms such as fatigue and weight loss, and a fall in pulmonary function. Although no consensus definition exists for these episodes, they are collectively referred to as pulmonary exacerbations (PExs).(1-4) Pulmonary exacerbations are associated with reduced health-related quality of life,(5-7) accelerated pulmonary function decline,(8, 9) and decreased survival.(10-13) Treatment of PExs usually involves antibiotics with frequent airway clearance and varies between individual clinicians and clinical care centers.(14) Standardized recommendations for PEx management have been limited by a lack of objective evidence for optimal therapy.(15, 16)
Pulmonary exacerbations are defined in a variety of ways.(17) Clinical trials frequently have used the clinician’s decision to treat with intravenous (IV) antibiotics as the definition for a PEx.(18-23) Studies with IV antibiotics used to treat PExs generally show the patient’s pulmonary function returning to baseline in 7-10 days.(24, 25) However a sub-population of patients fails to completely respond and many patients experience multiple exacerbations over the following year.(24, 26-28) Furthermore, studies of IV treatment fail to recognize that clinician-defined PExs may be treated only with oral or inhaled antibiotics. Although clinical and microbiologic improvement has been demonstrated following IV treatment for a PEx, little is known of the outcomes from oral or inhaled treated exacerbations.
The Epidemiologic Study of Cystic Fibrosis (ESCF) is a multicenter, prospective, encounter based, longitudinal study of therapy and the natural history of CF.(29) Beginning in 2003 an electronic data collection form related to PExs was initiated in ESCF by which clinicians could report treatment with antibiotics including IV, inhaled or oral. We analyzed these ESCF data to evaluate clinical use of antibiotics for treating PExs, measure pulmonary function change associated with treatment, and determine the association of PExs and PFT change over time.
METHODS
All 18,140 patients enrolled in the ESCF between 2003 and 2005 were eligible for this analysis. A PEx was defined prospectively as any new or increased respiratory symptoms or any clinical worsening in pulmonary status for which the clinician decided to initiate new antibiotic therapy. All therapies were determined by the clinician and all antibiotics used to treat the PEx were recorded. The onset of the PEx was defined as the date at the start of new antibiotic therapy. Age at exacerbation was defined as the patient’s age at the onset of the exacerbation and all reported PExs separated by at least 14 days were included in the analysis. Our initial analysis focused on the antibiotic treatment of an exacerbation. We then looked at a sub-group of exacerbations where pulmonary function test (PFT) results were available to better understand the impact of a PEx. The forced expiratory volume in 1 second (FEV1) was normalized to percent predicted values according to the method of Wang et al.(30) for female patients <16 years old and male patients <18 years old, and Hankinson et al.(31) for older patients. Two analyses of FEV1 were performed. To evaluate the short-term impact of an exacerbation, the FEV1 value closest to the PEx onset (within the 30 days prior) was compared with the FEV1 value closest to the end of antibiotic treatment (within the 90 days following onset). Long-term pulmonary function impact was evaluated by comparing the highest FEV1 within one year prior to the PEx and the highest FEV1 within 180 days following the PEx onset.
A separate analysis was undertaken to better define the pulmonary impact of a single exacerbation on an individual patient. For this analysis, patients with only one PEx during the 3 year study period were compared with patients who had no recorded exacerbations. Only patients with pulmonary function results were included since the aim was to study the impact of an exacerbation on pulmonary function. Similar criteria for pulmonary function testing were used, including both short-term and long-term impact.
All analyses were conducted using SAS 9.1 (SAS Institute, Inc., Cary NC). Due to the large number of exacerbations in the dataset, statistical significance was defined as p<0.001 unless otherwise noted.
RESULTS
During 2003-2005 45,374 exacerbations were reported involving 13,194 unique patients (25.3% had one, 20.0% had two, 15.8% had three, 11.4% had four, and 27.5% had >4 exacerbations over three years). The mean patient age at the time of an exacerbation was 16.3±11.0 years, with 15.8% of reported PExs occurring in patients <6 years old, 25.1% in 6-12 year olds, 20.0% in 13-17 year olds, 20.2% in 18-24 year olds, and 18.8% in patients 25 years old or older (Table 1). The average number of PExs reported per patient year varied by age group (Figure 1), but was highest in the youngest patients (< 6 years of age with 1.35 PExs per patient year) and lowest in older adults (1.17 PExs per patient year).
Table 1.
Exacerbation demographics by age group including gender, weight, and pulmonary function.
| Age Group at onset of PEx (years) |
<6 | 6-12 | 13-17 | 18-24 | 25+ |
|---|---|---|---|---|---|
| Number of PExs | 7,182 | 11,394 | 9,067 | 9,184 | 8,547 |
| Gender (% female) | 53.5 | 52.7 | 51.0 | 52.7 | 50.4 |
| Weight for age percentile at baseline* |
33.5±28.7 | 31.0±26.9 | 27.7±26.1 | 24.6±25.0 | 36.0±29.1 |
| Best stable FEV1 prior to PEx (within 1 year) # |
NA | 93.4±20.7 | 83.5±22.4 | 68.0±23.0 | 63.1±25.2 |
| FEV1 prior to PEx (within 30 days) # |
NA | 77.8±22.6 | 66.7±23.1 | 54.2±22.0 | 45.4±19.3 |
| Best FEV1 after PEx (within 90 days) # |
NA | 81.5±22.4 | 70.4±23.6 | 58.3±22.9 | 47.4±20.1 |
| Best stable FEV1 after PEx (within 180 days) # |
NA | 90.2±21.3 | 78.6±23.1 | 63.7±23.5 | 52.1±21.5 |
Definition of Abbreviation: FEV1 = forced expiratory volume in 1 second; PEx = pulmonary exacerbation; NA = not applicable
mean ± SD
percent predicted mean ± SD
Figure 1.
Incidence of PExs stratified by age group. Significant comparison differences (p<0.001) include: <6 vs 25+, 6-12 vs 13-17, 6-12 vs 18-24, 13-17 vs 25+, and 18-24 vs 25+.
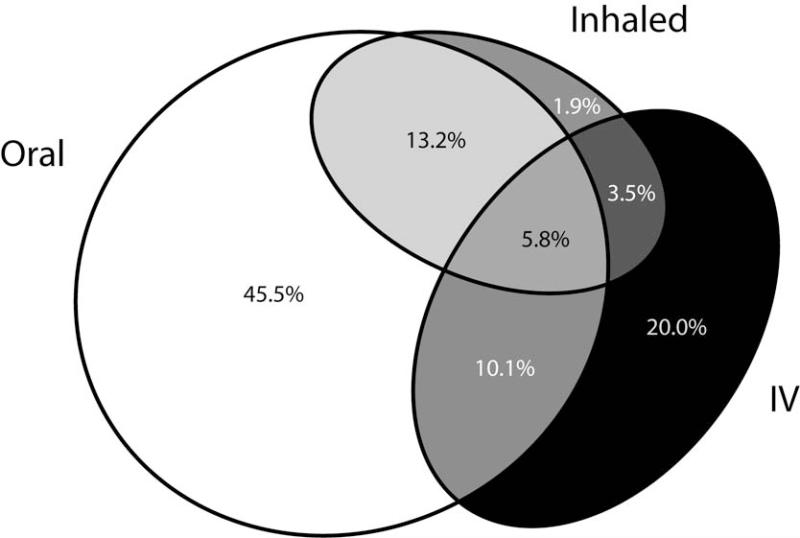
Antibiotics were used to treat 44,512 PExs (see supplemental table), with oral antibiotics used in 73.2%, inhaled antibiotics used in 23.9%, and IV antibiotics used in 38.7 %, with frequent overlap of more than one route of administration (Figure 2). Forty-four percent of exacerbations were treated with oral antibiotics alone and 15% were treated with inhaled ± oral antibiotics. For exacerbations treated with IV antibiotics, tobramycin was used 69.6% of the time. When tobramycin was used, it was almost always used in combination with a second IV antibiotic. Of the 17,544 IV-treated exacerbations, 2% were treated with tobramycin alone, 24% tobramycin plus ceftazidime (± a third antibiotic), 8% tobramycin plus cefepime, 8% tobramycin plus meropenem, 28% tobramycin with other IV antibiotics, and 30% included other IV antibiotic combinations without tobramycin.
Figure 2.
Oral, inhaled, and IV antibiotics prescribed for PExs from 2003 through 2005. Route of administration for 88,839 antibiotic treatments (Supplement Table) during 45,374 clinician-diagnosed pulmonary exacerbations. Areas reflect the number of treatments by route of administration: Oral only – 45.5%, Inhaled only – 1.9%, Inhaled plus oral – 13.2%, IV only – 20.0%, IV plus oral – 10.1%, IV plus inhaled – 3.5%, and IV plus oral and inhaled – 5.8%. (Figure created with eulerAPE http://www.eulerdiagrams.org/eulerAPE/).
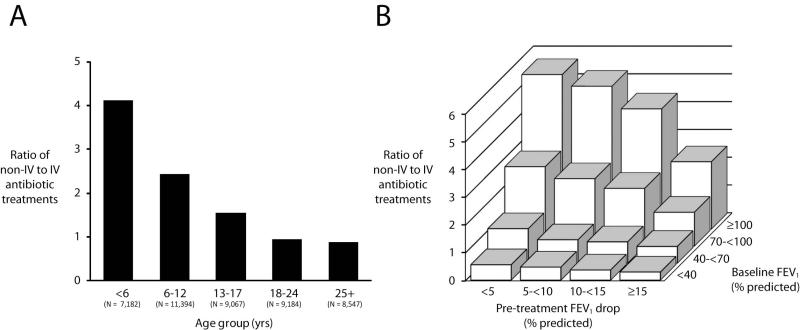
The proportion of non-IV to IV treated PExs decreased with patient age, with under 6 year olds being 4.1 times more likely to receive oral and/or inhaled antibiotics than IV antibiotics, while adult patients were slightly more likely to be treated with IV antibiotics (Figure 3A). The decision to treat with IV antibiotics or not was also associated with the degree of lung function limitation and the magnitude of decline in lung function prior to the PEx (Figure 3B). Exacerbations occurring in patients with less advanced disease (FEV1>100 % predicted) were 4.0 times more likely to be treated with inhaled and/or oral than IV antibiotics whereas PExs in patients with the most advanced disease (FEV1<40 % predicted) were treated with IV antibiotics 52% of the time.
Figure 3.
Ratio of non-IV to IV antibiotic treatment routes. Panel A: Ratio of treatment without or with IV antibiotics stratified by the patient age. Panel B: Ratio of treatment without or with IV antibiotics stratified by lung disease stage (based on best FEV1 within the year prior to the PEx) and the drop in FEV1 during the year prior to the exacerbation.
PFT results for evaluating the short-term impact of treatment were available for 13,673 exacerbations. Overall, treatment with antibiotics was associated with an average short-term improvement in FEV1 of 3.4±12.3 % predicted (mean ± SD) (Table 2, Figure 4). Treatment with IV antibiotics was associated with a significantly lower FEV1 prior to the PEx compared with non-IV treated exacerbations (53.6±22.8 vs. 71.5±24.1 % predicted, p<0.001). Average short-term improvement in FEV1 was significantly greater for events treated with IV antibiotics than for those that were not (5.1±12.7 vs. 2.0±11.6 % predicted, p<0.001).
Table 2.
Short-term Pulmonary Function Data for PExs with PFT Results Within 30 Days Prior to and 90 Days after Antibiotic Treatment
| All | IV antibiotics |
Inhaled ± Oral antibiotics |
Oral antibiotic only |
|
|---|---|---|---|---|
| Sample Size | 13,653 | 6,375 | 2,227 | 4,862 |
| Closest FEV1 within 30 days prior to treatment* |
63.1±25.2 | 53.6±22.8 | 69.1±23.9 | 72.9±24.0 |
| Closest FEV1 within 90 days after treatment* |
66.6±25.7 | 58.7±24.4 | 72.3±24.8 | 74.4±24.7 |
| Absolute change* | 3.4±12.3 | 5.1±12.7 | 3.2±11.3 | 1.5±11.6 |
| Relative change | 5.4% | 9.5% | 4.6% | 2.1% |
Definition of Abbreviation: FEV1 = forced expiratory volume in 1 second; IV = intravenous;
SD = standard deviation.
FEV1 % predicted ± SD
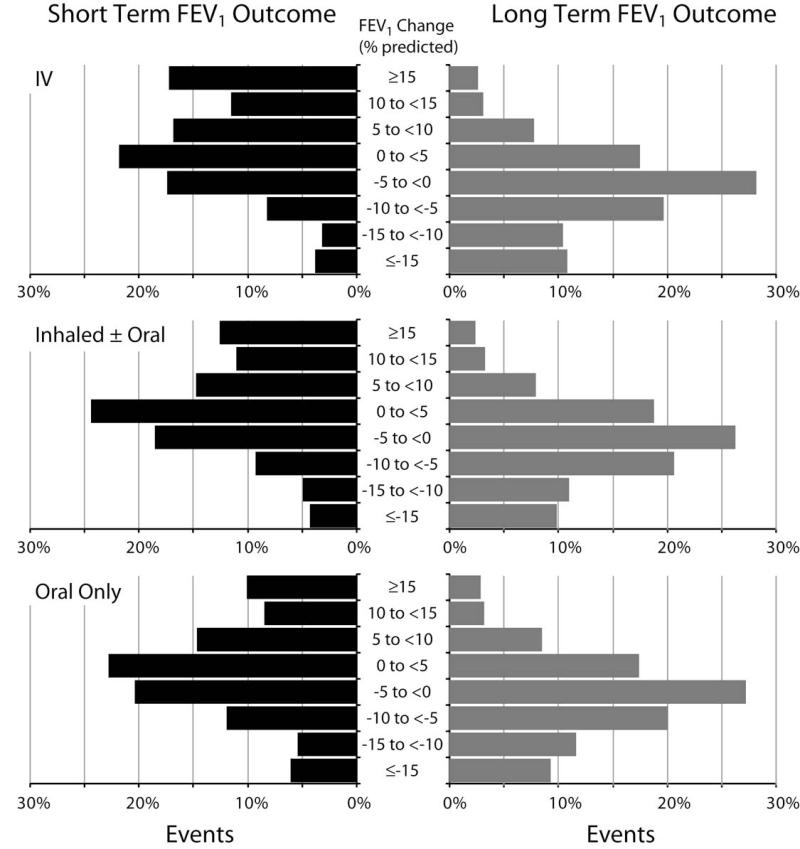
Figure 4.
Distribution of short-term (left panel) and long-term (right panel) absolute % predicted change in FEV1 associated with antibiotic treatment of PExs, separated by route of administration (IV on top, oral on bottom, and inhaled ± oral in the middle). Bars represent percent of PEx events divided into groups of 5 % predicted change in FEV1. Short-term and long-term patient groups are not identical.
There were 27,251 exacerbations with related PFT results available for evaluating long-term impact of therapy (Table 3, Figure 4). The average long-term loss of FEV1 associated with a treated PEx was 3.8±10.5 % predicted. Similar to the short-term results, use of IV antibiotics was associated with lower baseline lung function (FEV1 66.8 % predicted for IV treated vs. 84.9 % predicted for non-IV treated PExs), however over the long term both IV and non-IV treated exacerbations were associated with similar FEV1 losses (−4.0 and −3.7 % predicted).
Table 3.
Long-term Pulmonary Function Data for PExs with PFT Results Within One Year Prior to and 180 Days after Antibiotic Treatment
| All | IV antibiotics |
Inhaled ± Oral antibiotics |
Oral antibiotics only |
|
|---|---|---|---|---|
| Sample Size | 27,251 | 11,252 | 4,225 | 11,339 |
| Best FEV1 within 1 year prior to treatment* |
77.4±26.2 | 66.8±25.0 | 82.0±24.8 | 86.3±23.9 |
| Best stable FEV1 within 180 days after treatment* |
73.6±26.6 | 62.7±25.4 | 78.3±24.9 | 82.7±24.3 |
| Absolute change* | −3.8±10.5 | −4.0±11.1 | −3.7±10.1 | −3.6±10.1 |
| Relative change | −4.9% | −5.9% | −4.5% | −4.2% |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; IV = intravenous;
SD = standard deviation.
FEV1 % predicted ± SD
There were 2016 patients with long-term PFT results included in the sub-group with only one PEx during the 3-year study. Comparing these patients with the 4139 patients who had PFT results and no reported exacerbations, the long-term fall in FEV1 was almost identical (−2.4±9.9 vs. −2.4±10.6 % predicted). Similar to the results for all exacerbations, patients treated with IV antibiotics had significantly lower baseline lung function (77.2±23.7 vs. 90.6±22.3 % predicted FEV1, p<0.001) compared with non-IV treated patients. Short-term PFT results demonstrated an average FEV1 increase of 6.2±12.4 % predicted, with patients whose treatments included IV antibiotics improving an average of 8.6±13.2 % predicted, whereas those treated without IV antibiotics averaged only 4.2±11.3 % predicted (p< 0.001).
DISCUSSION
Patients with CF experience frequent PExs and based on our data, most clinician-defined exacerbations are treated with oral antibiotics. Only one third of reported exacerbations were treated with IV antibiotics, and as expected, this treatment was more likely to occur in older patients with more advanced lung disease and/or a greater recent fall in lung function. Immediately following treatment for a PEx there was generally an improvement in pulmonary function, however over a longer time period there was a drop in lung function associated with the average exacerbation. One important finding was that there was no difference in the drop in pulmonary function between patients with only a single treated exacerbation during the 3-year study and patients with no recorded exacerbations.
This study differs from most previous studies in that we include all clinician declared exacerbations, regardless of whether the exacerbation was treated with IV antibiotics or not. Much debate has arisen over the definition of a pulmonary exacerbation in patients with CF.(1, 4, 32) Using the ESCF dataset, Rabin and coworkers(33) identified specific risk factors associated with IV treated PExs, but noted that even in patients experiencing all of these risk factors, clinicians did not always initiate IV antibiotic therapy. Previous studies, both epidemiologic and randomized controlled trials, have employed IV antibiotic administration as a marker for a PEx.(8, 9, 17-19, 22, 23) Although these past observations are important, our results suggest that including IV therapy to define an exacerbation will bias the results towards the minority of clinician-defined PExs which occur predominately in the older patient population with more advanced lung disease. Our results indicate that more PExs are treated without IV antibiotics than are treated with IV antibiotics and the use of non-IV antibiotics is strongly weighted towards younger children who are earlier in their lung disease process. This is particularly important since children less than 6 years old have a greater incidence of reported antibiotic-treated PExs than any other age group.
There are likely multiple reasons that IV antibiotic use increases with age. Although viral infections are associated with PExs at all ages, they are reported more frequently in younger patients and would be less likely treated with antibiotics, particularly IV.(34-36) Furthermore, the composition of a CF patient’s microbial community changes over time, with bacteria such as Pseudomonas aeruginosa being more common in older patients and necessitating a different selection of antibiotics.(37) Finally, clinical experience is greater for the older patient and prior response to antibiotics will influence antibiotic choice and route for treating a PEx. It is important to note that while our data is limited to age and lung function, it suggests that the clinician’s decision to use IV antibiotics is influenced by the patient’s baseline FEV1 and the degree of their pulmonary function drop prior to presenting with a PEx. One risk of this apparent reliance on FEV1 and lung function loss to gauge therapy is that clinicians may inaccurately estimate the severity of a PEx in patients too young to reliably perform PFTs.
There have been several, relatively small prospective studies looking at antibiotic treatment of PExs in CF.(38-41) Most have involved the use of IV antibiotics for varying durations and in varying combinations. We did not analyze the duration of treatment, but did find that most of the time when IV therapy was used, the clinician decided to treat with multiple antibiotics. This was not true when oral or inhaled antibiotics were used; they were generally used alone and to treat younger patients and patients with better lung function. Of interest, there were a few reported exacerbations that were not treated with any antibiotics. Given the epidemiologic nature of this study, we are unable to know whether these patients were considered to have viral infections (ie. not requiring antibiotic therapy) or to have mainly an obstructive problem due to secretions (ie. possibly treated with increased airway clearance therapy without antibiotics), or whether an error was made in reporting. The existence of these events, however, suggests that the definition of a PEx should probably not be limited by requiring the use of antibiotics.
When we separated the antibiotic choices into three groups: 1) oral only, 2) inhaled with/without oral, and 3) IV with/without other antibiotics, the resulting groups were at different stages of lung disease and clinicians treated the patients with more advanced disease more aggressively. However, the difference in short-term improvement was a little surprising. As shown in Table 2, oral antibiotics were associated with a relatively small improvement in pulmonary function compared with IV, with inhaled ± oral being intermediate. This was true for both absolute and relative changes. The degree of pulmonary function improvement may be related to many aspects of treatment for a PEx. Patients with the largest drop in lung function prior to a PEx are the most likely to show the largest improvement, and they were also the most likely to be treated with IV antibiotics. Additionally the size of improvement could be related to the availability of more effective antibiotics for IV use, or could possibly indicate that other therapies (ie. inpatient care) used at the time of IV therapy have added benefit.(16) The long-term average change in lung function was not different between treatment groups and showed a discouraging average 3.8±10.5 % predicted absolute and 4.9% relative drop in FEV1 associated with each exacerbation. This average drop may indicate the harmful impact of a PEx in all patients or more likely represents improvement in some and failure to improve in others (Figure 4). Recently, Sanders and colleagues(26-28) reported that about one-fourth of patients fail to recover lost lung function to within 10% of baseline following treatment with IV antibiotics. Our data on long-term pulmonary function shows a similar finding with 21.1% of patients failing to regain within 10% of their prior year best FEV1. However this observation, as well as others describing health outcomes associated with PExs, may be complicated by the inclusion of patients who experience multiple exacerbations. Patients who have multiple IV-treated exacerbations in a prior year are at greater risk for a near-term decline in FEV1 as well as future PExs.(10, 42) In our analysis we treat each exacerbation as a unique event and we determined the average change associated with that one event. Our results may show a more severe average drop in lung function associated with a PEx than previously reported since in some cases there was a second PEx during the 180 day follow-up period. However in general this impact is small since these patients averaged just over three exacerbations during the 3-year period.
To further eliminate the contribution of patients experiencing multiple PExs, we studied a subset of patients that had only one exacerbation during the 3-year study. Average FEV1 increased 6.2±12.4 % predicted over the short term following treatment and the average improvement in patients treated with IV antibiotics was greater than in patients treated with oral and/or inhaled antibiotics (13.4% vs. 5.3% relative improvement). These results are consistent with the changes in FEV1 % predicted in the overall study involving all exacerbations. However the average long-term drop in FEV1 of 2.4±9.9 % predicted over 18 months was almost identical to the 2.4±10.6 % predicted fall seen in a comparator group of patients with no exacerbations. Importantly, these patients were at an early stage of their disease with baseline FEV1 values frequently in the normal range. This result suggests that PExs are not the only reason for pulmonary deterioration in patients with CF and that, although antibiotic treatment is associated with improved short-term pulmonary function, it does not prevent a drop in long-term lung function. It is also possible that the patients with no reported exacerbations may have experienced acute events similar to the treated patients, but these events were not recognized, or if recognized were not treated or reported. Regardless of the reason, it appears there is an opportunity for better or more frequent therapy to reduce this fall in lung function, a conclusion consistent with the findings of Johnson and colleagues(43) which indicate that CF care sites with greater reporting of exacerbations and greater use of antibiotics have overall better lung function in their patients.
As with all epidemiologic studies, these results are limited by the data collected and what the clinician reports. Conclusions must be considered hypothetical and not proven. However the strength of an epidemiologic study is that it more closely represents “real life” experience than is the case with a highly selected randomized controlled trial. In this study we chose to be descriptive in our analyses and avoid constructing a multivariate model since we had limited data about other patient characteristics. Additionally, we felt that this descriptive approach better represented the clinician’s decision making. Even with these limitations we identified interesting differences between routes of antibiotic use and were able to compare pulmonary function outcomes following PExs.
In summary we found that PExs are frequent in patients with CF at all ages. Antibiotic treatment choice varies greatly between clinicians, with oral antibiotics used to treat the majority of PExs and used most commonly in younger patients with less advanced lung disease. There is an acute improvement in FEV1 following treatment with antibiotics, however PExs are associated with a long term loss of lung function. In patients with infrequent PExs, this loss is not greater than the general loss of lung function in patients who are not reported as having an exacerbation and who are at a comparable early stage of lung disease. Importantly our results show that limiting the definition of a PExs to those treated with IV antibiotics will bias the results toward older patients in a later stage of disease. A better understanding of the impact from these less aggressively treated PExs may aid in developing improved, earlier care in CF.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the more than 400 site investigators and coordinators in ESCF who collected this comprehensive database, and the patients who consented to have their data submitted.
All sources of support for the Epidemiologic Study of Cystic Fibrosis in the form of grants, case report forms, and data analysis were provided by Genentech, Inc., South San Francisco, CA.
Portions of this research were presented at the 2008 North American Cystic Fibrosis Conference.
Footnotes
Disclosure of Conflict of Interest
Jeffrey Wagener, Donald VanDevanter, Warren Regelmann, Wayne Morgan, and Michael Konstan have received honoraria from Genentech to attend meetings as members of the North American Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF) and their respective institutions previously received grant support from Genentech, Inc. for participating in the study. No honoraria, grant, or other form of payment was given to any of these authors to produce this manuscript. Jeffrey Wagener was previously employed by Genentech, Inc. Lawrence Rasouliyan and David Pasta are employed by ICON Late Phase & Outcomes Research. ICON Late Phase & Outcomes Research was paid for providing biostatistical and analytical services for ESCF. The decision to submit the manuscript was made by the authors.
REFERENCES
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148:259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Dakin C, Henry RL, Field P, Morton J. Defining an exacerbation of pulmonary disease in cystic fibrosis. Pediatr Pulmonol. 2001;31:436–442. doi: 10.1002/ppul.1072. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BC. Pulmonary exacerbations in cystic fibrosis: It’s time to be explicit! Am J Respir Crit Care Med. 2004;169:781–782. doi: 10.1164/rccm.2401009. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139:359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 5.Orenstein DM, Pattishall EN, Nixon PA, Ross EA, Kaplan RM. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98:1081–1084. doi: 10.1378/chest.98.5.1081. [DOI] [PubMed] [Google Scholar]
- 6.Bradley J, McAlister O, Elborn S. Pulmonary function, inflammation, exercise capacity and quality of life in cystic fibrosis. Eur Respir J. 2001;17:712–715. doi: 10.1183/09031936.01.17407120. [DOI] [PubMed] [Google Scholar]
- 7.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 8.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. doi: 10.1016/j.jpeds.2007.03.006. 139 e131. [DOI] [PubMed] [Google Scholar]
- 9.VanDevanter DR, Wagener JS, Pasta DJ, Elkin E, Jacobs JR, Morgan WJ, Konstan MW. Pulmonary outcome prediction (pop) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45:1156–1166. doi: 10.1002/ppul.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 12.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 13.Ellaffi M, Vinsonneau C, Coste J, Hubert D, Burgel PR, Dhainaut JF, Dusser D. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:158–164. doi: 10.1164/rccm.200405-667OC. [DOI] [PubMed] [Google Scholar]
- 14.Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across us cf care centers. Pediatr Pulmonol. 2011;46:870–881. doi: 10.1002/ppul.21442. [DOI] [PubMed] [Google Scholar]
- 15.Smyth A. Update on treatment of pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med. 2006;12:440–444. doi: 10.1097/01.mcp.0000245711.43891.16. [DOI] [PubMed] [Google Scholar]
- 16.Flume PA, Mogayzel PJ, Jr., Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC. Cystic fibrosis pulmonary guidelines: Treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 17.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human dnase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The pulmozyme study group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 20.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW., 3rd. Azithromycin in patients with cystic fibrosis chronically infected with pseudomonas aeruginosa: A randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 21.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, Colin AA. Treatment with tobramycin solution for inhalation reduces hospitalizations in young cf subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 22.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. A cftr potentiator in patients with cystic fibrosis and the g551d mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collaco JM, Green DM, Cutting GR, Naughton KM, Mogayzel PJ., Jr. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med. 2010;182:1137–1143. doi: 10.1164/rccm.201001-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDevanter DR, O’Riordan MA, Blumer JL, Konstan MW. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010;11:137. doi: 10.1186/1465-9921-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, Goss CH. Return of fev1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010;45:127–134. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 28.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent fev1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46:393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 29.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, et al. Epidemiologic study of cystic fibrosis: Design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the u.S. And canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Dockery DW, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG., Jr. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148:1502–1508. doi: 10.1164/ajrccm/148.6_Pt_1.1502. [DOI] [PubMed] [Google Scholar]
- 31.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general u.S. Population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 32.Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med. 17:442–447. doi: 10.1097/MCP.0b013e32834b8c04. [DOI] [PubMed] [Google Scholar]
- 33.Rabin HR, Butler SM, Wohl ME, Geller DE, Colin AA, Schidlow DV, Johnson CA, Konstan MW, Regelmann WE. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2004;37:400–406. doi: 10.1002/ppul.20023. [DOI] [PubMed] [Google Scholar]
- 34.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7:320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifton IJ, Kastelik JA, Peckham DG, Hale A, Denton M, Etherington C, Conway SP. Ten years of viral and non-bacterial serology in adults with cystic fibrosis. Epidemiol Infect. 2008;136:128–134. doi: 10.1017/S0950268807008278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth AR, Smyth RL, Tong CY, Hart CA, Heaf DP. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73:117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubert D, Le Roux E, Lavrut T, Wallaert B, Scheid P, Manach D, Grenet D, Sermet-Gaudelus I, Ramel S, Cracowski C, et al. Continuous versus intermittent infusions of ceftazidime for treating exacerbation of cystic fibrosis. Antimicrob Agents Chemother. 2009;53:3650–3656. doi: 10.1128/AAC.00174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumer JL, Saiman L, Konstan MW, Melnick D. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest. 2005;128:2336–2346. doi: 10.1378/chest.128.4.2336. [DOI] [PubMed] [Google Scholar]
- 40.Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M. Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis. J Cyst Fibros. 2008;7:142–146. doi: 10.1016/j.jcf.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, Moss R, Ramsey B, Redding G, Rubio T, et al. Comparison of a beta-lactam alone versus beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr. 1999;134:413–421. doi: 10.1016/s0022-3476(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 42.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, Paterson N, Jackson M, Lougheed MD, Kumar V, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66:680–685. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 43.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: A center-based analysis. Chest. 2003;123:20–27. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.