Abstract
In this review, we address the following question: Are modifications at the level of sarcomeric proteins in acquired heart failure early inducers of altered cardiac dynamics and signaling leading to remodeling and progression to decompensation? There is no doubt that most inherited cardiomyopathies are caused by mutations in proteins of the sarcomere. We think this linkage indicates that early changes at the level of the sarcomeres in acquired cardiac disorders may be significant in triggering the progression to failure. We consider evidence that there are rate-limiting mechanisms downstream of the trigger event of Ca2+ binding to troponin C, which control cardiac dynamics. We discuss new perspectives on how modifications in these mechanisms may be of relevance to redox signaling in diastolic heart failure, to angiotensin II signaling via β-arrestin, and to remodeling related to altered structural rigidity of tropomyosin. We think that these new perspectives provide a rationale for future studies directed at a more thorough understanding of the question driving our review.
Keywords: Troponin, Tropomyosin, Myosin-binding protein C, Posttranslational modifications
Introduction and scope
Heart failure (HF) and other disorders of the heart are commonly studied after disease progression to decompensation, especially in human heart studies [28, 71]. This makes sense in view of the need for therapies in patients in which HF is extant or clearly in progress, for example, following survival of a myocardial infarction (MI). Yet, understanding the sequence of events that underlie the transition from the compensated to the decompensated state remains an important and challenging objective. In considering the timing of the mechanisms in the transition, factors extrinsic to the heart muscle (for example, hypertension, coronary artery disease, viral infection) are common instigators of the disease process. However, prevalent cardiomyopathies (CMs) are also instigated by modifications intrinsic to the heart muscle cells, especially in the form of missense or deletion mutations in proteins of the sarcomere [20, 67]. In this case, the progression to hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) and sudden death starts at the level of the sarcomeres. Moreover, arrythmogenic sudden death has been directly linked to altered sarcomeric properties [4]. We think that this linkage of disease progression to the sarcomeres provides lessons relevant to an understanding on acquired HF. Therefore, our review addresses the following major question: Are modifications at the level of sarcomeric proteins in acquired HF early inducers of altered cardiac dynamics and signaling leading to remodeling and progression to decompensation?
There have been excellent reviews [14, 22, 23, 37, 54, 61, 64] of the roles of cardiac sarcomeres in familial and acquired disorders of the heart leading to HF and in chapters of a recent monograph [62] on “Biophysics of the failing heart.” Mechanisms that control the heartbeat have been usefully categorized into either membrane-controlled mechanisms upstream of Ca2+ binding to troponin C (TnC) or sarcomere-controlled mechanisms downstream of Ca2+ binding to TnC [7, 39, 63]. In the present review, we focus on relatively new findings, which provide new perspectives in whether downstream myofilament modifications may be among the earliest changes leading to HF progression. Moreover, our review emphasizes the importance of fully integrating altered myofilament properties together with altered growth factor, nuclear, neurohumoral, and Ca2+ signaling into the consideration of mechanisms of HF progression.
Primary alterations in sarcomere proteins affect systolic and diastolic states by altering cardiac dynamics
If, as we propose, causal and early modifications in sarcomeric proteins occur in the progression to HF, then these modifications should perturb myofilament functions that are significant determinants of cardiac dynamics. While there is no doubt that levels and kinetics of Ca2+ transients trigger contraction and are critical determinants of the level and rate of rise and fall of systolic pressure, there appears to be a significant role for processes at the level of the myofilaments as determinants of the duration of systolic elastance, and the time course of isovolumic relaxation. We [27] have previously summarized arguments for this assertion, and since then, more supportive documentation [30, 63] has been published. In our assessment of the role of downstream sarcomere mechanisms in cardiac dynamics, we employed results of indirect evidence and modeling to predict the time course of Ca2+ bound to TnC during a beat of the heart. We concluded from this speculation that the fall in bound Ca2+ occurred well before the fall in ventricular pressure and that control mechanisms downstream of Ca2+ bound to TnC have a prominent role in determining the duration of systole and the time course of fall in isovolumic pressure. We emphasized that one mechanism for this maintenance of ejection and pressure appears to be a time-dependent cooperative activation of the thin filaments. There is also abundant evidence to support the idea of control of contraction/relaxation kinetics by alterations in crossbridge kinetics [27, 63]. Gain and loss of function studies with variations in crossbridge kinetics have been relatively straight-forward. However, gain and loss of cooperative activation in the sarcomeres and determination of the impact of this modification of the dynamics of the heartbeat have been more difficult to attain with high fidelity and specificity. We summarize one approach below in the section discussing altered tropomyosin (TM) flexibility and cardiac function.
Further support for the idea that mechanisms at the level of the myofilaments may be rate limiting has come from studies of Stehle and Iorga [63], who measured the kinetics of downstream and upstream mechanisms in preparations with and without removal of the membranes. A conclusion they state from their modeling and measurements is that “…the dynamics of cardiac contraction and relaxation are determined mainly by sarcomeric downstream mechanisms, in particular by the kinetics of the cross-bridge cycle.” Moreover, they emphasize that sarcomere lengthening is an essential element in cardiac relaxation and the early phase of diastolic filling. Janssen [30] also report a strong support for an essential role of downstream mechanisms in a control of the dynamics of the heartbeat. They carried out experiments at physiological temperatures in preparations with little or no diffusion barriers and have provided convincing evidence that the time course and amplitude of Ca2+ transients do not explain changes in force development occurring during both the force-frequency relation and length-dependent activation (Frank-Starling relation). In the case of the force-frequency relation, the data indicate that relaxation occurs despite high levels of diastolic Ca2+. The mechanism appears to be an increase in troponin I (TnI) phosphorylation at sites well known to desensitize the myofilaments to Ca2+. The exact mechanism relating frequency to TnI phosphorylation remains to be determined. A reduction in force with increases in frequency in the myocardium from failed human hearts has been known to occur for many years [2]. With regard to Starling’s law and length-dependent myofilament activation, while the mechanism remains a topic of research and debate [12, 15, 17, 34], studies by Monasky et al. [50] have emphasized a role for downstream mechanisms in control of the prolonged relaxation occurring with preload-dependent increases in muscle length. Posttranslational modifications of myofilament proteins have been invoked as a cause for the length-dependent modulation of contractile dynamics [49]. Moreover, as pointed out by ter Keurs [66], the loss of adrenergic signaling in HF induces an increased reliance of the heart on the Frank-Starling relation with the potential of the elevated end-diastolic pressures to exacerbate coronary flow abnormalities.
Hypertension-induced redox signaling to myofilaments induces diastolic dysfunction independent of altered cellular Ca2+ fluxes
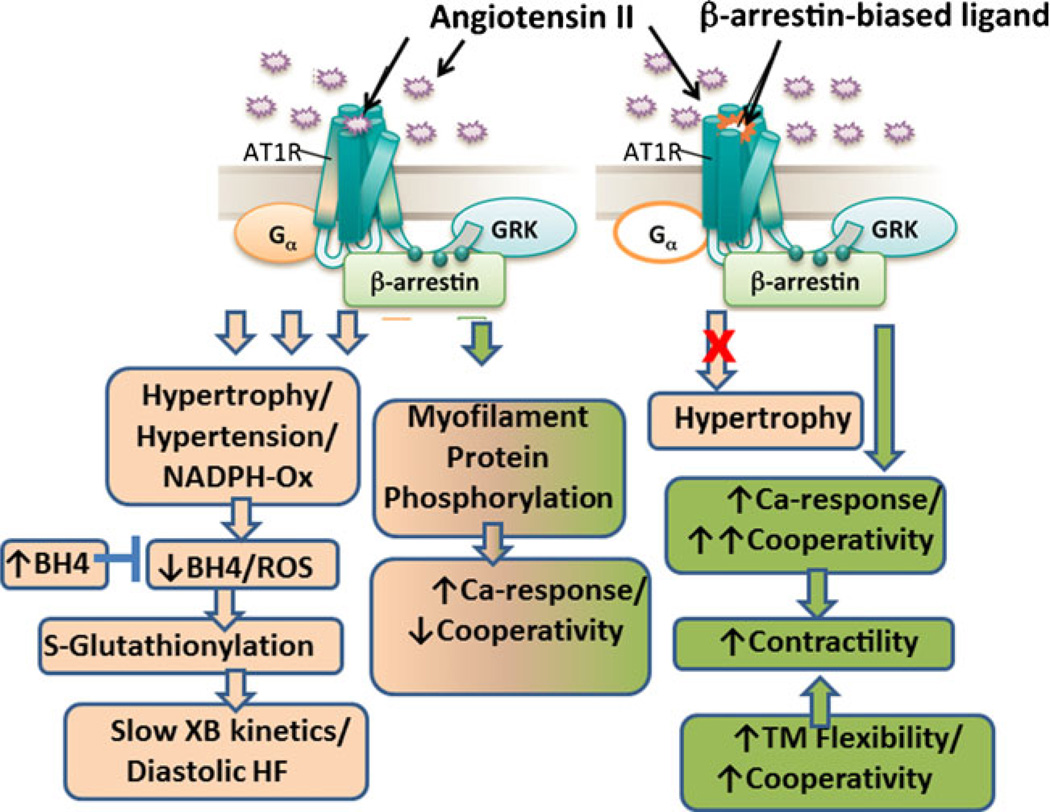
The release of reactive oxygen species (ROS) occurs as a general response to high angiotensin II (Ang II) levels in hypertension [38]. In this and the next section, we discuss new perspectives in acquired HF in terms of a potential role of posttranslational modifications of myofilament proteins in response to hypertension and Ang II. Figure 1 illustrates a scheme summarizing these perspectives. The role of oxidative damage to sarcomeric proteins has been reviewed before [5], and here we focus on significant recent advancements to our understanding on sarcomeric oxidative stress in the last few years. We have employed a well-established model of arterial hypertension and diastolic dysfunction to understand how the sarcomeric proteins are modified following an oxidative insult [41]. It has been shown that the deoxycorticosterone acetate (DOCA)-salt hypertension mouse model is characterized by an increase in the production of ROS from the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to oxidation of tetrahydrobiopterin (BH4), a cofactor of endothelial nitric oxide synthase (eNOS) in the production of nitric oxide from l-arginine [35]. This oxidation of BH4 to dihydrobiopterin, and thus its depletion, leads to uncoupling of eNOS and the production of superoxide anions instead of nitric oxide [6, 18]. In this mouse model, recent work in our laboratories has identified a myofilament redox-related modification as a primary cause of diastolic abnormalities [31, 41, 51]. In fact, as indicated in Fig. 1, in the DOCA-salt mouse model, cardiac oxidation leads to cardiac diastolic dysfunction, which can be ameliorated by BH4 treatment [31, 57]. Concomitantly, we observed that together with a reversal of diastolic dysfunction, there was a reversal of an increase in S-glutathionylation of cardiac myosin-binding protein C (cMyBP-C), and the slowed crossbridge kinetics, which occurred in hearts from DOCA-salt mice [31]. Increased S-glutathionylation is one indicator of oxidative stress since oxidative stress leads to reversible glutathione binding to thiols of proteins to protect them from irreversible oxidative damage [69].
Fig. 1.
Angiotensin II signaling and control of cardiac myofilament kinetics and response to Ca2+. The Ang II signaling cascade on the left emphasizes evidence that in the presence of Ang II, (1) redox signaling (reversed by BH4) via S-glutathionylation of myosin binding protein C slows crossbridge (XB) kinetics and may be significant in diastolic heart failure, and (2) kinase/phosphatase modifications alter myofilament protein phosphorylation and response to Ca2+. The signaling scheme on the right emphasizes evidence that the biased ligand, TRV120023, which binds to the Ang II receptor type 1 (ATR1), unlike conventional blockers, blocks only G protein signaling and activates β-arrestin 2-mediated signaling. Thus, Ang II effects on contractility are increased and preserved, whereas hypertrophy is blocked. Also shown in the lower right hand panel is the influence of tropomyosin (TM) flexibility on cooperative activation and cooperativity. See text for further discussion
We have also reported an improvement of the impaired ventricular relaxation in hearts of DOCA-salt mice with administration of Ranolazine [41], a late INa blocker used clinically to treat angina [24]. In this study, the time course and amplitude of Ca2+ transients in ventricular myocytes were not different from sham, suggesting a mechanism by which relaxation is altered on the level of the myofilaments independent of Ca2+ signaling [41]. Detergent-extracted fiber bundles from DOCA-salt hearts demonstrated an increased myofilament Ca2+ sensitivity and slowing of cross-bridge kinetics, which were also reversed with Ranolazine treatment [41]. These results strongly indicate a role for altered myofilament relaxation dynamics and/or response to Ca2+ as a significant factor in this model of a diastolic disorder with preserved systolic function and suggest a mechanism involving an oxidative modification on MyBP-C [31, 41].
Since oxidative stress during hypertension can lead to an increase in the posttranslational modification of S-glutathionylation and, indeed, an increase in S-glutathionylation of MyBP-C in the hypertensive DOCA-salt mouse model has been observed, a later study probed the role of MyBP-C and the different sites, which may be involved in regulating the sarcomeric response to Ca2+ [51]. S-glutathionylation of MyBP-C C655, C479, and C627 was associated with an increase in myofilament Ca2+ sensitivity in isolated detergent-extracted force-generating fiber bundles treated with oxidized glutathione (GSSG). This increase in myofilament Ca2+ sensitivity and S-glutathionylation of MyBP-C were reversed with the reducing agent, dithiothreitol (DTT) [51]. These results provide a novel mechanism involving the sarcomeric protein MyBP-C in the regulation of the sarcomeric response to Ca2+ and suggest that alterations to sarcomeric proteins may occur before changes in Ca2+ fluxes in hypertension and the progression to acquired HF. This is a significant advancement in identifying one of the first modifications to oxidative stress and opens a new focus of investigation, since sarcomeric alterations during oxidative stress can occur before and independently of alterations in Ca2+ signaling. This may apply not only to hypertensive heart disease but also to other diseases in which severe oxidative stress is central to the pathology. For example, the release of ROS occurs as an early response to MI and reperfusion [76]. In a short-term response to MI in a mouse model, we concluded that titin may also be a site of S-glutathionylation [3]. More recent work [1], positively identified titin as a substrate for S-glutathionylation and determined that with stretch cryptic cysteines, become available as substrates. The effect of S-glutathionylation of titin was to increase its extensibility in vitro and in situ in cardiac myocytes.
Ang II induces an increase in myofilament Ca2+ response that is preserved by a biased ligand acting as an Ang I-type 1 receptor blocker
Studies of biased ligands, which selectively activate signal transduction pathways of a given receptor, suggest an exciting new role for myofilaments in HF (Fig. 1). These biased ligands can partially activate downstream signaling cascades of a particular receptor by activating one cascade while blocking another. Such precise signaling cascade targeting may lead to the development of pharmaceuticals with less undesired side effects [16]. Two such biased ligands, namely, TRV120023 and TRV120027, have been described previously to bind Ang II-type 1 receptors (AT1R) to block G protein signaling yet preserve β-arrestin signaling and contractility [32, 48, 73]. In this way, the biased ligand TRV120027 inhibits Ang II-mediated vasoconstriction via blockade of G protein signaling yet increases cardiomyocyte contractility via β-arrestin signaling [73]. TRV120027 can reduce blood pressure in humans [60] and dogs [8, 9] and is in development as a pharmaceutical treatment for acute HF.
When administered to cardiomyocytes, TRV120023, a drug very similar to TRV120027, did not lead to the accumulation of inositol monophosphate 1 (IP1) and diacylglycerol (DAG) [32], nor did administration of the beta-arrestin-biased AT1R agonist [Sar1, Ile4, Ile8]-angiotensin II (SII) raise intracellular Ca2+ levels [55], suggesting an increase in the Ca2+ sensitivity of sarcomeric proteins as the mechanism of the inotropic effect of TRV120023 [32]. Since sarcomeric proteins can already regulate differences in force production in healthy myocardium despite the same intracellular Ca2+ concentration [46, 50], it stood to reason that changes at the myofilament level independent of Ca2+ signaling could alter contractile parameters. In fact, even in a rabbit model of acutely altered Ca2+ hemodynamics, there was still a significant dissociation between the rate of force relaxation and intracellular Ca2+ decline, indicating that the sarcomeric proteins are the final regulators in the development of force [47].
A recent study [48] investigated the effect of Ang II and TRV120023 on myofilament Ca2+ sensitivity. In this study, TRV120023 increased maximal Ca2+-activated isometric tension, pCa50, and the Hill coefficient, which is a determinant of crossbridge cooperative activation. Ang II increased maximal Ca2+-activated isometric tension and pCa50 but decreased the Hill coefficient, suggesting a different mechanism from TRV120023 for myofilament regulation [48]. The effect of Ang II on maximal Ca2+-activated isometric tension and pCa50 was still evident with simultaneous treatment with TRV120023 while apparently abolished with simultaneous treatment with losartan, an AT1R antagonist [48]. This is consistent with another study in which cardiomyocyte fractional shortening was increased after administration of Ang II, TRV120027, or TRV120023 [73]. The effects of both TRV120027 and TRV120023 were blocked by coadministration of valsartan, another Ang II receptor blocker with high affinity for AT1Rs [73]. Further investigation into sarcomeric changes revealed an increase in TM phosphorylation during both Ang II and TRV120023 administrations [48]. Additionally, other effects during administration of Ang II or TRV120023 were observed on β-myosin heavy chain isoform expression and phosphorylation of cardiac TnI and myosin-binding protein C S282 [48]. These studies suggest that during Ang II signaling or biased ligand signaling by TRV120027 and TRV120023, the final regulation of contraction is mediated by early changes at the level of the sarcomeric proteins independent of Ca2+ signaling.
Alterations in structural flexibility of tropomyosin affect cardiac function
As summarized above, whereas the cooperative activation of the myofilaments appears to be controlled by β-arrestin signaling, its role in the control of the dynamics of the heartbeat remains unclear. TM is indispensable both in cooperative activation within a structural unit (seven actins controlled by Tn/TM) as well as in cooperative activation by interactions between structural units leading to an extended functional unit. There is evidence that the flexibility of TM might be important for the role TM plays in cooperative activation. In this section of the review, we analyze new findings on structural and functional implications of alterations in TM’s structural rigidity or flexibility and discuss whether the alteration in the flexibility of TM could be an initial molecular abnormality that leads to disease phenotype. Support for this idea comes from studies indicating that alterations in structural flexibility of TM may be an initial trigger for inherited HCM and DCM that are linked to TM mutations [25, 33, 40, 43, 45, 78].
α-TM has an α-helical coiled coil structure along its entire length in which two α-helices associate to form a supercoil [13, 53, 77]. Coiled coils form a heptad repeat motif (abcdefg) where hydrophobic residues at the core positions a and d stabilize the molecule, while residues at positions e and f can supplement this stabilization [74]. Although destabilizing breaks in the heptad repeat structure of coiled coil proteins is a common and functionally important characteristic of these proteins, α-TM is unique as it has no discontinuity in its coiled coil repeats [10]. Alternatively, large hydrophobic residues at positions a and d are often interrupted by small hydrophobic, charged, or polar residues in α-TM. This interruption creates in TM’s structurally distinct domains that differ in their structural stability, which introduces an alteration in structural flexibility [11, 29, 52, 70]. Brown et al. was first to point out poorly packed regions around Ala residues of α-TM that are scattered along the molecule and located at a and d positions [11]. These so-called Ala clusters destabilize regions of TM to provide the molecule flexibility, which is considered important for binding of TM to actin [58, 59]. In 2008, Sumida et al. showed that a conserved Asp137 residue of α-TM in the d position contributes to another poorly packed region at the center of TM and provides flexibility to the molecule [65]. Mutation of this Asp137 to Leu, a hydrophobic residue, decreases the flexibility of TM and increases the ATPase activity of reconstituted filaments [65, 78]. Another flexible region around the noncanonical Gln263, which is also located at a d position, has been shown to have a significant function enabling complex formation between C-terminus and N-terminus of the adjacent TM molecules as well as between TM and troponin T [21]. It is previously reported that there is another poorly packed region around noncanonical and destabilizing residues (Tyr214, Glu218, Tyr221, and Tyr267) at the hydrophobic core of α-TM; nonetheless, its functional significance is not well understood [44]. These findings altogether suggest that destabilizing regions of α-TM are essential for its proper functioning in vitro and highlight the need for further studies to address functional significance of these regions in ejecting hearts.
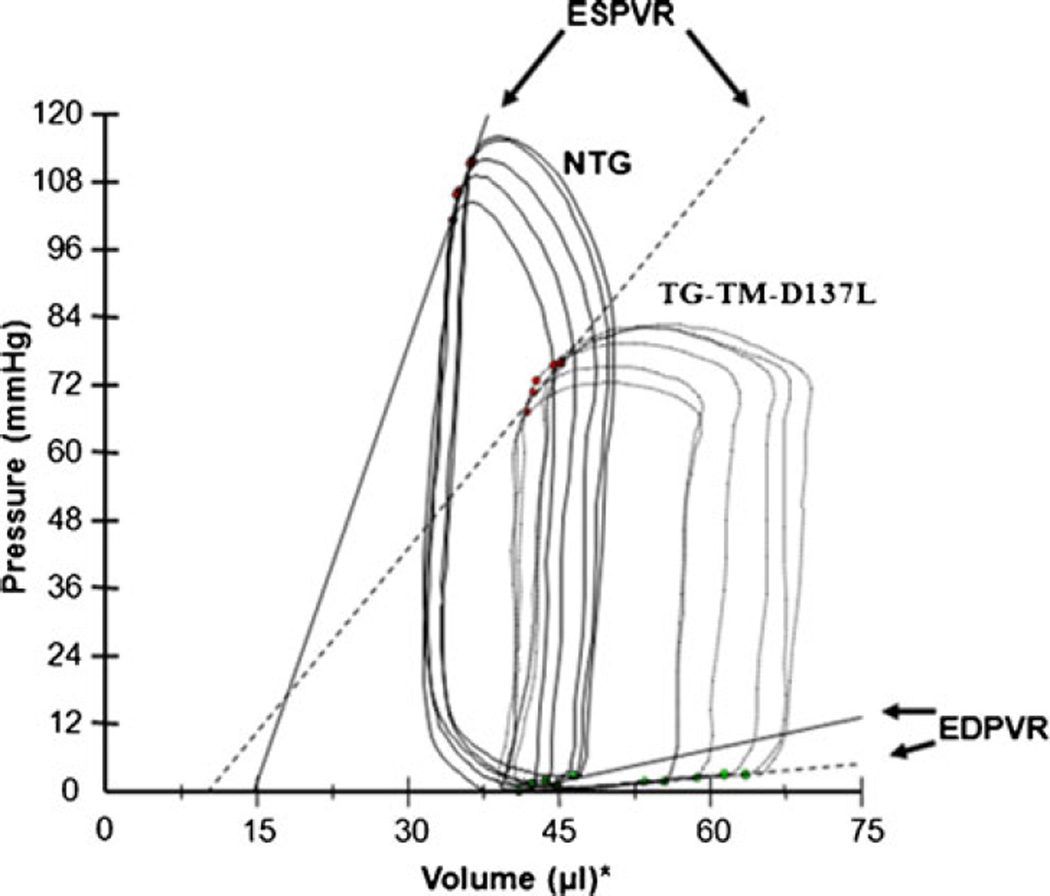
Recent studies have suggested a possible link between alterations in TM’s structural flexibility and inherited CMs. For example, TM’s increased flexibility has emerged as the characteristic of HCM-linked mutations (Glu180Gly, Asp175Asn, Lys70Thr, and Ala63Val) [25, 33, 40], whereas the DCM-linked Glu40Lys and Glu54Lys mutations have been shown to decrease the flexibility of TM [45]. In support of the causal link between altered flexibilities of TM and CMs, findings from our recent study showed that an expression in transgenic (TG) mouse hearts of a decreased flexibility mutant α-TM-Asp137Leu, which is not yet associated with CMs, depresses cardiac contractility and leads to a phenotype similar to DCM [78]. In this study, depressed contractile function and mild ventricular dilation of TG mice hearts was detected with echocardiography. As an extension to this previous study, our recent pressure-volume (PV) loop measurements (Fig. 2) confirmed depressed pump function in α-TM-Asp137Leu TG mice hearts. As shown in the representative baseline PV loops, during an inferior vena cava (IVC) occlusion, there was a loss of intrinsic inotropy in TG mice hearts as evident from a significant decrease in the slope of end-systolic pressure-volume relation (ESPVR). The significant increase in end-systolic volume (ESV) and end-diastolic volume (EDV), the decrease in the slope of ESPVR and end-diastolic pressure-volume relation (EDPVR), and the decrease in dP/dt max corroborate a phenotype similar to DCM. Interestingly, this depression in contractility occurred in the absence of any changes in intracellular Ca2+ transients. This result suggests a mechanism intrinsic to the myofilaments.
Fig. 2.
Representative left ventricular pressure-volume loops under baseline conditions, following an inferior vena cava occlusion. The black solid loops and solid slope line represent the ESPVR in the non-transgenic (NTG) group. The dotted black loops and dotted slope represent the depressed ESPVR in the TG-TM-D137L group
DCM-linked sarcomeric mutations are generally considered to cause a decrease in myofilament Ca2+ sensitivity, whereas HCM-linked sarcomeric mutations lead to an increase. Although our laboratory findings (i.e., TG mice that expresses a decreased flexibility mutant α-TM-Asp137Leu in the heart shows a DCM phenotype and a decreased myofilament Ca2+ sensitivity [78]) support this observation, there are still significant exceptions. For example, one study reports that DCM-linked α-TM Asp230Asn mutation increases myofilament Ca2+ response. Another study shows that α-TM Glu54Lys mutation does not change Ca2+ sensitivity [43]. A recent study demonstrates that there is no increase in Ca2+ sensitivity in a human HCM heart sample with an α-TM Ile284Valmutation [56]. Therefore, change in Ca2+ sensitivity may not be the major trigger in pathogenesis. Considering the findings summarized before, alterations in structural flexibility of TM may be an essential initial molecular abnormality that leads to disease phenotype. However, it is likely that additional factors contribute to the disease mechanism.
At the sarcomere level, TM regulates Ca2+- and ATP-dependent interactions between thin and thick filament proteins [75]. During this process, TM transmits the information from TnC, the Ca2+ receptor, to the actin-myosin interaction. We argue that variations in the flexibility of α-TM affect information transmission along the length of the molecule and thus lead to dysfunction. According to a widely accepted three-state model by McKillop and Geeves [42], during muscle contraction, thin filaments exist in a rapid equilibrium between three states. Ca2+ binding to Tn triggers relocation of TM from a blocked thin filament state to a weak crossbridge binding or closed state, and full activation requires an open state induced by strong crossbridge binding [36, 42, 72]. In addition to its effect on information transmission along the molecule, structural flexibility of TM is considered one of the main mediators of TM fluctuations relative to actin filaments during thin filament transition from activation (systole) to relaxation (diastole). Due to a small value for the equilibrium constant between blocked and open states of the thin filaments [19], relatively small changes in the thermal fluctuations of TM could easily alter its rapid regulatory movements on the surface of actin. There is no doubt that disruptions in the molecular switch mechanism would eventually alter physiological functioning of TM and result in a disease phenotype. Findings from our previous study support this hypothesis. We showed that decreasing structural flexibility of TM depresses Ca2+-dependent activation of cardiac thin filaments from TG mouse without causing any changes in the strongly bound crossbridge-dependent activation of skinned fiber preparations [78]. This result implies that decreasing flexibility of TM impedes Ca2+-dependent transition of TM between blocked and closed states. Yet, we could not find any changes in strong crossbridge-dependent transition of TM between closed and open states. Therefore, the decrease in TM’s flexibility is likely to have impeded Ca2+-dependent relocation of TM between blocked and closed states, resulting in a delay in time-sensitive contraction and relaxation processes of a cardiac myocyte. This proposed delay could eventually induce cardiac dysfunction, as was observed in TG α-TM-Asp137Leu mouse hearts.
Cooperative activation process of cardiac myofilaments involves Ca2+ binding to regulatory units, cooperative interactions between thin and thick filament proteins within a single regulatory unit, and long-range cooperative interactions between neighbor regulatory units along the filaments [19, 26, 68]. Although individual contribution of each component has not been clearly identified, TM molecules are known to play a crucial role in this cooperative activation process, possibly through end-to-end interactions between adjacent molecules leading to the spread of activation signal [19, 26]. However, little is known about the likely influence of changes in structural flexibility of TM on this cooperative activation process. Loong et al. recently argue that alterations in TM’s rigidity affect the cooperative spread of activation signal along TM molecule and across thin filaments [39, 40]. In their model, they particularly propose that increased flexibility of the HCM mutant α-TM-Glu180Gly significantly impedes signal transmission along the molecule, causing a higher Ca2+ sensitivity as well as a reduction in cooperative transmission of activation signal along the thin filaments. This explains prolonged systolic activation and diastolic relaxation of cardiac thin filaments from HCM hearts that express a more flexible TM. In support of these studies, our recent findings demonstrated an increase in Ca2+-dependent cooperative activation of thin filaments from TG mouse hearts expressing a decreased flexibility mutant α-TM-Asp137Leu [78]. On the contrary, a previous in vitro study by Heller et al. shows that HCM mutations Lys70Thr and Ala63Val increase N-domain flexibility of TM while having no effect on Ca2+-dependent cooperative activation of thin filaments [25]. Therefore, more studies are needed to reveal the effect of alterations in structural flexibility of TM on cooperative activation of thin filaments.
Future directions in investigating integration of modifications in myofilament proteins with cardiac dynamics
A critical question for the future is the time course of the relation between Ca2+ bound to the regulatory site of TnC and the time course of pressure and volume in heartbeats in various states. Probes for this determination in real time under load would serve to answer many questions concerning the relative role of upstream and downstream control of cardiac function (for example, those illustrated in Fig. 1). A related future direction is the determination of the dynamics of posttranslational modifications of sarcomeric proteins in relation to altered functional state of the myocardium. The development of probes to address this question should be a major consideration. While much has been theorized regarding the importance of cooperative activation of the sarcomeres, little is known regarding the in situ role for this mechanism or the relevance of variations in cooperative activation on cardiac function. Exciting future directions of research also involve continual unraveling of the many functions of the sarcomeric proteins in mechano-signaling including the functional effects of altered flexibility, and the influence of folded state on posttranslational modifications.
Acknowledgments
Work presented here was supported by grants from the NIH NHLBI (RJS) and by an American Heart Association pre-doctoral fellowship to S.Y. MMM was supported by a Marie Curie fellowship: Call FP7-PEOPLE-2010-COFUND No 267264-INVEST.
Contributor Information
Sumeyye Yar, Department of Physiology and Biophysics, College of Medicine, University of Illinois at Chicago, M/C 901, Chicago, IL 60612, USA; Department of Biochemistry and Molecular Genetics, College of Medicine, University of Illinois at Chicago, 835 S. Wolcott Ave, M/C 901, Chicago, IL 60612-7342, USA.
Michelle M. Monasky, Università Vita-Salute San Raffaele, Via Olgettina 58, 20132 Milan, Italy
R. John Solaro, Email: solarorj@uic.edu, Department of Physiology and Biophysics, College of Medicine, University of Illinois at Chicago, M/C 901, Chicago, IL 60612, USA; Center for Cardiovascular Research, College of Medicine, University of Illinois at Chicago, 835 S. Wolcott Ave, M/C 901, Chicago, IL 60612-7342, USA.
References
- 1.Alegre-Cebollada J, Kosuri P, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernanadez JM. S-glutathionylation of cryptic cysteins enhances titin elasticity by blocking protein folding. Cell. 2014 doi: 10.1016/j.cell.2014.01.056. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert NR, Mulieri LA. Human heart failure: determinants of ventricular dysfunction. Adv Exp Med Biol. 1997;430:97–108. doi: 10.1007/978-1-4615-5959-7_9. [DOI] [PubMed] [Google Scholar]
- 3.Avner BS, Shioura KM, Scruggs SB, Grachoff M, Geenen DL, Helseth DL, Jr, Farjah M, Goldspink PH, Solaro RJ. Myocardial infarction in mice alters sarcomeric function via posttranslational protein modification. Mol Cell Biochem. 2012;363(1–2):203–215. doi: 10.1007/s11010-011-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118(12):3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayeva M, Ardehali H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr Hypertens Rep. 2010;12(6):426–432. doi: 10.1007/s11906-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 6.Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, Yokoyama M, Kawashima S, Channon KM. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97(9):864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 7.Blinks JR, Endoh M. Modification of myofibrillar responsiveness to Ca++ as an inotropic mechanism. Circulation. 1986;73(3 Pt 2):III85–III98. [PubMed] [Google Scholar]
- 8.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC., Jr Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4(6):770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 9.Boerrigter G, Soergel DG, Violin JD, Lark MW, Burnett JC., Jr TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ Heart Fail. 2012;5(5):627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- 10.Brown JH, Cohen C, Parry DA. Heptad breaks in alpha-helical coiled coils: stutters and stammers. Proteins. 1996;26(2):134–145. doi: 10.1002/(SICI)1097-0134(199610)26:2<134::AID-PROT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-DeGregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci U S A. 2001;98(15):8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J: Off Publ Fed Am Soc Exp Biol. 2005;19(1):88–90. doi: 10.1096/fj.04-2066fje. [DOI] [PubMed] [Google Scholar]
- 13.Crick F. The packing of alpha-helices. Simple coiled-coils. Acta Crystallogr. 1953;6:689–697. [Google Scholar]
- 14.Davidoff AW, Boyden PA, Schwartz K, Michel JB, Zhang YM, Obayashi M, Crabbe D, ter Keurs HE. Congestive heart failure after myocardial infarction in the rat: cardiac force and spontaneous sarcomere activity. Ann N Y Acad Sci. 2004;1015:84–95. doi: 10.1196/annals.1302.007. [DOI] [PubMed] [Google Scholar]
- 15.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res. 2011;109(2):205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda N, Terui T, Ohtsuki I, Ishiwata S, Kurihara S. Titin and troponin: central players in the frank-starling mechanism of the heart. Curr Cardiol Rev. 2009;5(2):119–124. doi: 10.2174/157340309788166714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao YT, Roman LJ, Martasek P, Panda SP, Ishimura Y, Masters BS. Oxygen metabolism by endothelial nitric-oxide synthase. J Biol Chem. 2007;282(39):28557–28565. doi: 10.1074/jbc.M704890200. [DOI] [PubMed] [Google Scholar]
- 19.Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J. 1994;67(1):273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield NJ, Palm T, Hitchcock-DeGregori SE. Structure and interactions of the carboxyl terminus of striated muscle alpha-tropomyosin: it is important to be flexible. Biophys J. 2002;83(5):2754–2766. doi: 10.1016/S0006-3495(02)75285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77(4):649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 23.Hanft LM, Korte FS, McDonald KS. Cardiac function and modulation of sarcomeric function by length. Cardiovasc Res. 2008;77(4):627–636. doi: 10.1093/cvr/cvm099. [DOI] [PubMed] [Google Scholar]
- 24.Hawwa N, Menon V. Ranolazine: clinical applications and therapeutic basis. Am J Cardiovasc Drugs. 2013;13(1):5–16. doi: 10.1007/s40256-012-0003-2. [DOI] [PubMed] [Google Scholar]
- 25.Heller MJ, Nili M, Homsher E, Tobacman LS. Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility. J Biol Chem. 2003;278(43):41742–41748. doi: 10.1074/jbc.M303408200. [DOI] [PubMed] [Google Scholar]
- 26.Hill TL, Eisenberg E, Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinken AC, Solaro RJ. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology. 2007;22:73–80. doi: 10.1152/physiol.00043.2006. [DOI] [PubMed] [Google Scholar]
- 28.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92(4):350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 29.Ishii Y, Hitchcock-DeGregori S, Mabuchi K, Lehrer SS. Unfolding domains of recombinant fusion alpha alpha-tropomyosin. Protein Sci. 1992;1(10):1319–1325. doi: 10.1002/pro.5560011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol. 2010;299(6):H1741–H1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC, Jr, Solaro RJ. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–H1010. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremneva E, Boussouf S, Nikolaeva O, Maytum R, Geeves MA, Levitsky DI. Effects of two familial hypertrophic cardiomyopathy mutations in alpha-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin. Biophys J. 2004;87(6):3922–3933. doi: 10.1529/biophysj.104.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46(4):490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302(3):593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 37.LeWinter MM. Functional consequences of sarcomeric protein abnormalities in failing myocardium. Heart Fail Rev. 2005;10(3):249–257. doi: 10.1007/s10741-005-5254-4. [DOI] [PubMed] [Google Scholar]
- 38.Lijnen PJ, van Pelt JF, Fagard RH. Stimulation of reactive oxygen species and collagen synthesis by angiotensin II in cardiac fibroblasts. Cardiovasc Ther. 2012;30(1):e1–e8. doi: 10.1111/j.1755-5922.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 39.Loong CK, Badr MA, Chase PB. Tropomyosin flexural rigidity and single Ca(2+) regulatory unit dynamics: implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy. Front Physiol. 2012;3:80. doi: 10.3389/fphys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loong CK, Zhou HX, Chase PB. Familial hypertrophic cardiomyopathy related E180G mutation increases flexibility of human cardiac alpha-tropomyosin. FEBS Lett. 2012;586(19):3503–3507. doi: 10.1016/j.febslet.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mckillop DFA, Geeves MA. Regulation of the interaction between actin and myosin subfragment-1—evidence for 3 states of the thin filament. Biophys J. 1993;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Memo M, Leung MC, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Familial dilated cardiomyopathy mutations uncouple troponin I phosphorylation from changes in myofibrillar Ca(2)(+) sensitivity. Cardiovasc Res. 2013;99(1):65–73. doi: 10.1093/cvr/cvt071. [DOI] [PubMed] [Google Scholar]
- 44.Minakata S, Maeda K, Oda N, Wakabayashi K, Nitanai Y, Maeda Y. Two-crystal structures of tropomyosin C-terminal fragment 176–273: exposure of the hydrophobic core to the solvent destabilizes the tropomyosin molecule. Biophys J. 2008;95(2):710–719. doi: 10.1529/biophysj.107.126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirza M, Robinson P, Kremneva E, Copeland O, Nikolaeva O, Watkins H, Levitsky D, Redwood C, El-Mezgueldi M, Marston S. The effect of mutations in alpha-tropomyosin (E40K and E54K) that cause familial dilated cardiomyopathy on the regulatory mechanism of cardiac muscle thin filaments. J Biol Chem. 2007;282(18):13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- 46.Monasky MM, Biesiadecki BJ, Janssen PM. Increased phosphorylation of tropomyosin, troponin I, myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J Mol Cell Cardiol. 2010;48(5):1023–1028. doi: 10.1016/j.yjmcc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monasky MM, Janssen PM. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol B Biochem Syst Environ Physiol. 2009;179(4):469–479. doi: 10.1007/s00360-008-0331-3. [DOI] [PubMed] [Google Scholar]
- 48.Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, Violin JD, Solaro RJ. The beta-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305(6):H856–H866. doi: 10.1152/ajpheart.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monasky MM, Taglieri DM, Jacobson AK, Haizlip KM, Solaro RJ, Janssen PM. Post-translational modifications of myofilament proteins involved in length-dependent prolongation of relaxation in rabbit right ventricular myocardium. Arch Biochem Biophys. 2013;535(1):22–29. doi: 10.1016/j.abb.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monasky MM, Varian KD, Davis JP, Janssen PM. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium. Pflugers Arch Eur J Physiol. 2008;456(2):267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 51.Patel BG, Wilder T, Solaro RJ. Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front Physiol. 2013;4:336. doi: 10.3389/fphys.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pato MD, Mak AS, Smillie LB. Fragments of rabbit striated muscle alpha-tropomyosin. II. Binding to troponin-T. J Biol Chem. 1981;256(2):602–607. [PubMed] [Google Scholar]
- 53.Phillips GN, Jr, Fillers JP, Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 54.Poggesi G, Ferrantini C, Servettini E, Girolami F, Cecchi F, Olivotto I. Disease progression and systolic dysfunction in patients with hypertrophic cardiomyopathy: genetic basis, pathophysiology and clinical presentation. G Ital Cardiol (Rome) 2011;12(12):815–823. doi: 10.1714/996.10826. [DOI] [PubMed] [Google Scholar]
- 55.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103(44):16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sequeira V, Wijnker PJ, Nijenkamp LL, Kuster DW, Najafi A, Witjas-Paalberends ER, Regan JA, Boontje N, Ten Cate FJ, Germans T, Carrier L, Sadayappan S, van Slegtenhorst MA, Zaremba R, Foster DB, Murphy AM, Poggesi C, Dos Remedios C, Stienen GJ, Ho CY, Michels M, van der Velden J. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013;112(11):1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121(4):519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh A, Hitchcock-DeGregori SE. Local destabilization of the tropomyosin coiled coil gives the molecular flexibility required for actin binding. Biochemistry. 2003;42(48):14114–14121. doi: 10.1021/bi0348462. [DOI] [PubMed] [Google Scholar]
- 59.Singh A, Hitchcock-DeGregori SE. Dual requirement for flexibility and specificity for binding of the coiled-coil tropomyosin to its target, actin. Structure. 2006;14(1):43–50. doi: 10.1016/j.str.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Soergel DG, Subach RA, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV027: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2013;53(9):892–899. doi: 10.1002/jcph.111. [DOI] [PubMed] [Google Scholar]
- 61.Solaro RJ. Sarcomere control mechanisms and the dynamics of the cardiac cycle. J Biomed Biotechnol. 2010;2010:105648. doi: 10.1155/2010/105648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solaro RJ, Tardiff JC. Biophysics of the failing heart. New York: Springer; 2013. [Google Scholar]
- 63.Stehle R, Iorga B. Kinetics of cardiac sarcomeric processes and rate-limiting steps in contraction and relaxation. J Mol Cell Cardiol. 2010;48(5):843–850. doi: 10.1016/j.yjmcc.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Steinberg SF. Oxidative stress and sarcomeric proteins. Circ Res. 2013;112(2):393–405. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sumida JP, Wu E, Lehrer SS. Conserved Asp-137 imparts flexibility to tropomyosin and affects function. J Biol Chem. 2008;283(11):6728–6734. doi: 10.1074/jbc.M707485200. [DOI] [PubMed] [Google Scholar]
- 66.ter Keurs HE. Heart failure and Starling’s law of the heart. Can J Cardiol. 1996;12(10):1047–1057. [PubMed] [Google Scholar]
- 67.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77(5):701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 68.Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000;275(36):27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- 69.Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7(6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ueno H. Local structural changes in tropomyosin detected by a trypsin-probe method. Biochemistry. 1984;23(20):4791–4798. doi: 10.1021/bi00315a040. [DOI] [PubMed] [Google Scholar]
- 71.van der Velden J. Diastolic myofilament dysfunction in the failing human heart. Pflugers Arch Eurn J Physiol. 2011;462(1):155–163. doi: 10.1007/s00424-011-0960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266(1):8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 73.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335(3):572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 74.Wagschal K, Tripet B, Lavigne P, Mant C, Hodges RS. The role of position a in determining the stability and oligomerization state of alpha-helical coiled coils: 20 amino acid stability coefficients in the hydrophobic core of proteins. Protein Sci Publ Protein Soc. 1999;8(11):2312–2329. doi: 10.1110/ps.8.11.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang CL, Coluccio LM. New insights into the regulation of the actin cytoskeleton by tropomyosin. Int Rev Cell Mol Biol. 2010;281:91–128. doi: 10.1016/S1937-6448(10)81003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 2012;8(6):863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitby FG, Phillips GN., Jr Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins. 2000;38(1):49–59. [PubMed] [Google Scholar]
- 78.Yar S, Chowdhury SA, Davis RT, 3rd, Kobayashi M, Monasky MM, Rajan S, Wolska BM, Gaponenko V, Kobayashi T, Wieczorek DF, Solaro RJ. Conserved Asp-137 is important for both structure and regulatory functions of cardiac alpha-tropomyosin (alpha-TM) in a novel transgenic mouse model expressing alpha-TM-D137L. J Biol Chem. 2013;288(23):16235–16246. doi: 10.1074/jbc.M113.458695. [DOI] [PMC free article] [PubMed] [Google Scholar]