Abstract
Two independent studies show that, if push comes to shove, differentiated cells of the stomach and lung can act as adult stem cells generating various cell types of the tissue, including a pool of stem cells.
When functional cells die, they are soon replenished. In most cases, the replacement cells arise either from division and differentiation of adult stem cells, which reside in the tissue, or from division of surviving mature cells of the same class. In other cases, when extreme injury or widespread dysfunction limits the capacity of normally regenerative mature cells, differentiated cells of a different class are believed to revert to a less mature state and function as stem cells. This particular kind of stem cell, not detected normally or even after routine injury, has been referred to as a facultative stem cell1, so called because it is only active in special circumstances. The term ‘facultative’ may also be appropriate to describe mature cells that normally function as progenitors, in the sense they are not restricted to a differentiated function but can also play a role in generating new cells. We therefore refer to the former class as a ‘reserve’ progenitor or stem cell, since it performs a back-up function when primary replacement mechanisms fail, and the latter as a ‘bi-functional’ progenitor or stem cell, since it normally executes both a differentiated and replacement function. Two papers2,3, including one published on Nature’s website today, investigate replacement mechanisms of differentiated cells in vivo, in the stomach and lung, providing surprising insights into how cellular renewal is achieved.
The passages of stomach and lung are lined by a single layer of different cell types that are continually replaced throughout life as they become damaged or die. In the central part of the stomach, the cells lining periodic outpouchings called crypts are found in a stereotyped distribution, organized by class (Fig. 1a). It is believed that cells in individual crypt units are maintained by rapidly dividing stem cells that reside above the mid-point of the crypt and whose daughters spread in both directions, differentiating into cells of various classes4.
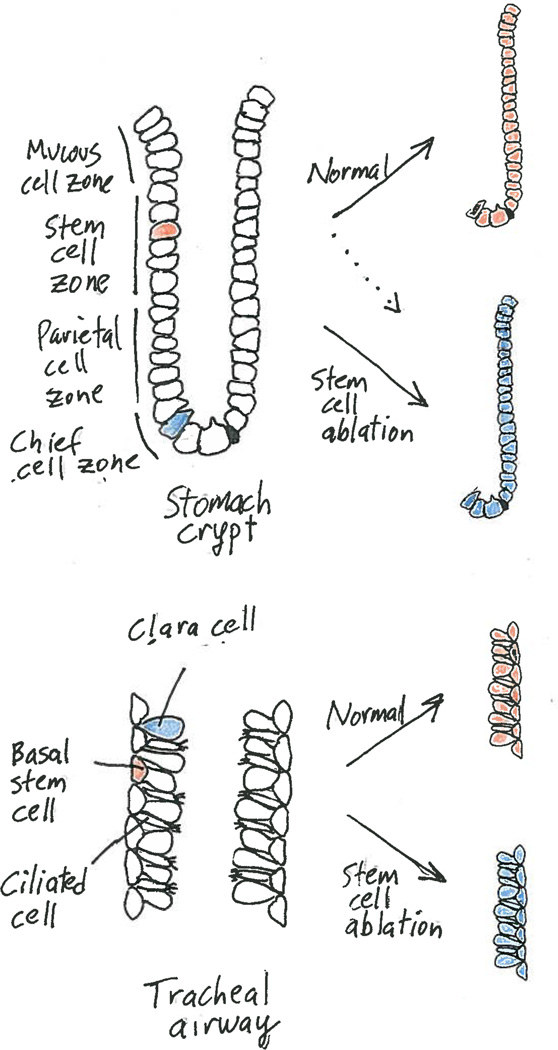
Figure 1. Two tissues, similar dedifferentiation process.
a, In the crypts of the stomach body, undifferentiated adult stem cells (asterisk) occupy the stem-cell zone, whereas functional, enzyme-secreting chief cells (blue) reside at the base. During cellular turnover, stem cells generate all cell types of the crypt (Normal, stem cell descendants in orange). But Stange et al.2 find that if stem cells are destroyed, mature chief cells assume a stem cell role, renewing even the depleted stem cells (Stem cell ablation, chief cell descendants in blue). Note, chief cells infrequently renew crypts in the absence of obvious injury (dotted line). b, The adult tracheal stem cells called basal cells (asterisk) replenish secretory Clara cells and multiciliated cells under physiological and injury conditions (Normal, basal cell descendants in orange). Rao Tata et al.3 report that if these stem cells are ablated, differentiated Clara cells become activated to regenerate basal cells, which resume the task of maintaining tracheal cell types (Stem cell ablation, Clara cell descendants in blue).
Stange and colleagues find that at the base of the crypts in mice there are cells that express Troy, a marker of intestinal stem cells. Using genetic techniques to ‘pulse-label’ these cells in a permanent and heritable manner at a low frequency, they find rare crypts in which all cells derive from a Troy-expressing cell whose progeny slowly spread up from the base. When the rapidly dividing stem cells are ablated, however, Troy-expressing cells execute this stem cell function much more rapidly and in many more crypts. Remarkably, this cell is a fully mature secretory cell type called a chief cell that maintains its differentiated identity even while executing its stem-cell behaviour. Because their regenerative function is activated following depletion of the primary stem cell population, chief cells can be considered ‘reserve’ stem cells.
Rao Tata et al. independently demonstrate that differentiated airway secretory cells called Clara cell scan contribute to regeneration in the lung. Previous work showed5,6 that undifferentiated basal cells in the mouse trachea renew secretory and multiciliated cells, which produce and clear airway mucous, respectively. In the present paper, the investigators pulse-labelled mature secretory cells en masse before specifically killing basal cells. Surprisingly, they could trace the lineage mark they introduced before basal-cell destruction in newly arising basal cells. Note that, Rao Tata and co-workers’ bulk-labelling strategy could be a caveat, because it may have inadvertently marked some original basal cells that escaped destruction. It would be valuable to conduct studies using a sparse-labelling strategy, to trace the behaviour of individual secretory cells.
These authors also report that the marked basal cells, presumably descendants of labelled mature secretory cells, function as stem cells, renewing both multiciliated and secretory cell types. Because their progenitor activity is only elicited following elimination of basal stem cells, tracheal Clara cells can also be considered ‘reserve’ stem cells.
Although the differentiated Clara cells of the lung and chief cells of the stomach each give rise to multiple cell types, the routes they take are quite different. Clara cells directly generate replacement stem cells, whereas chief cells apparently bypass this requirement and are themselves stem cells. However, lower in the airway tract, Clara cells have been implicated as ‘bi-functional’ stem cells, renewing themselves and multiciliated cells without the presence of basal cells7. Conversely, chief cells also seem to generate stem cells, albeit indirectly, since their descendants eventually replace entire crypt units, including the resident stem cell populations. Thus, despite taking different routes, these mature cells share the potential to generate both differentiated cells as well as stem cells.
The two papers challenge the primacy of undifferentiated tissue-specific stem cells, given that mature cells can substitute for their function and even make new ones. They also raise questions such as what reprogramming factors regulate stem cell behaviour in mature cells, and is reversion to an undifferentiated state an obligate step? Other questions include, what cells generate the initial adult-stem-cell population in a tissue? And how is an appropriate balance between mature cells and different types of stem cells within a tissue maintained? In the trachea, Rao Tata et al. provide evidence that contact or short-range inhibition of Clara cell dedifferentiation by basal cells may play a role.
Pursuing these questions may have implications for regenerative medicine, given that there is an intrinsic appeal to the shorter path in re-directing differentiation of a mature cell instead of starting from scratch with an undifferentiated stem cell. Equally important is the possibility that these ‘reserve’ programs can be activated in differentiated cells in vivo by extrinsic signals. This would eliminate the need for introducing cellular reprogramming factors, and thereby should avoid the attendant risk of promoting cancer through this form of potential therapy.
Contributor Information
Tushar J. Desai, Department of Medicine, Division of Pulmonary and Critical Care, Stanford University School of Medicine, Stanford, California 94305-5307, USA
Mark A. Krasnow, Email: krasnow@stanford.edu, Department of Biochemistry, Stanford University School of Medicine, Stanford, California 94305-5307, USA.
References
- 1.Yanger K, Stanger BZ. Dev Dyn. 2011;240:521–529. doi: 10.1002/dvdy.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stange DE, et al. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao Tata P, et al. Nature. 2013 doi:x0x0x0x. [ref correct?] [Google Scholar]
- 4.Karam SM, Leblond CP. Anat. Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 5.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 6.Rock JR, et al. Proc. Natl Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawlins EL, et al. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]