Abstract
Underlying mechanisms by which air pollutants adversely affect human health remain poorly understood. Oxidative stress has been considered as a potential mechanism that may promote lipid peroxidation by reactive oxygen species, leading to the formation of malondialdehyde (MDA) that is excreted in biofluids (e.g., urine and exhaled breath condensate (EBC)). A panel study was conducted to examine whether concentrations of MDA in EBC and urine were associated, respectively, with changes in air pollution levels brought by the Beijing Olympic air pollution control measures. EBC and urine samples from 125 healthy adults were collected twice in each of the pre-, during-, and post-Olympic periods. Period-specific means of MDA and changes in MDA levels associated with increases in 24-h average pollutant concentrations were estimated using linear mixed-effects models. From the pre- to the during-Olympic period, when concentrations of most pollutants decreased, EBC MDA and urinary MDA significantly decreased by 24% (P < 0.0001) and 28% (P = 0.0002), respectively. From the during-Olympic to the post-Olympic period, when concentrations of most pollutants increased, EBC MDA and urinary MDA increased by 28% (P = 0.094) and 55% (P = 0.046), respectively. Furthermore, the largest increases in EBC MDA associated with one interquartile range (IQR) increases in all pollutants but ozone ranged from 10% (95% CI: 2%, 18%) to 19% (95% CI: 14%, 25%). The largest increases in urinary MDA associated with IQR increases in pollutant concentration ranged from 9% (95%: 0.3%, 19%) to 15% (95% CI: 3%, 28%). These findings support the utility of EBC MDA as a biomarker of oxidative stress in the respiratory tract and urinary MDA as a biomarker of systemic oxidative stress in relation to air pollution exposure in healthy young adults. Both EBC and urine samples can be collected noninvasively in the general population.
Keywords: The Beijing Olympics, lipid peroxidation, malondialdehyde, oxidative stress, exhaled breath condensate
INTRODUCTION
Oxidative stress has been linked to various adverse health outcomes and even natural aging.1–4 Lipids are the most targeted molecules of oxidative stressors such as reactive oxygen species (ROS).5 Peroxidation of lipids by ROS has been linked to cardiovascular and/or respiratory diseases in several epidemiological studies.6–11 Malondialdehyde (MDA) is a principal stable product of lipid peroxidation and has been recognized as a relevant biomarker of oxidative stress.5,12–14 Higher levels of MDA, in plasma, serum, and exhaled breath condensate (EBC), have been observed in subjects with diseases characterized by oxidative stress, for example, cancer, diabetes, cardiovascular, and respiratory diseases.15–18
Oxidative stress has been regarded as an important mechanism by which air pollution exposure leads to adverse health effects.19–23 Several biomarkers (e.g., 8-hydroxydeoxyguanosine and 8-isoprostane) have been used in studies of air pollution exposure associated with oxidative stress. However, only a limited number of epidemiological studies employed MDA as a biomarker of oxidative stress in relation to air pollution.24–26 For example, Barregard et al.24 reported an increased level of MDA in EBC both immediately and 20 h after exposure to wood smoke. Romieu et al.26 studied associations of MDA in EBC with PM2.5 and O3 in asthmatic children living in Mexico City. Urinary MDA has also been measured to assess health effects of air pollution exposure in epidemiological studies.27,28 However, these studies used subjects with respiratory diseases, children or the elderly, who were believed to be more susceptible to air pollution exposure than young and healthy adults. Hence, it is necessary to examine whether MDA can be used as a biomarker of ambient air pollution-induced oxidative stress in young and healthy adults.
The 2008 Beijing Olympics provided a unique opportunity to study the acute health effects of air pollution. Aggressive air pollution control measures were implemented to temporarily improve Beijing’s air quality, including reducing cars running on the road through an odd/even plate number rule, relocating heavily polluting factories, installing or improving pollutant control devices, reducing the production capacity of various industries, and suspending all construction projects during the Olympic period, and so on.29 The control measures resulted in substantial reductions in air pollution levels during the Olympic period from 20 July 2008 to 19 September 2008. Taking advantage of this air pollution intervention, we conducted a panel study to test our hypotheses (1) that concentrations of MDA in young and healthy adults would reduce in response to the reduction in ambient air pollution during the Olympic period, and increase with increases of air pollution concentrations after the Olympics, and (2) that concentrations of MDA were associated with concentrations of some individual air pollutants in the same or previous few days before the MDA measurement. By testing these hypotheses, we aim to evaluate the utility of MDA measured in EBC or urine as a biomarker of oxidative stress induced by air pollution in healthy and young adults.
METHODS
Study Protocol
Based on the air pollution control measures described earlier,29 three Olympic periods were defined in our study30,31 as: the pre-Olympics (2 June 2008–19 July 2008) when some relatively mild controls were implemented, the during-Olympics (20 July 2008–19 September 2008) when the full-scale control measures were implemented, and the post- Olympics (20 September 2008–30 October 2008) when the majority of the control measures were relaxed. We collected EBC and urine samples from the participants twice in each of the three Olympic periods, i.e., two samples/participant from 10 June to 7 July (pre-Olympics), two samples/participant from 4 August to 29 August (during-Olympics), and two samples/participant from 6 October to 30 October (post-Olympics). Air pollution was monitored in each of the three Olympic periods one week before the starting date of the biological sample collections. The study protocol was approved by both the Institutional Review Board of University of Medicine and Dentistry of New Jersey and the Ethics Committee of the Peking University Health Sciences Center and the Peking University First Hospital.
Study Participants
Study participants have been described previously.30,31 Briefly, we recruited 128 nonsmoking individuals, 22–27 years of age, from the pool of the medical residents at Peking University First Hospital. The Hospital was located in the center of Beijing, within the second Ring Road. All study participants worked on the campus of the hospital and resided in dormitories of either the hospital or the nearby (< 5 km) Peking University Health Sciences Center. Ninety-three percentage (119 out of 128) of participants completed all of the six planned clinic visits, with six participants finishing five of the six visits. Three participants withdrew from the study after the first two clinic visits and were excluded from the data analysis. These 125 subjects, as shown in Table 1 for their demographical characteristics, were used in the statistical analyses.
Table 1.
Characteristics of subjects in the study.
| Male | Female | Total | |
|---|---|---|---|
| Sample size | 64 | 64 | 128 |
| Age: median (range), years | 24 (19–33) | 24 (22–29) | 24 (19–33) |
| Height: mean ± SD, m | 1.66 ± 0.05 | 1.71 ± 0.06 | 1.66 ± 0.07 |
| Weight: mean±SD, kg | 53.74 ± 7.23 | 66.25 ± 10.76 | 60.05 ± 11.09 |
| BMI: mean ± SD, kg/m2 | 22.5 ± 2.9 | 20.6 ± 2.4 | 21.5 ± 2.8 |
Air Pollution Monitoring
Air pollution measurements have been described previously.30,31 Briefly, air samplers and monitors were collocated on the top of a seven-story building located in the center of the hospital campus. Fine particles (PM2.5) and elemental carbon (EC) were measured on a 24-h basis. Sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2), and ozone (O3) were monitored continuously throughout the entire study period. Ambient temperature and relative humidity (RH) were monitored continuously and concurrently at the same site.
Sample Collection and Analysis for MDA
EBC were collected using a Jaeger EcoScreen condensing device (Jaeger, Wurzburg, Germany). The machine was turned on at least 30 min before collection to allow the cooling cuff to reach operating temperature (−20 °C). Each EBC sample was collected for 20 min, during which the participants were seated, wearing nose clips, and instructed to breathe tidally. From each participant, ~ 2.5 ml of condensate was obtained per collection. Aliquots of EBC samples were then stored at −70 °C before analyses. On each day of the clinical visit, a 50-ml morning urine sample was collected from each participant for MDA and creatinine analyses. The method for analyzing MDA in EBC and urine samples was modified from a published method that used an HPLC system with fluorescent detection.32 Briefly, a 100 µl aliquot of EBC or urine sample was added to a mixture of 500 µl phosphoric acid (440 mM) and 250 µl of thiobarbituric acid (TBA, 42 mM). A derivative reaction between MDA and TBA occurred and lasted for 1 h at 80 °C in an oven. After the reaction, a 20 µl aliquot of the solution with MDA–TBA derivatives was injected into the HPLC system with a fluorescence detector set at 532 nm for the excitation wavelength, and 553 nm for the emission wavelength. A Nova-Pak C18 column (Waters, USA) was used along with a mobile phase that was composed of 40% methanol and 60% water containing 50 mM KH2PO4 (pH = 6.8) at a flow rate of 0.8 ml/min. The detection limit, extraction recovery, and analytical precision of this method were 1.8 nM, 75.9%, and 2.2% (measured as RSD from eight replicate injections), respectively.
Statistical Methods
Linear mixed-effects models were described in a related, recently published paper,31 to examine differences in MDA levels between periods (pre-, during-, and post-Olympics) as well as, separately, associations between pollutants and MDA. Models adjusted for temperature, RH, gender, and day of week for biomarker measurement. Equicorrelation (i.e., compound symmetry) induced by a random effect for subject was chosen to account for the correlation between all observations within subject after comparison of several covariance structures, including unstructured, compound symmetry, and autoregressive. Best fits for the effects of average temperature and RH in 24 h before biomarker measurements were obtained using the natural splines function with up to 3 degrees of freedom. After estimating the effects of 24 h temperature and RH, cumulative averages of between 2 and 7 days of temperature and/or RH were added into the models if indicated by a reduced value of Akaike information criterion.
To examine period effects, indicator variables for period were added to the initial model. We estimated the mean concentrations of EBC MDA and urinary MDA for each of the three Olympic periods, as well as the absolute and percentage changes in the mean concentrations between each of the two periods. Air pollution concentrations were averaged over various time periods before when EBC/urine samples were collected, categorized as lag 0 (0–23 h), lag 1 (24–47 h), lag 2 (48–71 h), lag 3 (72–95 h), lag 4 (96–119 h), lag 5 (120–143 h), or lag 6 (144–167 h). Single-pollutant models were used to estimate the associations between MDA and each pollutant by adding the concentration of air pollution at each lag to the initial model. As the air pollutants have different variation scales, we standardized the association of MDA with air pollutants to their interquartile ranges (IQRs). In order to make the changes in EBC MDA and urinary MDA comparable, we calculated the percentage changes in MDA associated with one IQR changes in pollutants. The percentage change in MDA was calculated through dividing the absolute change associated with one IQR change in each air pollutant by its median concentration.
RESULTS
Changes in Air Pollution Levels
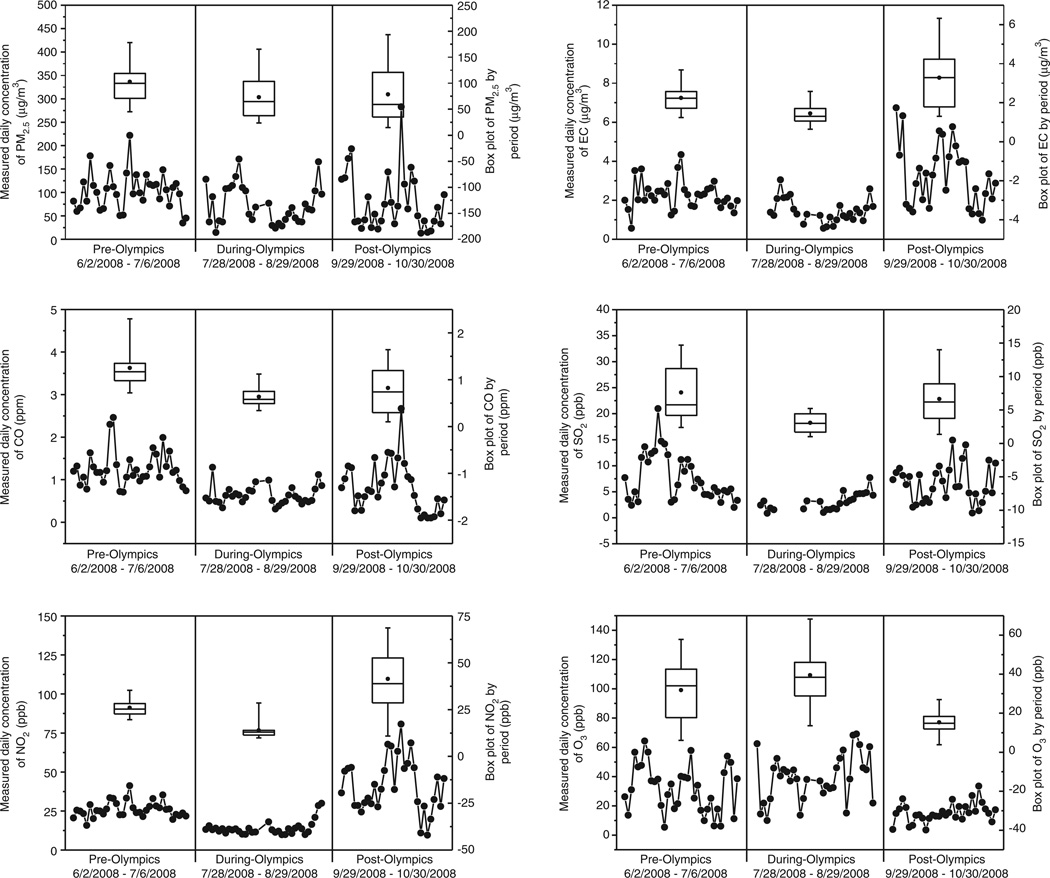
As reported previously, 27–60% reductions in period mean concentrations of CO, NO2, SO2, PM2.5, and EC were observed from the pre- to the during-Olympic period.31 Here, we show time series and box plots of daily average concentrations of the six measured pollutants (Figure 1). The time series and box plots of EC, CO, SO2, and NO2 showed decreases in daily concentrations from the pre- to the during-Olympic and increases from the during- to the post-Olympic period. We also observed large day-to-day variations within and across the three periods. In contrast, concentrations of O3 increased from pre- to during-Olympics, but decreased from during- to post-Olympics. Ozone showed the lowest concentration and day-to-day variation in the post-Olympic period (early autumn) when the photochemical activity was substantially lower than the summer months of the pre- and during-Olympic periods.
Figure 1.
Box plots of daily average concentrations of PM2.5, elemental carbon, CO, SO2, NO2, and O3, in each of the three Olympic periods.
Concentrations of EBC MDA and Urinary MDA
Period-specific means and between-period differences of MDA are given in Table 2. Both EBC MDA and urinary MDA were significantly decreased from the pre- to the during-Olympic period, and then increased from the during- to the post-Olympic period with marginal significance. The mean post-Olympic concentrations of MDA were larger (but not statistically significant) than those in the pre-Olympic period (see Table 2). The mean concentration of EBC MDA decreased by 24% (P < 0.0001) from the pre-Olympic to the during-Olympic period and increased by 39% (P = 0.094) from the during-Olympic period to the post-Olympic period. The mean concentration of urinary MDA decreased by 28% (P = 0.0002) from the pre-Olympic to the during-Olympic period, and increased by 55% (P = 0.046) from the during-Olympic period to the post-Olympic period.
Table 2.
Period-specific means (± SE) of MDA in EBC and urine samples and between-period differences.
| Period-specific means | Between-period differences | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Olympics | During-Olympics | Post-Olympics | During–Pre | Post–During | Post–Pre | ||||||||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | Diff | P-value | 95% CI | Diff | P-value | 95% CI | Diff | P-value | 95% CI | |
| EBC MDA (nM) | 212 | 21.0 ± 1.5 | 205 | 16.0 ± 1.9 | 198 | 22.3 ± 2.4 | −5.0 | <0.0001 | −7.3, −2.7 | 6.3 | 0.094 | −1.1,13.6 | 1.3 | 0.70 | −5.3, 7.9 |
| Urinary MDA (nM)a | 246 | 435.3 ± 1.1 | 248 | 311.1 ± 1.1 | 236 | 483.0 ± 12.8 | 0.72 | 0.0002 | 0.60, 0.85 | 1.55 | 0.046 | 1.01,2.39 | 1.11 | 0.54 | 0.79, 1.57 |
The statistics were estimated by mixed-effects models, adjusted for temperature, relative humidity, gender, and day of week.
Period-specific mean values of urinary MDA indicate its geometric mean, and between-period difference values indicate the ratio of the geometric means of two periods.
As urinary MDA was highly skewed, the Spearman correlation between EBC MDA and urinary MDA was examined. Small correlation coefficients (0.038–0.10) were observed between EBC MDA and urinary MDA in the entire study period and the three Olympic periods.
MDA–Pollutant Associations
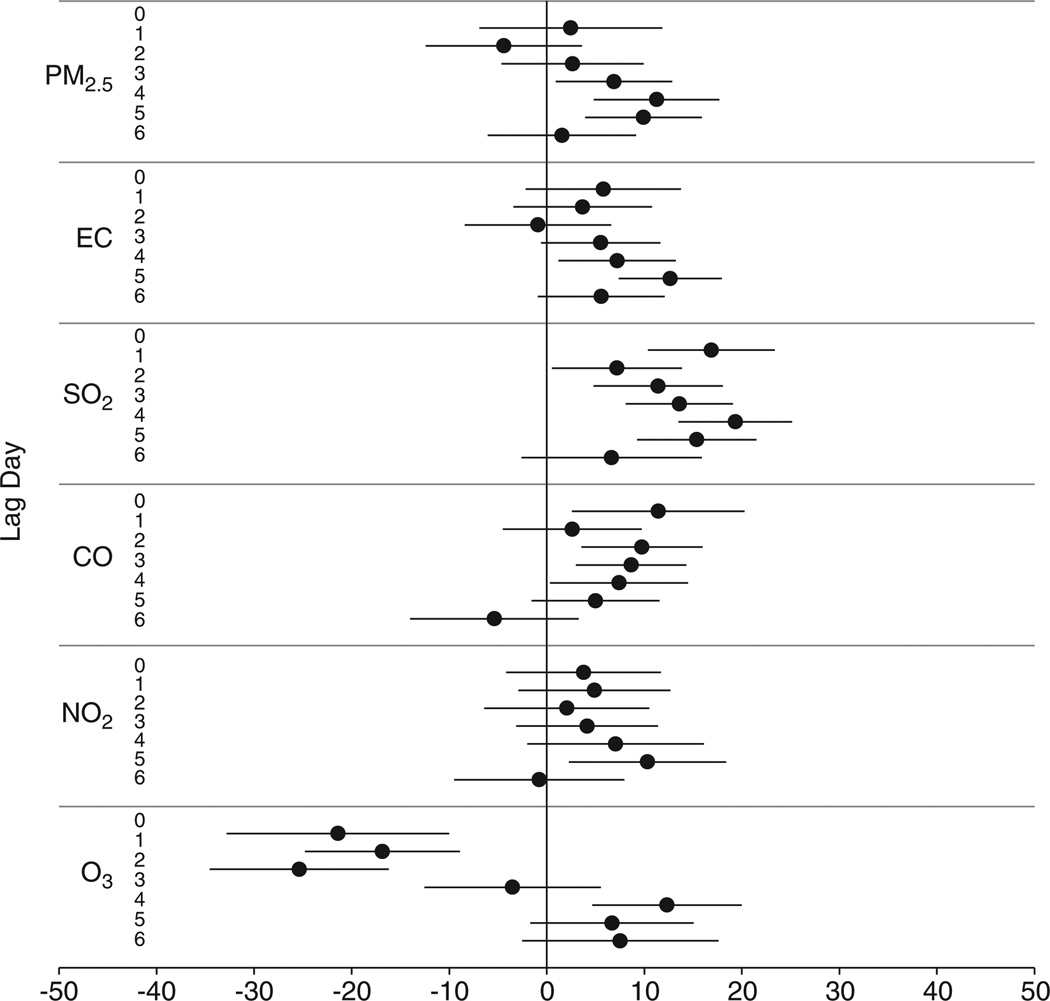
Consistent with the hypothesis, both EBC MDA and urinary MDA were significantly and positively associated with all pollutants except O3 at one or more lag days (Figure 2). Among the seven associations of EBC MDA with each individual air pollutant from lag 0 to lag 6, the largest increases (in percentage) in EBC MDA associated with each IQR increases in pollutants occurred at lag 4 for PM2.5 (11%), at lag 5 for EC (13%), at lag 4 for SO2 (19%), at lag 0 for CO (11%), and at lag 5 for NO2 (10%). A similar pattern was observed for the associations of EBC MDA with PM2.5, EC, and SO2 across lags, which decreased within first few lag days. The association of EBC MDA with CO showed a different pattern, which decreased from lag 0 to lag 6 except from lag 1 to lag 2. The association of EBC MDA with ozone was negative on the first four lags, and then increased to become positive on lag days 4, 5, and 6.
Figure 2.
Percentage change in EBC MDA concentration associated with each interquartile range (IQR) increase in pollutant concentration, by lag day. Generated using mixed-effects models controlling for temperature (degree of freedom in natural spline = 1), RH (df = 1), 6-day moving average of temperature (df = 3), 3-day moving average of RH (df = 3), gender, and day of week.
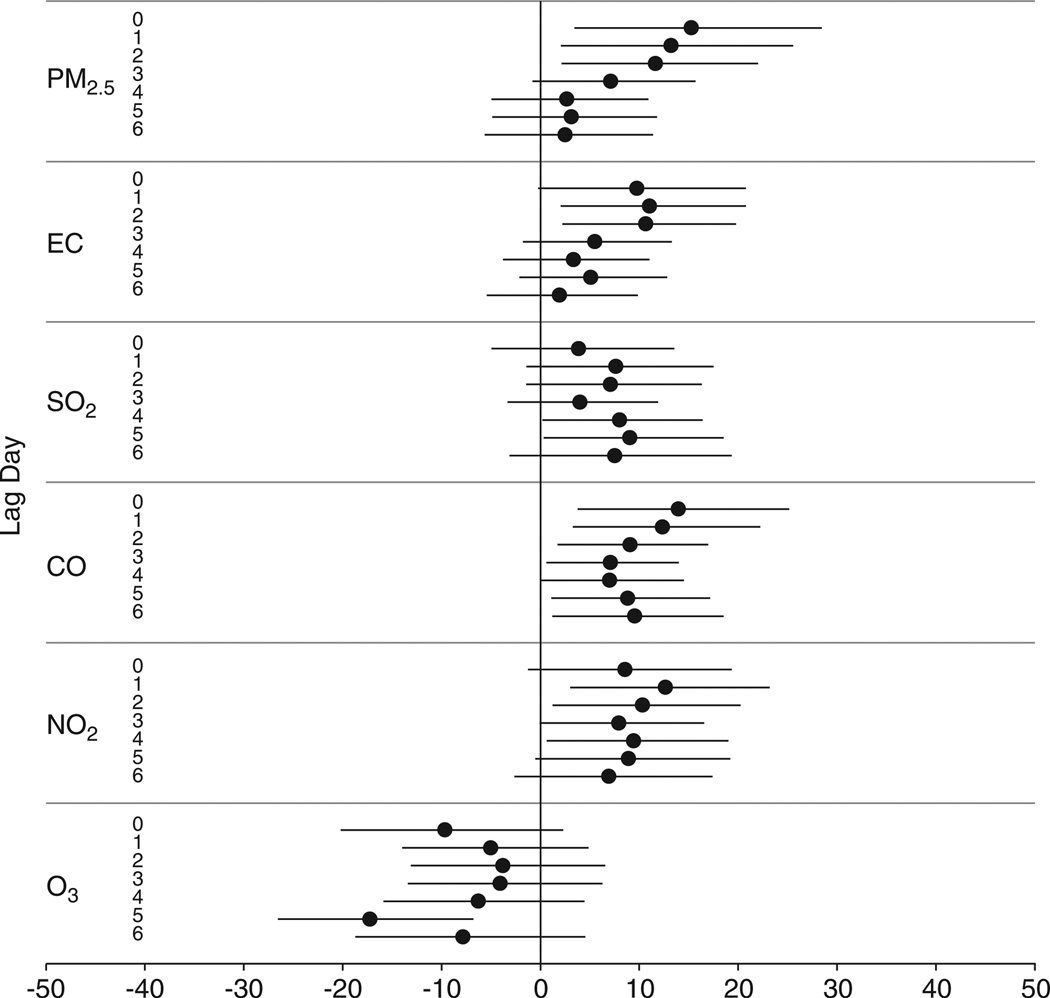
Figure 3 shows the associations between urinary MDA and PM2.5, EC, SO2, NO2, and CO. Among the seven associations with each pollutant at lag 0 through lag 6, the largest increases in urinary MDA per IQR increases in pollutants occurred at lag 0 for PM2.5 (15%), at lag 1 for EC (11%), at lag 5 for SO2 (9%), at lag 0 for CO (14%), and at lag 1 for NO2 (13%). For PM2.5 and CO, the association decreased from lag 0 to lag 4. For EC and NO2, the associations increased from lag 0 to lag 1, and then decreased along with increasing lag. Urinary MDA showed decreases associated with increases in ozone at all seven lags, with the largest decrease occurring at lag 5.
Figure 3.
Percentage change in urinary MDA concentration associated with each interquartile range (IQR) increase in pollutant concentration, by lag day. Generated using mixed-effects models controlling for temperature (degree of freedom in natural spline = 1), 6-day moving average of temperature (df = 1), gender, and day of week.
DISCUSSION
The quasi-experimental design of the current study allowed us to perform both the “period” comparison and the pollutant–biomarker association analysis. Both analyses consistently found a significant association between MDA and air pollution either as a mixture (in the period comparison) or as individual pollutant concentrations (in the biomarker–pollutant association analysis). These results demonstrate oxidative stress effects of air pollution in the respiratory tract and in the systemic system of young and healthy adults.
Reductions in ambient concentrations of air pollutants that were observed in the during-Olympic period, when extensive air pollution control measures were implemented, have been consistently reported in several other studies.29,31,33 After the air pollution control measures were relaxed, some of the measured pollutants increased to the pre-Olympic level (PM2.5, CO, and SO2) or a higher level (NO2 and EC). Concomitantly, concentrations of EBC MDA and urinary MDA changed across the three Olympic periods following the same increasing and decreasing pattern for most of the pollutants (except ozone). Furthermore, we observed associations between concentrations of MDA and concentrations of pollutants measured within 24 h (lag 0) and several days (lags 1–6) before MDA measurements, confirming that the reduction in MDA levels during the Olympic period was most likely owing to the reduction in air pollution. EBC MDA and urinary MDA showed similar patterns of change across the three Olympic periods and similar associations with air pollutants. This suggests that both EBC MDA and urinary MDA may serve as biomarkers of oxidative stress in response to changes in air pollution in healthy young adults.
The association between EBC MDA and PM2.5 exposure has been examined in a previous study.26 Romieu et al. reported a 1.12 nM increase in EBC MDA associated with a 14.2 µg/m3 (IQR) increase in PM2.5 (8-h moving average) on lag day 0, a larger increase in MDA per unit increase in PM2.5 than in the current study (0.079 nM vs 0.026 nM per 1 µg/m3). The larger per-unit-PM2.5 effect estimate in the previous study may reflect the differences in subject susceptibility to oxidative stress as asthmatic children were used in the Romieu et al. study as opposed to healthy young adults as in the present study. It may also reflect differences in PM2.5 composition for Mexico City, the location of the Romieu study, vs Beijing, or dietary differences among the subjects.
Figure 2 shows that the increases in EBC MDA with increases in particulate pollutants, that is, PM2.5 and EC, were not statistically significant on the first three lag days. In contrast, the increases in EBC MDA associated with increases in gaseous pollutants, CO and SO2, were significant at lag 0. The finding suggests that gaseous pollutants may lead to lipid peroxidation in the respiratory tract more rapidly than particulate pollutants. This is plausible because respirable particles (PM2.5) may need an extra step (time) to first deposit into the respiratory tract and then interact with cellular lipids to form MDA and other products of lipid peroxidation.22
The negative association between MDA and ozone in the first few lag days (Figures 2 and 3) needs to be regarded with caution as there is no evidence to support protective effects for ozone. At least two other studies found negative associations between ozone exposure and respiratory illness in children and adults.34,35 One explanation of the negative associations, raised in the paper of Hajat et al.,35 was that ozone was negatively associated with other air pollutants that were positively associated with the health outcomes. In the current study, ozone was negatively associated with NO2, EC, and OC with Spearman correlation coefficients ranging from −0.13 to −0.58.31
In biomonitoring studies, as the urinary concentrations of biomarkers can be affected by the variable dilution of urine samples, the concentration of creatinine is often used to standardize the concentrations of the urinary biomarkers.36 In the current study, we also analyzed the period changes and the associations of the creatinine-adjusted MDA in urine with air pollutants, and the results are given in the supplemental material (SM). We found nonsignificant decreases and increases across the three Olympic periods (SM1) and nonsignificant associations with air pollutants (SM2) for the creatinine-adjusted urinary MDA. The fact that unadjusted urinary MDA showed more statistically significant associations with air pollutants perhaps reflects that the adjustment for creatinine increased day-to-day variations (larger SDs in SM1 and larger 95% confidence intervals in SM2) in urinary MDA measurements. The current study uses within-subject period comparisons, and urine samples for each subject were collected at the same time of day. Hence, creatinine levels for the same individuals are expected to be similar across repeated measurements. Adjusting MDA levels with creatinine appeared to unnecessarily introduce additional errors into the final statistical analysis.
In the current study, we collected EBC and urine samples noninvasively and measured MDA in EBC and urine using an HPLC–fluorescence method, which was sensitive, reliable, and relatively inexpensive. The EBC MDA concentrations measured in the current study were consistent with the concentrations in control groups reported in other studies, but lower than concentrations in subjects with respiratory disease, asthma, or chronic obstructive pulmonary diseases (COPD).18,37,38 In previous studies, Corradi et al.18,38 found higher EBC MDA concentrations in asthmatic children (30.2 ± 2.4 nM) and patients with COPD (57.2 ± 2.4 nM). The EBC MDA concentrations for the control group were 19.4 ± 1.9 and 17.7 ± 5.5 nM in the two studies, which is comparable with the concentration of the current study (18.4 ± 8.9 nM).18,38 As the creatinine-adjusted urinary MDA were reported in other studies, the urinary MDA concentrations were 89.21 ± 55.30 µmol/mol creatinine in the current study (SM1), lower than those measured in children (407.2 ± 214.9 µmol/mol creatinine) and in the elderly (452.5 ± 181.0 µmol/mol creatinine).39 Lower concentrations of EBC MDA and urinary MDA obtained in the current study might be the result of having young and healthy adults as study participants, who might have been more resistant to the formation of or effects of ROS, compared with persons with respiratory diseases, children, or the elderly.40
Factors that might confound the level of MDA in EBC or urine samples were considered in the study design and throughout the entire study period. The baseline exposure questionnaires and the time–activity records suggest a similar opportunity for traffic exposure for the majority of the participants and that the lifestyle of participants was not markedly changed across three Olympic periods (add the time activity table as a SM3). As all participants we enrolled were nonsmokers and healthy, the medication history was rarely identified, and the physician determined that rescheduling owing to upper respiratory illness was only needed for two subject visits (seven cases were identified in total) during the entire study (one visit was rescheduled for 2 days later and the other for 6 days later).
This study had several limitations. First, common in this type of panel study, potential confounding from other factors than air pollution may be a concern. For this reason, we constrained our study to a relatively short time period, including summer and early autumn, and controlled for temperature and RH in the statistical analysis. However, a simple “linear adjustment” for temperature and RH would also prove problematic, because the post-Olympic period would essentially determine the slopes for the effect of temperature/RH (as this period has a quite different range of values relative to the first two periods). Second, owing to day-to-day variations in pollutant concentrations, we were not able to assess “true” reproducibility (i.e., inter-person variability) of the biomarkers. The utility of MDA in either EBC or urine as a biomarker to reflect steady-state routine exposure, hence, cannot be assessed in this study design. Third, the large day-to-day variation indicates that some potential confounders of urinary MDA other than the air pollution, such as diet, might need to be considered in future studies.
A significant decrease in EBC MDA concentration in healthy young adults was associated with a substantial improvement in air quality during the Beijing Olympic air pollution control period. When air pollution control measures ended, concentrations of EBC MDA correspondingly increased. In addition, EBC MDA concentration increases were significantly associated with increased air pollutant concentrations, measured on the same or previous few days. Urinary MDA showed similar changes across Olympic periods and also increased with an increase in pollutant concentrations measured on the same or previous few days, although not statistically significant. These findings demonstrate oxidative stress effects of air pollution in both the respiratory tract and systemically for healthy young adults. These findings, along with noninvasiveness of EBC and urine sample collection, support the utility of MDA as a biomarker of oxidative stress in the respiratory tract and urinary tract in population studies of air pollution health effects.
ACKNOWLEDGEMENTS
We thank all the students and staff from Dr Tong Zhu and Dr Min Hu’s labs for their assistance in air pollution monitoring. This research was jointly funded by NIEHS (1R01 ES0158640, P30 ES05022, and 5P30ES007048) and the Health Effects Institute (4760-RPFA05-3). T.Z. is partly funded by Beijing Environmental Protection Agency (OITC-G08026056). The views expressed in this manuscript are solely of the authors and do not necessarily reflect those of the funding agencies.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
REFERENCES
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21:5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 5.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Moison RM, Palinckx JJ, Roest M, Houdkamp E, Berger HM. Induction of lipid peroxidation of pulmonary surfactant by plasma of preterm babies. Lancet. 1993;341:79–82. doi: 10.1016/0140-6736(93)92557-a. [DOI] [PubMed] [Google Scholar]
- 7.Davies SW, Ranjadayalan K, Wickens DG, Dormandy TL, Timmis AD. Lipid peroxidation associated with successful thrombolysis. Lancet. 1990;335:741–743. doi: 10.1016/0140-6736(90)90866-4. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 9.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–1737. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero FJ, Bosch-Morell F, Romero MJ, Jareno EJ, Romero B, Marin N, et al. Lipid peroxidation products and antioxidants in human disease. Environ Health Perspect. 1998;106(Suppl 5):1229–1234. doi: 10.1289/ehp.98106s51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel EN, Neff WE. Formation of malonaldehyde from lipid oxidation-products. Biochim Biophys Acta. 1983;754:264–270. [Google Scholar]
- 13.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007;380:50–58. doi: 10.1016/j.cca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 15.Dierckx N, Horvath G, van Gils C, Vertommen J, van de Vliet J, De Leeuw I, et al. Oxidative stress status in patients with diabetes mellitus: relationship to diet. Eur J Clin Nutr. 2003;57:999–1008. doi: 10.1038/sj.ejcn.1601635. [DOI] [PubMed] [Google Scholar]
- 16.Gonenc A, Ozkan Y, Torun M, Simsek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26:141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamer L, Sucu N, Polat G, Ercan B, Aytacoglu B, Yucebilgic G, et al. Decreased serum total antioxidant status and erythrocyte-reduced glutathione levels are associated with increased serum malondialdehyde in atherosclerotic patients. Arch Med Res. 2002;33:257–260. doi: 10.1016/s0188-4409(01)00381-2. [DOI] [PubMed] [Google Scholar]
- 18.Corradi M, Rubinstein I, Andreoli R, Manini P, Caglieri A, Poli D, et al. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1380–1386. doi: 10.1164/rccm.200210-1253OC. [DOI] [PubMed] [Google Scholar]
- 19.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 23.Kelly FJ, Sandstrom T. Air pollution, oxidative stress, and allergic response. Lancet. 2004;363:95–96. doi: 10.1016/s0140-6736(03)15308-1. [DOI] [PubMed] [Google Scholar]
- 24.Barregard L, Saellsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65:319–324. doi: 10.1136/oem.2006.032458. [DOI] [PubMed] [Google Scholar]
- 25.Isik B, Hamamci C, Isik R. Effect of winter air pollution on lipid peroxidation product levels of patients with chronic obstructive pulmonary disease. Asian J Chem. 2006;18:1433–1436. [Google Scholar]
- 26.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Almstrand AC, Diaz-Sanchez D, Sly PD, et al. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol. 2008;121:903–909. e906. doi: 10.1016/j.jaci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect. 2010;118:579–583. doi: 10.1289/ehp.0901077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan CH, Chan CC, Huang YL, Wu KY. Urinary 1-hydroxypyrene and malondialdehyde in male workers in Chinese restaurants. Occup Environ Med. 2008;65:732–735. doi: 10.1136/oem.2007.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Zhu T, Zheng J, Zhang RY, Zhang SQ, Xie XX, et al. Use of a mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 Summer Olympics. Atmos Chem Phys. 2009;9:8247–8263. [Google Scholar]
- 30.Kipen H, Rich D, Huang W, Zhu T, Wang G, Hu M, et al. Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: a panel study approach. Ann N Y Acad Sci. 2010;1203:160–167. doi: 10.1111/j.1749-6632.2010.05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. J Am Med Assoc. 2012;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larstad M, Ljungkvist G, Olin AC, Toren K. Determination of malondialdehyde in breath condensate by high-performance liquid chromatography with fluorescence detection. J Chromatogr B. 2002;766:107–114. doi: 10.1016/s0378-4347(01)00437-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Xie SD. Assessment of traffic-related air pollution in the urban streets before and during the 2008. Beijing Olympic Games traffic control period. Atmos Environ. 2009;43:5682–5690. [Google Scholar]
- 34.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Emberlin J, Strachan DP. Air pollution, pollens, and daily admissions for asthma in London 1987–92. Thorax. 1998;53:842–848. doi: 10.1136/thx.53.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajat S, Haines A, Goubet SA, Atkinson RW, Anderson HR. Association of air pollution with daily GP consultations for asthma and other lower respiratory conditions in London. Thorax. 1999;54:597–605. doi: 10.1136/thx.54.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauwerys RR, Hoet P. Industrial Chemical Exposure: Guidelines for Biological Monitoring. 2nd edn. Boca Raton: Lewis Publishers; 1993. [Google Scholar]
- 37.Bartoli ML, Novelli F, Costa F, Malagrino L, Melosini L, Bacci E, et al. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediat Inflamm. 2011;2011:891752. doi: 10.1155/2011/891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corradi M, Folesani G, Andreoli R, Manini P, Bodini A, Piacentini G, et al. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Park EY, Lee KH, Park JD, Kim YD, Hong YC. Differential oxidative stress response in young children and the elderly following exposure to PM(2.5) Environ Health Prev Med. 2009;14:60–66. doi: 10.1007/s12199-008-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]