Abstract
Epidemiological and clinical data suggest that selenium may prevent prostate cancer, however the cellular effects of selenium in malignant prostate cells are not well understood. We previously reported that the activity of the tumor suppressor PTEN is modulated by thioredoxin (Trx) in a RedOx-dependent manner. In this study, we demonstrate that the activity of Trx Reductase is increased by seven-fold in the human prostate cancer cell line, DU-145, after five days of sodium selenite (Se) treatment. The treatment of DU-145 cells with increasing concentrations of Se induced an increase in PTEN lipid phosphatase activity by two-fold, which correlated with a decrease in phospho-Ser473-Akt, and an increase in phospho- Ser370-PTEN levels. Se also increased CK2 activity and the use of apigenin, an inhibitor of CK2, revealed that the regulation of the tumor suppressor PTEN by Se may be achieved via both the Trx-TR system and the RedOx control of the kinase involved in the regulation of PTEN activity.
Keywords: Thioredoxin reductase, PTEN, Akt, CK2, sodium selenite
Introduction
Selenium (Se) is an essential dietary nutrient for all mammalian species, including humans (1). The mechanism of action of Se compounds, either via a pro-oxidant pathway (as seen in cytotoxicity and apoptosis) or an antioxidant pathway (as proposed in cancer chemoprevention), is still unclear, but is nonetheless intriguing. Several studies have shown an effect of seleno-compounds on phospho-Akt in human prostate cancer cells (2–5). However, the mechanisms for such effects on the survival pathway remain unclear.
Thioredoxin (Trx) is a 12-kDa protein thiol-disulfide oxidoreductase which, in mammalian cells, has a variety of biological functions related to cell proliferation and apoptosis (6). The oxidized form (Trx-S2) contains a disulfide bridge in the active site that is bioreduced to a dithiol by NADPH and the flavoprotein Thioredoxin Reductase (TR) (7). Sodium selenite increases the activity of TR and stabilizes TR mRNA leading to an increase in TR expression in several cancer cell lines, including MCF-7 breast, HT-29 colon and A549 lung cancer cells (8). Trx has been implicated in many cellular functions such as DNA synthesis as electron donors (9), RedOx regulation of transcription factors such as NF-κB (10) and AP1 (11) as well as the modulating the activity of several proteins including PKC (12), ASK1 (13) and PTEN (14–15). In fact, we recently reported that Trx is a physiological inhibitor of the tumor suppressor protein PTEN/MMAC1 (phosphatase and tensin homolog deleted on chromosome ten)/(mutated in multiple advanced cancer), the Phosphatidylinositol (PtdIns)-3-phosphate phosphatase which regulates Akt activation in normal cells (14). In PTEN-deficient mouse embryo fibroblasts, constitutively elevated activity of Akt together with decreased sensitivity to apoptosis suggests a role for PTEN as a negative regulator of cell survival.
PTEN is a 54kDa protein, which exhibits a dual specificity tyrosine/serine/threonine and lipid phosphatase, but the main substrate of PTEN, both in vitro and in living cells, is the lipid PtdIns-(3,4,5)-trisphosphate. PTEN is also a phospho-protein and this post-transcriptional modification of the protein plays an important role in its stability (16). Specifically, the phosphorylation of Ser380 and Thr382 has been shown to affect the half-life of PTEN in T lymphocytes (17). Thus, PTEN is phosphorylated at several serine and threonine residues in the C-terminal of the protein, particularly in the PDZ (PSD95, Dlg and ZO1)-binding motif. Interestingly, the phosphorylation of the PTEN tail results in an inhibition of PTEN activity, at least in part, by preventing its interaction with PDZ domain-containing proteins such as MAGI-2. Moreover, the phosphorylation on Thr366 by Glycogen Synthase Kinase 3 (GSK3) was recently shown to destabilize PTEN (18). Taken together, PTEN activity may be regulated via its phosphorylation on the C-terminus and its association with PDZ binding partners.
The activation and the regulation of the kinases involved in PTEN phosphorylation and their effects on PTEN activity in prostate cancer cells remain an important topic to be investigated. Our laboratory is interested in understanding the possible mechanisms for PTEN regulation in normal and cancer cells. We have shown that the small RedOx protein, Trx inhibits PTEN activity in MCF-7 breast cancer cells (14–15). Trx, by binding directly to the C-terminal part of PTEN, inhibits PTEN lipid phosphatase activity in vitro and in cells. The interaction between Trx and PTEN is dependent on the RedOx status of Trx in that the interaction is observed only under reducing conditions (14). Our observations strongly suggest that Trx regulates PTEN activity in the cells and that this interaction may partially clarify the anti-apoptotic functions of Trx in the cells (14). Therefore, in this study, we have investigated the effects of the endogenous modulation of the Trx-TR RedOx system using sodium selenite and tested our hypothesis that PTEN activity can be modulated via the Trx system. We show that sodium selenite increase the activity of the tumor suppressor PTEN in prostate cancer cells. Interestingly, at similar concentration, Se increases the activity of casein kinase-2 (CK2) involved in PTEN phosphorylation thereby also regulating the tumor suppressor's activity.
Materials and Methods
Materials
Rabbit anti-PTEN, rabbit anti-phospho-(Ser380-Thr382/383) PTEN, rabbit anti-phospho-Ser473-Akt, rabbit total Akt and rabbit PARP antibodies were purchased from Cell Signaling-Technology (Danvers, MA). Rabbit anti-phospho-Ser370-PTEN and rabbit anti-phospho-Ser209-CK2 were obtained from Novus Biochemicals (Littletown, CO). Mouse anti-CK2 (clone mAb 6D5) antibody was purchased from Calbiochem (La Jolla, CA). Pleurotin (i.e. NSC131233) was recently identified as an irreversible inhibitor of TR with a Ki of 0.28 μM (19). Pleurotin and rabbit polyclonal antibodies against Trx were obtained as a gift from Dr. Garth Powis (MD Anderson Cancer Center, Texas). TR polyclonal antibodies were obtained from Upstate (Lake Placid, NY). Apigenin was obtained from Tocris Bioscience (Ellisville, MO). All other biochemicals were purchased from Sigma (St Louis, MO).
Cell culture, sodium selenite and drug treatments
Human DU-145, LNCaP and PC-3 prostate cancer cell lines were obtained from the American Type Culture Collection (Rockville, MD). The cells were maintained in bulk culture in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were passaged using 0.25% trypsin and 0.02% EDTA. The cells were confirmed to be mycoplasma free using an ELISA kit (Roche-Boehringer Mannheim, Indianapolis, IN). Sodium selenite was dissolved in distilled water at the stock concentrations of 10mM and added every other day at the indicated concentrations to the cell culture media for 5 days. For experiments using Pleurotin or Apigenin, cells were pre-incubated for 5 days with sodium selenite followed by one hour of drug at the concentrations indicated.
PTEN lipid phosphatase activity
Lipid phosphatase activity of PTEN was measured as described by Georgescu et al. (20). Briefly, PTEN was immunoprecipitated from DU-145 cells using anti-PTEN antibody and the lipid phosphatase activity was measured by detecting the release of phosphate from the water soluble substrate diC8-PdtIns-(3,4,5)-trisphosphate (Echelon, Salt Lake City, UT). Briefly, after immunoprecipitation the protein A/G-agarose beads (Santa Cruz Laboratories) were washed twice; once in a low-stringency buffer containing 20 mM HEPES (pH 7.7), 50 mM NaCl, 0.1 mM EDTA and 2.5 mM MgCl2, and once in phosphatase assay buffer lacking the substrate. The reaction was incubated for 40 min at 37°C and transferred to a 96-well plate. The release of phosphate from the substrate was measured in a colorimetric assay by using the Biomol Green® Reagent (Biomol, Plymouth Meeting, PA) in accordance with the instructions of the manufacturer. The absorbance at 650 nm was recorded in an ELISA plate reader. A standard curve was performed in each assay, and the amount of free phosphate was calculated from the standard curve line-fit data. Each assay was performed in triplicate and the data are reported as nmoles of phosphate liberated/mg of total protein.
Akt and CK2 activity and expression in control and selenite treated-DU-145 cancer cells
Cells were grown in 6-well plates to 75% confluency in DMEM/10% heat-inactivated FBS, 4.5g/l glucose, 100U/ml penicillin and 100μg/ml streptomycin in a 5% CO2 atmosphere. Following drug and/or selenite treatment, the culture media was aspirated and cells were lyzed in RIPA lysis buffer made up of 0.05 M Tris, pH 7.4, 0.15M NaCl, 1%Triton-X 100, 0.1% SDS and 1% Sodium deoxycholate supplemented with protease and phosphatase inhibitors. Thirty micrograms of the lysates were diluted into a 4X reducing sample buffer and boiled for 5 min. Samples were then loaded on a 7.5% SDS-PAGE. Proteins were transferred to PVDF membranes, pre-incubated in blocking buffer [137mM NaCl, 2.7mM KCl, 897μM CaCl2, 491μM MgCl2, 3.44mM Na2HPO4, 593mM KH2PO4 and 5% Bovine serum albumin (BSA)], and incubated with the antibodies of interest. Immunoreactive bands were detected using an anti-rabbit or anti-mouse coupled to peroxidase (Amersham, Arlington Heights, IL) and the Renaissance chemilluminescence kit (NEN™, Life Science Products, Inc., Boston, MA). Bands corresponding to phospho-CK2 were quantified using Eagle Eye software (BioRad, Richmond, CA) and Kodak X-Omat™ Blue XB (NEN™, Life Science Products). Actin or CK2 were used as respective loading controls.
Thioredoxin reductase activity and expression in control and selenite-treated DU-145 cells
TR activity was measured as previously described (21). Cell lysates were briefly incubated with adenosine 2'5' di-phosphate beads that were pre-swollen in 15ml of water and rotated for an hour at 4°C. The lysates mixed with the beads were rotated for one hour at 4°C. Lysates were washed twice in 1ml 0.1M NaCl, 50mM HEPES, 5mM EDTA pH 7.6 and 50μl of 1M NaCl, 50mM HEPES, 5mM EDTA pH 7.6 was added to the beads and rotated for one hour at 4°C. The supernatants obtained after centrifugation at 10,000g for five minutes, were used to measure TR activity. TR activity was determined spectrophotometrically at room temperature by the oxidation of NADPH at 339nm in the presence of 15 μM recombinant human Trx and 1mg/ml bovine insulin.
Measurement of Trx and Glutathione (GSH) bioreduction capacity in DU-145 cells
To measure Trx and GSH bioreduction capacity in live cells a modified method described by Biaglow et al. (22) was used. Briefly, DU-145 cells were treated with various concentrations of sodium selenite for 5 days. In some experiments prior to the bioreduction assay cells were treated with 1mM buthionine sulphoximine (BSO) for 2 hours to deplete GSH. To perform the assay, cells were washed twice with incubation buffer (1X PBS containing 5mM CaCl2, 10mM KCL, and 5mM MgCl2). Cells were then incubated at 37°C in the incubation buffer supplemented with 5mM glucose plus vehicle, 5mM oxidized lipoate or 5 mM hydroxyethyl disulfide (HEDS). Lipoate or HEDS freely diffuses into the cells and are bioreduced primarily by the Trx or glutathione systems, respectively (23–26). Following bioreduction the compounds diffuse back into the supernatant. Samples of the supernatant were taken every 10 minutes for 1 hr and added to 5mM of 5,5'-dithiobisnitrobenzoic acid (DTNB), which reacts rapidly with the reduced compounds. Disappearance of DTNB was read on a spectrophotometer at 412 nm. This technique is able to measure the following reactions as illustrated below as described in reference (22):
-
[1]
-
[2]
-
[3]
Apoptosis measurement
Apoptosis was ascertained microscopically using Ethidium Bromide and Acridine Orange (27). Briefly, cells were seeded in a 30mm plates at 1×105 cells for 24 hours until they reached confluency. They were then treated with various concentrations of Sodium selenite for 5 days. Both the floating and adherent cells were collected and centrifuged. They were then washed in Phosphate Buffered Saline, pH 7.0 (PBS) and re-suspended in 1 ml of DMEM media. To detect apoptosis, 10 μl of cells were mixed with Ethidium bromide and Acridine Orange solution (100 μg/ml each in DMEM) and visualized for morphological changes. Acridine orange stains the nuclei green in viable cells, and the propidium iodide is excluded. However, in apoptotic or necrotic cells, the nuclei are stained red by the propidium iodide, and the morphology of the nucleus easily determines whether the cell is apoptotic or necrotic. A minimum count of 200 cells was counted and the percentage of apoptotic cells determined. All experiments were performed in triplicates.
Statistical Analysis
Data are presented as means ± standard error. Significance was achieved with p-values referred as * for p<0.01; ** for p<0.005 and *** for p<0.001.
Results
Sodium selenite increases thioredoxin reductase activity in prostate cancer cells
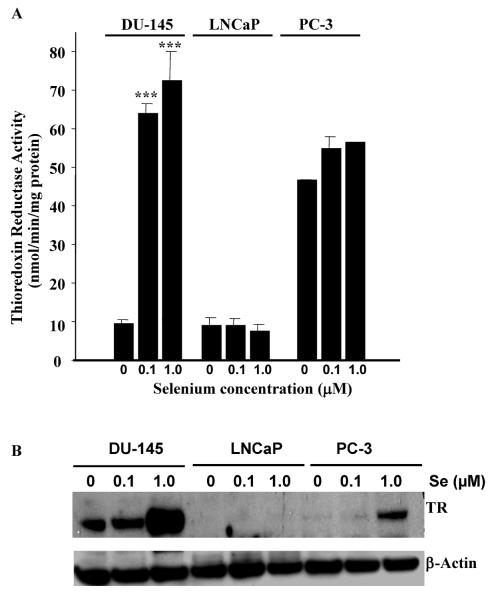
TR is a known selenoprotein and its activity can be regulated by seleno-compounds (8). We have measured TR activity in control and sodium selenite-treated prostate cancer cells. DU-145, LNCaP and PC-3 cells lines were grown in the presence of the indicated concentration of sodium selenite (Se) for five days. Following this treatment, TR activity was measured as described previously (8). TR activity was increased in a dose-dependent manner in presence of increasing concentrations of sodium selenite in DU-145 cells (Figure 1A). In the presence of 10 μM selenite, TR was inhibited (data not shown), potentially suggesting some level of toxicity. TR was not significantly modulated in the presence of increasing concentrations of selenite in LNCaP and PC-3 cells. In fact the level of enzyme activity was low in LNCaP as already observed by Lim and collaborators (18) who reported a TR activity of three times lower than in PC-3 cells. TR activity was also constitutively high in PC-3 (Figure 1A). Figure 1B is a representative western blot of TR protein expression in DU-145, PC-3 and LNCaP cells after sodium selenite treatment. TR protein expression was considerably increased in the presence of increasing concentration of selenite in DU-145 cells. However, LNCaP cells did not express detectable levels of TR and sodium selenite treatment had no significant effect on TR expression and activity (Figure 1A and B). In PC-3 cells, although a marked increase in the expression of TR was observed following 1 μM treatment, the measure of TR activity did not correlate with this increase. These results may reflect other RedOx systems being measured using this technique. Nonetheless, we show TR expression and activity is significantly increased by sodium selenite in DU-145 cells. The increase in thioredoxin reductase activity has been shown to occur over a range of Se concentrations from <0.01 to 10 μM (28), which encompasses the range of Se concentrations found in human serum (between 1 and 5 μM) (29).
Figure 1. Thioredoxin reductase activity is increased by sodium selenite in DU-145 prostate cancer cells.
Prostate cancer cells (DU-145, PC-3 and LNCaP) were treated for 5 days with 0.1 or 1.0μM sodium selenite. TR activity was measured as described in the Materials and Methods Section (Panel A). TR was increased significantly in a dose dependent manner by sodium selenite in DU-145 cells. Bars represent means ± SE from two independent experiments performed in triplicate (n=6). Significance was achieved with ** for p<0.01 and *** for p<0.001. Panel B represents the expression of TR in DU-145, PC-3 and LNCaP prostate cancer cells following 0.1 and 1.0 μM sodium selenite treatment. Note the large increase in TR protein expression in DU-145 cells as compared to LNCaP and PC-3 upon sodium 1.0 μM selenite treatment.
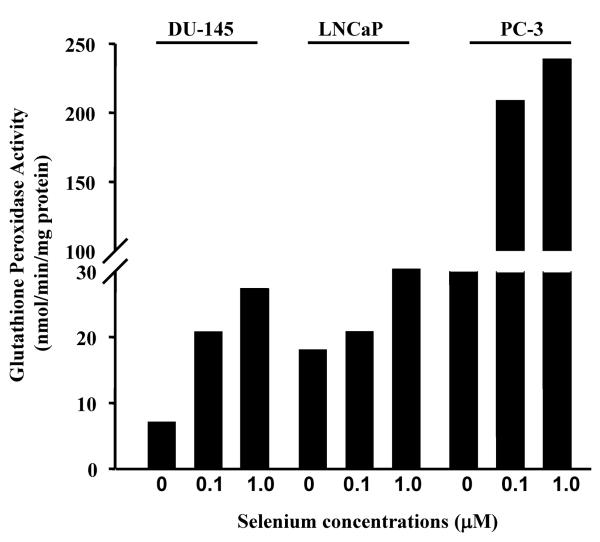
We next measured glutathione (GSH) peroxidase activity in these same cells. GSH peroxidase activity was increased by a factor of 8 in PC-3 upon increasing concentrations of sodium selenite (Figure 2). This observation could explain, at least in part, the high TR activity measured in Figure 1A. In DU-145 and LNCaP cells, GSH peroxidase was slightly increased by sodium selenite in a dose dependent manner. However, this increase was considered as negligible when compared to the increase observed in PC-3 cells. In all the three cell lines tested, higher concentrations of sodium selenite (10 μM) were shown also to become toxic (data not shown). In conclusion, we showed that sodium selenite increases TR in DU-145 cells and that increase in not due to GST peroxidase activity.
Figure 2. Effect of sodium selenite on glutathione peroxidase activity.
DU-145, PC-3 and LNCaP prostate cancer cells were treated with various concentrations of sodium selenite for 5 days at the indicated concentrations. The graph represents the activity of glutathione peroxidase as measured by the amount of reduced NADPH produced/min/mg of total protein/sample. Note that the level of glutathione peroxidase activity remains low in DU-145 and LNCaP cells as compared to the level in PC-3 cells.
Sodium Selenite increases the RedOx rate of Trx in DU-145 prostate cancer cells independently of GSH
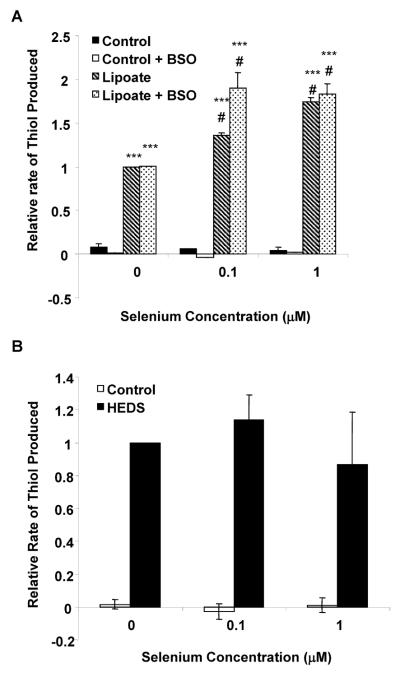
We have shown previously that the covalent interaction between Trx and PTEN occurs via disulfur bridges between Cys-32 of Trx and Cys-212 of the C2 domain of PTEN (14). Because TR activity was increased by sodium selenite, the bioreduction capacity of the Trx system was determined using lipoate as a substrate in DU-145 cells (Figure 3A). The rate of lipoate bioreduction is known to be primarily coupled to Trx as described previously (23–26). As observed with TR activity, increasing concentrations of sodium selenite increases the bioreduction rate of lipoate in a dose dependent manner in DU-145 cells (Figure 3A). Incubation of DU-145 cells with 0.1 or 1.0 μM sodium selenite increased the rate of lipoate bioreduction by 36% and 75%, respectively as compared to control. Depletion of GSH by BSO did not affect the rate of lipoate bioreduction, confirming that GSH was not contributing to the reaction and that selenite specifically acts on the Trx system. These data strongly suggest that the RedOx rate of Trx is increased and that Trx may not be available to bind PTEN. The specificity of the selenite was further confirmed using the HEDS in the bioreduction assay (Figure 3B). The bioreduction of HEDS, which is primarily mediated by GSH was unaffected by selenite (Figure 3B) and further indicates that sodium selenite specifically increased TR expression and its activity in DU-145 prostate cancer cells. Taken together, selenite increases TR expression and activity leading to an increase in the RedOx rate of Trx.
Figure 3. Effect of sodium selenite on Trx and GSH bioreduction capacity.
DU-145 cells were treated with various concentrations of sodium selenite for 5 days at the indicated concentrations. Panel A, DU-145 cells were treated with sodium selenite for 5 days and BSO at 1mM for 1 hour prior to the bioreduction assay. Cells were incubated with buffer containing 5mM glucose plus vehicle, or 5mM oxidized lipoate. The rate of Trx bioreduction was determined using 6 measurements over a 1hr period in duplicate treatment samples. Note that Trx's bioreduction potential increased significantly upon sodium selenite treatment. Bars represent means ± SE from three independent experiments performed in triplicate. Significance was achieved with *** represents significance at p< 0.001, when compared to respective vehicles or # represents significance at p< 0.01, when compared to lipoate reduction in control. Panel B, DU-145 cells were treated with sodium selenite for 5 days then cells were incubated with buffer containing 5mM glucose plus vehicle or 5mM HEDS. Note that there was no affect of selenite on GSH bioreduction. Bars represent means ± SE from three independent experiments performed in duplicate.
Sodium selenite increases PTEN lipid activity in DU-145 cells
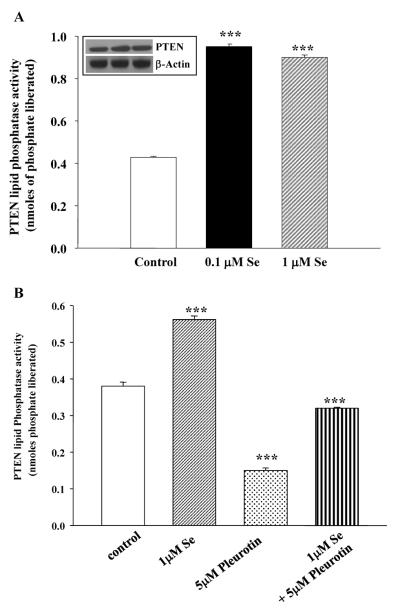
We have recently reported that Trx binds to PTEN in a RedOx-dependent manner and inhibits its lipid phosphatase activity (14,15). Moreover, TR is a selenium-dependent enzyme and increasing concentrations of sodium selenite increase TR expression and activity (Figure 1 and reference 8). Consequently, we hypothesized that by increasing TR activity via increasing amounts of sodium selenite, the RedOx rate of Trx increases in the cells. Trx would then not have been able to interact with PTEN and thus would allow the lipid phosphatase to be active. In order to test this hypothesis, DU-145 cells were treated with increasing amounts of sodium selenite and PTEN lipid phosphatase activity was measured as previously described (20). Figure 4A summarizes the effects of sodium selenite in DU-145 cells on PTEN lipid phosphatase activity using phosphatidyl-myo-inositol(3,4,5)-trisphosphate as substrate. PTEN activity from these cells was significantly increased by sodium selenite in a dose-dependent manner. There was no change in PTEN protein expression in DU-145 cells in presence of selenite as compared to control cells (Figure 4A, insert).
Figure 4. PTEN lipid phosphastase is increased by sodium selenite via the Trx-TR system.
Panel A represents PTEN lipid phosphatase activity as measured by the release of nmoles of phosphate from the substrate PtdIns-3,4,5-P3 as described in the Materials and Methods section. Note that increasing concentrations of sodium selenite stimulates PTEN lipid phosphatase activity in DU-145 cells. Inset shows the total PTEN protein expression in the corresponding samples as compared to internal control, β-actin. Panel B represents PTEN activity following 1μM sodium selenite, 5μM pleurotin (an inhibitor of TR) or the combination treatment. The combination treatment restores PTEN activity back to basal activity as compared to that in control cells. Bars represent means ± SE from three independent experiments performed in triplicate. Significance was achieved with *** for p<0.001.
To test whether this interaction is also dependent of TR activity, we used pleurotin (i.e. NSC131233, reference 19), a known inhibitor for TR and measured PTEN lipid phosphatase activity in DU-145 cells (Figure 4B). Cells were pre-treated with sodium selenite (1μM), pleurotin (5μM) or the combination, and PTEN lipid phosphatase was measured in DU-145 cells. As shown in Figure 4A, sodium selenite increased PTEN activity. Pleurotin decreased PTEN activity and the combination was able to restore PTEN activity to its basal activity as compared to control non-treated cells. Therefore, sodium selenite regulates PTEN activity which may be in part via the activation of the Trx-TR system in DU-145 prostate cancer cells.
Sodium selenite inhibits Akt phosphorylation and induces apoptosis in prostate cancer cells
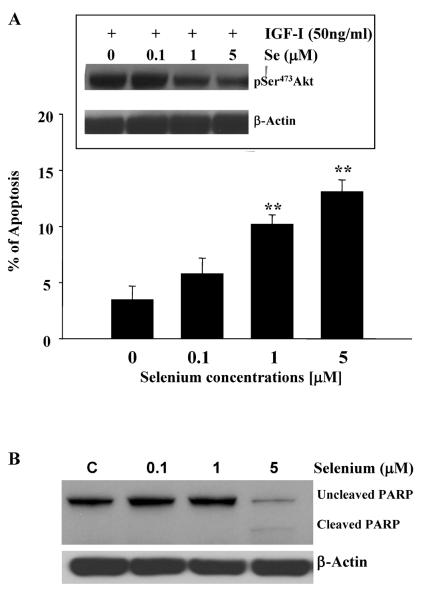
We have shown that PTEN lipid phosphatase is activated following sodium selenite treatment in DU-145 prostate cancer cells. Activation of PTEN has been correlated with a decrease in Akt activation in cells and an increase in apoptosis (30). In order to correlate the activity of PTEN with that of Akt, we have treated DU-145 cells with sodium selenite for five days and measured sodium-selenite induced apoptosis as well as the phosphorylation status of Akt. The results are summarized in Figure 5. A statistically significant increase in apoptosis was observed following 1μM and 5μM sodium selenite treatments in DU-145 cells (Panel A). As already shown by Menter et al (31), apoptosis was also confirmed by monitoring PARP cleavage following 5μM sodium selenite treatment in these cells (Panel B). A dose dependent decrease in Akt phosphorylation on Ser473 was also observed in sodium selenite-treated DU-145 cells (Insert, Panel A). These observations are in agreement with the increase in PTEN activity, correlating with a decrease in Akt phosphorylation leading to the induction of apoptosis in DU145 cells. In conclusion, we showed that sodium selenite treatment caused a decrease in Akt phosphorylation and a significant increase in apoptosis in DU-145 cells.
Figure 5. Sodium selenite inhibits Akt activation, induces apoptosis and PARP cleavage in DU-145 prostate cancer cells.
The insert of Panel A represents a typical western blot for the inhibition of Akt phosphorylation by increasing concentrations of sodium selenite in DU-145 cells. Briefly, cells were lysed and phosphorylated Akt was detected using specific anti-phospho-Ser473-Akt. Akt phosphorylation was decreased in the presence of increasing concentration of sodium selenite in DU-145 cells, correlating with the increase in PTEN lipid phosphastase activity. The graph in Panel A represents the induction of apoptosis in DU-145 cells by selenite as measured by the morphological assay described in the Materials and Methods section. Bars represent means ± SE from two independent experiments performed in triplicate. Significance was achieved with ** for p<0.01. Panel B represents a typical western blot for the cleavage of PARP by increasing concentrations of sodium selenite in DU-145 cells. Actin was used a loading control. Note the lower band appearing in cells treated with 5 μM selenite, which corresponds to cleaved PARP and induction of apoptosis (Panel A).
Sodium selenite modulates PTEN activity also via Casein Kinase 2 in DU-145 prostate cancer cells
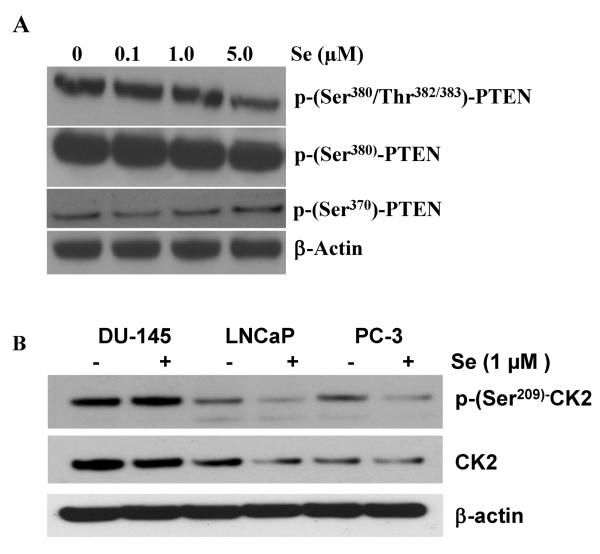
PTEN activity has been recently shown to be strongly regulated by its phosphorylation status (16–17). Because sodium selenite is also known to affect the expression as well as the activity of several enzymes, we have also tested the effects of sodium selenite on PTEN phosphorylation status in DU-145 cells using specific phospho-antibodies as well as the expression of the kinases involved in the phosphorylation of PTEN. Phosphorylation on Ser380/Thr382/383 was slightly decreased in a dose dependent manner but phospho-Ser380-PTEN remained unchanged in the presence of increasing concentrations of selenite (Figure 6A). Phospho-Ser370-PTEN appeared to increase slightly upon selenium treatment. Therefore we hypothesized that the activity of the kinases involved in the phosphorylation and regulation of PTEN activity may be also affected by sodium selenite.
Figure 6. Sodium selenite modulated PTEN phosphorylation in DU-145 cells.
DU-145 cells were treated with increasing concentrations of sodium selenite for 5 days. Cells were lysed and samples were probed for PTEN phosphorylation using specific phospho-antibodies. Panel A represents a typical Western blot for PTEN phosphorylation on residues Ser380/Thr382/383, PTEN phosphorylation on Ser380 and on Ser370 as compared to loading control with β-actin. Note the decrease in PTEN phosphorylation with 5μM of Sodium selenite on Ser380/Thr382/383 and a slight increase in PTEN phosphorylation on Ser370. Panel B represents the expression and the phosphorylation status of CK2 involved in the phosphorylation of PTEN following increasing concentrations of sodium selenite in DU-145, LNCaP and PC-3 prostate cancer cells. Cells were treated with 1μM of Se. Note the high levels of CK2 and phospho-(Ser209)-CK2 in DU-145 cells as compared to the other cell lines.
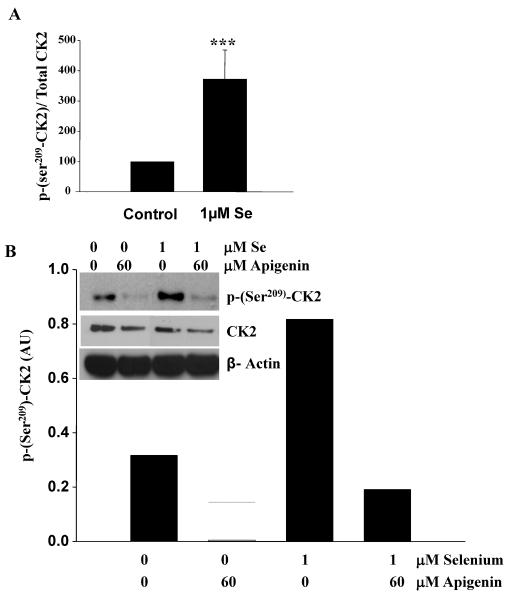
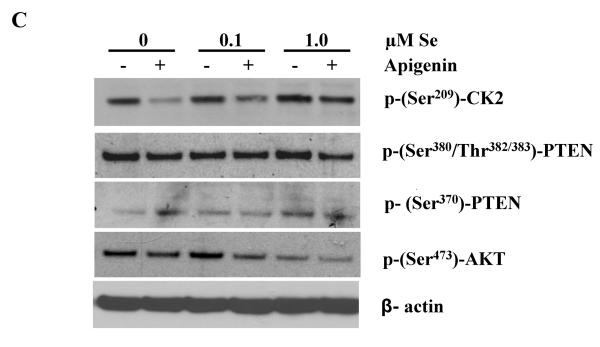
More specifically, we investigated the expression levels as well as the activity of CK2, a kinase known to phosphorylate PTEN (32). DU-145 cells expressed high levels of CK2 as compared to LNCaP and PC-3 cells (Figure 6B). Moreover, the addition of 1μM of Se increased pSer209-CK2 levels only in DU-145 cells and reduced it in the other cell lines. The activity of CK2, as measured by the ratio of phospho-Ser209-CK2 over total CK2, was significantly increased in DU-145 following 1μM of selenite treatment (Figure 7A). Apigenin, a known inhibitor of CK2 (33), was also shown to reduce phospho-Ser209-CK2 and confirmed its inhibitory effects on the kinase (Figure 7B). Apigenin-induced inhibition could partially be rescued by 1μM of selenite. CK2 is one of the kinase that phosphorylates PTEN and stabilizes the protein in an active form (32). Thus, to test the hypothesis that by increasing the activity of CK2 sodium selenite could also modulate the activity of PTEN, DU-145 cells were pre-treated with Se and then incubated with apigenin or vehicle control. Panel C represents a representative western blot correlating the phosphorylation status of PTEN, AKT and CK2. We show that phospho-Ser209-CK2 remains high in presence of 1 μM selenite and that consequently phospho-Ser370-PTEN is also high correlating with a low phosphor-Ser473-AKT. Apigenin decreased CK2 phosphorylation, which in turns also decreased Ser380/Thr382/383-PTEN phosphorylation as well as Ser473-Akt. Although these phosphorylation sites on PTEN have been suggested to act to facilitate PTEN phosphorylation on other residues but do not affect the lipid phosphatase activity, they may play a role in selenite-mediated effects on the PTEN/Akt pathway in prostate cancer cells.
Figure 7. Sodium selenite increases the activity of CK2 in DU-145 cells.
DU-145 cells were treated with different concentrations of sodium selenite for 5 days. Cells were lysed and samples were probed for phospho-Ser209CK2 and total CK2 using specific phospho-and total antibodies. Panel A represents the quantification of five separate experiments. Note the significant increase in the ratio phospho-Ser209CK2/Total CK2 following Se treatment in DU-145 cells. Bars represent means ± SE from five independent experiments performed in triplicate. Significance was achieved with *** for p<0.001. The inset in Panel B represents a typical western blot, where phospho-Ser209-CK2, total CK2 and β-actin were probed following treatment of the cells with Se with or without 60μM of apigenin. The graph represents the quantification of the typical western blot shown in the inset. Note that apigenin counteracts the effects of Se on CK2 phosphorylation. Panel C represents a typical western blot from DU-145 cells that were pre-treated with increasing concentrations of Se and then incubated with apigenin or vehicle control. Specific anti-phospho-Ser209CK2, phospho-Ser473Akt, phospho-Ser370PTEN and phospho-Ser380/Thr382/383PTEN antibodies were used to probe the membrane. Note that Se induces CK2 and PTEN phosphorylation (Ser380/Thr382/383) and decrease Akt phosphorylation. Se can also partially rescue the effects of apigenin on CK2.
Discussion
Epidemiological studies have shown that there is a consistent trend for populations residing in geographic areas that have low sodium selenite levels in the soil to have a high mortality from cancer. Other studies showed a lower risk for prostate cancer in individuals with selenium supplementation (34) and higher serum selenium concentrations correlates with a moderately reduced risk of prostate cancer (35). These cancers include cancers of the bladder, pancreas, thyroid, stomach, lung, esophagus, melanoma, head and neck tumors, and brain tumors. More specifically, several studies have suggested a protective role of sodium selenite against several cancers including prostate cancer (reviewed in 36). In an attempt to better understand the protective effects of Se on prostate cancer cells, we have studied the effects of sodium selenite on the regulation of the cell survival pathway, phosphatidylinositol-3 kinase/Akt/PTEN. Previously, we have shown in cell culture systems that human Trx binds in a RedOx dependent manner to PTEN and inhibits its PtdIns-3-phosphatase activity, resulting in increased Akt activation (14). Molecular docking and site-specific mutation studies showed that the binding of hTrx to PTEN occurs through a disulfide bond between the active site Cys32 of hTrx and Cys218 of the C2 domain of PTEN leading to steric interference by bound Trx of the catalytic site of PTEN and of the C2 lipid membrane-binding domain (14). Se is an essential element in RedOx pathways and acts as an effective oxidant of bioreduced Trx and bioreduced TR, which diminishes the Trx-TR pool. This important role of sodium selenite may explain its inhibitory influence on cell growth and cancer progression (31). Consequently, we have hypothesized that by modulating the Trx-TR RedOx system endogenously with sodium selenite, PTEN activity can be regulated in prostate cancer cells. We show that sodium selenite decreases Akt phosphorylation by increasing PTEN lipid phosphatase activity in DU-145 cells. Consistent with others, it has been shown that sodium selenite induces a dose-dependent growth inhibition of prostate cancer cells such as DU-145, PC-3, and LNCaP (31) and an induction of apoptosis in selenite-treated prostate cancer cells (31). Our observations are in agreement with these studies and confirm the inhibitory effects of sodium selenite on Akt phosphorylation, leading to the increase in apoptosis. However, we show here that it is by releasing PTEN from the inhibitory binding of Trx, that Se treatment leads to a decrease in Akt phosphorylation and consequently to apoptosis in DU-145 cells.
In this study, we also showed that selenite increased the activity of a kinase involved in PTEN phosphorylation and thereby regulating the tumor suppressor's activity in these cells. In the DU-145 prostate cancer cells which express an active PTEN (37) we expected a bigger effect on Akt, however Akt phosphorylation was only slightly decreased in the presence of sodium selenite. The reason for these observations may reflect the dual role of CK2. CK2 is a ubiquitous serine/threonine kinase which regulation and function is not well understood yet (38). Originally CK2 was defined as a constitutively active protein kinase though recently several reports suggest that CK2 acts as a regulated kinase participating in signal transduction. Indeed, CK2 was recently identified as the kinase that phosphorylates the C-terminus of PTEN on residues Ser385 and Ser370 (32, 39–40). CK2 has been demonstrated to also phosphorylate PTEN on Ser380 and thereby affects its stability (32). The phosphorylation by CK2 hinders the cleavage of PTEN by caspases. On the other hand, CK2 also phosphorylates at Ser129 of Akt and up-regulates Akt activity in Jurkat cells (41). In this study, we showed that CK2 activity is increased upon selenite treatment. The increase in phosphorylation of CK2 may translate itself in an increase in Ser129 phosphorylation of Akt, leading to an increase in Akt activity, and thereby counterbalancing the effects of selenite on PTEN activity via the Trx-TR system. Interestingly, using apigenin, an inhibitor for CK2, we showed that the decrease in Akt phosphorylation due to the increase in PTEN activity can be enhanced. This suggests that up-regulation of CK2 activity with selenite may contribute to the observed decrease in the effects of selenite on Akt. Therefore, although we observed an increase in phosphorylation status of CK2, overall the effects of sodium selenite are counteracted by the fact that CK2 directly phosphorylates Akt to increase its activity and its phosphorylation status at Ser473.
Sodium selenite may modulate the activity of protein kinases and other enzymes through phosphorylation-independent mechanisms such as RedOx modification. In this study, we show that sodium selenite increased CK2 activity in prostate cancer cells. The regulation of this enzyme is then affecting PTEN activity because it has been recently found to phosphorylate PTEN and influence the stability and the activity of the tumor suppressor. Interestingly, recent studies using apigenin showed that the down-regulation of CK2 in prostate cancer cells promotes apoptosis (42). These observations correlate well with our study presented in this paper. Finally, although apigenin's mechanism of action remains to be clearly established and probably extends beyond the inhibition of CK2, the novel finding that Se increases CK2 activity, which in turn can affect PTEN activity is of interest for the treatment of prostate cancer.
In conclusion, we have demonstrated that the regulation of PTEN activity may be achieved at least in part via the modulation of the Trx-TR system by selenite as well as the RedOx properties of the kinases involved in PTEN phosphorylation. These results may explain at least in part the chemopreventive effects of sodium selenite in prostate cancers. However, they also suggest the importance of PTEN status (expression as well as phosphorylation) in predicting the therapeutic effects of sodium selenite.
References
- 1.Linder MC. Nutrition and metabolism trace elements. In: Linder MC, editor. Nutritional biochemistry and metabolism with clinical applications. Elsevier; New York: 1990. [Google Scholar]
- 2.Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1:1059–1066. [PubMed] [Google Scholar]
- 3.Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–12. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–252. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. doi: 10.1074/jbc.M304095200. [DOI] [PubMed] [Google Scholar]
- 6.Freemerman AJ, Powis G. A RedOx-inactive thioredoxin reduces growth and enhances apoptosis in WEHI7.2 cells. Biochim Biophys Res Comm. 2000;274:136–141. doi: 10.1006/bbrc.2000.3091. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 8.Gallegos A, Berggren M, Gasdaska JR, Powis G. Mechanisms of the regulation of thioredoxin reductase activity in cancer cells by the chemopreventive agent selenium. Cancer Res. 1997;57:4965–4970. [PubMed] [Google Scholar]
- 9.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of RedOx regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 10.Schenk H, Klein M, Erdbrugger W, Droge W, Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abate C, Patel L, Rauscher FJ, 3rd, Curran T. RedOx regulation of fos and jun DNA binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 12.Watson JA, Rumsby MG, Wolowacz RG. Phage display identifies thioredoxin and superoxide dismutase as novel protein kinase C-interacting proteins: thioredoxin inhibits protein kinase C-mediated phosphorylation of histone. Biochem J. 1999;343:301–305. [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada S, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Song Z, Brabant M, Saghafi N, Brabant M, Meuillet EJ. The Regulation of the Tumor Suppressor Activity, PTEN by Thioredoxin Controls Cell Number and Cell Size. Exp Cell Res. 2007;313:1161–371. doi: 10.1016/j.yexcr.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 17.Birle D, Bottini N, Williams S, Huynh H, deBelle I, Adamson E, Mustelin T. Negative feedback regulation of the tumor suppressor PTEN by phosphoinositide-induced serine phosphorylation. J Immunol. 2002;169(1):286–291. doi: 10.4049/jimmunol.169.1.286. [DOI] [PubMed] [Google Scholar]
- 18.Maccario H, Perera NM, Davidson L, Downes P, Leslie NR. PTEN is destabilized by phosphorylation on Thr366. Biochem J. 2007;405:439–444. doi: 10.1042/BJ20061837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick DL, Watson S, Kunkel M, Fletcher S, Ulhaq S, Powis G. Parallel syntheses of disulfide inhibitors of the thioredoxin RedOx system as potential antitumor agents. Anticancer Drug Des. 1999;14:421–432. [PubMed] [Google Scholar]
- 20.Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oblong JE, Berggren M, Powis G. Biochemical, structural and biological properties of human thioredoxin active site peptides. FEBS lett. 1993;343:81–84. doi: 10.1016/0014-5793(94)80611-x. [DOI] [PubMed] [Google Scholar]
- 22.Biaglow JE, Donahue J, Tuttle S, Held K, Chrestensen C, Mieyal J. A (method for measuring disulfide reduction by cultured mammalian cells: relative contributions of glutathione-dependent and glutathione-independent mechanisms. Anal Biochem. 2000;281:77–86. doi: 10.1006/abio.2000.4533. [DOI] [PubMed] [Google Scholar]
- 23.Constantinescu A, Pick U, Handelman GJ, Haramaki N, Han D, et al. Reduction and transport of lipoate by human erythrocytes. Biochem.Pharmacol. 1995;50:253–261. doi: 10.1016/0006-2952(95)00084-d. [DOI] [PubMed] [Google Scholar]
- 24.Bustamante J, Lodge JKM, Marcoccl L, Tritschler HJ, Packer L, Rihn BH. a-Lipoate in liver metabolism and disease. Free Radic. Biol. Med. 1998;24:1023–1039. doi: 10.1016/s0891-5849(97)00371-7. [DOI] [PubMed] [Google Scholar]
- 25.Packer L, Roy S, Sem CK. a-Lipoate: A metabolic antioxidant and potential RedOx modulator of transcription. Adv. Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 26.Arner ESJ, Nordberg J, Holmgren A. Effect of reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase, thioredoxin. Biochem. Biophys. Res. Commun. 1996;225:268–274. doi: 10.1006/bbrc.1996.1165. [DOI] [PubMed] [Google Scholar]
- 27.Powell AA, LaRue JM, Batta AK, Martinez JD. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem J. 2001;356:481–486. doi: 10.1042/0264-6021:3560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berggren M, Gallegos A, Gasdaska J, Powis G. Cellular thioredoxin reductase activity is regulated by selenium. Anticancer Res. 1997;17:3377–3380. [PubMed] [Google Scholar]
- 29.Kitaoka Y, Sorachi K, Nakamura H, Masutani H, Mitsui A, Kobayashi F, Mori T, Yodoi J. Detection of adult T-cell leukemia-derived factor/human thioredoxin in human serum. Immunol Lett. 1994;41:155–161. doi: 10.1016/0165-2478(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 30.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–79. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 31.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidem Biomark and Prevent. 2000;9:1171–1172. [PubMed] [Google Scholar]
- 32.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 33.Kuo ML, Yang NC. Reversion of v-H-ras-transformed NIH3T3 cells by apigenin through inhibiting mitogen activated protein kinase and its downstream oncogenes. Biochem Biophys Res Commun. 1995;212:767–775. doi: 10.1006/bbrc.1995.2035. [DOI] [PubMed] [Google Scholar]
- 34.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 35.Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, Schoenberg JB, Swanson GM, Hayes RB, Mayne ST. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 36.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 37.Vliestra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 38.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115:3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 40.Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3 beta. J Biol Chem. 2005;280:35195–35202. doi: 10.1074/jbc.M503045200. [DOI] [PubMed] [Google Scholar]
- 41.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad KA, Wang G, Ahmed K. Intracellular hydrogen peroxide production is an upstream event in apoptosis induced by down-regulation of CK2 in prostate cancer cells. Mol Cancer Res. 2006;4:331–338. doi: 10.1158/1541-7786.MCR-06-0073. [DOI] [PubMed] [Google Scholar]