Abstract
Purpose/Objectives
To assess the validity of filter paper (FP) against the gold standard of passive drool (PD) for collecting salivary alpha amylase as a surrogate biomarker of psychological stress in adolescents with cancer.
Design
Part of a longitudinal, descriptive study of symptoms in adolescents with cancer during chemotherapy.
Setting
A pediatric hematology/oncology treatment center.
Sample
33 saliva sample pairs from nine adolescents with cancer, aged 13–18 years.
Methods
Salivary alpha amylase was collected by PD and FP at four time points during a cycle of chemotherapy: days 1 (time 1) and 2 (time 2) of chemotherapy, day 7–10 (time 3), and day 1 of the next cycle (time 4). A random effects regression was used to assess the correlation between PD and FP values, and a Bland Altman analysis was conducted to assess agreement between the values.
Main Research Variables
Salivary alpha amylase.
Findings
The estimated correlation between PD and FP values was r = 0.91, p < 0.001. Regression results were also used to rescale FP values to the levels of the PD values because the FP values were on a different scale than the PD values. The Bland Altman analysis revealed that the agreement between the rescaled FP values and PD values was not satisfactory.
Conclusions
Eluted FP may not be a valid method for collecting salivary alpha amylase in adolescents with cancer.
Implications for Nursing
Psychological stress in adolescents with cancer may be linked to negative outcomes, such as greater symptom severity and post-traumatic stress disorder. Nurses need valid, efficient, biobehavioral measures to assess psychological stress in the clinical setting.
A cancer diagnosis poses many challenges for adolescents, and the psychological stress associated with some of these challenges may have detrimental effects. Stressors that have been identified by children and adolescents with cancer include painful procedures (Hedstrom, Ljungman, & von Essen, 2005; McCaffery, 2006; Woodgate, Degner, & Yanofsky, 2003), frequent and extended hospital stays (McCaffery, 2006), alopecia, difficult symptoms (Hedstrom et al., 2005; McCaffery, 2006; Woodgate, 2005), and uncertainty (Stewart, Lynn, & Mishel, 2010; Woodgate et al., 2003). The few studies on psychological stress in children and adolescents with cancer have noted both short- and long-term effects. In the short-term, psychological stress has been linked to greater symptom severity (Docherty, Sandelowski, & Preisser, 2006; McCaffery, 2006) and higher levels of anxiety (Hedstrom et al., 2005; Hockenberry-Eaton, Dilorio, & Kemp, 1995). In the long-term, psychological stress in survivors of cancer in childhood and adolescence has been associated with post-traumatic stress disorder (PTSD) (Rourke, Hobbie, Schwartz, & Kazak, 2007; Schrag, McKeown, Jackson, Cuffe, & Neuberg, 2008). Psychological stress in adolescents with cancer may be a targeted area for oncology nurse intervention, but the prevalence and degree have not been studied extensively. A biobehavioral approach to investigating psychosocial functioning, including psychological stress, is highly recommended for adolescents with cancer (Moore, 2004). However, validated biologic measures of pyschological stress are limited. The purpose of this study was to examine the validity of a method for collecting one biologic marker of psychological stress: salivary alpha amylase (sAA). Efficient, easy-to-obtain biologic markers of stress may enhance development of clinical research and lead to future use in clinical settings by oncology nurses.
Background
An individual’s response to psychological stress is multifactorial, encompassing such dimensions as psychosocial and physical responses, indicating a need for a biologic and behavioral approach to the measurement of stress. In the few studies conducted on stress in children with cancer, cortisol has been used as a biologic marker of psychological stress (Hockenberry-Eaton, Kemp, & Dilorio, 1994; Walco, Conte, Labay, Engel, & Zeltzer, 2005). However, steroids, specifically corticosteroids, can cause hypothalamic-pituitary-adrenal axis suppression (Taketomo, Hodding, & Kraus, 2010) and alter cortisol levels. Many of the childhood cancer treatment protocols include high-dose corticosteroids. Consequently, the use of cortisol as a measure of stress can be problematic. A promising alternative to cortisol is sAA, a noninvasive, surrogate biologic marker of psychological stress not affected by corticosteroid use.
sAA is a digestive enzyme involved in the breakdown of starch. Unlike salivary cortisol, which is produced in endocrine glands and diffused into saliva, sAA is produced by the acinar cells of the salivary glands. The three major salivary glands are the parotid, submandibular, and sublingual glands, with the parotid gland secreting the largest amount of amylase—about 80% of the total sAA. Levels of sAA are very low in young infants, who do not have to digest complex carbohydrates yet. Adult levels of sAA are reached by ages 5 or 6, when diet is similar to that of an adult (O’Donnell & Miller, 1980).
The research on sAA as a surrogate biomarker of psychological stress is growing. The stress response involves two primary systems: the hypothalamic-pituitary-adrenal axis and the locus ceruleus/autonomic nervous system (ANS), which includes the sympathetic (SNS) and parasympathetic nervous system (PNS). In a number of studies, investigators found that psychological stress was associated with increases in sAA activity (Nater et al., 2005; Nierop et al., 2006; Wetherell et al., 2006). However, initially, which system was responsible for activating sAA was unclear. Additional investigation revealed that, during psychological stress, the salivary glands are innervated by the ANS, with SNS activation increasing secretion of sAA (Ehlert, Erni, Hebisch, & Nater, 2006; Nater et al., 2006; Speirs, Herring, Cooper, Hardy, & Hind, 1974; van Stegeren, Rohleder, Everaerd, & Wolf, 2006). Activation of the PNS increases salivary flow rate and, therefore, salivary volume (Proctor & Carpenter, 2007).
As the use of sAA as a measure of psychological stress in research has increased, the methods of collecting sAA have come under scrutiny, indicating that additional validation and comparison of collection methods is needed. Several of the methods that have been used to collect saliva to measure sAA include passive drool (PD), Salivette®, and eluted filter paper (FP). PD is considered the gold standard for obtaining many components of saliva, including sAA (Munro, Grap, Jablonski, & Boyle, 2006). To obtain PD, individuals are instructed to expectorate or drool into a collection device (e.g., specimen cup, straw). Salivette is a commercially available cotton roll used to collect saliva that individuals roll or chew in their mouth for as long as five minutes. Alternatively, some investigators have used FP, which is a thin piece of tasteless paper placed in the mouth (usually in the cheek pocket) until saturated. Eluted FP is appealing because it requires a minute amount of saliva (about 20 mcl) and is quick and easy for individuals to use. Each of the three collection methods has advantages and disadvantages (see Table 1), particularly depending on the analyte of interest. Although validation studies have been conducted on the use of Salivette for sAA collection (DeCaro, 2008; Granger et al., 2006), none have focused on the validation of FP for sAA collection. The specific aim of this study was to evaluate the validity of FP for sAA collection by assessing agreement (replaceability) between sAA levels collected by PD and FP in adolescents with cancer.
Table 1.
Advantages and Disadvantages of Salivary Alpha Amylase by Collection Method
| Collection Method | Advantages | Disadvantages |
|---|---|---|
| Filter paper | Easy to learn and use, minimally intrusive, and easy to transport and store | May have issues with eluting similar to cotton, yet to be validated, and most expensive (eluting takes about 24 hours) |
| Passive drool | Unstimulated whole saliva, reflects saliva from all glands, and least expensive because of least amount of technician time (no eluting) | Technique requires practice, less desirable by participants, and unfiltered sample |
| Salivette® | Easy to learn and use, and cotton acts as filter | Cannot avoid some stimulation of saliva, not all analytes elute well from cotton, and more expensive (eluting takes about one hour) |
Methods
Design and Sample
As part of a larger longitudinal descriptive study of the symptom experience in adolescents with cancer during a cycle of chemotherapy, the authors evaluated the validity of FP compared to PD for collection of sAA. Adolescents with cancer from the pediatric oncology clinic at the Virginia Commonwealth University Medical Center participated. Inclusion criteria for the main study were being aged 12–20 years, diagnosed with cancer for at least one month, and receiving anywhere from the second through sixth cycle of chemotherapy. Exclusion criteria were diagnosis of a brain tumor because of the uniqueness of the disease trajectory, participant’s or minor’s parent’s inability to read and write in English, or known cognitive disabilities.
Procedure
The institution’s review board approved the study. Adolescents with cancer were recruited from the pediatric oncology clinic. At the initial meeting, the researcher obtained written assent from participants 17 years of age and younger with consent from a parent, and consent from participants 18 years of age and older. For the main study, data were collected at four time points: day 1 of a cycle of chemotherapy, before the chemotherapy was started (time 1); day 2 of chemotherapy, when symptoms related to chemotherapy are expected to be intense (time 2); day 7–10, when bone marrow suppression is typically most severe (time 3); and day 1 of the next cycle, again before the chemotherapy was started (time 4). At each time point, participants first completed questionnaires pertaining to their symptom experience and then provided salivary samples by PD and FP. Lastly, they completed brief interviews on their symptom experience. For the sAA collection, participants were instructed to avoid caffeine on the morning of sample collection and to refrain from drinking any liquids 15 minutes prior to sample collection.
Salivary Alpha Amylase
Collection Methods and Assay
Filter paper
Whatman® grade 42 FP (2.4 cm × 9 cm) was used for the collection of saliva. The FP was placed in the participant’s cheek pocket until saturated to a marked line, taking about 20 seconds total. At each of the data collection time points, participants were instructed to place the FP in the same location in the mouth (cheek pocket), so to obtain the same proportion of contribution of sAA from the different glands. The FP was air dried and then single packaged in a plastic bag to prevent cross contamination of specimens. FP samples were stored at room temperature until assayed.
Passive drool
PD was collected in a sterile specimen cup. Participants were asked to briefly (for 30 seconds) refrain from swallowing and expectorate however much saliva was in the mouth from a single expectoration into the sterile cup. The PD samples were frozen until assayed. No samples were stored longer than 15 months.
Assay
Because sAA is an enzyme, the assay is a kinetic assay, which measures activity of the enzyme rather than amount of enzyme present. This method uses a substrate for alpha amylase which produces a color change when acted on by alpha amylase. The amount of alpha amylase activity present in the sample is directly proportional to the increase in absorbance for one minute at 405 nm. PD and FP at the same time point were treated as separate samples, not as duplicates of the same sample. Each of the samples was assayed in duplicate, on the same assay plate, to decrease the plate-to-plate variability, and the values of the two were averaged to obtain a value for that sample. Values are reported in units of enzyme activity per milliliter (U/ml). Values for the duplicates were close, such that variability between duplicates in the raw data was very low—values differed by 0.03 U/ml or less. The assay was done using a commercial kit from Salimetrics, LLC, and the kit has a less than 8% coefficient of variation for both intra- and inter-assay precision.
Data Analysis Approach
The aim was to evaluate validity by assessing agreement between sAA values collected by PD and FP. First, to determine the degree to which the values were correlated, the authors computed a random effects regression using the FP values to predict the PD values. Because this was a longitudinal design, a random effects model was used to account for the correlated data within subjects. The regression results were also used to rescale the FP sAA values to the levels of the PD sAA values. The rescaling was necessary because the FP was not going to yield replaceable values for the PD. Next, to assess agreement, a Bland Altman analysis (Bland & Altman, 2010) was conducted to determine if the rescaled FP values could be used in place of the actual PD values. The authors set the limits of considering values to be replaceable if they fell in the range of 25 to −25 from the true value (i.e., the value of the PD) based on the range of sAA values and what is clinically significant.
Results
Nine adolescents with cancer, aged 13–18 years, participated in the study (see Table 2). A total of 33 sAA sample pairs of PD and FP were collected. Missing data (n = 3) were from attrition (n = 1) and missed clinic appointments (n = 2). The adolescents did not have any problems sufficiently saturating the FP with saliva—all of the FP samples were completely saturated to the pre-marked level within 20 seconds. For the PD samples, all of the adolescents reported that spitting into a cup was not a bother. The FP sAA values ranged from 0.5–24.4 U/ml, and the PD values ranged from 10.5–325.4 U/ml, which is within the normal adult range of 3.1–423.1 U/ml.
Table 2.
Sample Characteristics
| Characteristic | X̅ | SD | Range |
|---|---|---|---|
| Age (years) | 15.3 | 1.7 | 13–18 |
| Time since diagnosis (months) | 2.6 | 1.9 | 1–7 |
| Characteristic | n | ||
| Gender | |||
| Male | 5 | ||
| Female | 4 | ||
| Race | |||
| African American | 5 | ||
| Caucasian | 3 | ||
| Asian | 1 | ||
| Cancer diagnosis | |||
| Bone tumor | 3 | ||
| Lymphoma | 3 | ||
| Leukemia | 2 | ||
| Soft tissue tumor | 1 | ||
N = 9
In the first step of validation, a regression analysis was performed to obtain a measure of correlation between PD and FP values. The estimated correlation was r = 0.91, p < 0.001. However, the PD and FP values were clearly on a different scale. To assess agreement between methods (i.e., conduct a Bland Altman analysis), both values had to be on the same scale. Therefore, the regression model was used to formulate an equation that rescaled FP values to the same scale as the PD values.
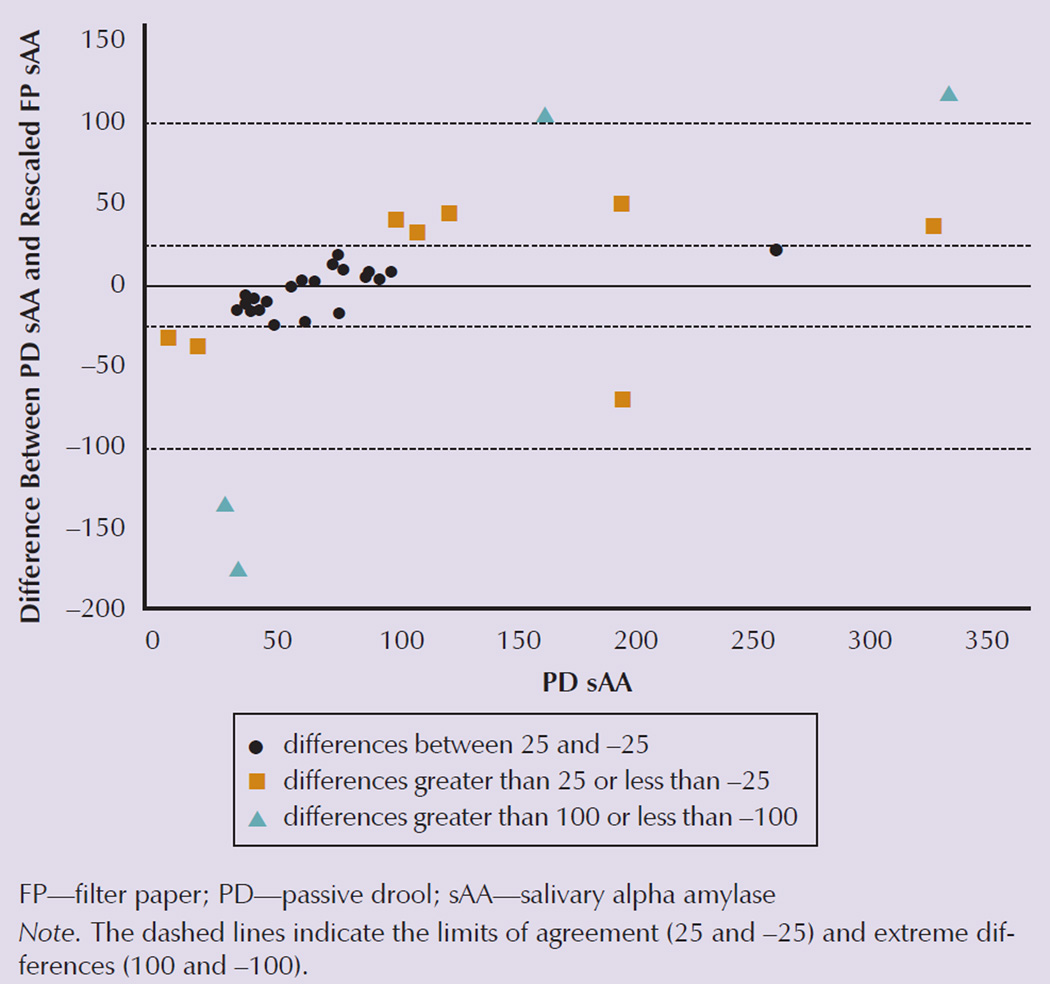
A Bland Altman analysis was conducted using the rescaled FP values. The results indicated that agreement between the rescaled FP values and PD values was not satisfactory—FP values were not replaceable for PD values (see Figure 1). The rescaled FP sAA values ranged as much as 100 U/ml above and below the PD values, with 36% of the values outside of the acceptable range of 25 to −25.
Figure 1. Bland Altman Results of the Rescaled FP sAA Values Plotted Against the PD sAA Values.
Discussion
In this study, the validity of FP as a method for collecting sAA was evaluated by assessing agreement between sAA values collected by FP and the gold standard of PD. The sAA levels obtained in this study were within the normal adult range and are consistent with findings from studies with other young adults (Gordis, Granger, Susman, & Trickett, 2008; Takai et al., 2004; Wetherell et al., 2006). However, results suggest that eluted FP may not be a valid method for collecting sAA since rescaled FP values were not replaceable for PD values.
Comparison of these findings to other research findings is limited because very few validation studies of collection methods for sAA and FP have been conducted. In one study, investigators compared sAA levels in PD to levels recovered from a cotton roll Salivette (DeCaro, 2008). Recovery of sAA from the Salivette was significantly less than 100%, even when the Salivette was completely saturated with saliva. Recovery was even lower when the Salivette was partially saturated. Overall, most experts agree that PD remains the gold standard for collecting sAA (Bosch, Veerman, de Geus, & Proctor, 2011; DeCaro, 2008; Rohleder & Nater, 2009).
Unlike sAA, more validation studies have been conducted of collection methods for salivary cortisol, but results have been mixed. Four validation studies were identified: In the only FP study, FP was compared to whole saliva (Neu, Goldstein, Gao, & Laudenslager, 2007). In three studies, other methods for collecting salivary cortisol (rope, Salivette, sponge, and eye spears) were compared to PD (Harmon, Hibel, Rumyantseva, & Granger, 2007; Shirtcliff, Granger, Schwartz, & Curran, 2001; Strazdins et al., 2005). In the only validation study found on FP (Neu et al., 2007), investigators compared whole saliva to FP for salivary cortisol collection in preterm infants, using the same type of FP as was used in the current study, Whatman grade 42 FP. Neu et al. (2007) obtained 92% recovery of salivary cortisol from the FP, and an inadequate saturation of saliva occurred in less than 2% of the samples. These results indicate that FP may be useful for salivary cortisol collection in populations in which saliva can be challenging to obtain.
In one of the three studies on salivary cortisol and PD, Shirtcliff et al. (2001) found that cortisol levels of saliva that was filtered through a cotton swab (same material used in a Salivette) did not differ significantly from PD. In a second study using PD, Strazdins et al. (2005) compared cortisol levels obtained by Salivette and cellulose cotton-tipped applicators (eye spears) to PD and found that eye spears yielded similar levels of cortisol to PD but concentrations of cortisol by Salivette were lower. In a third study using PD, Harmon et al. (2007) compared cotton rope, Salivette, and hydrocellulose microsponge to PD for collecting salivary cortisol. They found that those methods provided comparable cortisol levels when an adequate amount of saliva was present and volume recovery was adequate. However, cortisol levels by those methods were not comparable to cortisol levels by PD when saliva amounts were lower. Drawing from these cortisol validation studies, eye spears (which have similar advantages to FP, such as ease of use, require minute amounts of saliva, and obtain unstimulated saliva) may be worthwhile to investigate for sAA collection.
Limitations
The current study had several limitations. The sample size was small, although it was large enough to show the non-reproducibility of the PD values. The PD and FP samples were collected separately but in immediate succession. That approach may have contributed to differences in values. However, because both methods collect unstimulated drool, and were collected consecutively, the sAA levels were not expected to differ. The samples were collected separately to evaluate validity based on how the samples would actually be used in the clinical setting and to determine whether the adolescents would have any difficulty adequately saturating the FP. The validity of FP was assessed in adolescents with cancer only; therefore, findings are limited to this population. Evaluating the validity in other populations may yield different results.
Research Implications
Psychological stress in adolescents with cancer has been understudied. Given that findings have indicated that psychological stress may have a negative influence on short-term outcomes, such as symptom severity, and on long-term outcomes, such as PTSD, more research on the biologic and behavioral responses of stress is needed. As the research on stress in adolescents with cancer moves forward, the authors suggest that sAA may be a more appropriate biologic measure of psychological stress instead of cortisol when corticosteroids are used. Methods of collecting sAA are limited because few have been adequately validated. In comparing sAA collected by FP to PD, the current study’s findings suggest FP may not be suitable for collecting sAA in adolescents with cancer. Agreement between the values was not acceptable and, in addition, sAA values obtained by PD reflected the normal range of adult values more closely than those obtained by FP. The adolescents in this study were easily able to produce the required amount of PD. Currently, PD has the strongest evidence of validity for measuring sAA in this population and is a relatively inexpensive and easy collection method. Therefore, in research including sAA as a biologic marker of psychological stress in adolescents with cancer, PD is recommended over FP.
Clinical Implications
Many of the stressors identified by adolescents with cancer, such as painful procedures, bothersome symptoms, and even active hair loss, occur during hospital or clinic visits. Therefore, nurses in the clinical setting are in key positions to assess adolescents and identify those who may be at risk for or are experiencing psychological stress related to these situations or to cancer in general. In addition, nurses can implement strategies to reduce stress or link these adolescents to the appropriate resources. A number of strategies have been shown to reduce some of the stressors related to cancer. For example, in children and adolescents with cancer, hypnosis has been effective for reducing stress related to lumbar punctures (Liossi & Hatira, 2003); social support (e.g., hand holding) was reported to be helpful during times of physical and mental stress (Woodgate, 2006); and yoga during hospitalization was found to decrease anxiety (Thygeson, Hooke, Clapsaddle, Robbins, & Moquist, 2010). However, a key initial step is to develop valid multidimensional measures of stress, such as measures used to assess pain. Biologic measures used in vulnerable populations, such as adolescents, should be easy to use and as painless and noninvasive as possible (Moore, 2004). Future use of efficient measures of biologic markers in the clinical setting has potential for detecting psychological stress in adolescents with cancer. sAA has the potential to be used as a biologic marker of stress in the clinical setting, although additional investigation of other collection methods suitable for adolescents is needed.
Conclusion
This study on the examination of the validity of FP demonstrated that the absolute values of FP were about ten-fold lower than PD values and, therefore, not an accurate representation of the PD enzyme activity for this particular analyte. Neither additive nor multiplicative manipulation could adjust for the inaccuracy of the FP measure. Although FP is convenient and easy to use, these findings suggest that the data are likely to be less reliable than PD. On a positive note, these adolescents reported that expectorating into a cup for the PD was not a bother. When considering collection of salivary biomarkers of psychological stress, findings from this and other studies bring to light a critical point: method does matter. Additional investigation of the validity of sAA collection methods is warranted to advance the research on psychological stress in adolescents with cancer.
Acknowledgments
Funding for this research was provided, in part, by a grant from the National Institute of Nursing Research (P20 NR008988).
Footnotes
Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Ameringer can be reached at swameringer@vcu.edu, with copy to the editor at ONFEditor@ons.org.
References
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. International Journal of Nursing Studies. 2010;47:931–936. [Google Scholar]
- Bosch JA, Veerman ECI, de Geus EJ, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology. 2011;36:449–453. doi: 10.1016/j.psyneuen.2010.12.019. [DOI] [PubMed] [Google Scholar]
- DeCaro JA. Methodological considerations in the use of salivary α-amylase as a stress marker in field research. American Journal of Human Biology. 2008;20:617–619. doi: 10.1002/ajhb.20795. [DOI] [PubMed] [Google Scholar]
- Docherty SL, Sandelowski M, Preisser JS. Three months in the symptom life of a teenage girl undergoing treatment for cancer. Research in Nursing and Health. 2006;29:294–310. doi: 10.1002/nur.20143. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Salivary α-amylase levels after yohimbine challenge in healthy men. Journal of Clinical Endocrinology and Metabolism. 2006;91:5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Schwartz EB. Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships. 2006;23:267–290. [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, Granger DA. Measuring salivary cortisol in studies of child development: Watch out—What goes in may not come out of saliva collection devices. Developmental Psychobiology. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Hedstrom M, Ljungman G, von Essen L. Perceptions of distress among adolescents recently diagnosed with cancer. Journal of Pediatric Hematology Oncology. 2005;27:15–22. doi: 10.1097/01.mph.0000151803.72219.ec. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton M, Dilorio C, Kemp V. The relationship of illness longevity and relapse with self-perception, cancer stressors, anxiety, and coping strategies in children with cancer. Journal of Pediatric Oncology Nursing. 1995;12:71–79. doi: 10.1177/104345429501200206. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton M, Kemp V, Dilorio C. Cancer stressors and protective factors: Predictors of stress experienced during treatment for childhood cancer. Research in Nursing and Health. 1994;17:351–361. doi: 10.1002/nur.4770170506. [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure-related pain in pediatric oncology patients. International Journal of Clinical and Experimental Hypnosis. 2003;51:4–28. doi: 10.1076/iceh.51.1.4.14064. [DOI] [PubMed] [Google Scholar]
- McCaffery CN. Major stressors and their effects on the wellbeing of children with cancer. Journal of Pediatric Nursing. 2006;21:59–66. doi: 10.1016/j.pedn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Moore IM. Advancing biobehavioral research in childhood cancer. Journal of Pediatric Oncology Nursing. 2004;21:128–131. doi: 10.1177/1043454204264400. [DOI] [PubMed] [Google Scholar]
- Munro CL, Grap MJ, Jablonski R, Boyle A. Oral health measurement in nursing research: State of the science. Biological Research for Nursing. 2006;8:35–42. doi: 10.1177/1099800406289343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater U, Lamarca R, Florin L, Moses A, Langhans W, Koller M, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity—Associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. International Journal of Psychophysiology. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Neu M, Goldstein M, Gao D, Laudenslager M. Salivary cortisol in preterm infants: Validation of a simple method for collecting saliva for cortisol determination. Early Human Development. 2007;83:47–54. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary α-amylase responses to psychosocial stress in human pregnancy. Journal of Clinical Endocrinology and Metabolism. 2006;91:1329–1335. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- O’Donnell MD, Miller NJ. Plasma pancreatic and salivary type amylase and immunoreactive trypsin concentrations: Variations with age and reference ranges for children. Clinica Chimica Acta. 1980;104:265–273. doi: 10.1016/0009-8981(80)90384-8. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Autonomic Neuroscience. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34:469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rourke MT, Hobbie WL, Schwartz L, Kazak AE. Posttrauamatic stress disorder (PTSD) in young adult survivors of childhood cancer. Pediatric Blood and Cancer. 2007;49:177–182. doi: 10.1002/pbc.20942. [DOI] [PubMed] [Google Scholar]
- Schrag NM, McKeown RE, Jackson KL, Cuffe SP, Neuberg RW. Stress-related mental disorders in childhood cancer survivors. Pediatric Blood and Cancer. 2008;50:98–103. doi: 10.1002/pbc.21285. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: Cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Speirs RL, Herring J, Cooper WD, Hardy CC, Hind CR. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Archives of Oral Biology. 1974;19:747–752. doi: 10.1016/0003-9969(74)90161-7. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Lynn MR, Mishel MH. Psychometric evaluation of a new instrument to measure uncertainty in children and adolescents with cancer. Nursing Research. 2010;59:119–126. doi: 10.1097/NNR.0b013e3181d1a8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazdins L, Meyerkort S, Brent V, D’Souza RM, Broom DH, Kyd JM. Impact of saliva collection methods on sIgA and cortisol assays and acceptability to participants. Journal of Immunological Methods. 2005;307(1–2):167–171. doi: 10.1016/j.jim.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Archives of Oral Biology. 2004;49:963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Taketomo CK, Hodding JH, Kraus DM. Pediatric dosage handbook. 17th ed. Hudson, Ohio: Lexi-Comp.; 2010. [Google Scholar]
- Thygeson MV, Hooke MC, Clapsaddle J, Robbins A, Moquist K. Peaceful play yoga: Serenity and balance for children with cancer and their parents. Journal of Pediatric Oncology Nursing. 2010;27:276–284. doi: 10.1177/1043454210363478. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Walco GA, Conte PM, Labay LE, Engel R, Zeltzer LK. Procedural distress in children with cancer: self-report, behavioral observations, and physiological parameters. Clinical Journal of Pain. 2005;21:484–490. doi: 10.1097/01.ajp.0000146166.15529.8b. [DOI] [PubMed] [Google Scholar]
- Wetherell MA, Crown AL, Lightman SL, Miles JNV, Kaye J, Vedhara K. The four-dimensional stress test: Psychological, sympathetic-adrenal-medullary, parasympathetic and hypothalamic-pituitary-adrenal responses following inhalation of 35% CO2. Psychoneuroendocrinology. 2006;31:736–747. doi: 10.1016/j.psyneuen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Woodgate R, Degner LF, Yanofsky R. A different perspective to approaching cancer symptoms in children. Journal of Pain and Symptom Management. 2003;26:800–817. doi: 10.1016/s0885-3924(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Woodgate RL. A different way of being: Adolescents’ experiences with cancer. Cancer Nursing. 2005;28:8–15. doi: 10.1097/00002820-200501000-00002. [DOI] [PubMed] [Google Scholar]
- Woodgate RL. The importance of being there: Perspectives of social support by adolescents with cancer. Journal of Pediatric Oncology Nursing. 2006;23(3):122–134. doi: 10.1177/1043454206287396. [DOI] [PubMed] [Google Scholar]