Abstract
Melatonin in the mammalian eye is synthesized by the photoreceptors and its levels show a clear daily pattern with high levels at night and lower levels during the day. It is synthesized in the ciliary body and secreted into the aqueous humor with a pattern similar to what has been reported for the retina. It acts by interacting with a family of G-protein coupled receptors that are negatively coupled with adenylate cyclase. Melatonin receptor subtypes MT1 and MT2 have been identified in the retina. Both are found in the inner nuclear layer (horizontal and amacrine cells), in the inner plexiform layer, ganglion cells (RGC) and retinal pigmented epithelium. They are also present in the ciliary body. Several studies implicate melatonin in the rhythmic regulation of intraocular pressure. MT1 and MT2 melatonin receptors are expressed in many parts of the eye. Melatonin receptors are expressed in the iris and ciliary body. Recent studies showed that mice lacking MT1 receptors have elevated IOP during the night and show a significantly reduced number of retinal ganglion cells. These new studies suggest that dysfunctional melatonin signaling may be considered a possible risk factor in the pathogenesis of glaucoma and that mice deficient in MT1 receptors may be an animal model of glaucoma.
The neurohormone melatonin, which is synthesized primarily in the pineal gland, mediates rhythmic physiology in many species, including man. Emerging experimental evidence also indicates that melatonin is the key regulator of ocular circadian rhythms.1,2 Glaucoma is associated with dysregulation of circadian IOP rhythms and sleep disorders.3 Plasma and urine levels of melatonin decline with age. Systemic or topical treatment with melatonin or its receptor agonists lowers IOP in mammals, including humans.4-8 These observations suggest that the melatonin system in part regulates rhythmic IOP changes and that melatonin and its receptors may be pharmacological targets for glaucoma management.3 If true, animal models of glaucoma may be created by disrupting the melatonin system.
The physiological effects of melatonin and its putative endogenous role in IOP regulation may not be solely systemic. In the mammalian eye, melatonin is synthesized rhythmically by retinal photoreceptor cells and the ciliary body, with high levels at night and lower levels during the day, and is secreted into the aqueous humor.1,2,9,10 Melatonin exerts its action by interacting with a family of G-protein coupled receptors that are negatively coupled with adenylate cyclase.11 Melatonin receptor subtypes MT1 and MT2 reside in the neural retina, the iris, and the ciliary body.12,13 and these subtypes are directly implicated in IOP regulation.7 In addition, a recent study using mice lacking the melatonin receptor type 1 (MT1-/-) have demonstrated that MT1-/- mice have higher nocturnal IOP than wild type or melatonin receptor type 2 knock-out (MT2-/-) mice at 3 and 12 months of age. Administration of exogenous melatonin in wild-type, but not in MT1-/-, can significantly reduce IOP.14 Furthermore, MT1-/- mice showed a significantly reduced number of retinal ganglion cells at the age of 18 months compared with the number of cells observed in aged-matched congenic wild-type mice.15 The loss of these cells appears to be a direct consequence of MT1 receptor removal, since age matched MT2-/- mice did not show any significant change in the number of retinal ganglion cells.14 Therefore, MT1-/- mice could prove to be a model for some forms of glaucoma. Conditional knockout of the melatonin receptor gene may more closely model the human condition by permitting expression during development and extinguishing it in adulthood.
In conclusion, the data indicate that two key characteristics of high-tension primary open-angle glaucoma (i.e., loss of retinal ganglion cells and elevated IOP) are present in mice deficient in the MT1 receptor. We showed that increase in IOP preceded loss of RGCs and that a 4-6 mmHg nocturnal increase in IOP over a long period of time may induce a significant loss (20-30%) of retinal ganglion cells. These studies suggest that dysfunctional melatonin signaling and an associated increase in nocturnal IOP should be considered possible risk factors in the pathogenesis of glaucoma.
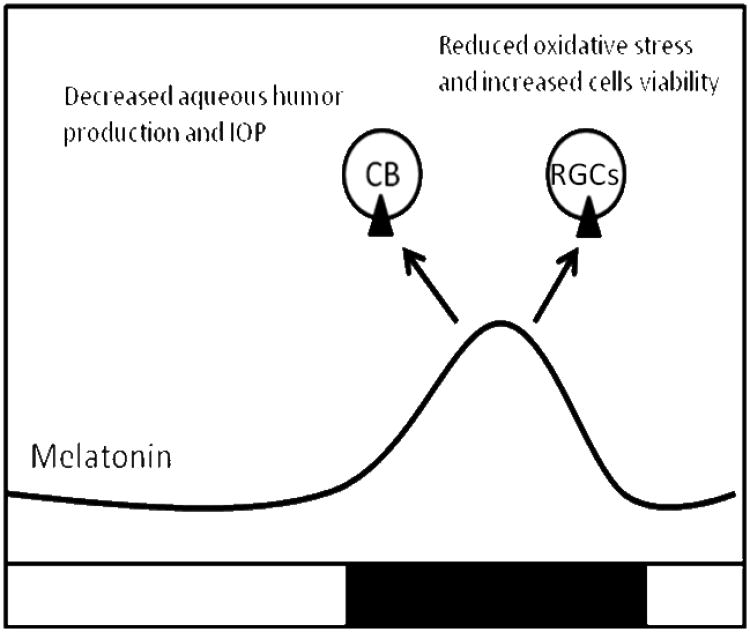
Figure 1. Schematic drawing illustrating hypothetical role of melatonin and MT1receptor in glaucoma pathogenesis.
Increased levels of melatonin during the night (black line) activate MT1 receptors (black triangles) located in the ciliary body (CB) to reduce aqueous humor production and consequently intraocular pressure. Further investigation is needed on neuroprotective role of melatonin via MT1 receptors against glaucomatous damage.
Footnotes
Disclosures: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iuvone PM, Tosini G, Haque R, Klein DC, Chaurasia SS. Circadian Clocks, Clock-Controlled Genes and Melatonin Biosynthesis in the Retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The Circadian Clock System in Mammalian Retina. BioEssays. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agorastos A, Huber CG. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res. 2011;50:1–7. doi: 10.1111/j.1600-079X.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Samples JR, Krause G, Lewy AJ. Effect of melatonin on intraocular pressure. Curr Eye Res. 1988;7:649–653. doi: 10.3109/02713688809033192. [DOI] [PubMed] [Google Scholar]
- 5.Osborne NN, Chidlow G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp Eye Res. 1994;59:3–9. doi: 10.1006/exer.1994.1076. [DOI] [PubMed] [Google Scholar]
- 6.Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur J Pharmacol. 2001;416:251–254. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- 7.Alarma-Estrany P, Crooke A, Mediero A, Peláez T, Pintor J. Sympathetic nervous system modulates the ocular hypotensive action of MT2-melatonin receptors in normotensive rabbits. J Pineal Res. 2008;45:468–475. doi: 10.1111/j.1600-079X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 8.Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth Analg. 2009;108:1146–1151. doi: 10.1213/ane.0b013e3181907ebe. [DOI] [PubMed] [Google Scholar]
- 9.Martin XD, Malina HZ, Brennan MC, Hendrickson PH, Lichter PR. The ciliary body—the third organ found to synthesize indoleamines in humans. Eur J Ophthalmol. 1992;2:67–72. doi: 10.1177/112067219200200203. [DOI] [PubMed] [Google Scholar]
- 10.Criquet C, Claustrat B, Thuret G, Brun J, Cooper HM, Denis P. Melatonin concentrations in aqueous humor of glaucoma patients. Am J Ophthalmol. 2006;142:325–327. doi: 10.1016/j.ajo.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Reppert SM. Melatonin receptors: molecular biology of a new family of G-protein-coupled receptors. J Biol Rhythms. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 12.Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–522. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Alcantara-Contreras S, Baba K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cell death in the mouse. Neuroscience Letters. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]