Abstract
Reliable prognostic biomarkers for chordoma have not yet been established. Recent studies revealed that expression of miRNA-1(miR-1) is frequently downregulated in several cancer types including chordoma. The goal of this follow-up study is to investigate the expression of miR-1 as a prognostic biomarker and further confirm the functional role of miR-1 in chordoma cell growth and proliferation. We determined the relative expression levels of miR-1 and Met in chordoma tissue samples and correlated those to clinical variables. The results showed that miR-1 was downregulated in 93.7% of chordoma tissues and expression was inversely correlated with Met expression. miR-1 expression levels also correlated with clinical prognosis. To characterize and confirm the functional role of miR-1 in the growth and proliferation of chordoma cells, miR-1 precursors were stably transfected into chordoma cell lines UCH-1 and CH-22. Cell Proliferation Assay and MTT were used to evaluate cell growth and proliferation. Restoring expression of miR-1 precursor decreased cell growth and proliferation in UCH-1 and CH-22 cells. These results indicate that suppressed miR-1 expression in chordoma may in part be a driver for tumor growth, and that miR-1 has potential to serve as prognostic biomarker and therapeutic target for chordoma patients.
Keywords: Chordoma, miR-1, Met, Prognostic Biomarker
Chordomas are rare tumor of bone but represent the common primary malignant tumor of the mobile spine and of the sacrum. The clinical management of chordoma is very challenging. Surgical resection is the primary curative maneuver for chordoma but it is often not possible to perform adequately due to the anatomic location of the tumor 1-3. Currently there are no effective drugs useful in treating patients with chordoma, although radiation has been more commonly used as an adjuvant both in situations if complete resection is not possible, as well as when non-contaminated surgical margins are achieved1; 2; 4; 5. Furthermore, no prognostic biomarker exists to predict treatment results of chordoma. Therefore, identification of prognostic and therapeutic biomarkers is critical in advancing treatments for patients with chordoma.
It is commonly accepted that cancer is caused by genetic changes leading to activation of multiple cellular signaling pathways. miRNAs (miRs) are non-coding RNA chains of 21-25 nucleotides that regulate gene expression, influencing many cellular functions such as proliferation, differentiation, apoptosis, oncogenesis, and drug sensitivity in tumor cells6-9. miRs are known to undergo genetic alterations, such as amplification, deletion, and epigenetic silencing, which can ultimately activate oncogenes and inactivate tumor suppressors in cancer cells10. Certain miRs are consistently dysregulated across many cancers. Functional studies have directly documented the potent pro- and antitumorigenic activity of specific miRs in almost all human cancers both in vitro and in vivo 6;10. Recently, we have identified miR-1 as a gene that has significantly lower expression or and may even be absent in chordoma cells 11. Re-introduction of miR-1 mimics by transient transfection suppresses Met expression and inhibits growth of chordoma cells. There are two different precursors of miR-1 in human, miR-1-1 and miR-1-2. miR-1-1 and miR-1-2 are located in two distinct chromosomal regions in human genome, −20q13.33 and 18q11.2, respectively, but both are processed into an identical mature form of miR-1. The decrease or loss of miR-1 expression and the overexpresson of Met have been related to invasive growth or increased tumor stages in several human cancers 12-17. However, whether the expression of miR-1 can be a prognostic biomarker in chordoma has not been investigated. In this follow-up study, we seek to gain a better understanding of the miR-1 expression and clinical outcome in chordoma. Correlating expression of miR-1 and clinical parameters will allow us to assess the possibility that miR-1 status may predict disease outcomes in patients. The functional role of miR-1 was further studied by stable tranfection into chordoma cells.
MATERIALS AND METHODS
Chordoma Samples and Chordoma Cell Lines
Chordoma tissues samples were selected from previously collected materials requested from the Massachusetts General Hospital (MGH) Sarcoma Tissue Bank and from prospectively collected chordoma tissue samples obtained from 35 patients with histologically confirmed sporadic chordomas at the time of surgical resection. This study was conducted with the approval of the MGH Institutional Review Board (IRB protocol #:2007-P-002464/5). Histopathologic confirmation and grading were scored by pathologists at MGH. The characteristics of chordoma patients were recorded. Chordoma cell line UCH-1 was generously supplied to our group by Dr. Silke Bruderlein (University Hospitals of Ulm, Germany)18. Chordoma cell line CH-22 was established in our laboratory as previously reported19.
miRs Extraction
Total RNA was extracted from frozen chordoma tissue samples and chordoma cell lines UCH-1, CH-22 using miRNANeasy Mini Kit (Qiagen GmbH, Hilden, Germany) by following the manufacturer’s instructions. The purity and quantity of the isolated miRs were assessed using 1% formaldehyde-agarose gel electrophoresis and by spectrophotometer measurement. miRs from three human normal skeletal muscle RNAs and three human osteoblast cell lines RNAs were used as normal controls. Normal muscle tissues and normal osteoblast cells have been widely used as controls in several previous studies on gene (miR and mRNA) expression in different sarcomas, including chordoma 11; 20-23. Normal skeletal muscle RNAs were purchased from Ambion (Applied Biosystems, Foster City, CA) and Invitrogen (Carlsbad, CA). Human osteoblast cell line HOB-c was purchased from PromoCell GmbH (Heidelberg, Germany), osteoblast cell lines NHOST was purchased from Lonza Wallkersville Inc.(Walkersville, MD), and osteoblast cell lines hFOB was purchased from the American Type Culture Collection (ATCC, Rockville, MD).
Real-time qRT-PCR Analysis of miR-1 Expression
The expression of miR-1 was quantitatively determined by Real-time PCR with the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). miR-RNU48 (one of the most highly abundant and stably expressed miR across the human tissues) was used as a endogenous control. In brief, cDNA reverse transcription was carried out from total RNA samples using specific miR-1 primers from the TaqMan MicroRNA Reverse Transcription Kit. The resulting cDNA was amplified by PCR using TaqMan miR-1 (Mature miR-1 sequences: 5′-UGGAAUGUAAAGAAGUAUGUAU-3′) and miR-RNU-48 MicroRNA Assay primers with the TaqMan Universal PCR Master Mix and analyzed with a StepOnePlus Real-time PCR System (Applied Biosystems) according to the manufacturer’s instructions. The relative levels of miR-1 expression were calculated from the relevant signals by normalization with the signal for miR-RNU48 expression. PCR reaction mixtures contained TaqMan human miR-1 probe or miR-RNU48 probe and Universal PCR Master Mix in a total volume of 20 ul. Cycling variables were as follows: 95°C for 10 minutes followed by 40 cycles at 95°C (15 seconds) and annealing/extension at 60°C (1 minute). All reactions were carried out in triplicate.
Immunohistochemistry of Met Expression
Chordoma tissue microarray (TMA) slides have been used and validated for immunohistochemical studies in our previous studies of Stat3 and brachyury5; 24 The expression of Met was determined on a chordoma TMA by immunohistochemistry as previously described5. Met staining intensity was graded into four groups: no staining (0), weak staining (1+), moderate staining (2+) and intense staining (3+). The correlation between Met and miR-1 expression levels was analyzed by GraphPad Prism software 5.0 (GraphPad Software, San Diego, CA).
miR-1 Precusor Stable Transfection
The miR-1 precursor vector, miRSelect pEP-miR-1(miR-1 sequence: 5′-UGGAAUGUAAAGAAGUAUGUAU-3′), was purchased from Cell Biolab, Inc. (San Diego, CA). This precursor vector expresses miR-1 precursor in its native context while preserving putative hairpin structures to ensure biologically relevant interactions with endogenous processing machinery and miR targeted genes. The vector also contains a red fluorescence protein (RFP) for evaluating the transfection efficiency. The miR-1 precursor is cloned between BamHI and Nhe I sites. A control miR vector, miRSelect pEP-miR-Null (Also from Cell Biolab, Inc.), was used as a negative control. Both the miR-1 precursor expression vector and the control vector were purchased as bacterial glycerol stocks. Individual colonies were picked from the cultured bacteria on ampicillin LB plates. The plasmid was extracted by EndoFree Plasmid Kit (QIAGEN). Transfections of miR-1 precursor into UCH1 and CH22 chordoma cells were carried out with Lipofectamine LTX and Plus Reagent (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours post-transfection, the stable clones were selected in 2 mg/ml of puromycin (Sigma-Aldrich, St. Louis, MO) containing medium until the stable tranfected cells were established.
Cell-proliferation Assay of miR-1 Stable Tranfected Cells
Cell proliferation was measured by MTT assay as previous described6. The miR-1 precursor and miR-Null control vectors stable transfected cells (4,000 cells per well) were plated in 96-well plates and incubated in RPMI 1640 containing 10% FBS. After 2, 3, 4, 5, 6 and 7 days of culture, 10 ml of MTT (5 mg/ml in PBS, purchased from Sigma-Aldrich) was added to each well and the plates were incubated for 4 hours. The resulting formazan product was dissolved with acid-isopropanol, and the absorbance at a wavelength of 490 nm (A490) was read on a SPECTRAmax Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA). Experiments were carried out in triplicate. Cell growth curves were fitted with use of GraphPad Prism software.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software 5.0. Kaplan-Meier survival curves were generated to examine the relationship between the expression levels of miR-1 and patients’ survival rate. Survival time was calculated from the date of tumor diagnosis to the date of death or last follow-up. The statistical significance between two groups was determined using unpaired Student’s t-test. Data were expressed as mean ± standard error of the mean (SEM). The statistical significance is described in figures and in legends.
RESULTS
Patient’s Clinical Data and Specimens
A retrospective study of 35 chordoma patients from the Massachusetts General Hospital cancer registry and orthopedic oncology databases was identified for miR-1 expression analysis. The data of each patient’ age, gender, histologic subtype, metastasis, recurrence tumor location(s), follow up months, and disease status were collected (Table 1). RNAs were extracted from the frozen tissues of Massachusetts General Hospital sarcoma tissue bank. The expression of miR-1 in these 35 chordoma samples was then determined by the TaqMan miR assay.
Table 1.
Clinical Data on miRs Specimens from Chordoma Tissues
| Sample number | Age/Gender | Histologic Subtype | Metastasis | Recurrence | Location | Tumor size (cm) | Surgical margin | Follow up months | Disease status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56/F | Conventional | None | Yes | Sacrum | 3.0 × 3.0 × 1.0 | Positive | 27 | NS |
| 2 | 28/M | Conventional | None | None | Humerus | 4.5 × 3.0 × 1.5 | Negative | 168 | S |
| 3 | 51/M | Conventional | Yes | None | Sacrum | 1.5 × 1.2 × 1.0 | Positive | 1 | S |
| 4 | 58/M | Conventional | None | Yes | Sacrum | 6 × 6 × 1 | Positive | 1 | S |
| 5 | 55/M | Conventional | None | Yes | L1 | 4.5 × 4.0 × 4.0 | Positive | 66 | S |
| 6 | 60/F | Conventional | None | None | Sacrum | N/A | N/A | 61 | S |
| 7 | 75/F | Conventional | None | Yes | T12-L1 | 4.0 × 3.0 × 0.5 | Pegative | 4 | NS |
| 8 | 89/M | Conventional | None | Yes | Sacrum | 6 × 4 × 5 | Pegative | 9 | NS |
| 9 | 68/M | Conventional | None | Yes | Pelvis | N/A | N/A | 24 | NS |
| 10 | 53/M | Conventional | None | Yes | Sacrum | 9.5 × 8.5 × 4.5 | Negative | 1 | S |
| 11 | 64/M | Conventional | None | None | Sacrum | 15.0 × 14.2 × 12.0 | Negative | 91 | N/A |
| 12 | 60/F | Conventional | None | None | Sacrum | 14.0 × 7.0 × 5.0 | Negative | 72 | N/A |
| 13 | 50/M | Conventional | None | Yes | T12-L1 | 11.0 × 7.0 × 3.0 | Negative | 79 | S |
| 14 | 52/F | Conventional | None | Yes | Sacrum | 1.0 × 0.8 × 0.3 | Positive | 47 | S |
| 15 | 60/M | Conventional | None | Yes | Sacrum | 18.0 × 15.0 × 11.0 | Negative | 4 | NS |
| 16 | 35/M | Conventional | Yes | None | L1-L2 | 7.9 × 2.8 × 4.2 | Negative | 8 | NS |
| 17 | 77/M | Conventional | None | None | Sacrum | 6.0 × 5.0 × 4.5 | Negative | 111 | NS |
| 18 | 74/M | Conventional | None | Yes | Sacrum | 23.0 × 6.0 × 4.0 | Positive | 20 | NS |
| 29 | 55/M | Conventional | None | None | Sacrum | 5.0 × 3.7 × 3.0 | Negative | 192 | S |
| 20 | 58/M | Conventional | Yes | None | Left scapula | 11.0 × 7.0 × 7.0 | Negative | N/A | S |
| 21 | 60/M | Conventional | Yes | None | Chest wall | 10.0 × 7.0 × 5.0 | Negative | 39 | S |
| 22 | 48/M | Conventional | None | None | Sacrum | 4.5 × 4.5 × 1.3 | Positive | 154 | S |
| 23 | 62/M | Conventional | None | None | Sacrum | 9 × 7.5 × 7.0 | Negative | 145 | S |
| 24 | 52/F | Conventional | None | Yes | L3 | 5.0 × 4.0 × 0.8 | Negative | 4 | NS |
| 25 | 63/M | Conventional | None | None | Sacrum | 5.5 × 5 × 4.5 | Negative | 6 | S |
| 26 | 71/F | Conventional | None | None | L4 | N/A | N/A | 145 | S |
| 27 | 50/F | Conventional | None | None | Sacrum | 9.7 × 9.5 × 6.3 | Negative | 88 | NS |
| 28 | 46/M | Conventional | None | None | Sacrum | 12.5 × 10.2 × 9.5 | Negative | 129 | S |
| 39 | 74/M | Conventional | None | Yes | Sacrum | 5.5 × 5.5 × 4.0 | Positive | 69 | NS |
| 30 | 70/M | Conventional | None | Yes | Sacrum | 4.5 × 4.0 × 4.5 | Negative | 145 | NS |
| 31 | 73/M | Conventional | None | Yes | Sacrum | 12.0 × 8.5 × 6.5 | Positive | N/A | N/A |
| 32 | 83/M | Conventional | None | Yes | Sacrum | 10.0 × 8.5 × 7.0 | Positive | 39 | NS |
| 33 | 37/M | Conventional | None | Yes | Sacrum | 4 × 1.8 × 2.4 | Positive | 118 | S |
| 34 | 74/M | Conventional | None | Yes | Sacrum | 6.0 × 3.5 × 2.5 | Positive | 90 | S |
Abbreviations: M=Male, F=Female, S=Survival, NS=Non-survival, N/A=Not available
The Expression of miR-1 is Significant Decreased in Chordoma
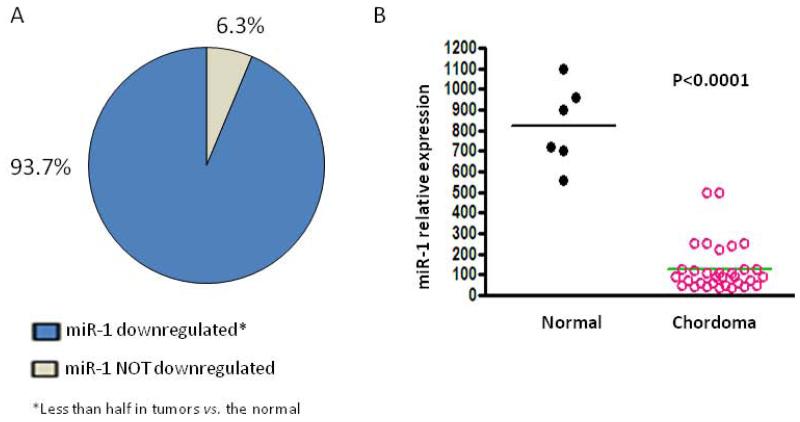
The expression of miR-1 was analyzed by real-time RT-PCR in clinical chordoma tissues and in normal tissues. The results showed miR-1 was down regulated (less than half in tumors vs. the normal) in 93.7% of chordoma tissues (Fig. 1A); the median expression level of miR-1 was significantly decreased in chordoma tissues (level of 129) as compared with normal tissues (level of 823, P < 0.0001; Fig. 1B).
Figure 1.
miR-1 expression is decreased in chordoma. A. Expression of miR-1 was evaluated by real-time RT-PCR. As shown, miR-1 was downregulated (less than half in chordoma tumors vs. the normal) in 93.7% of the chordoma tissues. B. Comparison and distribution of miR-1 expression between normal tissues and chordoma tissues. A two-sided Student’s t-test was used to compare the differences between groups. Results are given as mean and values with P < 0.05 were considered as statistically significant.
miR-1 Decrease Correlates with Met Increase in Chordoma
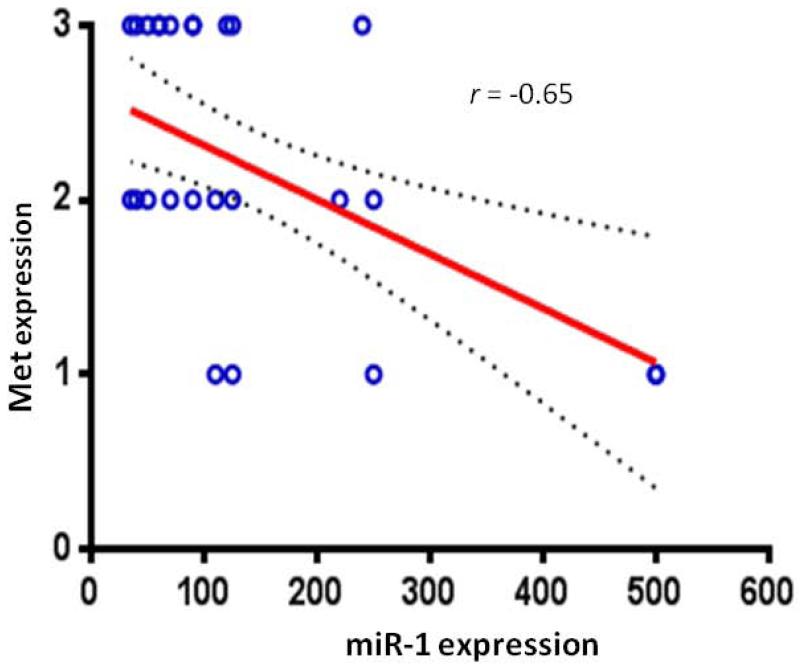
Previous studies have shown that miR-1 is involved in the control of Met expression in several tumor cells including osteosarcoma and chordoma 6; 7; 13-15. Because Met overexpression is not due to gene amplification in the examined chordoma samples, and it is known that miR-1 negatively regulates Met, we evaluated if down regulation of miR-1 in chordoma samples was correlated to Met up-regulation. Indeed, a concomitant decrease of miR-1 and increase of MET was observed in 78% of the samples (Fig. 2). The correlation coefficient of −0.67 with P<0.001 indicates that miR-1 expression was highly and inversely correlated with that of Met expression. These data strongly suggest a role for miR-1 in controlling Met expression during progression of chordoma.
Figure 2.
miR-1 expression in chordoma samples were inversely correlated with Met protein expression. Expression of miR-1 was evaluated by real-time PCR and expression of Met was determined by immunohistochemistry. The correlation coefficient of −0.67 with P<0.001 indicates that miR-1 expression was highly and inversely correlated with that of Met expression.
miR-1 Expression Levels Correlates with Clinical Prognosis in Patients with Chordoma
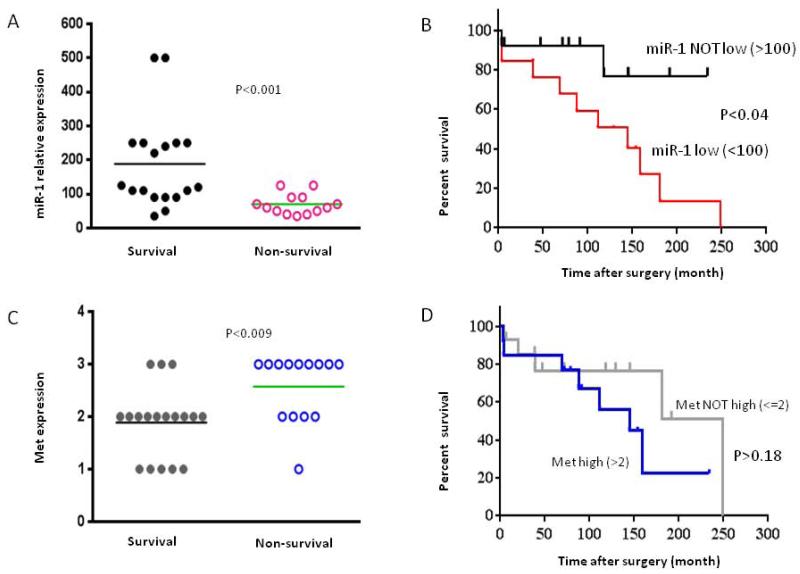
To further validate the clinical relevant of miR-1 expression in patients with chordoma, we determined miR-1 expression levels and analyzed the correlation by a two-sided Student’s t-test to compare the differences between the survival and nonsurvival groups of chordoma patients. A total of 18 (58%) samples from survivors and 13 (42%) samples from non-survivors were collected. Comparison of miR-1 expression between 2 group patients revealed that miR-1 expression for samples from non-survivors were significantly lower than that of survivors. The average miR-1 expression levels for survivors and non-survivors were 188 to 70, respectively (Fig 3.A). By comparing the clinical characteristics of miR-1 low (<100) and miR-1 not low (>100) chordoma, Kaplan-Meier survival analysis showed that the outcome for patients in the miR-1 low group was significantly worse than for those in the miR-1 not low group (Fig. 3B). We further analyzed the correlation between Met expression and the prognosis of chordoma patients. The average Met expression levels for survivors and non-survivors were 1.9+ to 2.6+, respectively (Fig. 3C). The results also revealed although there is a trend of higher Met expression in patient with short-term survival than patient with long-term survival, no statistical significant difference in Met expression was identified between these groups of patients (Fig.3D).
Figure 3.
Correlation of miR-1 and Met with chordoma patient survival. A. Distribution of miR-1 expression in survival and non-survival of chordoma patient samples as determined by real time RT-PCR. B. Analyses of association between expression of miR-1 (miR-1 level low < 100 and miR-1 level not low > 100 and survival in chordoma patients. C. Distribution of Met expression in survival and non-survival of chordoma patient samples as determined by immunohistochemistry. D. Association between expression of Met (Met level <=2+ and Met level >2+) and survival in chordoma patients. Kaplan–Meier survival analysis was used to analyze the correlation between the level of miR-1 or Met expression and survival.
miR-1 Expression Inhibits Chordoma Cell Growth and Proliferation
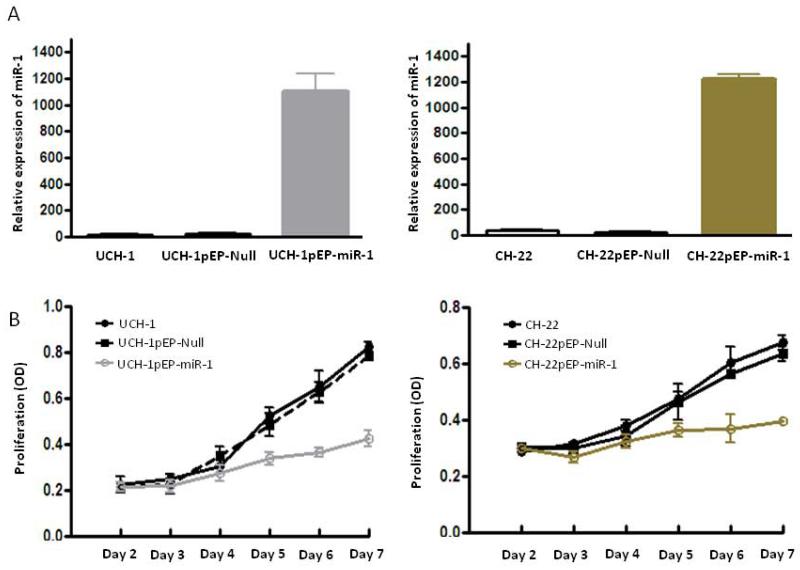
To further study the biologic activity of miR-1 in chordoma, we investigated its effects on chordoma cell growth and proliferation in 2 chordoma cell lines. We stably transfected either miR-1 precursor vector or control vector with into UCH-1 and CH-22 chordoma cell lines. Successful transfections were evaluated with red fluorescence, and then further selected with puromycin to obtain stable clones. We confirmed by real-time RTPCR that miR-1 was highly expressed in puromycin-selected clones as compared with cells transfected with the empty control vector pEP-Null (Fig. 4A). Significant reduction of growth and proliferaion was observed in both in both UCH-1 and CH-22 cell lines after restoration of miR-1 expression (Fig.4B).
Figure 4.
Transfection of miR-1 into chordoma cells decreases cell growth and proliferation. A. Relative expression of miR-1 in transfected chordoma cell lines UCH-1 and CH-22 was assessed by real-time RT-PCR with total RNA isolated from the indicated cell lines. B. The growth and proliferation of chordoma cells were determined by MTT between day 2 and day 7 after transfection of miR-1 precursor into UCH-1 and CH-22 cells.
DISCUSSION
Our previous studies have shown that miR-1 and miR-206 expression are decreased in chordoma cell lines and miR-1 is one of the first to be found down-regulated in chordoma tissues as compared with normal tissues11. More recently studies have found several other deregulated miRs in chordoma, including miR-31, miR-222, miR-140-3p, miR-148a and miR-149-3p 1; 25; 26. Among these miRs with deregulated expression in chordoma, miR-1 is a validated and frequently down-regulated in various types of cancer7; 8; 12-17. In the present study, we characterized 35 chordoma specimens and extracted RNAs to quantify the relative expression level of miR-1. Our analysis results showed that miR-1 was downregulated in 93.7% of chordoma tissues. We further analyzed whether miR-1 expression level could affect chordoma patients’ survival. Patient survival was analyzed by Kaplan-Meier survival analyses with miR-1 expression. Comparison of miR-1 expression showed that miR-1 expression for samples from non-survivors were significantly lower than that of survivors. The outcome for patients in the miR-1 low group was significantly worse than for those in the miR-1 non low group. The data confirmed that miR-1 expression levels also correlated with clinical prognosis, low expression level of miR-1 is a predictor of poor prognosis in patients. These results are consistent with previous reports in that miR-1 level was found to be significantly lower in shorter-survival group as compared with longer-survival group of patients with lung or prostate cancer17; 27.
It has been reported that miR-1 regulates cell growth and proliferation in various cancer types, including lung, gastrointestinal, genitourinary, head/neck cancer and chordoma12; 17; 28-31. In these different cancers, one of the targets of miR-1 is Met, an important oncogene coding for a tyrosine kinase receptor that binds to the HGF and drives the malignant progression of several tumor types, including chordoma, by promoting signaling cascades that mainly result in alterations of cell growth, proliferation and invasion 32. Overexpression of Met has been found in many human cancers, including chordoma. We determined the correlation between expression of miR-1 and Met in chordoma tissues. The results showed miR-1 expression was inversely correlated with Met expression; expression of miR-1 was lower in chordoma tissues and was coupled with high expression of Met. These results are consistent with studies in other types of human cancers such as colorectal tumors which also show that miR-1 and Met are often concomitantly deregulated14; 15.
miR-1 expression is consistently decreased in many cancers as compared with normal tissues. The dysregulation of miR-1 is not limited to a particular tumor type and in some cases, the aberrantly expressed miRs correlate with the clinical status, such as the tumor stage, and patient survival. Additionally, recent studies have observed a functional contribution of miR-1 to cellular transformation, tumorgenesis, apoptosis and drug sensitivity28. miR-1 provides critical functions downstream of classic oncogenic signaling pathways such as those controlled by Met, HDAC4, PIM-1, Wnt, Cyclin D, FOXP1, Slug and TAGLN2. Finally, functional studies have directly documented the potent antitumorigenic activity of miR-1 both in vitro and in vivo26; 28; 33. These exciting findings not only improve our understanding of the molecular mechanisms of miR-1 in human cancer, but also provide a new class of potential molecular targets. In this study, with stable tranfect miR-1 expression vectors in chordoma cells, we have shown restoration of miR-1 significantly inhibits chordoma cell growth and proliferation. These data provide novel evidence that miR-1 has tumor suppressor activity in chordoma. In addition to the above listed putative miR-1 target gene interactions, we investigated the role of miR-1 in controlling Met expression in chordoma in this and previous studies, and we currently are also working on other miR-1 target genes in chordoma.
In conclusion, we discovered expression of miR-1 was correlated with patient survival in chordoma, implicating miR-1 as a new candidate prognostic biomarker in chordoma patients. Furthermore, as Met is highly expressed in majority of chordoma and Met is a confirmed target of miR-1, either miR-1 restoration or Met inhibitors could be tested in clinical trials to treat chordoma.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the Stephan L. Harris Fund, the Gattegno and Wechsler funds. Support has also been provided by the Chordoma Foundation. Dr. Duan is supported, in part, through a grant from Sarcoma Foundation of America (SFA), a grant from National Cancer Institute (NCI)/National Institutes of Health (NIH), UO1, CA151452-01, and a grant from an Academic Enrichment Fund of MGH Orthopedic Surgery.
REFERENCES
- 1.Bydon M, Papadimitriou K, Witham T, et al. Novel therapeutic targets in chordoma. Expert opinion on therapeutic targets. 2012;16:1139–1143. doi: 10.1517/14728222.2012.714772. [DOI] [PubMed] [Google Scholar]
- 2.Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. The lancet oncology. 2012;13:e69–76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 3.DeLaney TF, Duan Z, Hornicek FJ. Proteomic profiling of chordoma. Journal of surgical oncology. 2010;102:719. doi: 10.1002/jso.21766. [DOI] [PubMed] [Google Scholar]
- 4.Diaz RJ, Cusimano MD. The biological basis for modern treatment of chordoma. Journal of neuro-oncology. 2011;104:411–422. doi: 10.1007/s11060-011-0559-8. [DOI] [PubMed] [Google Scholar]
- 5.Yang C, Schwab JH, Schoenfeld AJ, et al. A novel target for treatment of chordoma: signal transducers and activators of transcription 3. Molecular cancer therapeutics. 2009;8:2597–2605. doi: 10.1158/1535-7163.MCT-09-0504. [DOI] [PubMed] [Google Scholar]
- 6.Di Leva G, Garofalo M, Croce CM. MicroRNAs in Cancer. Annual review of pathology. 2013 doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi E, Hornicek FJ, Duan Z. MicroRNA Involvement in Osteosarcoma. Sarcoma. 2012;2012:359739. doi: 10.1155/2012/359739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong YW, Ferland-McCollough D, Jackson TJ, et al. microRNAs in cancer management. The lancet oncology. 2012;13:e249–258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 9.Holleman A, Chung I, Olsen RR, et al. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene. 2011;30:4386–4398. doi: 10.1038/onc.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nature reviews Genetics. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Z, Choy E, Nielsen GP, et al. Differential expression of microRNA (miRNA) in chordoma reveals a role for miRNA-1 in Met expression. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28:746–752. doi: 10.1002/jor.21055. [DOI] [PubMed] [Google Scholar]
- 12.Yan D, Dong Xda E, Chen X, et al. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. The Journal of biological chemistry. 2009;284:29596–29604. doi: 10.1074/jbc.M109.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migliore C, Martin V, Leoni VP, et al. MiR-1 downregulation cooperates with MACC1 in promoting MET overexpression in human colon cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:737–747. doi: 10.1158/1078-0432.CCR-11-1699. [DOI] [PubMed] [Google Scholar]
- 14.Reid JF, Sokolova V, Zoni E, et al. miRNA profiling in colorectal cancer highlights miR-1 involvement in MET-dependent proliferation. Molecular cancer research: MCR. 2012;10:504–515. doi: 10.1158/1541-7786.MCR-11-0342. [DOI] [PubMed] [Google Scholar]
- 15.Fleming JL, Gable DL, Samadzadeh-Tarighat S, et al. Differential expression of miR-1, a putative tumor suppressing microRNA, in cancer resistant and cancer susceptible mice. PeerJ. 2013;1:e68. doi: 10.7717/peerj.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novello C, Pazzaglia L, Cingolani C, et al. miRNA expression profile in human osteosarcoma: role of miR-1 and miR-133b in proliferation and cell cycle control. International journal of oncology. 2013;42:667–675. doi: 10.3892/ijo.2012.1717. [DOI] [PubMed] [Google Scholar]
- 17.Hudson RS, Yi M, Esposito D, et al. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic acids research. 2012;40:3689–3703. doi: 10.1093/nar/gkr1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheil S, Bruderlein S, Liehr T, et al. Genome-wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U-CH1. Genes, chromosomes & cancer. 2001;32:203–211. doi: 10.1002/gcc.1184. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Nielsen GP, Rosenberg AE, et al. Establishment and characterization of a novel chordoma cell line: CH22. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30:1666–1673. doi: 10.1002/jor.22113. [DOI] [PubMed] [Google Scholar]
- 20.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nature reviews Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian S, Lui WO, Lee CH, et al. MicroRNA expression signature of human sarcomas. Oncogene. 2008;27:2015–2026. doi: 10.1038/sj.onc.1210836. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri A, Pezzetti F, Graziano A, et al. Comparison between osteoblasts derived from human dental pulp stem cells and osteosarcoma cell lines. Cell biology international. 2008;32:733–738. doi: 10.1016/j.cellbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Duan Z, Choy E, Harmon D, et al. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Molecular cancer therapeutics. 2011;10:1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Guo S, Schwab JH, et al. Tissue microarray immunohistochemical detection of brachyury is not a prognostic indicator in chordoma. PloS one. 2013;8:e75851. doi: 10.1371/journal.pone.0075851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long C, Jiang L, Wei F, et al. Integrated miRNA-mRNA analysis revealing the potential roles of miRNAs in chordomas. PloS one. 2013;8:e66676. doi: 10.1371/journal.pone.0066676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayrak OF, Gulluoglu S, Aydemir E, et al. MicroRNA expression profiling reveals the potential function of microRNA-31 in chordomas. Journal of neuro-oncology. 2013 doi: 10.1007/s11060-013-1211-6. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:1721–1726. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 28.Nasser MW, Datta J, Nuovo G, et al. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. The Journal of biological chemistry. 2008;283:33394–33405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Tominaga E, Yuasa K, Shimazaki S, et al. MicroRNA-1 targets Slug and endows lung cancer A549 cells with epithelial and anti-tumorigenic properties. Experimental cell research. 2013;319:77–88. doi: 10.1016/j.yexcr.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Datta J, Kutay H, Nasser MW, et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer research. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Wang F, Song G, Liu M, et al. miRNA-1 targets fibronectin1 and suppresses the migration and invasion of the HEp2 laryngeal squamous carcinoma cell line. FEBS letters. 2011;585:3263–3269. doi: 10.1016/j.febslet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nature reviews Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 33.Patel A, Tiwari AK, Chufan EE, et al. PD173074, a selective FGFR inhibitor, reverses ABCB1-mediated drug resistance in cancer cells. Cancer chemotherapy and pharmacology. 2013 doi: 10.1007/s00280-013-2184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]