Abstract
Background and Objective
Hypothesizing that stress dysregulation may worsen cocaine dependence, we investigated the effect of diurnal cortisol secretion profile, suppression of cortisol secretion, and total cortisol secretion on retention, abstinence-based voucher earnings, days of cravings, and mood status of participants at the end of a 2-week medication-free lead-in prior to randomization in a clinical trial of mirtazapine (60 mg vs. placebo) for depressed cocaine-dependent patients.
Methods
We measured saliva cortisol levels at 9am, 2pm, and 5pm on the first two consecutive days of a 2-week medication-free lead-in period. Results from saliva samples were used to estimate the total daily level of cortisol, the diurnal profile of secretion (typical vs. atypical), and response to dexamethasone suppression (0.1 mg). Seventy-seven patients collected saliva samples at baseline, and 65 (85%) were suitable for profile analysis.
Results
Patients with typical profiles (52%) collected significantly more abstinence-based voucher earnings during the lead-in (U = 299.50, p = .025). Diurnal secretion profile did not significantly affect mood status, days of craving, or retention. There were no significant effects of suppression of cortisol secretion or of total cortisol levels on any outcome measures.
Conclusion
In a subgroup of cocaine-dependent patients, deviation of cortisol secretion away from the homeostatic diurnal pattern was associated with reduced success at achieving early abstinence, an important determinant of treatment success.
Keywords: stress, cocaine, addiction, cortisol, clinical trial, drug treatment
Introduction
The relapsing nature of cocaine addiction may relate to the stress response of individuals with cocaine dependence. Cocaine-dependent patients expressing high stress sensitivity are more likely to relapse in human laboratory studies (1, 2, 3, 4). Stress is involved in cocaine addiction because cocaine acts as a stressor (6, 7), and its effect on the stress response may play a role in the transition from intermittent use of cocaine to addiction (7). When a stressor like cocaine becomes chronic, normal regulation of stress is lost. Indications of stress dysregulation include hyper- or hypo-secretion of cortisol, altered diurnal secretion profile, and alteration in the regulation of cortisol secretion. These types of dysregulations are found, together or separately, in a host of medical, psychiatric, and addictive conditions. In depression, increased diurnal cortisol secretion, a flattened diurnal secretion profile, and unresponsiveness of cortisol secretion to the corticosteroid analog dexamethasone is reported (8). In cocaine dependence, similar dysregulations are reported also (9). These signs of stress dysregulation are associated with greater physical and psychiatric morbidity, and diminished treatment effects (8, 10, 11, 12, 13, 14, 15, 16).
Simple measures of stress regulation would be valuable, especially if they could signal risks of early relapse. Since early abstinence from cocaine is associated with treatment success in outpatient settings (17), understanding what may prevent patients from achieving it is important.
In this study, we investigated whether altered stress regulation could relate to worse outcomes at the end of a two-week, medication-free, lead-in phase of a pharmacological trial during which high-value vouchers were dispensed for evidence of abstinence from cocaine. The goal of this preliminary study was to correlate (1) diurnal cortisol secretion profile, (2) total diurnal cortisol secretion, and (3) results from the dexamethasone suppression test (DST); with collection of abstinence-based vouchers, retention, number of days cravings for cocaine, and mood status at the end of the lead-in. We hypothesized that patients with altered stress regulation would collect fewer abstinence-based vouchers, drop out earlier, register more days with cocaine cravings, and remain depressed by the end of the lead-in.
Material and Methods
This study took place during a 10-week, randomized, placebo-controlled, double-blind, clinical trial of mirtazapine in depressed cocaine-dependent individuals. The New York State Psychiatric Institute Institutional Review Board approved it, and all participants gave informed consent.
The screening assessment included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID Axis1/P version 2.0; 18), and a clinical interview on substance abuse. Medical assessment included a history, laboratory tests, electrocardiogram, physical, and psychiatric evaluation. Eligible individuals had to be 18 to 60 years old, meet DSM-IV criteria for cocaine dependence and major depression, used at least 4 days in the previous month, and provide one urine sample positive for cocaine. Baseline cocaine use was estimated by self-reported number of days of cocaine use and dollars spent on cocaine in 30 days prior to study consent. Patients were excluded if they suffered from Schizophrenia or Bipolar Disorder, medical conditions, or suicidality that would jeopardize participation. Patients with a diagnosis of drug dependence other than nicotine or alcohol not requiring detoxification were excluded. To date, 99 individuals consented, 78 have been randomized, and 77 were included in this study. The trial is listed in ClinicalTrials.gov (NCT00249444), is ongoing, and the blind is in effect.
Following study consent, patients began a 2-week medication-free lead-in period consisting of three visits per week. Urine toxicology was collected at every visit. To be randomized, patients had to attend at least 2 of 4 clinic visits, and submit at least 4 of 6 urine samples for toxicology.
Lead-in Contingency Reinforcement Behavioral Strategy
At the first meeting, participants were introduced to the voucher incentive schedule. The goal of this schedule was to encourage abstinence during the 2-week lead-in. Patients earn $20 for their first cocaine-negative urine. Each subsequent negative urine adds a $5 dollar value to the earned voucher, for a potential total of $195. Urine toxicology for cocaine was determined by semi-quantitative analysis of benzoylecognine (BE) concentration with results available in 24 hours. During the first week, vouchers can be earned either if the BE concentration is reduced by at least 50% compared to the previous sample, suggesting no use since the last visit, or if the BE concentration fell below 300 ng/ml (19). For a positive urine sample, the voucher is not earned, but the patient can reinstate the previous voucher level by producing a negative urine at the next visit. Hence, voucher earnings are a sensitive measure of the cumulation of abstinence days. Hamilton Depression Scale (Ham-D 25, 20) and craving scale (Likert scale from 0 to 10, 10 = highest frequency per 24 hours) scores were computed 3x/week during the lead-in. HamD-25 item # 9, 10, 11, early, middle, late insomnia were separately computed to evaluate sleep disturbance. At the end of the lead-in, voucher gains and number of cocaine craving days are totaled for each patient. Full completion of the lead-in determined ‘retained’ status. A 50% drop in Ham-D 25, from a study-entry score that had to exceed 12, determined a mood responder status. Three negative cocaine urines during the second week defined abstinent status.
Salivary Cortisol with Dexamethasone Suppression Test
At the beginning of the lead-in, patients were given two kits of salivettes™ with a diary for the salivary cortisol collection. Collections were done at home on two consecutive days (14). Patients were instructed on how to collect the samples between 8 to 9 am before eating, at 1 to 2 pm, at 5–6 pm, then refrigerate them until returned. At bedtime before the second collection day, patients took 0.1 mg of dexamethasone to test for suppression of cortisol secretion (DST). Suppression was noted when the AM cortisol levels was 50% lower than pre-suppression level. In the diaries, patients recorded what they consumed and at what time, and any stressor. Cortisol concentration was measured by ELISA methodology at the Nathan Kline Analytic Psychopharmacology Laboratory in Orangeburg, NY. Intra-assay and inter-assay coefficients of variation were 2.9% and 5% respectively. 77 participants completed saliva collection at baseline, 65 samples (85%) were analyzed for secretion profile and total secretion; and 64 (83%) for dexamethasone suppression.
Data Analysis
Sixty-five diurnal cortisol secretion profiles were coded categorically (Typical vs. Atypical) according to clinically accepted criteria (21, 22) by two independent raters. In a typical pattern, cortisol levels peak in the early morning hours (usually 6 to 9 am), then decline sharply from the mid-day hours until it increases again in the pre-waking hours (n = 34). Deviations from this profile, in which cortisol levels remain unchanged or increased through the daytime hours, were termed atypical (n = 31). Inter-rater reliability (κ) was 0.94.
Suppression of cortisol secretion (≥ 50% decrease in AM cortisol) by 0.1 mg of dexamethasone was coded categorically (positive/suppressed vs. negative/non-suppressed). Total cortisol level was calculated by summing the three levels on the first day of lead-in (continuous).
Because of the skewed distribution of the data, we used the nonparametric Mann-Whitney U test to assess the unadjusted effect of variables ‘Diurnal cortisol secretion profile’, ‘suppression of cortisol secretion’ on two outcome variables, ‘Abstinence Voucher Earnings’, ‘Cocaine Craving Days’. The dichotomous outcome variables ‘Retention’ and ‘Mood Status’ were analyzed using either a chi-square or Fisher’s Exact test. Unadjusted regression models were used to assess the unadjusted effect of variable ‘total cortisol level’ on the four outcome variables.
Secondly, we modeled the outcome variables ‘Retention’, ‘Abstinence Voucher Earnings’, ‘Cocaine Craving Days’, and ‘Mood Status’ to assess the effect of baseline cocaine use, using non-normal skewed distributions, such as the negative binomial or logistic model, with appropriate link function using GENLIN in SPSS. Possible factors confounding cortisol secretion (food, tobacco, caffeine, sleep disturbance) were compared alone (unadjusted) and in combination (adjusted). Cocaine use during collection was analyzed using a test of proportions. All statistical tests were two-tailed with an α significance level of .05, unless otherwise stated.
Results
Sample Description
There were no significant differences in baseline demographic data, severity of cocaine use, route of cocaine use, depression type or severity (Ham-D), Addiction Severity Index scores (ASI; 23), Symptom Checklist-90 scores (SCL-90; 24), or prevalence of Post Traumatic Stress Disorder (PTSD) between groups (Table 1).
Table 1.
Group demographics, depression type and severity, and drug use frequency by secretion profile type, of patients entered in a clinical trial of mirtazapine for depressed cocaine-dependent patients.
| Typical n=34 | Atypical n=31 | P | |
|---|---|---|---|
| Age, years | 41.1 ± 7.2 | 42.6 ± 8.4 | .434 |
| Education, years | 13.4 ± 2 | 14.3 ± 2.2 | .086 |
| Gender, % male | 88.2 (30) | 80.6 (25) | .397 |
| Race, % | .844 | ||
| Hispanic | 32.4 (11) | 29 (9) | |
| Black | 26.5 (9) | 25.8 (8) | |
| White | 26.5 (9) | 35.5 (11) | |
| Other | 14.7 (5) | 9.7 (3) | |
| Employment, % | .173 | ||
| Full-time | 61.8 (21) | 51.6 (16) | |
| Part-time | 11.8 (4) | 3.2 (1) | |
| Out of work | 23.5 (8) | 45.2 (14) | |
| Student | 2.9 (1) | 0 | |
| Marital, % | .931 | ||
| Single | 35.3 (12) | 35.5 (11) | |
| Married | 20.6 (7) | 25.8 (8) | |
| Separated | 11.8 (4) | 12.9 (4) | |
| Divorced | 32.4 (11) | 25.8 (8) | |
| Cocaine, route, % IN | 55.9 (19) | 54.8 (17) | .933 |
| Depression, % Primary | 61.8 (21) | 58.1 (18) | .761 |
| HAM-D Baseline | 18.4 | 18.6 | .820 |
| HAM-D end of Lead-In | 12.8 | 13.8 | .511 |
| ASI scores, ETOH | 0.23 | 0.27 | .526 |
| ASI scores, Drug | 0.22 | 0.23 | .538 |
| SCL-90, Total | 2.08 | 2.04 | .811 |
| SCL-90, Anxiety | 1.94 | 1.95 | .980 |
| SCL-90, Paranoid ideation | 2.04 | 2.14 | .641 |
| SCL-90, Psychotism | 1.82 | 1.76 | .730 |
| PTSD prevalence % | 10.7 | 14.3 | .686 |
|
| |||
| Typical n=29 | Atypical n=28 | ||
|
| |||
| Cocaine, use | |||
| Dollar value/day | 149.1 ± 230.1 | 92.6 ± 76.2 | .229 |
| Days used/30 | 16.6 ± 10 | 17.3 ± 8.2 | .786 |
Note. Eight (8) participants were excluded from cocaine use analysis because of incomplete or inaccurate baseline assessments.
Effect of Cortisol Secretion Profile
Compared to patient with typical cortisol secretion, patients with atypical profiles earned significantly fewer abstinence-based dollars (U = 299.50, p = .025). At the end of lead-in, no significant difference in retention (Fisher’s p = .413), craving (U = 557, p = .094) or mood status (Χ2 = .176, p = .675) was noted between groups (Table 2). No effects of suppression, or total cortisol secretion were noted.
Table 2.
The effect of Typical vs. Atypical cortisol secretion profile on abstinence-based voucher collection, cocaine craving days, retention, and mood status during a 2-week lead-in of a clinical trial for depressed cocaine-dependent patients.
| Secretion Profile: Atypical vs. Typical N = 65 |
Typical Median (IQR) or n (%) N=34 |
Atypical Median (IQR) or n (%) N=31 |
Mann-Whitney U or χ2 | P-value |
|---|---|---|---|---|
| Abstinence-based voucher collection* | 102.50 (135) | 52.50 (100) | U = 299.50 | 0.025 |
| Cocaine craving days* | 7 (4.25) | 10 (4.25) | U = 557.00 | 0.094 |
| Retention | 32 (94%) | 27 (87%) | Fisher’s Exact | 0.413 |
| Mood status** (Depressed) | 15 (50%) | 15 (56%) | X2 = 0.176 | 0.675 |
With regard to abstinence-based voucher collection and cocaine craving days, there were 2 missing values in the typical group and 3 missing values in the atypical group due to noncompliance.
Regarding mood status, there were 4 missing values in the typical group and 4 missing values in the atypical group due to incomplete data or noncompliance.
IQR = Inter-Quartile Range.
Effect of cocaine use severity
A significant effect of the ‘number of cocaine days in the 30 days prior to study entry’ was noted on ‘days of cocaine craving’ during the lead-in (Χ2= 3.87, p = .049). ‘Cocaine dollar amount in the prior 30 days’ had no effect (Χ2= 0.325, p = .569). Adjusted for secretion profile, no difference in ‘days of cocaine craving’ could be found between typical and atypical groups (Χ2= 0.001, p = .982). Other outcome variables were not affected by cocaine severity.
Effect of confounding factors during saliva sample collection
Food, caffeine, or tobacco, singly or in combination, did not differ between cortisol secretion profile groups (F (7,35) = 1.04, p = .42); nor did early (Χ2 = 3.42, p = .09), middle (Χ2 = 1.51, p = .26), or late insomnia (Χ2 = 0.1, p = .92); or the prevalence of post-traumatic stress disorder diagnosis (Χ2 = .16, p = .686). Alcohol consumption was recorded in the diaries for 3 out of 70 participants with analyzed samples. No events that could affect cortisol secretion were recorded in the diaries.
Effects of cocaine use during lead-in saliva sample collection
Urine toxicology results were tabulated for up to three days before, during, and immediately after saliva collection to investigate the extent of cocaine use during collection. Out of 70 samples, 69% (48/70) could be analyzed; 31% (22/70) could not due to missing data. Twenty-three samples displayed a typical profile, and 78%(18/23) were collected during abstinence; 25 samples displayed an atypical profile, and 48% (12/25) were collected during abstinence (Z = 1.98, p = .050), suggesting a difference in abstinence level between the two groups.
Discussion
Cocaine-dependent patients with depression, displaying an atypical diurnal cortisol secretion profile, earned fewer abstinence vouchers during a two-week medication-free lead-in preceding a pharmacotherapy trial, even though both groups displayed the same baseline level of cocaine use severity. This difference in abstinence did not arise from depression-related morbidity. Baseline days of cocaine use in the 30 days, but not secretion profile, significantly affected ‘days of craving’ during the lead-in. No effect of secretion profile was seen on retention. Neither the DST results nor total cortisol secretion affected any of the lead-in outcomes.
Secretion profile exerted a significant effect on attaining abstinence, but other measures of stress dysregulation did not. Atypical secretion, with its unvarying level across the diurnal time span may have physiological significance. It may affect cellular immunity, and thus survival in breast cancer patients (14, 25), as well as the risk of cardiovascular disease (26). Atypical cortisol secretion is observed in cocaine dependent patients during early abstinence (27). As cortisol sensitizes Corticotropin-Releasing-Factor (CRF) and noradrenergic pathways to and from the extended amygdala, and since atypical secretion aborts the pm cortisol secretion trough, the relatively unvarying cortisol level may accentuate the effect of cortisol on CRF and noradrenergic pathways in a manner not achieved with typical secretion. Since activation of the central amygdala generates aversive states (28, 29), patients with atypical secretion may refrain from abstinence due to aversive states of greater intensity. These internal aversive states would heighten the positive reinforcing role of cocaine as a means to obtain relief (3). Our data supports an association between atypical secretion, lower abstinence and probable aversive states, but not with greater craving for cocaine. Analysis of the complete 8-week trial with a larger sample may clarify this, and other points such as the impact of secretion profile on mood and retention.
Like atypical cortisol secretion, a cocaine-positive baseline is associated with lower abstinence (17). Hence, both cocaine and stress are factors relating to persistent use during treatment, perhaps because the effects of stress and cocaine converge on ascending dopamine pathways (30). Some cocaine-dependent patients may bear a greater burden of morbidity as a result of individual propensity to stress dysregulation (7, 31, 32, 33). Thus patients coming to treatment, indistinguishable in terms of baseline cocaine severity, may have different likelihood of attaining abstinence due to differences in stress sensitivity, as did 48% of patients with atypical cortisol secretion in our sample. ‘Total cortisol’ at baseline, contrary to when measured during a stress challenge (4), may not be a sensitive marker of stress dysregulation given the wide range of ‘normal’ values (34). Non-suppression to the DST was only found in 19% (12/64) of patients, equally distributed between groups (6/12). Its significance in addiction is a matter for further study.
There are several limitations to this study. One is the procedure whereby patients collected saliva samples without direct supervision: 85% of the samples collected were usable. Reports using similar outpatient collection methods exist in the mental health, psychology, and cancer literature (35, 36, 14). Second, active cocaine use during saliva collection could simulate an atypical profile if it raised the afternoon cortisol levels. Since ½ of the atypical, and ¾ of the typical samples were collected during abstinence, the data likely represents an amalgam of chronic and acute effects of cocaine. That said, our report also shows that such collection procedures can be accomplished in outpatient settings, and can collect valuable information on patients coming to treatment. Third, our procedure of evaluating baseline cortisol secretion as an index of stress dysregulation was not compared to stress sensitivity measures in the laboratory. Such a comparison could possibly bring findings from cortisol secretion profile more in line with existing studies of stress sensitivity in cocaine dependent patients. Fourth, the data analysis used established categorical measures in keeping with the existing literature rather than continuous analyses, which may have revealed more details on the relationship between cocaine dependence and stress dysregulation.
Despite these limitations, we have presented evidence that depressed cocaine-dependent patients with atypical diurnal cortisol secretion face greater difficulty in reaching early abstinence, a key predictor of subsequent treatment success. We also propose that atypical cortisol secretion may indicate stress dysregulation in cocaine dependence. If stress dysregulation could be rectified when present, this may improve cocaine treatment outcome. Some evidence exists for this. Attenuation of the sympathetic nervous system arm of the stress response with alpha-2 adrenergic receptor agonists can reduce relapse risk following treatment for cocaine dependence (37). Results from ongoing investigations may provide supporting evidence for this approach to treat cocaine dependence.
Fig 1.
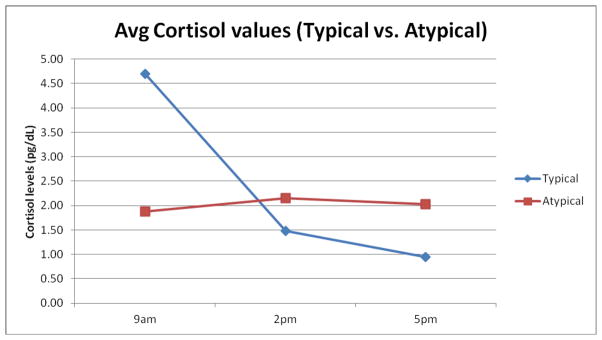
Average diurnal cortisol secretion profiles collected during daytime awake hours from depressed cocaine dependent patients at baseline, illustrating 1): ◆ patients with typical secretion profiles; 2): ■ patients with atypical secretion profiles.
Acknowledgments
Funding for this study was provided by NIDA P50 DA 09236 to Dr. Herbert D. Kleber, NIDA K24 DA022412 to Dr. Edward V. Nunes, and NIDA K23 DA277044 to Dr. Wilfrid N. Raby.
Footnotes
All authors do not have conflicts of interest to declare.
References
- 1.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adrenal-medullary responses during stress-induced and drug cue-induced craving states. Psychopharmacology. 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Brady KT, McRae AL, Moran-Santa Maria M, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to CRH infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66(4):422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106(1):21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ. Effects of cocaine on cortisol secretion in humans. Am J Med Sci. 1995;310:61–64. doi: 10.1097/00000441-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Mello NK, Mendelsohn JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity, and vulnerability to drug abuse and addiction. Nature Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 8.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 9.Gawin FH, Kleber HD. Neuroendocrine findings in chronic cocaine abusers: a preliminary report. Br J Psychiatry. 1985;147:569–573. doi: 10.1192/bjp.147.5.569. [DOI] [PubMed] [Google Scholar]
- 10.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prevention. Am J Psychiatry. 2001;158(5):748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- 11.Jokinen J, Carlborg A, Martensson B, Forslund K, Nordstrom AL, Nordstrom P. DST non-suppression predicts suicide after attempted suicide. Psychiatry Res. 2007;150(3):297–303. doi: 10.1016/j.psychres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Yehuda R, Golier J. Is there a rationale for cortisol-based treatments for PTSD? Expert Rev Neurother. 2009;9(8):1113–1115. doi: 10.1586/ern.09.79. [DOI] [PubMed] [Google Scholar]
- 13.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 14.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- 16.Yehuda R. Hypothalamic-pituitary-adrenal alterations in PTSD: are they relevant to understanding cortisol alterations in cancer? Brain Behav Immun. 2003;17 (Suppl 1):S73–S83. doi: 10.1016/s0889-1591(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 17.Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, Maany I. A cocaine-positive baseline predicts outpatient attrition and failure to attain initial abstinence. Drug Alcohol Depend. 1997;46:79–85. doi: 10.1016/s0376-8716(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis 1 Disorders – patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- 19.Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. J Consult Clin Psychol. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- 20.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatr. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 21.Despopoulos A, Silbernagl S. Color Atlas of Physiology. New York, Stuttgart: Georg Thieme Verlag; 1986. p. 261. [Google Scholar]
- 22.Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22(2):89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 23.McLelland AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Derogatis LR, Lipman Rs, Covi L. SCL-90: an outpatient psychiatric rating scale – preliminary report. Psychopharmacol Bull. 1973;9(1):13–28. [PubMed] [Google Scholar]
- 25.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system and cancer. The Lancet – Oncology. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 26.Holt-lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom Med. 2007;69:339–343. doi: 10.1097/PSY.0b013e318050d6cc. [DOI] [PubMed] [Google Scholar]
- 27.Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Fortner-Burton C, Hess J. Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Reproductive Neuroendocrinol. 2003;78:154–162. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- 28.Di Chiara G. A motivational learning hypothesis of the role of dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 29.Van Bockstaele EJ, Foote SL, Page ME. Amygadaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10(10):743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 30.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 31.Wust S, Van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- 32.Kreek MJ. Role of a functional human gene polymorphism in stress responsitivity and addictions. Clin Pharm Ther. 2007;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dynov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 34.Beer MH, Porter RS, Jones TV, Kaplan JL, Berkwits M. The Merck Manual of Diagnosis and Therapy. Merck Research Laboratories; Whitehouse Station, NJ: 2006. pp. 1207–1214. [Google Scholar]
- 35.Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D’Albenzio A, Di Nicola M, Fisher H, Handley R, Marques TR, Morgan C, Navari S, Taylor H, Papadopoulos A, Aitchison KJ, Murray R, Pariante CM. Abnormal cortisol levels during the day and cortisol awakening response in first episode psychosis: the role of stress and of antipsychotic treatment. Schizophrenia Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. PNAS. 2008;105(16):6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving, and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012 Jan 9; doi: 10.1177/0269881111430746. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


