Abstract
Excessive delay discounting (DD) has been related to various maladaptive behaviors, and may stem from a myopic focus on immediate gratification. Neuroimaging studies have shown that episodic future thinking (EFT) – vivid mental simulation of future experiences – may reduce DD by promoting consideration of delayed outcomes. However, the EFT manipulations in these experiments may have induced positive affect, which could independently enhance executive functions that facilitate self-regulation. To clarify the mechanism of this effect, 87 participants were randomized to visualize neutral- or positive-valenced events expected to occur in the present or in the future while completing a standardized DD questionnaire. Working memory capacity, inhibitory control, the genotypes of 3 functional dopaminergic polymorphisms (DRD1 rs686, DRD2 rs1800497 and COMT rs4680), as well as an additive dopamine genetic risk score were assessed as potential moderators. The results indicate that EFT reduces DD primarily by shifting the time perspective of intertemporal decision-making, and that this effect is moderated by working memory capacity. In addition, positive episodic thinking may independently attenuate the protective effects of high working memory capacity, high inhibitory control, and lower dopamine genetic risk scores on DD. The current findings dovetail with previous research to suggest that the time perspective and emotional valence of episodic thinking may dynamically shape intertemporal choice, perhaps in part by transiently modulating executive function and dopaminergic neurotransmission.
Keywords: episodic future thinking, delay discounting, executive function, time perspective, emotional valence
Introduction
Deciding among behaviors that have divergent future ramifications is a part of everyday life. When making these intertemporal choices, some people routinely forego small immediate pleasures for larger delayed benefits, while others desire immediate gratification even at great expense to future outcomes. The ability to delay gratification can be quantified as delay discounting (DD) – the rate at which individuals subjectively devalue future rewards as a function of temporal distance – and high DD has been associated with various maladaptive behaviors that provide immediate reinforcement but have negative consequences, including substance abuse and pathological gambling (Reynolds, 2006). Understanding factors that influence DD could contribute toward elucidating the mechanisms of intertemporal decision-making as well as the development of interventions for related adverse conditions.
Reframing of choice scenarios to shift attention toward delayed rewards has been shown to decrease DD in laboratory tasks. For instance, participants exhibited lower DD when choices between a smaller immediate reward and a larger delayed reward were explicitly presented as being mutually exclusive (Magen, Dweck, & Gross, 2008), when they were asked to first list possible reasons for waiting (Weber et al., 2007), or when delayed rewards were contextualized with dates of anticipated receipt (Read, Frederick, Orsel, & Rahman, 2005). Taken together, these findings suggest that DD could arise from a myopic focus on immediate rewards and may be reduced through the use of certain cognitive strategies.
A promising approach may be to broaden the temporal horizon of intertemporal decision-making by concurrently engaging in episodic future thinking (EFT) – the vivid episodic simulation of future events, including autobiographical, emotional and circumstantial details (Atance & O'Neill, 2001). EFT has been shown to reduce DD in several experiments (Benoit, Gilbert, & Burgess, 2011; Gellert, Ziegelmann, Lippke, & Schwarzer, 2011). Importantly, in two neuroimaging studies, changes in DD were correlated with enhanced coupling of brain regions previously implicated in subjective valuation of delayed rewards and construction of mental imagery, suggesting that EFT decreases DD by shifting the time perspective of decision-making and promoting consideration of the future utility of the available options (Benoit et al., 2011; Gellert et al., 2011). However, the effects of EFT were compared to those of no manipulation (Gellert et al., 2011) or a non-episodic thinking control task (Benoit et al., 2011), and participants were asked to visualize positive future events during EFT (Gellert et al., 2011) or rated EFT as being emotionally salient (Benoit et al., 2011). Though the literature is mixed (Gray, 2001; Rowe, Hirsh, & Anderson, 2007), positive affect may improve inhibitory control (Kuhl & Kazen, 1999) and working memory (Yang, Yang, & Isen, 2013) – executive functions that enable self-regulation by allowing for withholding of prepotent responses to and allocation of attention away from immediately-available tempting stimuli (Sarsour et al., 2011). Therefore, whether EFT modulates DD by altering the time perspective or emotional valence of intertemporal decision-making remains unclear.
In addition, the effect of EFT on DD seems to be contingent upon the vividness of episodic imagery (Gellert et al., 2011) and sensitivity toward the imagined future rewards (Benoit et al., 2011). Working memory and inhibitory control processes may also facilitate episodic memory retrieval (Baddeley, 2000; Levy & Anderson, 2002) and the subsequent generation of mental imagery (Guthrie, Butler, & Ward, 2009). Moreover, mesocorticolimbic dopamine neurotransmission underlies executive function (Cools & D'Esposito, 2011), episodic memory (Shohamy & Adcock, 2010), as well as future hedonic expectations (Sharot, Shiner, Brown, Fan, & Dolan, 2009), and functional dopaminergic polymorphisms have been shown to modulate DD (Eisenberg et al., 2007). Collectively, these data indicate that individual differences in working memory, inhibitory control and dopamine genetics may moderate the effect of EFT on DD.
To clarify how EFT shapes intertemporal choice, this study examined the effects of time perspective and emotional valence of episodic thinking on DD. Participants were randomized to engage in episodic simulation of neutral- or positive-valenced events expected to occur in the present (episodic present thinking, EPT) or the future (EFT) while completing a standardized DD questionnaire. Individual differences in working memory, inhibitory control and the genotypes of three functional dopaminergic polymorphisms were assessed as potential moderators.
Methods
Participants
The study utilized a 2×2 design, with time perspective (EPT vs. EFT) and emotional valence (neutral vs. positive) of episodic thinking as the between factors, and with DD as the dependent measure. Participants were recruited via an existing family database, flyers posted around the University at Buffalo campuses and in community settings, as well as electronic advertisements on Craig's List. Exclusion criteria included medications or conditions associated with changes in appetite, working memory or inhibitory control (e.g. methylphenidate and attention-deficit hyperactivity disorder), current tobacco use, diabetes, current or past history of a psychiatric disorder (e.g. anxiety or depression), current dieting and pregnancy. The analytic sample consisted of 87 of the 100 participants studied; individuals who did not follow experimenter instructions (n = 2) or had missing data (due to a computer error, n = 1; declined to provide saliva sample or genetic assay failed, n = 10) were excluded from analysis. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics.a
| Neutral EPT N = 20 |
Positive EPT N = 22 |
Neutral EFT N =23 |
Positive EFT N = 22 |
p-value | |
|---|---|---|---|---|---|
| Age (Years) | 37.8 ± 12.1 | 41.2 ± 13.3 | 43.4 ± 10.7 | 40.7 ± 11.9 | 0.51 |
| Sex (M/F) | 12/8 | 11/11 | 12/11 | 10/12 | 0.82 |
| Minority Status (Caucasian/Non-Caucasian) | 12/8 | 14/8 | 19/4 | 13/9 | 0.30 |
| Household Education Level (Years) | 16.4 ± 2.1 | 15.6 ± 2.5 | 16.8 ± 1.9 | 16.0 ± 2.6 | 0.38 |
| Working Memory Capacityb | 5.0 ± 0.7 | 5.4 ± 1.0 | 5.4 ± 0.8 | 5.3 ± 0.7 | 0.23 |
| Inhibitory Controlc | 5.6 ± 3.7 | 8.2 ± 5.5 | 5.2 ± 3.2 | 7.1 ± 4.6 | 0.09 |
| DRD1 rs686 Genotype (AA/GA/GG)d | 5/10/5 | 3/9/10 | 5/11/7 | 2/11/9 | 0.70 |
| DRD2 rs1800497 Genotype (CC/CT/TT) | 13/6/1 | 16/5/1 | 11/10/2 | 11/8/3 | 0.59 |
| COMT rs4680 Genotype (AA/GA/GG) | 3/10/7 | 5/10/7 | 3/9/11 | 2/10/10 | 0.84 |
| Dopamine Genetic Risk Score | 0.95 ± 0.18 | 1.05 ± 0.17 | 1.30 ± 0.16 | 1.36 ± 0.17 | 0.26 |
Plus-minus values are means ± SD
Length of the longest sequence accurately recalled on the Corsi Block Tapping Task, adjusted for the number of incorrect trials.
Number of errors of commission made on the Go/No-Go task.
None of the genotype distributions differed significantly from Hardy-Weinberg Equilibrium (p > 0.05).
Number of risk genotypes: DRD1 rs686 AA + DRD2 rs1800497 CT/TT + COMT rs4680 GG
Procedures
The study consisted of one session lasting about 2 hours, scheduled between 7AM-2PM. Prior to the session, participants completed sociodemographic questionnaires on a survey website. To standardize the potential effects of hunger on executive function, participants were asked to refrain from consuming anything except water for ≥8 hours before their appointment. In most cases, this requirement was satisfied by a simple overnight fast. Upon arrival at the laboratory, participants completed consent forms and were interviewed about their last eating experience to verify adherence to study protocol. Participants then completed tasks assessing working memory capacity and inhibitory control. Two-minute breaks were given after each task to reduce cognitive fatigue and carry-over effects.
During the episodic thinking manipulation, participants verbally described and mentally simulated neutral events (e.g. activities they neither want to avoid nor look forward to) or positive events (e.g. activities they would enjoy or look forward to) that could realistically happen in the present (during the next 24 hours; at non-overlapping intervals of 1 hour, 2 hours, 4 hours and 24 hours after the session) or in the future (during the next 6 months; at non-overlapping intervals of 1 week, 2 weeks, 1 month and 6 months after the session). Participants were asked to picture 3 events from each time interval actually occurring, and to include as many autobiographical, contextual, emotional and procedural details as possible. Participants rated their event simulations on positive valence, detail of context and vividness of imagery. They then generated word cues for the events they could visualize the most clearly from each time interval.
Afterwards, participants completed a computerized version of the Kirby Monetary Choice Questionnaire (Kirby & Marakovic, 1996) while being cued to engage in episodic simulation of neutral-present, positive-present, neutral-future or positive-future events. The timeframes of the future events approximated the time delays specified on the Kirby Monetary Choice Questionnaire (7 – 186 days), whereas the timeframes of the present events were chosen to match those of the future events at a 1 hour: 1 week ratio. For each question, participants were therefore cued to envision the most distal event expected to happen during the time they would have had to wait for the delayed reward. For instance, participants were asked to visualize a salient event they expect to happen 4 hours (neutral- and positive-present groups) or 1 month (neutral- and positive-future groups) after the session before deciding whether they would prefer receiving $49 now or $60 in 89 days. To verify that they were attending to the task, participants were asked to read the word cues aloud before choosing their preferred rewards. At the end of the session, participants indicated how much they were thinking about the events during the DD questionnaire and provided a saliva sample for genotyping of dopaminergic polymorphisms. The study protocol was approved by the University at Buffalo Social and Behavioral Sciences Institutional Review Board.
Measurements
Sociodemographics
Information about minority status (Caucasian/non-Caucasian) and household education level (highest educational attainment between the participant and his/her spouse, in years of formal education) was collected using a standardized questionnaire (Stewart, 2004).
Working memory capacity and inhibitory control
The Corsi Block Tapping Test was used to assess working memory capacity (Seligman & Schillinger, 2010). Nine blocks were presented on a computer screen. The blocks lit up briefly (for 500 milliseconds), one at a time (1000 milliseconds apart), in a random order and in sequences of increasing length. Participants were asked to reproduce the patterns shown, starting with a sequence of two blocks. Two non-identical trials were given at each sequence length, and if the pattern were correctly reproduced during at least one of the trials, the task continued with the sequence length increased by 1. Consecutive incorrect responses at any sequence length terminated the task. The length of the longest sequence accurately recalled, adjusted for the number of incorrect trials (-0.5 per error), provided an index of working memory capacity. Participants were given 3 practice trials using a sequence of 3 blocks before the test trials.
The Go/No-Go paradigm was used to assess inhibitory control (Brown & Landgraf, 2010). An array of 2×2 squares was presented on a computer screen. A letter (“P” or “R”) appeared briefly (for 500 milliseconds) in one of the 4 squares. Participants were instructed to press the shift key whenever they saw a “P” (Go trials, 80% of the trials) and to withhold responding whenever they saw an “R” (No-Go trials, 20% of the trials). Participants were instructed to pay attention and to respond as quickly but as accurately as possible. Participants were given 10 practice trials before completing 160 experimental trials spaced 1500 milliseconds apart. The number of incorrect responses during No-Go trials (errors of commission) provides a measure of inhibitory control. The Corsi Block Tapping and Go/No-Go tasks were implemented using Psychology Experiment Building Language, version 0.13 (http://pebl.sourceforge.net).
Episodic simulation ratings
During the episodic thinking manipulation, participants rated their simulations for positive valence, detail of context and vividness of imagery using Likert-type scales, with 1 indicating not at all and 5 indicating very much. Participants also rated how much they visualized the events while completing the DD questionnaire using Likert-type scales, with 1 indicating not at all and 9 indicating very much.
Delay discounting
A computerized version of the Kirby Monetary Choice Questionnaire was used to assess DD (Kirby & Marakovic, 1996). Participants were asked to make a series of hypothetical choices between a smaller reward available immediately (e.g. “$10 now”) and a larger reward available after a specified time delay (e.g. “$12 in 1 day”). The ratios of the immediate and delayed rewards and the length of the time delays were titrated across 27 conditions. The pattern of responses was used to estimate the DD parameter k, or the rate at which the subjective value (V) of a delayed reward (A) decays as a hyperbolic function of temporal distance (D) according to the formula: V = A/(1 + kD).
Saliva Collection, Genotyping and Dopamine Genetic Risk Scores
Participants were asked to provide a 2-mL saliva sample by spitting directly into a plastic collection vial (DNA Genotek, Kanata, Ontario, Canada). DNA extracted from these samples was then amplified by PCR, and 3 functional dopaminergic single nucleotide polymorphisms (SNPs) that have been previously associated with greater DD and/or related conditions such as substance dependence (DRD1 rs686, DRD2 rs1800497 and COMT rs4680) were genotyped using a mass spectroscopy array (Sequenom, San Diego, CA, USA). Previous research suggests that the rs686 AA (vs. rs686 AG/GG), the rs4680 GG (vs. rs4680 AG/AA) and the rs1800497 CT/TT (vs. rs1800497 CC) genotypes may be related to greater DD (Boettiger et al., 2007; Eisenberg et al., 2007; Zhu et al., 2013). Furthermore, functional polymorphisms influencing the same neurotransmitter pathway may have additive effects on cognitive function and behavior (Bertolino et al., 2006; Comings et al., 1996). Thus, the genotype of each polymorphism – as well as a cumulative genetic risk score indicating the number of high-DD genotypes (rs686 AA, rs4680GG, rs1800497 CT/TT) carried by a participant – were tested as potential moderators. The assay was designed and implemented by the Genomics Shared Resource (Roswell Park Cancer Institute, Buffalo, NY, USA).
Analytic Plan
The goals of the analyses were to assess the independent and interactive effects of time perspective (EPT vs. EFT) and emotional valence (neutral vs. positive) on DD, as well as to determine whether these relationships were moderated by individual differences in working memory capacity, inhibitory control, and dopamine genetics. Specifically, two-way ANCOVA, with time perspective and emotional valence as between-subject factors, were used to compare DD rates among the neutral EPT, positive EPT, neutral EFT and positive EFT groups. Potential moderating effects were assessed by inspecting the respective interaction terms. To explore significant interactions, the least-square mean DD rates of each group were estimated at tertile values of the moderator. DD rates were calculated and natural log-transformed to remove positive skew as previously described by Kirby (Kirby & Marakovic, 1996). Age, sex, minority status and household education level were included as covariates in all models. All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA, 2012).
Results
No group differences in participant characteristics were observed (Table 1). A manipulation check showed that, as intended, the positive EPT and positive EFT groups rated event simulations as being more positive than did the neutral EPT and neutral EFT groups (F(3, 83) = 50.07, p < 0.001). There were no group differences in ratings of event context (F(3, 83) = 2.62, p = 0.056), vividness (F(3, 83) = 1.95, p = 0.13) and visualization during the DD questionnaire (F(3, 83) = 2.40, p = 0.074).
Independent and interactive effects of time perspective and emotional valence
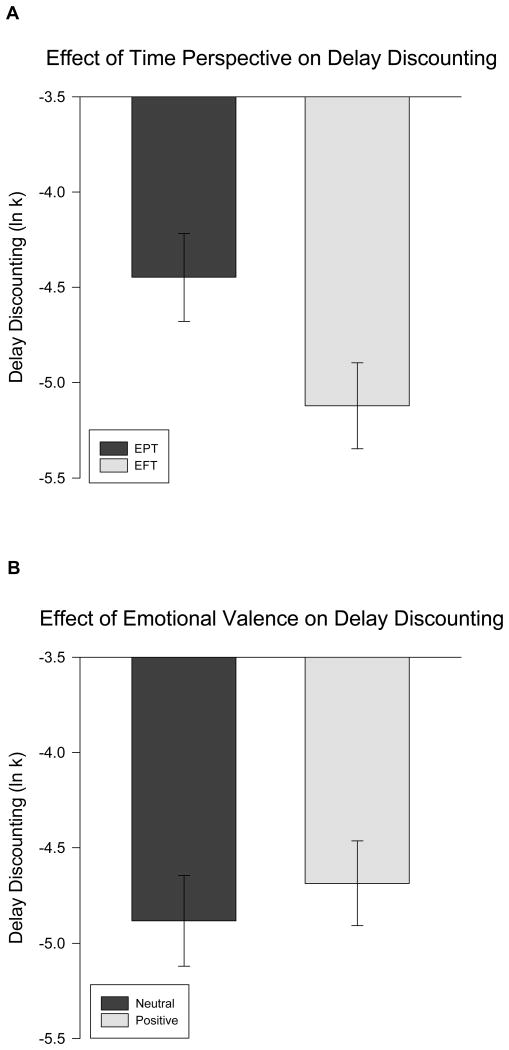
EFT decreased DD compared to EPT (F(7, 79) = 4.72, p = 0.033, Figure 1A), whereas there were no differences between the effects of positive episodic thinking and neutral episodic thinking on DD (F(7, 79) = 0.39, p = 0.54, Figure 1B). Time perspective and emotional valence did not interact to influence DD (F(7, 79) = 2.68, p = 0.11).
Figure 1.
Time perspective of episodic thinking independently influences delay discounting.
Two-way ANCOVA controlling for the effects of age, sex, minority status and household education level showed that EFT decreased DD compared to (F(7, 79) = 4.72, p = 0.033, Figure 1A), while positive episodic thinking and neutral episodic thinking had similar effects on (F(7, 79) = 0.39, p = 0.54, Figure 1B). Error bars indicate ±1 standard error of the mean.
Moderating effects of working memory capacity and inhibitory control
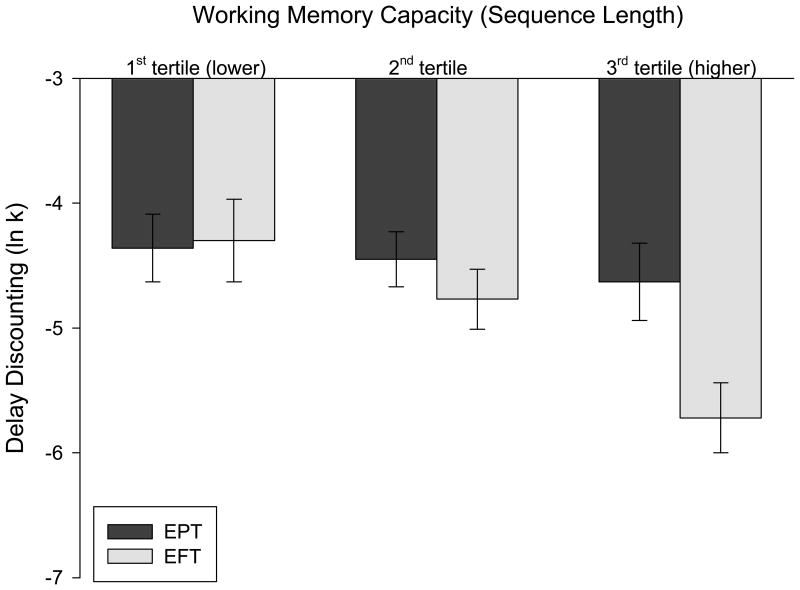
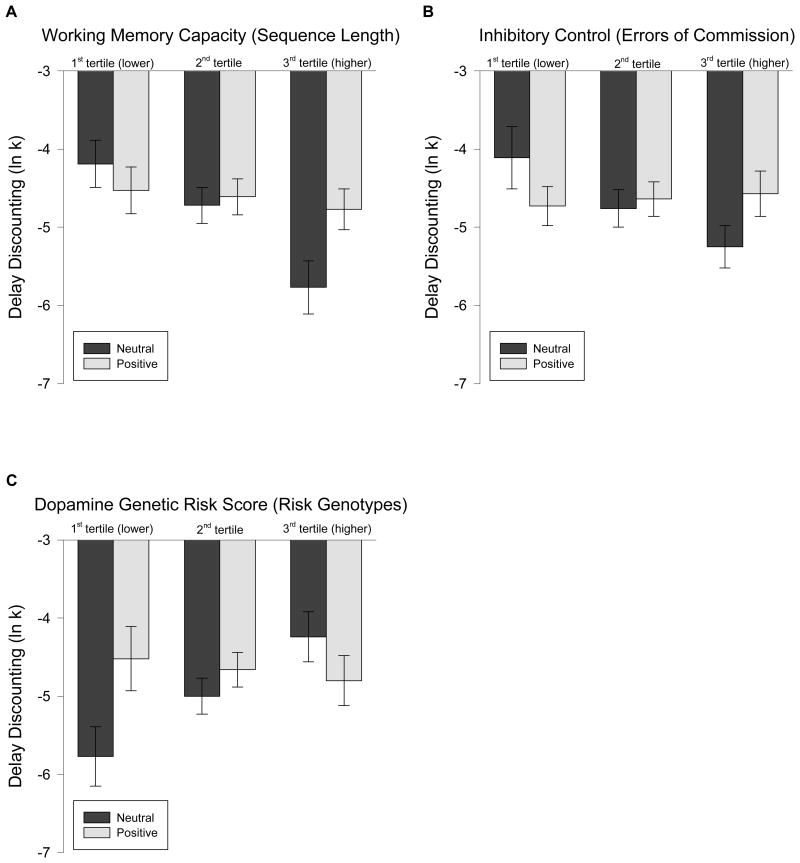
Working memory capacity moderated the effect of time perspective on DD (F(9, 77) = 4.57, p = 0.036), such that EFT reduced DD compared to EPT among participants with higher working memory capacity, but not among participants with lower working memory capacity (Figure 2). In addition, working memory capacity moderated the effect of emotional valence on DD (F(9, 77) = 5.97, p = 0.017). Specifically, neutral episodic thinking decreased DD compared to positive episodic thinking among participants with higher working memory capacity, but not among participants with lower working memory capacity (Figure 3A). There were no working memory × time perspective × emotional valence effects on DD (F(11, 75) = 0.08, p = 0.77).
Figure 2.
Working memory capacity moderates the effect of time perspective of episodic thinking on delay discounting.
Two-way ANCOVA controlling for the effects of age, sex, minority status and household education level showed that working memory capacity moderated the effect of time perspective on DD (F(9, 77) = 4.57, p = 0.036), such that EFT reduced DD compared to EPT among participants with higher working memory capacity, but not among participants with lower working memory capacity. To graph this relationship, the least-square mean DD rates of each group were estimated at tertile values of working memory capacity. Error bars indicate ±1 standard error of the mean.
Figure 3.
Working memory capacity and inhibitory control moderate the effect of emotional valence on delay discounting.
Two-way ANCOVA controlling for the effects of age, sex, minority status and household education level showed that working memory capacity (F(9, 77) = 5.97, p = 0.017, Figure 3A), inhibitory control (F(9, 77) = 5.82, p = 0.018, Figure 3B) and dopamine genetic risk scores (F(9, 77) = 5.41, p = 0.023, Figure 3C) moderated the effect of emotional valence on DD. Neutral episodic thinking reduced DD compared to positive episodic thinking among participants with higher working memory capacity, higher inhibitory control or lower dopamine genetic risk scores, but not among participants with lower working memory capacity, lower inhibitory control or higher dopamine genetic risk scores. To graph this relationship, the least-square mean DD rates of each group were estimated at tertile values of the moderators. Error bars indicate ±1 standard error of the mean.
Inhibitory control did not interact with time perspective to influence DD (F(9, 77) = 0.27, p = 0.60). However, inhibitory control also moderated the effect of emotional valence on DD (F(9, 77) = 5.82, p = 0.018). Contrasts suggest that neutral episodic thinking decreased DD compared to positive episodic thinking when participants had high inhibitory control, but not when they had low inhibitory control (Figure 3B). There were no inhibitory control × time perspective × emotional valence effects on DD (F(11, 75) = 1.15, p = 0.29).
Moderating effects of rs686, rs1800497 and rs4680 genotype
DRD1 rs686 genotype did not interact with time perspective (F(9, 77) = 1.06, p = 0.31), emotional valence (F(9, 77) = 1.60, p = 0.21), or time perspective × emotional valence (F(11, 75) = 0.63, p = 0.43) to influence DD. Similarly, DRD2 rs1800497 genotype did not moderate the effects of time perspective (F(9, 77) = 0.96, p = 0.33), emotional valence (F(9, 77) = 3.22, p = 0.077) or time perspective × emotional valence (F(11, 75) = 1.46, p = 0.23) on DD. COMT rs4680 genotype also did not influence the effects of time perspective (F(9, 77) = 0.46, p = 0.50), emotional valence (F(9, 77) = 1.26, p = 0.26) or time perspective × emotional valence (F(11, 75) = 1.19, p = 0.28) on DD.
Moderating effects of the additive dopaminergic risk score
Dopamine genetic risk scores interacted with emotional valence to influence DD (F(9, 77) = 5.41, p = 0.023). Compared to positive episodic thinking, neutral episodic thinking decreased DD among participants with lower dopamine genetic risk scores, but not among participants with higher dopamine genetic risk scores (Figure 3C). Dopamine genetic risk score did not interact with time perspective (F(9, 77) = 0.65, p = 0.42) or with time perspective × emotional valence (F(11, 75) = 0.03, p = 0.87) to influence DD.
Discussion
In this study, EFT decreased DD compared to EPT, while positive episodic thinking and neutral episodic thinking had similar effects on DD. Since there were no significant differences between the EPT groups and the EFT groups in terms of positive valence and attentiveness during the task, a lengthened temporal horizon was likely the primary mechanism underlying the effect of EFT on DD. Importantly, EFT influenced DD despite not being explicitly associated with the choice scenario; participants were instructed that they did not have to relate their decision to the event, but to just picture the event actually happening before going on to make their selection. The non-specificity of this effect, taken together with studies showing that incidental cues (Ungemach, Stewart, & Reimers, 2011) as well as mind-wandering (Smallwood, Ruby, & Singer, 2013) could trigger episodic memory retrieval and influence DD, invites the speculation that some people are more capable of delaying gratification because they are in an environment that encourages frequent EFT. Conversely, people experiencing strenuous life circumstances may habitually focus their attention on immediate needs rather than future outcomes (Shah, Mullainathan, & Shafir, 2012), resulting in greater DD. Whether training or prompting individuals to routinely engage in EFT reduces DD warrants investigation.
In addition, the effect of EFT on DD may be most robust among participants with high working memory capacity. Working memory has been implicated in the synthesis and interpretation of information from the sensory and long-term memory systems (Baddeley, 2000; Unsworth & Engle, 2007) – processes that may be pivotal to scene construction (Addis, Wong, & Schacter, 2008) and prediction of future emotions (Hoerger, Quirk, Lucas, & Carr, 2010) during EFT. Taken together with research showing that limitations in or loads on working memory increases DD (Hinson, Jameson, & Whitney, 2003; Shamosh et al., 2008), some people may be unable to delay gratification in part because they lack the cognitive resources to effectively engage in mental simulation of future outcomes via EFT (Schacter et al., 2012). Notably, individuals with high working memory capacity tend to engage in more EFT and planning when mind-wandering (Baird, Smallwood, & Schooler, 2011), and working memory training programs have been shown to decrease DD (Bickel, Yi, Landes, Hill, & Baxter, 2011). Thus, whether working memory training regimens also improve EFT should be assessed.
Moreover, participants with higher working memory capacity, higher inhibitory control, or lower dopamine genetic risk scores exhibited lower DD when they engaged in neutral episodic thinking, but not when they engaged in positive episodic thinking. This pattern of results suggests that positive episodic thinking may attenuate the protective effects of these factors on DD. In the context of the mixed literature on the effect of positive affect on working memory and inhibitory control (Gray, 2001; Kuhl & Kazen, 1999; Rowe et al., 2007; Yang et al., 2013), these findings could reflect that baseline differences moderate the relationships among emotional valence and these executive functions. Interestingly, positive affect or imagery may enhance mesocorticolimbic dopamine neurotransmission (Ashby, Isen, & Turken, 1999; Costa, Lang, Sabatinelli, Versace, & Bradley, 2010), which modulates executive function in an inverted U-shaped manner (Cools & D'Esposito, 2011). Individuals with high working memory capacity or high inhibitory control may already be experiencing peak dopaminergic activity. Positive episodic thinking could therefore push them over the threshold and impair their intertemporal decision-making. In accordance with this view, administration of a dopaminergic agonist during EFT has been shown to increase unrealistic optimism (Sharot, Guitart-Masip, Korn, Chowdhury, & Dolan, 2012), which may be related to greater DD (Berndsen & van der Pligt, 2001). The effect of a dopamine reuptake inhibitor on executive function has further been shown to be moderated by rs4680, such that the drug improved executive function among carriers of the GG genotype (previous associated with greater DD), but worsened executive function among carriers of the AA genotype (previously associated with lower DD) (Giakoumaki, Roussos, & Bitsios, 2008). The potential conflicting effects of time perspective and emotional valence of episodic thinking on intertemporal decision-making among such individuals – perhaps involving transient changes in dopaminergic signaling, working memory and inhibitory control – should therefore be further examined.
The study had several limitations. First, the exclusionary criteria included behaviors and conditions related to DD, such as current smoking, use of controlled substances and ADHD. Future research should test the effect of EFT on DD in the context of these factors. Second, DD was evaluated using the Kirby Monetary Choice Questionnaire. Though this instrument is convenient to administer and has been used to detect differences in DD rates among diverse participant populations, this questionnaire involves only hypothetical monetary choices. However, several studies have reported no systematic within-subject differences in DD rates assessed using hypothetical and actual monetary rewards (Lagorio & Madden, 2005). Third, with regards to genetic factors, only the role of dopaminergic polymorphisms was considered. Serotonergic polymorphisms have also been shown to influence executive function, affect regulation and DD (Homberg, 2012). Future experiments should test whether genetic variations in serotonergic signaling influences EFT and for epistasis between the two systems.
In summary, the current results demonstrate that EFT reduces DD by shifting the time perspective of intertemporal decision-making to promote consideration of delayed outcomes. This relationship was most apparent in individuals with high working memory capacity, suggesting that persons with limited cognitive resources may be less capable of delaying gratification because they have difficulties vividly visualizing future outcomes, as well as that working memory training programs may enhance the effect of EFT on DD. In addition, positive affect may temporarily nullify the protective effects of high working memory capacity, high inhibitory control or low dopamine genetic risk scores on DD, perhaps by transiently impairing dopaminergic signaling. Thus, the time perspective and emotional valence of episodic thinking could dynamically modulate intertemporal choice. In this sense, EFT is not only a process that allows people to predict the utility of delayed outcomes, but also the lens through which they could see the path that bridges the present and their desired future.
Acknowledgments
Appreciation is expressed to Travis Stewart, Shirin Aghazadeh and Morgan Pratte for assisting with implementation of the protocol and data management. This research was funded in part by a grant from the University at Buffalo Graduate Student Association Mark Diamond Research Fund (FA-12-05) awarded to Henry Lin, as well as a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK088380) awarded to Leonard Epstein.
References
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19(1):33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken U. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106(3):529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Atance CM, O'Neill DK. Episodic future thinking. Trends in Cognitive Sciences. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. S1364-6613(00)01804-0 [pii] [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4(11):417–423. doi: 10.1016/S1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Schooler JW. Back to the future: autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition. 2011;20(4):1604–1611. doi: 10.1016/j.concog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience. 2011;31(18):6771–6779. doi: 10.1523/Jneurosci.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndsen M, van der Pligt J. Time is on my side: Optimism in intertemporal choice. Acta Psychologica. 2001;108(2):173–186. doi: 10.1016/S0001-6918(01)00029-4. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. The Journal of Neuroscience. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the Future: Working Memory Training Decreases Delay Discounting Among Stimulant Addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. Journal of Neuroscience. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. 27/52/14383 [pii] 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad Med. 2010;122(5):42–51. doi: 10.3810/pgm.2010.09.2200. [DOI] [PubMed] [Google Scholar]
- Comings DE, Wu S, Chiu C, Ring RH, Gade R, Ahn C, Muhleman D. Polygenic inheritance of Tourette syndrome, stuttering, attention deficit hyperactivity, conduct, and oppositional defiant disorder: the additive and subtractive effect of the three dopaminergic genes--DRD2, D beta H, and DAT1. American Journal of Medical Genetics. 1996;67(3):264–288. doi: 10.1002/(SICI)1096-8628(19960531)67:3<264::AID-AJMG4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 2011;69(12):e113–125. doi: 10.1016/j.biopsych.2011.03.028. 10.1016/j.biopsych.2011.03.028S0006-3223(11)00278-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain's reward circuitry. Human brain mapping. 2010;31(9):1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behavioral and Brain Functions. 2007;3 doi: 10.1186/1744-9081-3-2. Artn 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert P, Ziegelmann JP, Lippke S, Schwarzer R. Future Time Perspective and Health Behaviors: Temporal Framing of Self-Regulatory Processes in Physical Exercise and Dietary Behaviors. Ann Behav Med. 2011 doi: 10.1007/s12160-011-9312-y. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Bitsios P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology. 2008;33(13):3058–3068. doi: 10.1038/npp.2008.82. 10.1038/npp.2008.82npp200882 [pii] [DOI] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130(3):436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Guthrie LC, Butler SC, Ward MM. Time perspective and socioeconomic status: A link to socioeconomic disparities in health? Social Science & Medicine. 2009;68(12):2145–2151. doi: 10.1016/j.socscimed.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hoerger M, Quirk SW, Lucas RE, Carr TH. Cognitive determinants of affective forecasting errors. Judgment and Decision Making. 2010;5(5):365–373. [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. Serotonin and decision making processes. Neuroscience and Biobehavioral Reviews. 2012;36(1):218–236. doi: 10.1016/j.neubiorev.2011.06.001. 10.1016/j.neubiorev.2011.06.001S0149-7634(11)00111-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN. Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychonomic Bulletin & Review. 1996;3(1):100–104. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kuhl J, Kazen M. Volitional facilitation of difficult intentions: Joint activation of intention memory and positive affect removes Stroop interference. Journal of Experimental Psychology: General. 1999;128(3):382–399. doi: 10.1037/0096-3445.128.3.382. [DOI] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes. 2005;69(2):173–187. doi: 10.1016/j.beproc.2005.02.003. S0376-6357(05)00031-8 [pii] 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences. 2002;6(7):299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Magen E, Dweck CS, Gross JJ. The hidden-zero effect: representing a single choice as an extended sequence reduces impulsive choice. Psychological Science. 2008;19(7):648–649. doi: 10.1111/j.1467-9280.2008.02137.x. PSCI2137 [pii] 10.1111/j.1467-9280.2008.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D, Frederick S, Orsel B, Rahman J. Four score and seven years from now: The date/delay effect in temporal discounting. Management Science. 2005;51(9):1326–1335. doi: 10.1287/mnsc.1050.0412. [DOI] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):383–388. doi: 10.1073/pnas.0605198104. 0605198104 [pii] 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family Socioeconomic Status and Child Executive Functions: The Roles of Language, Home Environment, and Single Parenthood. Journal of the International Neuropsychological Society. 2011;17(1):120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman HK, Schillinger D. Hunger and Socioeconomic Disparities in Chronic Disease. New England Journal of Medicine. 2010;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- Shah Anuj K, Mullainathan Sendhil, Shafir Eldar. Some consequences of having too little. Science. 2012;338(6107):682–685. doi: 10.1126/science.1222426. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. PSCI2175 [pii] 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sharot T, Guitart-Masip M, Korn CW, Chowdhury R, Dolan RJ. How dopamine enhances an optimism bias in humans. Current Biology. 2012;22(16):1477–1481. doi: 10.1016/j.cub.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Shiner T, Brown AC, Fan J, Dolan RJ. Dopamine enhances expectation of pleasure in humans. Current Biology. 2009;19(24):2077–2080. doi: 10.1016/j.cub.2009.10.025. 10.1016/j.cub.2009.10.025S0960-9822(09)01844-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. 10.1016/j.tics.2010.08.002S1364-6613(10)00186-5 [pii] [DOI] [PubMed] [Google Scholar]
- Smallwood J, Ruby FJM, Singer T. Letting go of the present: Mind-wandering is associated with reduced delay discounting. Consciousness and Cognition. 2013;22(1):1–7. doi: 10.1016/j.concog.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Stewart J. Notebook on the social and physical environment: John D and Catherine T. MacArthur Research Network on Socioeconomic Status and Health 2004 [Google Scholar]
- Ungemach C, Stewart N, Reimers S. How incidental values from the environment affect decisions about money, risk, and delay. Psychological Science. 2011;22(2):253–260. doi: 10.1177/0956797610396225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. 2006-23341-004 [pii] 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Weber EU, Johnson EJ, Milch KF, Chang H, Brodscholl JC, Goldstein DG. Asymmetric discounting in intertemporal choice - a query-theory account. Psychological Science. 2007;18(6):516–523. doi: 10.1111/j.1467-9280.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang S, Isen AM. Positive affect improves working memory: Implications for controlled cognitive processing. Cognition and Emotion. 2013;27(3):474–482. doi: 10.1080/02699931.2012.713325. [DOI] [PubMed] [Google Scholar]
- Zhu F, Yan CX, Wen YC, Wang J, Bi J, Zhao YL, Li SB. Dopamine d1 receptor gene variation modulates opioid dependence risk by affecting transition to addiction. PLoS One. 2013;8(8):e70805. doi: 10.1371/journal.pone.0070805. [DOI] [PMC free article] [PubMed] [Google Scholar]