Abstract
Food reinforcement, or the motivation to eat, has been associated with increased energy intake, greater body weight and prospective weight gain. Much of the previous research on the reinforcing value of food has focused on the role of dopamine, but it may be worthwhile to examine genetic polymorphisms in the serotonin and opioid systems as these neurotransmitters have been shown to be related to reinforcement processes and to influence energy intake. We examined the relationship among 44 candidate genetic polymorphisms in the dopamine, serotonin and opioid systems, and food reinforcement and body mass index (BMI) in a sample of 245 individuals. Polymorphisms in the Monoamine oxidase A (MAOA-LPR) and serotonin receptor 2A genes (rs6314) moderated the effect of food reinforcement on BMI, accounting for an additional 5-10% variance and revealed a potential role of the single nucleotide polymorphism, rs6314 in the serotonin 2A receptor as a differential susceptibility factor for obesity. Differential susceptibility describes a factor that can confer either risk or protection depending on a second variable, such that rs6314 is predictive of both high and low BMI based on the level of food reinforcement, while the diathesis stress or dual-gain model influences only one end of the outcome measure. The interaction with MAOA-LPR better fit the dual-risk or diathesis stress model, with the 3.5R/4R allele conferring protection for individuals low in food reinforcement. These results provide new insight into genes theoretically involved in obesity and support the hypothesis that genetics moderate the association between food reinforcement on BMI.
Keywords: Obesity, dopamine, serotonin, food reinforcement, differential susceptibility
Differential susceptibility of a functional polymorphism in the serotonin 2A receptor moderates the relationship between food reinforcement and Body mass index
Food reinforcement is a measure of the motivation to eat, or the effort one is willing to expend to obtain a desired food on a progressive ratio schedule (Epstein, Leddy, Temple, & Faith, 2007). Both obese children (Temple, Legierski, Giacomelli, Salvy, & Epstein, 2008) and adults (Giesen, Havermans, Douven, Tekelenburg, & Jansen, 2010; Saelens & Epstein, 1996) demonstrate increased food reinforcement compared to their leaner peers. High food reinforcement predicts increased weight gain in children (Hill, Saxton, Webber, Blundell, & Wardle, 2009) and sensitization of food reinforcement, or the increase in motivation to eat after prolonged exposure to specific high energy-dense foods, predicts weight gain in adults (Temple & Epstein, 2011). Research has shown that several behavioral phenotypes, including dietary restraint and disinhibition (Epstein, Lin, Carr, & Fletcher, 2012) and impulsivity (Appelhans et al., 2011; Rollins, Dearing, & Epstein, 2010) moderate the influence of food reinforcement on BMI or energy intake.
Genetic factors may also interact with food reinforcement to predict BMI. Dopamine is a key neurotransmitter associated with motivated behaviors involving both food and drug reinforcement and is found in brain regions associated with reward such as the ventral tegmental area and amygdala (Volkow, Fowler, Wang, Swanson, & Telang, 2007; Volkow, Wang, & Baler, 2011). Appetitive stimuli, including primary reinforcers such as food, increase dopamine release in brain reward centers (Cheng, De Bruin, & Feenstra, 2003) and it is thought that increases in dopamine release are directly related to motivation and reinforcement or the currency that determines a reinforcers' motivational value (Salamone & Correa, 2002). Polymorphisms in the dopamine D2 receptor (DRD2), including the ANKK1 or Taq1A allele, that lead to a reduction in D2 receptor protein levels, are associated with obesity (Spitz et al., 2000; Thomas, Critchley, Tomlinson, Cockram, & Chan, 2001), increased energy intake (Epstein, Temple, et al., 2007; Epstein et al., 2004) and high food reinforcement (Epstein, Temple, et al., 2007), in addition to associations with alcoholism, nicotine and opioid dependence (Noble, 2000). Research has implicated dopaminergic genes and receptor regulation in the risk of developing obesity as an adolescent, such that polymorphisms in the dopamine D2 or D4 receptors predicted weight gain in youth with weak striatal activation to food cues (Stice, Yokum, Bohon, Marti, & Smolen, 2010). However, unlike drugs of abuse, obesity and energy intake involve several additional neural systems involving hunger and satiety.
Other neurotransmitter systems that may interact with food reinforcement include the opioid and serotonin systems. Opioids have been shown to increase operant responding and breakpoint for food in animals (Peciña & Smith, 2010; Solinas & Goldberg, 2005). Opioid agonists increase operant responding, which is blocked by antagonists including naloxone (Solinas & Goldberg, 2005). Deficiencies in opioid receptors or neuropeptides decrease operant responding in animals (Peciña & Smith, 2010), suggesting a contribution of opioid neuropeptides to motivational processes. Research examining the influence of serotonin on motivation, however, has had mixed results. Animal models have shown that serotonin receptor agonists decrease operant responding for food (De Vry, Schreiber, Daschke, & Jentzsch, 2003), while chronic administration of selective serotonin reuptake inhibitors (SSRIs) increased responding for cocaine in rats (Sasaki-Adams & Kelley, 2001). One study showed reduced operant responding for cocaine in serotonin transporter knock-out mice (Sanders, Hussain, Hen, & Zhuang, 2007), but two other studies showed no changes in cocaine reinforcement (Thomsen, Hall, Uhl, & Caine, 2009) or effort to obtain food (Denk et al., 2005).
It is possible that these neurotransmitter systems interact with behavioral phenotypes to predict complex outcomes, including obesity. One influential theory of gene-environment interactions is the diathesis stress or dual-risk model, in which individuals with specific risk factors are disproportionately influenced by negative environmental factors, such as stress (Belsky & Pluess, 2009). Many studies examine only the negative aspects of the environment and new research has suggested that more informative relationships may be found when examining a wide range of positive and negative environmental factors (Belsky & Pluess, 2009). Another way to conceptualize gene-environment interactions include the dual-gain model, which suggests gene-environment interactions can be protective, rather than increase risk. Factors that confer plasticity, or an increase in sensitivity to the environment, show increased positive outcomes in supportive environments and more negative outcomes in stressful environments (Belsky, Bakermans-Kranenburg, & Van Ijzendoorn, 2007). This effect is termed differential susceptibility and re-examination of previous studies of diathesis-stress have identified that both genetic and behavioral factors fit the differential susceptibility model (Belsky & Pluess, 2009). For example, research has found the short allele of the 5-HTTLPR polymorphism in the serotonin transporter gene is associated with increased depression, anxiety and attention deficit hyperactivity disorder in a variety of stressful environments, including stressful life events, low socioeconomic status and childhood emotional abuse, but a lower propensity to exhibit these issues in supportive environments (Belsky & Pluess, 2009). Similarly, investigators have shown that the DRD4 7R allele confers risk for impulsivity, as measured by delay discounting, in children of low socio-economic status, but is a protective factor for less impulsive choice in children living in a non-disadvantaged environment (Sweitzer et al., 2012), providing evidence of differential susceptibility. This study aimed to examine the interactions between food reinforcement and genetic polymorphisms in the dopamine, opioid and serotonin systems on BMI, controlling for population stratification and reinforcing value of an alternative reinforcer.
Methods
Participants
A sample of 245 (119 males, 126 females) participants with a broad range of BMI (18.5-58.6) (134 non-obese, 111 obese) and twenty seven percent minority, were examined for single marker genetic associations. BMI can be categorized as obese, or BMI> 30 and non-obese BMI< 30 and our sample included 134 non-obese and 111 obese individuals. Participants were recruited from an existing family database, newspaper ads, flyers posted around the University at Buffalo campuses and in community settings, web based recruitment (e.g. ads on Craig's list and on the department's website) and direct mailings targeted to community residents between the ages of 18-50. Participants were excluded from the study if they were taking medications associated with loss of appetite, were smokers, had diabetes, had previously been diagnosed with an eating disorder or psychiatric disorder (e.g. anxiety, depression, attention deficit hyperactivity disorder), were allergic to the ingredients in the study foods, were currently dieting, and did not rate at least a moderate liking (≥4 on a 9 point Likert type scale) for five out of the six study foods. Participants received a $50 gift certificate to local stores for completing the study. The study was approved by the University at Buffalo Health Sciences Institutional Review Board. Participant characteristics by sex and obesity status are shown in Table 1.
Table 1.
Participant Characteristics (mean ± standard deviation).
| Variable | Sex | Food Reinforcement | Total | ||
|---|---|---|---|---|---|
|
| |||||
| Male | Female | Low | High | ||
| N | 119 | 126 | 177 | 68 | 245 |
| Age (years) | 33.6 ± 10.3 | 35.3 ± 11.0 | 34.4 ± 10.5 | 34.5 ± 10.9 | 34.5 ± 10.6 |
| BMI | 31.0 ± 7.6 | 29.1 ± 7.6 | 29.3 ± 7.1* | 31.9 ± 8.7* | 30.1 ± 7.6 |
| Restraint | 6.5 ± 4.3* | 8.7 ± 4.8* | 8.3 ± 4.7* | 6.0 ± 4.2* | 7.7 ± 4.7 |
| Disinhibition | 5.9 ± 3.2 | 6.6 ± 3.6 | 6.2 ± 3.5 | 6.4 ± 3.2 | 6.3 ± 3.4 |
| Hunger (TFEQ) | 5.6 ± 3.3 | 5.1 ± 3.1 | 5.0 ± 3.2* | 6.0 ± 3.3* | 5.3 ± 3.2 |
| Pmax food | 85.6 ± 154.1* | 38.0 ± 98.4* | 14.9 ± 10.0* | 181.6 ± 203.2* | 61.2 ± 130.4 |
| Pmax reading | 80.4 ± 107.4 | 81.2 ± 119.0 | 63.5 ± 90.3* | 126.0 ± 149.7* | 80.8 ± 113.2 |
| Education (n) | |||||
| Some college or less | 51 | 58 | 69 | 40 | 109 |
| Completed college | 68 | 68 | 108 | 28 | 136 |
| Income** (%) | |||||
| <$50,000 | 66.4 | 55.6 | 54.8* | 76.5* | 60.8 |
| Minority (%) | 27.7 | 26.1 | 21.4* | 41.1* | 27.0 |
p<0.05
One unreported value
Procedures
Participants visited the laboratory for two sessions, an ad libitum snack-eating session, and the food reinforcement session. Both experimental sessions were scheduled between the hours of 2PM and 5PM, during a normal period that individuals would consume additional calories outside of meal time. Participants were asked to refrain from eating or drinking, with the exception of water, for at least 3 hours prior to the test session and to refrain from consuming the experimental foods in the 24 hours prior to the test session. Upon initial arrival to the laboratory, participants read and signed consent forms, completed a same day and 24 hour food recall, hunger questionnaires and were asked to provide a saliva DNA sample. Participants were asked to rinse their mouth with water and then spit into a plastic vial. Participants who had difficulty providing 2mL of saliva were asked to place a small dab of sugar on the center of their tongue to increase saliva production. Prior to the start of each session participants were provided with a preload of a Luna Sunrise Blueberry Bliss, Strawberry Crumble or Vanilla Almond Breakfast bar (Clif Bar & Company; Berkeley, CA, 42g, 150kcal, 4g fat, 23g carbohydrates, 7g protein) to minimize the effects of hunger on energy intake and food reinforcement. The inclusion of a standard preload increases the ability to show individual differences in food reinforcement (Reiss & Havercamp, 1996). Demographic information, height and weight measurements and three dietary habits questionnaires were administered during the ad libitum eating session. Results relevant to the ad libitum eating task have been presented (Epstein, Carr, Lin, & Fletcher, 2011), and are not reported here.
Measurement
Demographics
Participant education level and income were assessed using a standardized questionnaire.
Height and weight
The participant's weight and height were measured using a digital scale (TANITA Corporation of America Inc, Arlington Heights, IL) and a digital stadiometer (Measurement Concepts & Quick Medical, North Bend, WA). On the basis of height and weight data, body mass index (BMI) was calculated according to the following formula: BMI=kg/m2. Individuals were considered obese if their BMI was at least 30kg/m2 and non-obese if their BMI was less than 30kg/m2 (NHLBI Obesity Education Initiative Expert Panel, 1998).
Eating Questionnaires
Participants completed the Three Factor Eating Questionnaire (TFEQ) (Stunkard & Messick, 1985), the Questionnaire of Eating and Weight Patterns (QEWP) (Spitzer et al., 1992), and the Binge Eating Scale (BES) (Gormally, Black, Daston, & Rardin, 1982). The TFEQ is a validated instrument to detect dietary restraint (Allison, Kalinsky, & Gorman, 1992; Laessle, Tuschl, Kotthaus, & Pirke, 1989) with three subscales that assess dietary restraint, hunger and disinhibition. The QEWP and BES are used to assess binge eating disorder. Participants were identified as potentially having binge eating disorder if they scored higher than 27 on the BES or were indicated as having the disorder by the QEWP. Any participant identified as potentially having binge eating disorder was required to complete the Eating Disorders Examination (Bryant-Waugh, Cooper, Taylor, & Lask, 1996), administered by a trained personnel in an additional session. No participants in this sample met the criteria for binge eating disorder.
Food liking, hunger
Subjective ratings of hunger were collected pre and post intake of the pre-load and after both test sessions using a 10-point Likert-type scale. Food hedonics were also measured pre and post intake of the preload and after the session for the food reinforcement session. For hunger and fullness, 1 indicated not at all hungry or not at all full and 10 indicated extremely hungry or extremely full, while for hedonics 1 indicated not liking at all and 9 indicated liking very much.
Food reinforcement task
The reinforcing value of food was measured by determining the number of responses participants made for food or food alternatives on progressive ratio schedules of reinforcement. The experimental environment included two computer stations that participants could go back and forth between. At one station, participants could earn points toward food and at the other station they could earn points for time to spend reading Time and Newsweek magazines. This alternative activity was provided to reduce the likelihood that participants would engage in responding out of boredom. Participants were instructed on how to use the computer task and given a practice session. Following the instructions for the task, the experimenter left the room. An intercom and closed circuit video system were present in the room so the experimenter could observe the participant and the participant could communicate with the experimenter.
The reinforcement task is similar to a slot machine with shapes that rotate on the screen and a point is earned each time the three shapes match in shape and color. For every five points earned, the subject was able to receive a 70-101 kcal (14 - 20 g) portion of his or her preferred snack food selected during the ad libitum eating session or 2 minutes of time to spend reading depending on which reward they were working for. The programmed reinforcement schedules for food and reading were progressive fixed ratio schedules with response requirements of 4,8, 16, 32, 64, 128, 256, 512, 1,024, 2,048 and so forth for each point. Participants were instructed to perform one activity at a time (i.e. play the computer game, eat or read), and that the session would end when they no longer wished to earn points for access to food or time to spend reading. The food chosen for the task was the most preferred food from a list of six palatable, high-energy density snack foods (amount of food presented (g) and energy density (kcal/g) shown in parentheses): Wavy Lay's Potato Chips (57 g, 5.4); Cooler Ranch Doritos (56 g, 5.4); M's (60 g, 5.0);Twix (48 g, 5.0); Kit Kat (42 g, 5.0); and Butterfinger (57g, 4.5). Water was provided ad libitum.
The food reinforcement task generates an overall response curve showing responding for food across the levels of reinforcement. The task also generates a total number of responses made for a reinforcer across all levels of reinforcement and was used to generate a breakpoint for responding, or PMAX, which was defined as the last reinforcement schedule (i.e. 4, 8, 16, 32, 64, 128, 256, 512, 1024, 2048) completed for access to the food (PMAX FOOD) or non food alternative (PMAXREADING).
Genotyping
DNA was collected from saliva samples collected in a plastic vial (Oragene, DNA Genotek Inc, Ottawa, Canada) and extracted using a commercially available genomic DNA quick preparation kit (Gentra Systems, Minneapolis, MN). DNA was extracted from the samples yielding 20µL of DNA at a concentration of 100-130ng/µL. After DNA purification, each sample was stored at -20°C for later analysis.
Gene and Single Nucleotide Polymorphism Selection
Candidate genes were chosen to represent polymorphisms of the dopamine, opioid and serotonin systems that have been associated with BMI, adiposity, food reinforcement, reward mechanisms or impulsivity. All types of gene products were considered including receptors, transporters and metabolic enzymes. Genes examined in the dopamine system include all 5 receptors (dopamine receptors DRD1, DRD2, DR D3, DRD4 and DRD5), tyrosine hydroxylase (TH), catecholamine O-methyltransferase (COMT) and monoamine oxidase-A (MAOA), the dopamine transporter (DAT1), dopamine beta-hydroxylase (DBH), dopa decarboxylase (aromatic L-amino acid decarboxylase) (DDC) and Sepiapterin reductase (SPR). Genes chosen in the serotonin system included receptors (5HT1A, 5HT1B, 5HT2A), and serotonin transporter (SERT) and Tryptophan hydroxylase 2 (TPH2). Mutations in the opioid delta receptor (OPRD) were also genotyped. Once candidate genes were established, single nucleotide polymorphisms (SNP) were chosen based on their previous associations with obesity, reward, impulsivity or drug abuse, or known effects on gene transcription or protein function. 161 SNPs were genotyped on an Illumina Golden gate platform (Illumina, San Diego, CA).
Dopamine Receptor D1 (DRD1)
The Ddel (A48G) polymorphism (rs4532) in the DRD1 gene is found on the 5′ untranslated region of exon 2 at base pair (bp) 48. The primers used were described by Limosin (Limosin, Loze, Rouillon, Adès & Gorwood, 2003) and were modified to sense 5′- ACT GAC CCC TAT TCC CTG CT-3′ and antisense 5′-AGC ACA GAC CAG CGT GTT C-3′ and amplified a 207 bp fragment. The restriction endonuclease Ddel digests the 207 bp fragment which was separated on a 10% polyacrylamide gel by electrophoresis. The polymorphism A48G creates two restriction sites for the A allele, forming three fragments of 146bp, 42bp and 19bp, while the G allele includes only one restriction site forming two fragments of 146bp and 61bp in size. The DRD1 gene was coded AA, AG and GG, (or A1/A1, A1/A2, A2/A2).
Dopamine Receptor D2 (DRD2)
For detection of the Taq1A polymorphism (rs1800497) in the ANKK1 gene, which is in linkage disequilibrium with DRD2 gene, a region of 304 base pairs (bp) was amplified. The primers first described by Grandy et. al (Grandy et al., 1989) were modified to sense 5′ –CCC TTC CTG AGT GTC ATC A-3′ and antisense 5′-CGG CTG GCC AAG TTG TCT-3′. The restriction endonuclease Taq1 digests the 304-bp amplicon, and subsequently the fragments are separated on a 6% polyacrylimide gel by electrophoresis. The Taq1A polymorphism in the DRD2 gene at Position 32806 T to C creates a restriction site resulting in partition of the 304 bp fragment of 177 and 127 bp. The A1/A1 or TT variant therefore is represented by an uncut amplicon of 304bp; the A1/A2 or TC heterozygous form digests in three fragments of 304, 177 and 127 bp; and the A2/A2 or CC variant is characterized by two fragments of 177 and 127 bp. The DRD2 was coded for the allele patterns of A2/A2, A2/A1, and A1/A1.
Dopamine Receptor D3 (DRD3)
The DRD3 gene includes a mutation (rs6280) of an A to G substitution resulting in a serine to glycine amino acid change. Primers were modified from Messas (Messas et al., 2005) and including the sense 5′-GCT CTA TCT CCA ACT CTC ACA-3′ and antisense 5′-AAG TCT ACT CAC CCT CCA GGT TA-3′ which amplified a 362bp fragment. The restriction enzyme Msc1 cuts the fragment results in one fragment of 304bp for the A1 or G allele and two fragments of 206bp and 98bp for A2 or the A allele. Restriction fragments were analyzed on a 10% polyacrylamide gel. The DRD3 polymorphism was coded A1/A1, A1/A2 and A2/A2, or GG, AG, AA.
Dopamine Receptor D4 (DRD4)
The polymorphism (rs4646984) examined in the dopamine receptor DRD4 gene included a 48 bp variable tandem repeat in exon 3. Methods are previously described by Kustanovich et al (Kustanovich et al., 2004) and the modified primers included the sense 5′-CTA CCC TGC CCG CTC ATG-3′ and antisense 5′-CCG GTG ATC TTG GCA CGC-3′. Briefly, PCR was used to amplify the region of interest and were analyzed after electrophoresis by comparing with molecular weight standards. The DRD4 was coded as absence of the 7R allele (non-7R/non-7R), presence of at least one 7R allele (7R/non-7R) and presence of two copies of the 7R allele (7R/7R). The dopamine D4 receptor included 2-9 tandem repeats with the two most common alleles being 4R (65%) and 7R (18%) and did not differ from reported allele frequencies (Vandenbergh et al., 2007).
Dopamine Receptor D5 (DRD5)
The dopamine receptor DRD5 contains a di-nucleotide (CT)n repeat 18.5 kb upstream of the gene. Methods have been previously described by Manor (Manor et al., 2004) and the modified primers included the sense 5′-CGT GTA TGA TCC CTG CAG-3′ and antisense 5′-GCT CAT GAG AAG AAT GGA GTG-3′. Briefly PCR products were amplified in a multiplex reaction and electrophoresed. The dopamine D5 receptor included 2-13 repeats with 9R (148 bp) being the most common allele in our data (41%) and 10R and 8R accounting for 13% of our sample each. Allele frequencies in our sample had similar frequencies to previous reports (Squassina et al., 2008). The DRD5 polymorphism was coded as absence of the 9R allele, presence of at least one 9R and presence of two 9R alleles.
Catecholamine-O-methyltransferase
The COMT gene examined results in a Val158Met, or G to A, substitution (rs4680) amplified using the primers described by (Yacubian et al., 2007) 5′-ACC CAG CGG ATG GTG GAT TTC-3′ 5′-GCC CTT TTT CCA GGT CTG AC-3′. The resulting product was digested using restriction enzyme N1alll and analyzed on a 10% polyacrylamide gel by electrophoresis. The COMT polymorphism was coded for patterns of GG, GA, and AA.
Monoamine Oxidase A (MAOA)
MAOA-LPR was amplified using the following primers as described by (Wiesbeck et al., 2006); sense 5′-CCC AGG CTG CTC CAG AAA C-3′ and antisense 5′-GGA CCT GGG CAG TTG TGC-3′. Digestion by Taq polymerase resulted in three possible fragments of varying copies of a 30-bp repeat, corresponding to the three alleles; 3 repeat (209bp), 4 repeat (239bp), 5 repeat (269bp). Our results included both a 2 repeat allele and a 3.5 repeat allele (227bp), distinguishable from the other more common alleles. Results were analyzed by electrophoresis on a 6% acrylamide gel. MAOA-LPR was coded for in the presence of two 3.5R or 4R alleles (indicating high activity of the enzyme, (Sabol, Hu, & Hamer, 1998), presence of one 3.5R or 4R and absence of any 3.5R or 4R alleles. The 4R (61%) and 3R (35%) occurred the most frequently in our sample and did not differ from previously reported frequencies (Sabol et al., 1998). The MAOA gene is located on the X chromosome, so analyses for this gene were stratified by sex.
Tyrosine Hydroxylase (TH)
Tyrosine hydroxylase contains a 4 bp (TCAT)n repeat (rs71029110). Methods have been previously described by Zhang et al (Zhang et al., 2004), however, briefly, sense 5′-GGT GTT TGA GTC CCT GTT GG-3′ and antisense 5′-GTA CAC AGG GCT TCC GAG TG-3′ primers were modified to amplify a 498 bp section. Exonuclease I and shrimp alkaline phosphatase were used to remove primers and dNTPs, before cycle sequencing with BigDye terminators. Sequenced alleles included between 6-10 TCAT repeats and the 9.3 repeat allele in which the 9 repeat allele includes an extra TCA repeat. The 9.3 allele was the most common (30%) which did not differ from previous reports (Wei, Ramchand, & Hemmings, 1997). Tyrosine hydroxylase, rs71029110, was coded for the absence of a 9.3R or 10R allele, presence of at least one 9.3R or 10R allele and presence of two 9.3R or 10R alleles.
Dopamine Transporter (DAT1)
Analysis of DAT1 gene focused on the VNTR of a 40bp sequence (rs28363170), which recurs 3 to 11 times. Primers first described by Vandenbergh et al. (Vandenbergh et al., 1992) were modified to sense 5′- GGT GTA GGG AAC GGC CTG AG-3′ and antisense 5′-CTG GAG GTC ACG GCT CAA GG-3′. The amplicon was analyzed on a 10% polyacrylamide gel by electrophoresis. The large increment of 40bp provides distinct typing of the VNTRs. The DAT1 included the 6R allele and 8-12 repeat alleles with the 10R (77%) and 9R (21%) being the most frequent (Kang, Palmatier & Kidd, 1999). The DAT1 genotype was coded for the allele patterns of non 10R (e.g. 9R/9R, 9R/11R), at least one 10R (e.g. 10R/any) and 10R/10R patterns.
Ancestry Informative markers
To control for individual ancestry (Halder, Shriver, Thomas, Fernandez, & Frudakis, 2008; Hoggart et al., 2003; Rosenberg et al., 2002), we genotyped a panel of 110 SNPs to estimate each individual's proportion of European, Asian and African ancestry (Kosoy et al., 2009). Proportions were estimated using the Bayesian clustering algorithms implemented in the program STRUCTURE v2 (Pritchard, Stephens, & Donnelly, 2000). Proportion of African ancestry was then used as a covariate in all regression analyses.
Analytic Plan
Participant characteristics were examined by sex and food reinforcement (mean breakpoint split of 62.5) using analysis of variance for continuous variables and chi-square for categorical variables to identify potential covariates.
The genetic dataset was cleaned by removing participants who were not successfully genotyped for at least 90% of the SNPs. SNPs were removed on the basis of a minor allele frequency (MAF) <0.05 and Hardy-Weinberg equilibrium <0.001. Due to the diversity of our sample, both minor allele frequency and Hardy-Weinberg equilibrium were examined for differences between racial populations, split into Caucasian, African and other based on self-identified ethnicity. For Hardy-Weinberg equilibrium, SNPs were excluded if they violated the criteria in any population as this may be indicative of a SNP that can differentiate between populations.
Genes with multiple allelic variations were examined for call rates, but not traditional measures of Hardy-Weinberg equilibrium. As variable tandem repeat genotypes can include many more than two alleles, cell frequencies for the less frequent alleles can be less than 5, which is inappropriate for analyzing Hardy-Weinberg equilibrium using the traditional Chi square analyses. Allele frequencies for the multi-allelic genes were examined for similarity to previously observed frequencies as reported in the methods section. For the SNPs, an additive genetic model was used, coded for the number of minor alleles, 0, 1 or 2. All subsequent genetic analyses were performed using PLINK (Purcell et al., 2007).
Regression analyses were conducted to determine if individual polymorphisms were associated with PMAXFOOD or BMI, while controlling for variables that differed by sex, including dietary restraint, or variables that differed by food reinforcement, including sex, income, dietary hunger and PMAXREADING and co-varying proportion African ancestry. Additional regression models were analyzed examining the potential interaction between PMAXFOOD and genes on BMI. P values, including those for the interaction terms, were corrected using False Discovery (FDR) estimates (Benjamini & Hochberg, 1995) for the 44 candidate polymorphisms studied. FDR is a multiple testing procedure that probabilistically controls for the proportion of false positive results in a set of results declared significant. Tests are rank-ordered based on the ascending p-value of interest and a significance cutoff is calculated by dividing the rank order by the total number of tests and multiplying by the desired FDR value (e.g. 0.05). FDR control methods of this type are somewhat more powerful than are traditional family-wide error control methods (Gadbury et al., 2008), which seemed desirable as false negatives are as important to avoid as false positives. An Incremental F test was used to determine if the interaction significantly increased the variance accounted for by the model (Wampold & Freund, 1987). Simple slopes analyses were conducted to determine the moderating association of the additive model on high PMAXFOOD (+1 Standard Deviation) and low PMAXFOOD (-1 Standard Deviation). To assess genes as potential differential susceptibility factors, the steps outlined by Belsky (Belsky et al., 2007) were followed. In brief, a true (cross-over) interaction was determined by plotting the predicted graph, and simple slopes were used to assess the relation between PMAXFOOD and BMI at each genotype. The non-risk allele should show no relation between BMI and PMAXFOOD, while the risk allele should show a strong relation that covers both the high and low range of BMI.
Separate exploratory analyses were conducted to examine the moderating effect of dietary restraint, hunger, disinhibition, minority status and income on the relationship between genes, food reinforcement and BMI. Three-way interactions were examined and, for the exploratory analysis, each model was only corrected for the number of genes, rather than both the number of genes and number of additional models. Any interaction that passed correction was examined using simple slopes.
Results
Participants
The average participant was 34.5 ± 10.6 (mean ± standard deviation) years of age with a BMI of 30.1 ± 7.6 (see Table 1). There were significant differences between males and females on dietary restraint and PMAXFOOD, with females reporting higher restrained eating and males having higher food reinforcement. There were significant differences in reading reinforcement, dietary restraint and hunger between the high and low food reinforcement groups, which were included as covariates in subsequent models. Chi-square analysis did not reveal any sex differences in education, income or race, but there were differences by food reinforcement group for income, so this was also included as a covariate in testing predictors of BMI. Significant differences in percentage minorities were observed between high and low food reinforcement groups, and proportion of African ancestry was included as a covariate to control for population stratification.
Genotyping
A total of 54 candidate polymorphisms were genotyped for analysis. The final dataset included only 44; 10 were removed based on minor allele frequency<0.05, and there were no violations of Hardy-Weinberg equilibrium (<0.001) (Table 2). For the non-SNP genes, minor allele frequencies and Hardy-Weinberg equilibrium are based on the coding schemes posited in the methods section.
Table 2.
List of candidate polymorphisms and minor allele frequency. EA, European; AA, African; Other, Asian, Hispanic
| Minor allele Frequency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| System | Gene | CHR | SNP/mutation | Minor | Major | n | TOTAL | EA | AA | other |
| Dopamine | SPR | 2 | rs2421095 | G | A | 245 | 0.153 | 0.128 | 0.405 | 0.143 |
| 2 | rs1876487 | A | C | 244 | 0.393 | 0.361 | 0.786 | 0.310 | ||
| DRD3 | 3 | rs6280 | A | G | 245 | 0.398 | 0.377 | 0.568 | 0.429 | |
| DRD1 | 5 | rs4532 | A | G | 245 | 0.358 | 0.406 | 0.091 | 0.167 | |
| DDC | 7 | rs2060762 | A | G | 243 | 0.156 | 0.152 | 0.143 | 0.214 | |
| 7 | rs11575543* | A | G | 245 | 0.043 | 0.032 | 0.167 | 0.023 | ||
| 7 | rs11575542* | A | G | 245 | 0.041 | 0.030 | 0.167 | 0.024 | ||
| 7 | rs11575522* | A | G | 244 | 0.037 | 0.027 | 0.143 | 0.024 | ||
| 7 | rs11238131 | A | G | 245 | 0.318 | 0.300 | 0.405 | 0.405 | ||
| TH | 11 | rs71029110 | 9.3R or 10R | Non 9.3R or 10R | 245 | 0.307 | 0.337 | 0.205 | 0.119 | |
| DAT1 | 5 | rs28363170 | Non 10R | 10R | 245 | 0.232 | 0.232 | 0.250 | 0.214 | |
| DRD5 | 4 | - | 9R | Non 9R | 226 | 0.398 | 0.431 | 0.237 | 0.237 | |
| DBH | 9 | rs77905 | A | G | 245 | 0.473 | 0.510 | 0.310 | 0.286 | |
| 9 | rs6271 | A | G | 245 | 0.073 | 0.079 | 0.024 | 0.071 | ||
| 9 | rs1611115 | A | G | 245 | 0.229 | 0.234 | 0.214 | 0.190 | ||
| 9 | rs1108580 | A | G | 244 | 0.439 | 0.438 | 0.350 | 0.524 | ||
| COMT | 22 | rs4580 | A | G | 245 | 0.573 | 0.532 | 0.818 | 0.714 | |
| DRD4 | 11 | rs4646984 | 7R | Non 7R | 243 | 0.200 | 0.205 | 0.227 | 0.119 | |
| DRD2 | 11 | rs1800497 | A2 | A1 | 245 | 0.203 | 0.187 | 0.318 | 0.238 | |
| Dopamine/Serotonin | MAOA | 23 | MAOA-LPR | 3.5R or 4R | Non 3.5R or 4R | 245 | 0.386 | 0.352 | 0.500 | 0.595 |
| Serotonin | HTR1A | 5 | rs6295 | C | G | 245 | 0.488 | 0.483 | 0.405 | 0.619 |
| 5 | rs1800044* | A | C | 245 | 0.006 | 0.007 | 0.000 | 0.000 | ||
| 5 | rs1799920* | - | G | 243 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 5 | rs10042486 | G | A | 245 | 0.471 | 0.502 | 0.357 | 0.286 | ||
| HTR1B | 6 | rs6296 | G | C | 245 | 0.251 | 0.236 | 0.262 | 0.381 | |
| 6 | rs13212041 | G | A | 245 | 0.288 | 0.264 | 0.548 | 0.262 | ||
| 6 | rs130058 | A | T | 245 | 0.263 | 0.288 | 0.095 | 0.190 | ||
| TPH2 | 12 | rs7963720 | G | A | 244 | 0.457 | 0.428 | 0.619 | 0.571 | |
| 12 | rs7305115 | A | G | 245 | 0.414 | 0.414 | 0.357 | 0.476 | ||
| 12 | rs4290270 | T | A | 245 | 0.394 | 0.360 | 0.571 | 0.548 | ||
| 12 | rs17110690 | A | G | 244 | 0.172 | 0.191 | 0.048 | 0.119 | ||
| 12 | rs1487275 | C | A | 245 | 0.284 | 0.266 | 0.262 | 0.476 | ||
| 12 | rs17110747 | A | G | 245 | 0.110 | 0.114 | 0.000 | 0.182 | ||
| HTR2A | 13 | rs927544 | G | A | 245 | 0.261 | 0.273 | 0.167 | 0.238 | |
| 13 | rs7997012 | A | G | 245 | 0.343 | 0.372 | 0.095 | 0.310 | ||
| 13 | rs6314 | A | G | 243 | 0.088 | 0.097 | 0.071 | 0.024 | ||
| 13 | rs6313 | A | G | 245 | 0.427 | 0.431 | 0.310 | 0.500 | ||
| 13 | rs6311 | A | G | 237 | 0.439 | 0.446 | 0.310 | 0.500 | ||
| 13 | rs2770296 | G | A | 244 | 0.283 | 0.295 | 0.238 | 0.214 | ||
| 13 | rs1923886 | G | A | 245 | 0.365 | 0.394 | 0.095 | 0.357 | ||
| SERT | 17 | rs2066713 | A | G | 244 | 0.338 | 0.354 | 0.238 | 0.286 | |
| 17 | rs2020933* | A | T | 245 | 0.010 | 0.007 | 0.048 | 0.000 | ||
| 17 | rs16965628 | C | G | 245 | 0.108 | 0.103 | 0.262 | 0.000 | ||
| 17 | rs1042173 | C | A | 245 | 0.420 | 0.441 | 0.190 | 0.452 | ||
| Opioid | OPRD | 1 | rs569356 | G | A | 244 | 0.117 | 0.134 | 0.024 | 0.048 |
| 1 | rs2236861 | A | G | 244 | 0.182 | 0.204 | 0.000 | 0.143 | ||
| 1 | rs204076 | T | A | 244 | 0.305 | 0.332 | 0.190 | 0.167 | ||
| 6 | rs7773995 | A | G | 245 | 0.153 | 0.158 | 0.167 | 0.095 | ||
| 6 | rs514980 | G | A | 245 | 0.204 | 0.214 | 0.119 | 0.190 | ||
| 6 | rs2281617* | A | G | 245 | 0.014 | 0.012 | 0.000 | 0.048 | ||
| 6 | rs1799971 | G | A | 245 | 0.118 | 0.131 | 0.000 | 0.119 | ||
| 6 | rs12205732 | A | G | 245 | 0.061 | 0.052 | 0.119 | 0.095 | ||
| 6 | rs10485057 | G | A | 241 | 0.110 | 0.109 | 0.143 | 0.079 | ||
| 6 | rs17174801* | G | A | 239 | 0.004 | 0.000 | 0.053 | 0.000 | ||
Excluded based on MAF<0.05
Regression Analysis
No individual genetic polymorphisms were significantly associated with BMI or PMAXFOOD after FDR correction. Thirty-nine genes showed non-significant results before correction for BMI and 43 genes showing non-significant results before correction for PMAXFOOD. After controlling for sex, income, dietary restraint and hunger, PMAXREADING and proportion African ancestry, two polymorphisms interacted with PMAXFOOD to predict BMI; rs6314 (B = 0.03, p = 0.0003, FDR = 0.01) and MAOA-LPR (B = 0.02, p = 0.0005, FDR = 0.02) and 29 were non-significant before correction. Both interactions significantly improved the fit of the model; rs6314 (FINC(1,235) = 12.2, p = 0.0006, FDR = 0.02) and MAOA-LPR (FINC(1,237) = 12.1, p = 0.0005, FDR = 0.03).
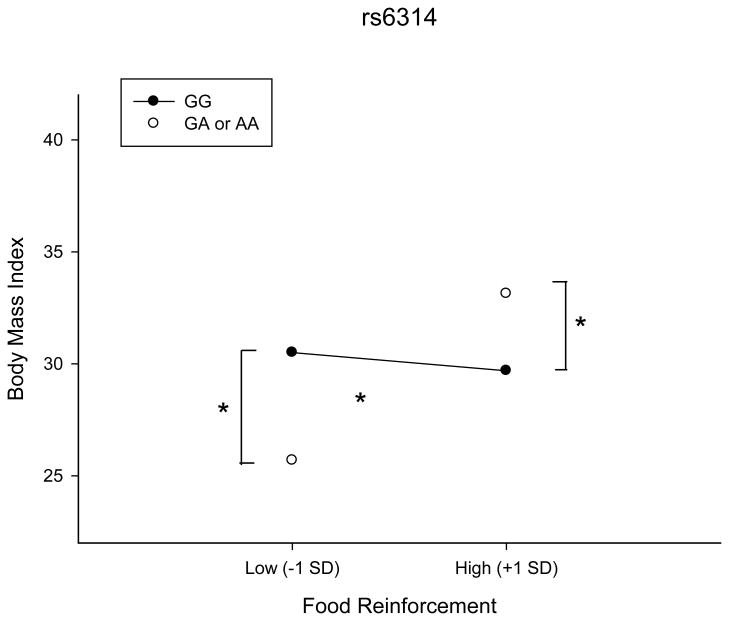
The rs6314 gene was re-coded for a dominant allele effect, as there was less than 5% of the sample with an AA genotype. The interaction between rs6314 and PMAXFOOD accounted for an additional 5.5% of the variance in BMI. For rs6314, there was a significant association between genotype and BMI at both high (B = 3.63, p = 0.03) and low (B = -5.06, p = 0.004) PMAXFOOD (See Figure 1). The presence of the minor allele A predicted a BMI of 33.1 kg/m2 for subjects with high PMAXFOOD and a BMI of 24.5 kg/m2 for subjects with low PMAXFOOD (Figure 1). There is also a significant association between PMAXFOOD and BMI for individuals with the AA or GA genotype (B = 0.03, p = 0.0001) but not the GG genotype (B = -0.003, p = 0.59). Presence of the A allele predicted the highest BMI when individuals were also high in food reinforcement and the lowest BMI when individuals were low in food reinforcement, while food reinforcement did not predict BMI for those with the GG genotype. These results indicate that the A allele is associated with both the highest and lowest BMI, suggesting that rs6314 may confer differential susceptibility towards food reinforcement, while the alternate genotype, GG, does not influence BMI as a function of food reinforcement.
Figure 1.
Effect of a mutation in RS6314 on the relationship between food reinforcement (PMAXFOOD) and Body mass index (BMI). Effects of genotype shown for ±1 Standard deviation of PMAXFOOD. *p-value<0.05
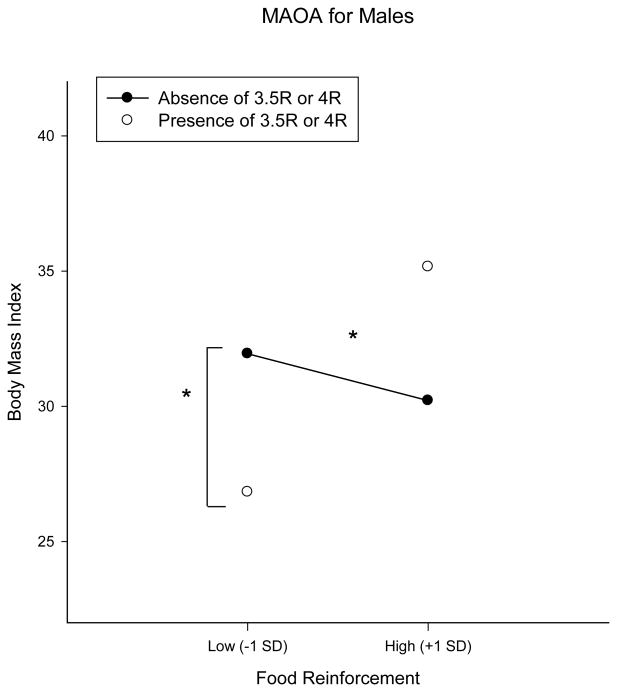
Analyses for MAOA-LPR were stratified by sex as this gene is located on the X chromosome. Males were coded for presence or absence of the risk alleles (3.5R and 4R) and females coded for the full additive model. There was a significant interaction with PMAXFOOD in males (B = 0.03, p = 0.0005, FDR = 0.02, n =119)(See Figure 2) which accounted for an additional 10.0% of the variance in BMI, but this interaction was not significant in females (B = 0.002, p = 0.92, n = 125). Simple slopes analysis for MAOA-LPR in males revealed a significant association between food reinforcement and BMI for individuals with a copy of the 3.5R or 4R high activity allele (B = 0.03, p = 0.0003), such that individuals with high PMAXFOOD had a BMI of 35.1 kg/m2 compared to a BMI of 26.8 kg/m2 for individuals with low PMAXFOOD, but no relationship for individuals with the low activity allele (B = -0.01, p = 0.30) (Figure 2). In addition there was a significant effect of genotype for individuals with low PMAXFOOD (B = -5.18 p = 0.01), but not high PMAXFOOD (B = 3.45, p = 0.05). These results are consistent with 3.5R/4R as a protective factor for individuals with low food reinforcement and fit a dual-risk model, in which genotype influences the outcome measure disproportionately in one environment, but not another.
Figure 2.
Effect of a mutation in Monoamine oxidase A, MAOA-LPR on the relationship between food reinforcement (PMAXFOOD) and Body mass index (BMI). Effects of genotype shown for ±1 Standard deviation of PMAXFOOD. MAOA-LPR is located on the X chromosome, so analyses were stratified by sex. *p-value <0.05
Exploratory Analysis
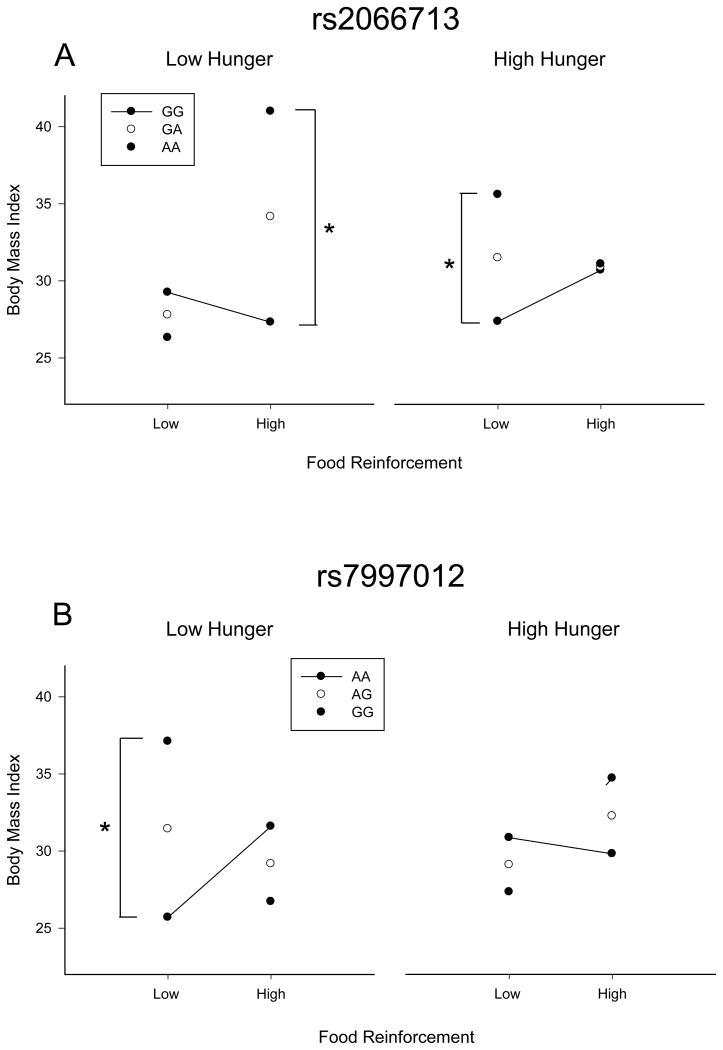
For the exploratory analysis, a median split of dietary hunger (<5.4, ≥5.3) revealed significant differences between the groups in age (F(1,243) = 5.05, p = 0.03) and was included as an additional covariate. There were significant three way interactions between genetics, PMAXFOOD and dietary hunger found with two SNPs; rs2066713 (B = -0.007, p = 0.0007, FDR = 0.03) located in the serotonin transporter SLC6A gene (Figure 3), and rs7997012 (B = 0.007, p = 0.0008, FDR = 0.03) located in the HTR2A gene (Figure 3). The three-way interaction with rs2066713 accounted for an additional 4.4 percent variance above the two-way interaction model (FINC(1, 230) = 11.9, p = 0.0006). The simple slopes for rs2066713 revealed a main effect of genotype at the combination of low hunger (-1 standard deviation; SD) and high food reinforcement (+1 SD) (B = 6.92, p = 0.003) and the combination of high hunger (+1 SD) and low food reinforcement (-1 SD) (B = 3.56, p = 0.005). In both cases, increasing number of copies of the A allele was associated with a dose-dependent increase in BMI. For individuals with low hunger and high food reinforcement there was an increase in BMI from 27.3 kg/m2 (GG) to 41.0 kg/m2 (AA) with increasing copies of the risk allele (Figure 3A). Individuals with a combination of high hunger and low food reinforcement showed similar results with increasing copies of the A risk allele was associated with increased BMI; GG (27.4 kg/m2), AG (31.5 kg/m2) and AA (35.6 kg/m2) (Figure 3B). The three way interaction with rs7997012 accounted for an additional 4.4 percent variance above the two-way interaction model (FINC(1, 231) = 11.70, p = 0.0007). For rs7997012, there was only a significant effect of genotype in individuals who were both low hunger (-1 SD) and low food reinforcement (-1 SD) (B = 5.34, p = 0.001) with increasing copies of the G allele associated with an increase in BMI; AA (25.7 kg/m2), AG (31.43 kg/m2) and AA (37.1 kg/m2) (Figure 3C).
Figure 3. Results of a three way interaction between Genes, Dietary Hunger and Food Reinforcement on BMI.
A. The effect of a mutation in rs2066713 and dietary hunger (±1 Standard Deviation) on the relationship between food reinforcement (PMAXFOOD) and Body Mass Index (BMI). The left side illustrates the interaction between food reinforcement (±1 Standard Deviation) and rs2066713 for low dietary hunger and the right side shows high dietary hunger. *p-value <0.05
B. The effect of a mutation in rs7997012 and dietary hunger (±1 Standard Deviation) on the relationship between food reinforcement (PMAXFOOD) and Body Mass Index (BMI). The left side illustrates the interaction between food reinforcement (±1 Standard Deviation) and rs7997012 for low dietary hunger and the right side shows high dietary hunger. *p-value <0.05
Discussion
Differential susceptibility describes the effect of some genetic or personality factors that confer greater responsivity to the environment such that individuals with these factors exhibit both the high and low points of the outcome measure (Belsky et al., 2007; Belsky & Pluess, 2009). The interaction between food reinforcement and rs6314, a single nucleotide polymorphism in the HTR2A gene, shows evidence of differential susceptibility, such that the “risk allele” seems to confer both increased and decreased risk of high BMI, at high and low food reinforcement, respectively. In our sample, individuals with the rs6314 risk allele had both the highest and lowest BMI's. The A allele may be associated with a blunted response to serotonin stimulation, perhaps related to decreased sensitivity to feelings of satiety. In combination with a high motivation to eat, this mutation is associated with increased BMI. However, a blunted response to serotonin and low food reinforcement is associated with significantly lower BMIs, which may suggest that this population's decreased obesity may be related to a different mechanism than rs6314's effects on satiety.
An examination of the interaction effect of MAOA-LPR suggests a dual-gain model, with protective effects of the genotype at low food reinforcement. The high activity MAOA-LPR risk allele is associated with decreases in brain serotonin, as MAOA is an enzyme involved in neurotransmitter breakdown. It is possible the decreased serotonin availability is potentially related to decreased feelings of satiety. MAOA-LPR risk allele also reduces synaptic dopamine availability, with theories of both hyper and hypo-functionality being associated with obesity (Stice, Spoor, Bohon, & Small, 2008; Stice, Yokum, Burger, Epstein, & Small, 2011). In our data, a combination of low food reinforcement and low synaptic dopamine levels is associated with lower BMIs than individuals with normal synaptic dopamine. The high activity allele for MAOA-LPR has also shown associations with both novelty seeking and reward dependence in humans (Shiraishi et al., 2006), in addition to a general influence of serotonin on impulsivity, including choices of delayed rewards (Bizot, Le Bihan, Puech, Hamon, & Thiebot, 1999; Schweighofer et al., 2008). Our theory of reinforcement pathology describes individuals most at risk for increased BMI as those who are high in food reinforcement and high in impulsivity (Carr, Daniel, Lin, & Epstein, 2011; Epstein, Salvy, Carr, Dearing, & Bickel, 2010), which has been tested in laboratory studies (Appelhans et al., 2011; Rollins et al., 2010). Consistent with this idea, the high-impulsivity risk alleles, 3.5R or 4R MAOA-LPR genotype show a significant interaction with food reinforcement, such that those with high food reinforcement and the risk allele show the highest BMIs. While this study did not measure impulsivity, future research may consider interactions between behavioral measures of impulsivity, food reinforcement and genotype.
MAOA-LPR and rs6314 are especially interesting polymorphisms, as both are associated with functional changes in their respective genes. The mutation examined in rs6314 results in charge differences in the second structure of the HTR2A receptor protein possibly influencing protein function (Heiser et al., 2007). The A allele is a tyrosine substitution that is related to slower calcium mobilization, which may cause a blunted response to serotonin stimulation (Wagner, Schuhmacher, Schwab, Zobel, & Maier, 2008). MAOA is involved in the breakdown of serotonin and dopamine and the 3.5R/4R alleles in the MAOA-LPR mutation have been associated with increased activity (Deckert et al., 1999; Sabol et al., 1998), which may increase the rate of neurotransmitter breakdown and decrease the amount of dopamine and serotonin available for release and stimulation of receptors. Many previous associations found between genes and obesity have been with non-functional SNPs that are likely in linkage disequilibrium with a functional locus. The identification of two functional alleles that interact with food reinforcement on BMI is an important contribution towards elucidating potential mechanisms that influence obesity.
The polymorphisms rs2066713 and rs7997012 were found in exploratory analyses to interact with food reinforcement and dietary hunger, and are not currently associated with any functional gene changes. Without any functional significance, it is difficult to speculate the mechanism by which these genes interact with food reinforcement and hunger to influence obesity. In the case of rs2066713, the A allele only influenced BMI when food reinforcement and hunger were low, suggesting that this gene is an obesity risk factor only for otherwise low-risk individuals. Either increased food reinforcement or increased hunger removed the effects of the gene. In the case of rs7997012, the gene influenced BMI when there was a mismatch between food reinforcement and dietary hunger, such that individuals with low hunger, but high food reinforcement and those with high hunger and low food reinforcement, had BMI's that were influenced by increased numbers of G alleles. It is possible that these genes influence an individual's ability to detect hunger or satiety signals as they are found in the serotonin system, either making them more or less sensitive to satiety or hunger. For example, it is possible that while individuals rate their hunger as low on the dietary questionnaire, the rs7997012 polymorphism makes them less sensitive to satiety signals; so even with low hunger levels individuals must ingest more food to feel full.
It is interesting that all the polymorphisms that interacted with food reinforcement or hunger to predict BMI are serotonin genes. Serotonin is hypothesized to be related to feelings of satiety, such that increases in the neurotransmitter's availability are associated with decreases in energy intake (Halford, Harrold, Lawton, & Blundell, 2005). Experiments with serotonergic agonists and antagonists show that increasing serotonergic activity in the brain can lead to decreased meal size in both animals and humans (Halford et al., 2005) and serotonin antagonists increase energy intake, particularly of carbohydrates (Stallone & Nicolaidis, 1989). Empirical evidence supports a role of hunger and satiety in food reinforcement, with greater hunger predicting an increase in the reinforcing value of food (Carr & Epstein, 2011; Raynor & Epstein, 2003). Food reinforcement is typically measured after a preload, to measure the influence of hedonic hunger, or energy intake above normal caloric needs (Saper, Chou, & Elmquist, 2002), rather than homeostatic hunger or the drive to consume minimum daily energy needs (Epstein, Truesdale, Wojcik, Paluch, & Raynor, 2003; Reiss & Havercamp, 1996). The reinforcing value of food is most informative when differences in hedonic hunger are considered, as this is the basis for excess energy intake (Lowe & Butryn, 2007).
The combination of low food reinforcement and previously identified “risk allele” in either MAOA-LPR or rs6314 are associated with lower BMI. While an examination of the contributions of serotonin towards energy intake and BMI would suggest that decreased serotonergic activity is associated with higher BMI and greater energy intake, this is not the case for individuals low in food reinforcement. An examination of the non-risk genotype suggests that normal or high serotonergic functioning effect on BMI is not influenced by food reinforcement. The previous mixed results regarding the effect of serotonin on motivation (De Vry et al., 2003; Sanders et al., 2007; Thomsen et al., 2009), may be due to the interaction between food reinforcement and serotonin.
Food reinforcement has been identified as a potential candidate for genetic study using the criteria outlined by Faith (Faith, 2008); including reliability (Epstein, Temple, et al., 2007), both cross-sectional (Giesen et al., 2010; Saelens & Epstein, 1996; Temple et al., 2008) and prospective associations with obesity (Hill et al., 2009; Temple & Epstein, 2011), heritability (Epstein, Dearing, Temple, & Cavanaugh, 2008), and differences in at risk individuals (Hill et al., 2009). Examining candidate genes associated with BMI and food reinforcement did not reveal any significant associations after FDR correction. While we were not able to identify any genes that predicted level of food reinforcement, it is still possible that the interactions between food reinforcement and the serotonin genes are the result of gene-gene effects that could not be identified in our sample.
Previous research from our laboratory on the relationship of food reinforcement and BMI has focused in the dopamine receptor D2, ANKK1 or Taq1A allele (Epstein, Dearing, & Erbe, 2010; Epstein, Temple, et al., 2007; Epstein et al., 2004). It is interesting to note that if the Taq1A allele had been one of a few polymorphisms examined, we would have replicated previous results (p < 0.05), but in a more comprehensive genetic analysis correcting for testing multiple genes, this association was not found.
This exploratory study provides a new approach to studying how gene-behavioral phenotype interactions may predict BMI, but the study is limited due to the small sample size and relatively large number of candidate genes. It should be noted that our variances may have been inflated due to the Beavis effect, which describes a situation in which samples of under 500 participants tend to have larger R2 (Xu, 2003). While dopamine has been implicated in energy intake and BMI, and responding for drug reinforcers, we examined a limited array of dopamine polymorphisms. Due to the size of the sample, this study did not have sufficient power to examine at gene-gene interactions as many of the genotype combinations would not be represented, but may have been particularly interesting between genes in the dopamine and serotonin systems.
Further research should examine the potential interaction of dopamine and serotonin genetics and food reinforcement. The data presented in this study suggests that serotonin may be a genetic moderator of food reinforcement, and distinguishing between serotonin's effect on satiety and impulsivity may provide mechanisms by which serotonin influences food reinforcement. Targeting mechanisms that influence food reinforcement and satiation may suggest novel or individualized treatments, similar to the principles of pharmaco-genomic therapies, perhaps by creating diets of satiating foods and limiting access to highly reinforcing foods for at risk individuals with low serotonin and high food reinforcement. The translation of these behavioral genetic interactions may represent a new frontier for improving behavioral interventions.
Acknowledgments
Appreciation is granted to Paul Juzdowski, Michelle Detwiller and to Jeffery Conroy at Roswell Park Cancer Institute for help in genotyping. Appreciation is expressed to Lora G. Roba, Vida Rostami, Lauren Angelucci, Nicole Gens, Caitlin Hart and Kirstie Clune for data collection and data entry and assisting in the implementation of protocol. This research was funded in part by a grant from the National Institute of Drug Abuse, R01DA024883 awarded to Dr. Epstein. The study sponsors had no role in study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Footnotes
Dr. Allison has, anticipates, or has had financial interests with the Frontiers Foundation; Vivus, Inc; Kraft Foods; University of Wisconsin; University of Arizona; Paul, Weiss, Wharton & Garrison LLP; and Sage Publications.
None of the other authors declared a conflict of interest.
References
- Allison DB, Kalinsky LB, Gorman BS. A comparison of the psychometric properties of three measures of dietary restraint. Psychological Assessment. 1992;4:391–398. [Google Scholar]
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) 2011;19:2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bizot J, Le Bihan C, Puech A, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berl) 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders. 1996;19:391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement pathology and obesity. Current Drug Abuse Reviews. 2011;4:190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, Epstein LH. Relationship between food habituation and reinforcing efficacy of food. Learning and Motivation. 2011;42:165–172. doi: 10.1016/j.lmot.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JJ, de Bruin JP, Feenstra MG. Dopamine efflux in nucleus accumbens shell and core in response to appetitive classical conditioning. European Journal of Neuroscience. 2003;18:1306–1314. doi: 10.1046/j.1460-9568.2003.02849.x. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Daschke A, Jentzsch K. Effects of serotonin 5-HT1/2 receptor agonists in a limited-access operant food intake paradigm in the rat. European Neuropsychopharmacology. 2003;13:337–345. doi: 10.1016/s0924-977x(03)00014-2. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Human Molecular Genetics. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton M, Jennings K, Sharp T, Rushworth M, Bannerman D. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology (Berl) 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. The American Journal of Clinical Nutrition. 2011;94:12–18. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Erbe RW. Parent-child concordance of Taq1 A1 allele predicts similarity of parent-child weight loss in behavioral family-based treatment programs. Appetite. 2010;55:363–366. doi: 10.1016/j.appet.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Temple JL, Cavanaugh MD. Food reinforcement and impulsivity in overweight children and their parents. Eating Behaviors. 2008;9:319–327. doi: 10.1016/j.eatbeh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Lin H, Carr KA, Fletcher KD. Food reinforcement and obesity: Psychological Moderators. Appetite. 2012;58:157–162. doi: 10.1016/j.appet.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology and Behavior. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiology and Behavior. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jr, Jaroni JL, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. The American Journal of Clinical Nutrition. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- Faith MS. Behavioral science and the study of gene–nutrition and gene–physical activity interactions in obesity research. Obesity (Silver Spring) 2008;16:82–84. doi: 10.1038/oby.2008.524. [DOI] [PubMed] [Google Scholar]
- Gadbury GL, Xiang Q, Yang L, Barnes S, Page GP, Allison DB. Evaluating statistical methods using plasmode data sets in the age of massive public databases: an illustration using false discovery rates. PLoS genetics. 2008;4:e1000098. doi: 10.1371/journal.pgen.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen JC, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity (Silver Spring) 2010;18:966–970. doi: 10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, et al. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. American Journal of Human Genetics. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Human Mutation. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Halford JCG, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: Effects on appetite expression and use for the treatment of obesity. Current Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- Heiser P, Dempfle A, Friedel S, Konrad K, Hinney A, Kiefl H, et al. Family-based association study of serotonergic candidate genes and attention-deficit/hyperactivity disorder in a German sample. Journal of Neural Transmission. 2007;114:513–521. doi: 10.1007/s00702-006-0584-5. [DOI] [PubMed] [Google Scholar]
- Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7-10-y-old children. The American Journal of Clinical Nutrition. 2009;90:276–281. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. American Journal of Human Genetics. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AM, Palmatier MA, Kidd KK. Global Variation of a 40-bp VNTR in the 3′-untranslated region of the Dopamine Transporter gene (SLC6A3) Biological Psychiatry. 1999;46:151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Human Mutation. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustanovich V, Ishii J, Crawford L, Yang M, McGough J, McCracken J, et al. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: Confirmation of association of ADHD with DRD4 and DRD5. Molecular Psychiatry. 2004;9:711–717. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- Laessle R, Tuschl R, Kotthaus B, Pirke K. A comparision of the validity of three scales for the assessment of dietary restraint. Journal of Abnormal Psychology. 1989;98:504–507. doi: 10.1037//0021-843x.98.4.504. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Rouillon F, Ades J, Gorwood P. Association between Dopamine Receptor D1 gene DdeI polymorphism and sensation seeking in alcohol dependant men. Alcoholism: Clinical and Experimental Research. 2003;27:1226–1228. doi: 10.1097/01.ALC.0000081624.57507.87. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiology and Behavior. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Manor I, Corbex M, Eisenberg J, Gritsenkso I, Bachner Melman R, Tyano S, et al. Association of the dopamine D5 receptor with attention deficit hyperactivity disorder (ADHD) and scores on a continuous performance test (TOVA) American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127:73–77. doi: 10.1002/ajmg.b.30020. [DOI] [PubMed] [Google Scholar]
- Messas G, Meira-Lima I, Turchi M, Franco O, Guindalini C, Castelo A, et al. Association study of dopamine D2 and D3 receptor gene polymorphisms with cocaine dependence. Psychiatric Genetics. 2005;15:171–174. doi: 10.1097/00041444-200509000-00006. [DOI] [PubMed] [Google Scholar]
- NHLBI Obesity Education Initiative Expert Panel. Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--The evidence report. Obesity Research. 1998;6(2):51S–209S. [PubMed] [Google Scholar]
- Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. European Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Peciña S, Smith KS. Hedonic and motivational roles of opioids in food reward: Implications for overeating disorders. Pharmacology Biochemistry and Behavior. 2010;97:34–46. doi: 10.1016/j.pbb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Reiss S, Havercamp S. The sensitivity theory of motivation: Implications for psychopathology. Behaviour Research and Therapy. 1996;34:621–632. doi: 10.1016/0005-7967(96)00041-1. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, et al. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Sanders AC, Hussain AJ, Hen R, Zhuang X. Chronic blockade or constitutive deletion of the serotonin transporter reduces operant responding for food reward. Neuropsychopharmacology. 2007;32:2321–2329. doi: 10.1038/sj.npp.1301368. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sasaki-Adams DM, Kelley AE. Serotonin-dopamine interactions in the control of conditioned reinforcement and motor behavior. Neuropsychopharmacology. 2001;25:440–452. doi: 10.1016/S0893-133X(01)00240-8. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, et al. Low-serotonin levels increase delayed reward discounting in humans. Journal of Neuroscience. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, et al. Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants. Psychiatric Genetics. 2006;16:55–58. doi: 10.1097/01.ypg.0000199447.62044.ef. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Detry MA, Pillow P, Hu Y, Amos CI, Hong WK, et al. Variant alleles of the D2 dopamine receptor gene and obesity. Nutrition Research. 2000;20:371–380. [Google Scholar]
- Spitzer RL, Devlin MJ, Walsh BT, Hasin D, Wing RR, Marcus MD, et al. Binge eating disorder: A multisite field trial of the diagnostic criteria. International Journal of Eating Disorders. 1992;11:191–203. [Google Scholar]
- Squassina A, Lanktree M, De Luca V, Jain U, Krinsky M, Kennedy JL, et al. Investigation of the dopamine D5 receptor gene (DRD5) in adult attention deficit hyperactivity disorder. Neuroscience Letters. 2008;432:50–53. doi: 10.1016/j.neulet.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Stallone D, Nicolaidis S. Increased food intake and carbohydrate preference in the rat following treatment with the serotonin antagonist metergoline. Neuroscience Letters. 1989;102:319–324. doi: 10.1016/0304-3940(89)90099-2. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. Journal of Neuroscience. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Halder I, Flory JD, Craig AE, Gianaros PJ, Ferrell RE, et al. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: Evidence for differential susceptibility. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss020. in press Epub ahead of print retrieved Mar 2 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple J, Epstein L. Sensitization of food reinforcement is related to weight status and baseline food reinforcement. International Journal of Obesity. 2011;36:1102–1107. doi: 10.1038/ijo.2011.210. [DOI] [PubMed] [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. The American Journal of Clinical Nutrition. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GN, Critchley JAJH, Tomlinson B, Cockram CS, Chan JCN. Relationships between the TaqI polymorphism of the dopamine D2 receptor and blood pressure in hyperglycaemic and normoglycaemic Chinese subjects. Clinical Endocrinology. 2001;55:605–611. doi: 10.1046/j.1365-2265.2001.01404.x. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. Journal of Neuroscience. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, O'Connor RJ, Grant MD, Jefferson AL, Vogler GP, Strasser AA, et al. Dopamine receptor genes (DRD2, DRD3 and DRD4) and gene–gene interactions associated with smoking-related behaviors. Addiction Biology. 2007;12:106–116. doi: 10.1111/j.1369-1600.2007.00054.x. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Archives of Neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schuhmacher A, Schwab S, Zobel A, Maier W. The His452Tyr variant of the gene encoding the 5-HT2A receptor is specifically associated with consolidation of episodic memory in humans. The International Journal of Neuropsychopharmacology. 2008;11:1163–1167. doi: 10.1017/S146114570800905X. [DOI] [PubMed] [Google Scholar]
- Wampold BE, Freund RD. Use of multiple regression in counseling psychology research: A flexible data-analytic strategy. Journal of Counseling Psychology. 1987;34:372–382. [Google Scholar]
- Wei J, Ramchand C, Hemmings G. Possible association of catecholamine turnover with the polymorphic (TCAT) n repeat in the first intron of the human tyrosine hydroxylase gene. Life sciences. 1997;61:1341–1347. doi: 10.1016/s0024-3205(97)00679-6. [DOI] [PubMed] [Google Scholar]
- Wiesbeck GA, Wodarz N, Weijers HG, Dursteler-MacFarland KM, Wurst FM, Walter M, et al. A functional polymorphism in the promoter region of the monoamine oxidase A gene is associated with the cigarette smoking quantity in alcohol-dependent heavy smokers. Neurospsychobiology. 2006;53:181–185. doi: 10.1159/000093782. [DOI] [PubMed] [Google Scholar]
- Xu S. Theoretical basis of the Beavis effect. Genetics. 2003;165:2259–2268. doi: 10.1093/genetics/165.4.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Gläscher J, Kalisch R, Leuenberger B, et al. Gene–gene interaction associated with neural reward sensitivity. Proceedings of the National Academy of Sciences. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: Prediction of catecholamines and response to stress in twins. Physiological Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]