Abstract
Background
ADV (adenovirus) is an important cause of viral mortality in hematopoietic stem cell transplantation (HSCT). T-cell depleted (TCD) HSCT are at increased risk of viral infections. We compared the rates and outcomes of ADV viremia and disease between TCD and conventional (CONV) HSCT at our institution.
Methods
This was an observational study of 624 adult and pediatric recipients of myeloablative HSCT at Memorial Sloan-Kettering Cancer Center from January 1, 2006, through March 11, 2011. Viral cultures and ADV polymerase chain reaction (PCR) were ordered as clinically indicated. ADV viremia by a quantitative PCR assay was defined as ≥1 positive value ≥1,000 copies/mL or ≥2 consecutive values at any value. Competing risk regression analyses were used to identify predictors for ADV viremia.
Results
Eight percent TCD and 4.0% CONV were noted to have ADV viremia at 1 year after HSCT (P=.041). Among TCD, 15% of children compared with 5% of adults developed ADV viremia (P=.008). Young age (Hazard Ratio [HR] 3.0; P<.001) and acute graft-versus-host-disease (GVHD) (HR 3.2; P=.001) were risk factors for ADV viremia. ADV viremia was a predictor of mortality (HR 6.0; P<.001). ADV disease developed in 3.5% TCD and 0.4% CONV HSCT (P=.022) with attributable mortality of 27%. Among TCD, grade 2–4 GVHD was a risk factor for ADV disease (HR 13; P<.001); age was not significant. More than 90% ADV disease cases had a viral load of ≥ 10,000 copies/mL.
Conclusions
Rates of ADV disease were 10-fold higher in TCD compared with CONV HSCT, predominantly in patients who developed acute GVHD. The benefit of preemptive therapy for ADV viral load ≥ 10,000 copies/mL for prevention of ADV disease in recipients of TCD grafts should be evaluated in prospective clinical trials.
Keywords: Adenovirus, polymerase chain reaction (PCR), risk factors, T-cell depletion, Hematopoietic stem cell transplantation (HSCT)
INTRODUCTION
Adenovirus (ADV) is an important viral pathogen in hematopoietic stem cell transplantation (HSCT). ADV-associated hepatitis, pneumonitis, and encephalitis are frequently fatal, while colitis and hemorrhagic cystitis cause substantial morbidity and may contribute to mortality (1–3). Over the last 10 years the relative contribution of ADV as a viral cause of mortality in HSCT has increased, possibly because of a striking decline in rates of CMV related mortality (4, 5).
Since 2004, quantitative polymerase chain reaction (PCR) assays have been commercially available for detection and quantification of ADV in the blood. Serial monitoring for ADV by PCR has been advocated by some groups during the first 6 months after HSCT or for the duration of severe immunosuppression and/or lymphopenia (6). This practice has not been widely adopted in clinical practice mainly due to the lack of safe and effective treatment for ADV. In addition, the natural history of ADV viremia in HSCT is not well defined. Rates of ADV viremia post-HSCT vary widely, ranging from 4.9% to 25.8%, depending on the surveillance strategy and patient population (2, 7–9). Ohrmalm et al found little utility in serial monitoring of plasma ADV PCR in a cohort of 97 HSCT comprised of 64% T-cell depleted allografts (7). High level or rising ADV viremia has been reported to predict disseminated ADV disease and death (10–13). Rising ADV viral load in the stool has also been reported as a useful predictor of ADV disease (12). T-cell depletion (TCD), younger age, and graft-versus-host disease (GVHD) have been associated with invasive ADV disease (1, 10–12). Although preemptive treatment for ADV viremia could potentially prevent ADV disease, currently there are no data from randomized clinical trials to support its use. Furthermore, at present there is no approved therapy for ADV. Cidofovir has been used in established ADV disease, yet its efficacy is not well documented. CMX001, a novel, orally administered, broad spectrum antiviral active against ADV, has shown promising results in case reports (10, 14, 15). A clinical trial of preemptive treatment of ADV viremia with CMX001 for prevention of ADV disease in HSCT is ongoing.
At our institution prior to the availability of ADV PCR, the rate of ADV disease was 1.3%, with the majority of cases occurring in TCD HSCT (2). Since 2006, we have used ADV PCR to test patients with clinical symptoms compatible with ADV infection or disease. We conducted a retrospective study to: 1) identify risk factors for ADV viremia and disease; and 2) compare rates and outcomes of ADV disease in TCD and conventional (CONV) HSCT.
METHODS
Study Patients
The study was reviewed and approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board (IRB). The cohort consisted of 624 adult and pediatric patients who first underwent myeloablative allogeneic HSCT at MSKCC from January 1, 2006, through March 11, 2011. Patients were followed up to September 2011 or death, whichever occurred first. Minimum follow-up was 6 months. The procedure for T-cell depletion (TCD), supportive care, and management of GVHD has been previously described (16–18). Since July 2006, the Miltenyi TV: MACS® was used for T-cell depletion for 106 allografts. Recipients of TCD grafts did not receive additional immunosuppression for GVHD prophylaxis.
Clinical and laboratory data were extracted from a computerized prospective database. Antiviral treatment and outcomes were extracted from pharmacy and medical records.
Definitions
There was no routine surveillance for ADV during the study period. Blood ADV PCR and viral cultures were ordered at the discretion of treating physicians. In this study, patients not tested by ADV PCR and those with negative ADV PCR were considered negative for ADV viremia. ADV infection was defined as a positive culture for ADV from a non-sterile site (such as stool, urine, or nasopharynx). ADV viremia was diagnosed by ADV PCR In the blood. Patients were considered have ADV viremia if they had at least 1 PCR value ≥1,000 copies/mL or >2 consecutive positive PCRs of any value. The time to ADV viremia was defined as the number of days from the date of stem cell infusion (d0) to the first positive ADV PCR report meeting definition criteria. High-level ADV viremia was defined as at least 1 PCR value ≥ 10,000 copies/mL, and very-high-level ADV viremia was defined as at least 1 PCR value ≥200,000 copies/mL at any time point. Viremia was early if it occurred ≤180 days after transplantation. ADV end-organ disease has been previously defined (1, 12). Definite disease was defined as presence of typical ADV nuclear inclusions or positive immunohistochemistry for ADV in tissue and/or positive culture from tissue (excluding the gastrointestinal [GI] tract). Probable ADV disease was defined as ≥2 positive ADV PCR, and clinical signs and symptoms compatible with ADV disease without other identified cause. Disseminated ADV disease was defined as presence of ADV by culture or histopathology, and clinical evidence of disease in ≥2 noncontiguous sites excluding blood. Death was attributed to ADV if ADV caused or significantly contributed to death.
Laboratory methods
Routine viral cultures for ADV were performed in the Clinical Microbiology Laboratory at MSKCC. Quantitative real-time ADV PCR in whole blood was performed by Focus (Cypress, CA, USA) until June 2009 and by Viracor-IBT (Lee’s Summit, MO, USA) thereafter. The linear range of quantitation was 250 – 1×108 copies/mL and 100 – 1×1010 copies/mL, respectively.
Statistics
Binary variables were compared using χ2 or Fisher’s exact test. Continuous variables were compared by the Mann-Whitney test. The cumulative incidence rates were estimated for ADV in the stool and ADV viremia using cumulative incidence analysis. Death was treated as a competing risk. Patients were censored on the date of second transplant or last follow-up, whichever occurred first. Cumulative incidence rates between TCD and CONV HSCT throughout the study period were compared using Gray’s test. The Wald test was used to compare rates at 1 and 2 years after HSCT. Permutation test was used when the number of event was less than 5 in a group (19).
Competing risk regression was used to identify risk factors for ADV viremia and disease. The hazard ratio (HR) and the 95% confidence interval (CI) were estimated. A time-dependent Cox regression was utilized to assess the association between ADV viremia and overall survival (OS). Statistical analyses were performed with software packages (SAS 9.2; SAS Institute Inc., Cary, NC, USA, and R version 2.13, The R Foundation for Statistical Computing). A P value of ≤.05 was considered statistically significant.
RESULTS
Patients
Among 624 myeloablative HSCT patients, 401 received T-cell depleted, 131 unmodified, and 92 umbilical cord blood stem cell allografts. In this study, the term “conventional (CONV)” includes unmodified bone marrow, peripheral blood, and umbilical cord stem cell allografts. Table 1 shows the clinical characteristics of the 624 study patients. Compared with CONV HSCT recipients, TCD HSCT recipients were older, received more peripheral blood mononuclear cell (PBMC) grafts, and had less GVHD.
Table 1.
Clinical Characteristics of HSCT Patients (N=624)
| HSCT Characteristics | T-cell depleted Total n=401 N (%) |
Conventionala Total n=223 N (%) |
P Value |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 47.5 (0.1 – 73) | 31.8 (0.4 – 69.9) | <.001 |
| Gender | |||
| Male | 230 (57.4) | 122 (54.7) | .52 |
| Female | 171 (42.6) | 101 (45.3) | |
| Underlying disease | |||
| Acute leukemia | 231 (57.6) | 135 (60.5) | |
| Lymphoma | 22 (5.5) | 37 (16.6) | |
| Myelodysplastic syndrome | 64 (16) | 18 (8.1) | |
| Multiple myeloma | 28 (7.0) | 0 | |
| Nonmalignant | 44 (11.0) | 20 (9.0) | |
| Chronic myelogenous leukemia | 9 (2.2) | 7 (3.1) | |
| Otherb | 3 (0.7) | 6 (2.7) | |
| Stem cell source | |||
| Peripheral blood | 372 (92.8) | 83 (37.2) | <.001 |
| Bone marrow | 29 (7.2) | 48 (21.5) | |
| Cord blood | 0 | 92 (41.3) | |
| Donor type | |||
| Matched related donor | 132 (32.9) | 69 (30.9) | <.001 |
| Matched unrelated donor | 136 (33.9) | 51 (22.9) | |
| Mismatched related donor | 16 (4) | 0 | |
| Mismatched unrelated donor | 117 (29.2) | 11 (4.9) | |
| Cord blood | 92 (41.3) | ||
| Acute GVHD | |||
| Grade 0 – 1 | 341 (85.1) | 124 (55.6) | <.001 |
| Grade 2 | 25 (6.2) | 46 (20.6) | |
| Grade 3 – 4 | 21 (5.2) | 40 (18) | |
| Non-evaluable | 14 (3.5) | 13 (5.8) | |
Conventional includes peripheral blood, bone marrow, and cord blood allografts.
Other: Sarcoma, chronic lymphocytic leukemia
Abbreviations: HSCT, hematopoietic stem cell transplantation; GVHD, Graft versus host disease
Adenovirus in the Stool
During the study period, 89.2% CONV and 72.8% TCD had ≥1 stool viral culture (P<.001) for evaluation of diarrhea and/or clinical symptoms of enteritis or colitis. At 1 year after transplant, 10.8% CONV and 12.7% TCD recipients had a positive stool culture for ADV (P=.479).
Eighty-two patients had ≥1 positive stool culture for ADV. Colonoscopy was done in 25 (30%). Six patients had ADV colitis by histopathology.
Adenovirus Viremia
During the study period, 362 (58%) patients had blood tested by ADV PCR. More CONV than TCD HSCT recipients were tested by ADV PCR (67.7% versus 52.6%; P<.001).
Forty-three (6.9%) patients had ADV viremia. At 1 year, 4% CONV, and 7.9% TCD HSCT recipients had ADV viremia (P=.04). At 2 years, the difference was not statistically significant (TCD 8.3% vs CONV 5.3%, P=.16). Among TCD HSCT recipients, ADV viremia was more frequent in children than adults (P=.008).
Figure 1 shows the distribution of ADV cases by month after transplant. Forty-three patients developed ADV viremia (32 TCD recipients and 11 CONV recipients) a median of 82 days post-transplant (range 10 – 722). Thirty-two (74%) cases occurred earlier than 6 months after HSCT. Among TCD recipients with early ADV viremia 1 (4%) had grade 3–4 GVHD. In contrast 50% of late ADV viremia cases had grade 3–4 GVHD.
Figure 1.
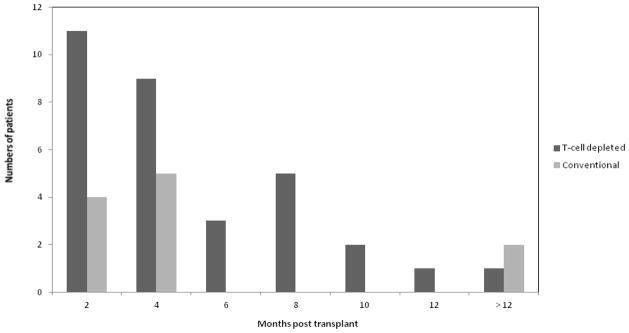
Distribution of cases of ADV viremia by month post transplant.
ADV Viremia and Survival
A time-dependent Cox regression model was used to estimate the impact of ADV viremia on overall survival (OS). Patients with ADV viremia had decreased OS compared to patients without ADV viremia (HR 6.0; 95% CI 4.1–8.6, P<.001).
Risk Factors for Adenovirus Viremia
We compared 43 patients of ADV viremia to 581 patients without documented ADV viremia. By competing regression analyses, young age and acute grade 2–4 GVHD were risk factors for ADV viremia, whereas TCD, stem cell source, and donor type were not statistically significant (Table 2). By multivariable models, young age (HR 2.8; 95% CI 1.5–5, P=.001) and acute grade 2–4 GVHD (HR 3.1; 95% CI 1.6–6.1, P=.001) remained independent risk factors for ADV viremia.
Table 2.
Risk Factors for ADV Viremia
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age at HSCT | ||
| Adult | 1.0 | <.001 |
| Pediatric | 3.0 (1.6–5.6) | |
| Stem cell source | ||
| Peripheral blood | 1.0 | |
| Bone marrow | 1.0 (0.4–2.4) | .96 |
| Cord blood | 1.4 (0.6–3.2) | .42 |
| T-cell depletion | ||
| Conventional | 1.0 | |
| T-cell depleted (TCD) | 1.8 (0.8–3.3) | .15 |
| Donor type | ||
| Matched related | 1.0 | |
| Matched unrelated | 0.8 (0.4–1.9) | .67 |
| Mismatched | 1.1 (0.6–2.2) | .78 |
| Acute GVHD | ||
| Grade 0 – 1 | 1.0 | |
| Grade 2 – 4 | 3.2 (1.6–6.3) | .001 |
| Grade 0 – 2 | 1.0 | |
| Grade 3 – 4 | 5.4 (2.7–11.0) | <.001 |
Abbreviations: ADV, adenovirus; CI, confidence interval; TCD, T-cell depleted; GVHD, graft versus host disease
ADV Disease
Fifteen (2.4%) patients developed end-organ ADV disease (14 TCD recipients and 1 CONV recipients). The incidence of ADV disease was higher in TCD compared with CONV HSCT recipients (3.5% vs 0.4% respectively, P=.022). Ten patients had definite and 5 had probable ADV disease. ADV colitis occurred in 6 patients, pneumonitis 6, hepatitis 4, and pyelonephritis/cystitis 4. Eight patients had involvement of ≥2 organs by ADV.
We performed competing regression analyses to identify risk factors for ADV disease in TCD HSCT recipients. Age, donor type, and GVHD were included as variables. Only grade 2–4 GVHD was statistically significant (HR 13.3; 95% CI 4.5–39.2, P<.001). Young age was not significant in our model (HR 1.5; 95% CI 0.5–4.4, P=.496).
Eighty-six CONV and 45 TCD recipients had GVHD (grade 2–4). ADV disease developed in 1 of 86 (1.2%) CONV compared with 7 of 45 (15.5%) TCD recipients. Among patients with GVHD (grade 2–4), ADV disease was 15 times more common in TCD compared to CONV HSCT recipients.
The characteristics of patients with ADV disease were compared to patients with ADV viremia without disease (Table 3). Among ADV disease cases, 93% were TCD HSCT recipients and 40% had grade 3–4 GVHD. The average maximum ADV viral load in ADV disease cases was 3 log10 higher compared to cases without disease, although 3 patients with autopsy-proven ADV disease had negative ADV PCR prior to death.
Table 3.
Comparison of Patients with ADV Viremia with and without ADV Disease
| Characteristics | ADV Viremia Only (N=28) No. (%) |
ADV Disease (N=15) No. (%) |
|---|---|---|
| Age at HSCT | ||
| Pediatric | 17 (61) | 6 (40) |
| Adult | 11 (39) | 9 (60) |
| HSCT Stem cell source | ||
| Peripheral blood | 16 (57) | 14 (93) |
| Bone marrow | 6 (21.5) | 1 (7) |
| Cord blood | 6 (21.5) | 0 (0) |
| Graft manipulation | ||
| T-cell depletion | 18 (64) | 14 (93) |
| None (Conventional) | 10 (36) | 1 (7) |
| Acute GVHD | ||
| Grade 0 – 2 | 22 (79) | 9 (60) |
| Grade 3 – 4 | 6 (21) | 6 (40) |
| Days to ADV viremia | ||
| Median (range) | 80 (10–722) | 125 (20–350) |
| Days to ADV disease | ||
| Median (range) | 125 (20 – 361) | |
| Maximum VL | ||
| Median copies/mL (range) | 6.9×104 (600–1×107) | 2×107 (100–>1× 1010) |
| Maximum VL >2×105 (copies/mL) | 9 (32) | 11 (73) |
| ALC, median (range) | 319 (0–1471) | 336 (17–1208) |
| Antiviral treatment | 13 (46) | 14 (93) |
| Resolution of viremia | 16 (57) | 4 (27) |
| OS at 3 months | 16 (57) | 8 (53) |
| OS at 6 months | 13 (46) | 2 (13) |
OS: Overall survival from diagnosis of ADV viremia
Abbreviations: ADV, adenovirus; HSCT, hematopoietic stem cell transplantation; ALC, absolute lymphocyte count (MCL); OS, overall survival; VL, viral load; GVHD, graft versus host disease
Cause of death of patients with ADV disease: attributed to ADV; 4 patients, GVHD; 6 patients, others (disease progression, other infections, and veno-occlusive disease); 4 patients
Ten (63%) patients with ADV disease had concomitant opportunistic infections including cytomegalovirus (CMV), polyoma BK virus, human herpes virus 6 (HHV-6), Epstein-Barr virus (EBV), toxoplasma, and invasive fungal infection.
Fourteen patients (93%) with ADV disease received antiviral treatment. Treatment was withheld in 1 patient because it was deemed futile. At 6 months after diagnosis of ADV viremia, 2 (13%) patients with ADV disease were alive compared to 13(46%) of patients with ADV viremia alone.
Overall, 14 patients (93%) with ADV disease died within the study period. Death was attributed to ADV in 4 (27%) patients, GVHD in 6 (40%), and other causes including progression of disease, other infection, and veno-occlusive disease in 4(26%) patients.
High ADV Viremia Associated with ADV Disease
Patients with ADV viremia were divided into 3 groups based on maximum ADV viral load: group 1 (< 10,000 copies/mL), group 2 (≥ 10,000 copies/mL), and group 3 (≥ 200,000 copies/mL). Table 4 shows clinical outcomes of the 3 groups. In Group 1, viremia resolved spontaneously in 7/8 (88%) patients; 1 patient with hemorrhagic cystitis and late onset of grade 4 acute GVHD was treated successfully with CMX001. The 3-month all-cause mortality was 13%, and no death was related to ADV. In contrast more patients in groups 2 and especially group 3 received antiviral treatments, developed ADV end-organ disease, and died of adenovirus (Table 4).
Table 4.
Comparison of ADV Viremia Cases by Maximum ADV Viral Load (N=43)
| Variables | Group 1 Total N=8 |
Group 2 Total N=15 |
Group 3 Total N=20 |
|---|---|---|---|
| Mx VL Median (range) | 3.6×103 (1×102–8866) | 1.8×104 (1.1×104–1.4×105) | 1.7×106 (2×105–> 1×1010) |
| Days to ADV | |||
| Median (range) | 76.5 (15 – 480) | 78 (19 – 674) | 91 (10 – 722) |
| Children (%) | 6 (75) | 19 (60) | 8 (40) |
| T-cell depleted (%) | 7 (88) | 9 (60) | 16 (80) |
| GVHD 3–4 (%) | 1 (13)a | 4 (27) | 7 (35) |
| ADV disease (%) | 1 (13) | 3 (20) | 11 (55) |
| Antiviral treatment (%) | 1 (13) | 9 (60) | 17 (85) |
| Resolution of viremia | 7 (88) | 9 (60) | 4 (20) |
| 3-month OS | 7 (87) | 7 (47) | 9 (45) |
Groups: group 1 low level ADV viremia <10,000 copies/mL, group 2 high level ADV viremia ≥10,000 copies/mL, group 3 very high level ADV viremia (≥200,000 copies/mL)
Abbreviations: Mx: Maximum; VL: viral load; GVHD, graft vs host disease; OS: overall survival from diagnosis of ADV viremia
Patient experienced late onset of severe acute GVHD of the skin.
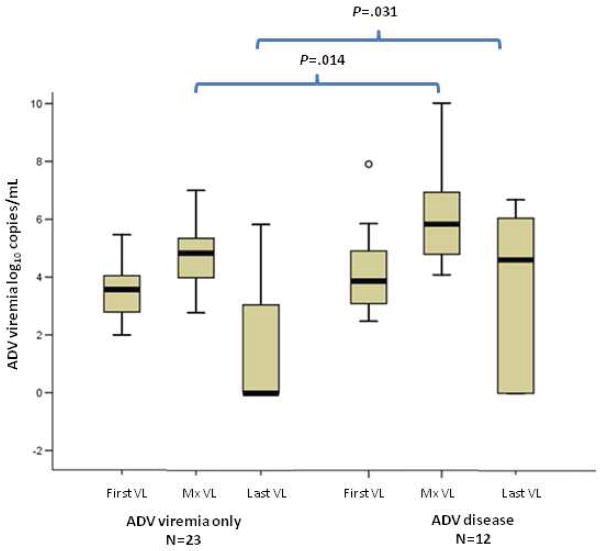
Thirteen of 28 (46%) patients with ADV viremia received antiviral treatment (ADV viremia resolved in 6 patients). In contrast, 14/15 patients (93%) with ADV disease received antiviral treatment (ADV viremia resolved in 4 patients). Figure 2 shows a comparison of first, maximum, and last available ADV viral load between patients with ADV viremia without disease and patients with ADV disease. The maximum viral load and last available viral loads were significantly higher in patients with ADV disease (P<.05).
Figure 2. Comparison of ADV viral load between patients with ADV viremia without disease (ADV viremia only) and with ADV disease.
First, maximum, and last available ADV viral loads were compared between the 2 groups. Patients with ≥3 positive ADV PCR were included.
First VL: First positive ADV PCR; Mx VL: Maximum ADV PCR, Last VL: Last available ADV PCR. Y axis: ADV viral load log10 (copies/mL). Boxplots: Shaded areas represent 5th to 95th percentile; Whiskers represent 95% confidence intervals. Circles represent outliers. Horizontal line within the boxes represents the median. Significant difference is marked: Maximum viral load was higher in ADV disease (P=.014); Last available viral load was higher in ADV disease (P=.031).
DISCUSSION
Adenovirus is an important infectious cause of mortality in HSCT (1–3, 10, 20). Reconstitution of virus-specific cytotoxic T-lymphocytes (CTLs) is essential for protection against viral pathogens after HSCT (21, 22). Recipients of T-cell depleted grafts experience lower rates of GVHD compared with those receiving conventional allografts, but because there is no transfer of virus specific CTLs from the donor, they are at increased risk for viral infections. T-cell depletion has been reported as a risk factor for ADV infections, but studies are limited by small size and variable degrees of T-cell depletion (7, 11, 22, 23). A limited number of studies have reported on the epidemiology and natural history of ADV infections in HSCT by prospective routine monitoring for ADV (23, 24). Because of the relative rarity of ADV disease and lack of effective and safe therapy, the majority of HSCT centers currently do not employ monitoring for ADV in clinical practice.
The main objective of our study was to describe the rates and risk factors for ADV disease in the era of ADV PCR in a cohort of 624 adult and pediatric patients comprised of 401 T-cell depleted, 131 conventional, and 92 cord blood allografts. During the study period, patients with clinical symptoms compatible with ADV infection were followed with viral cultures or ADV PCR, as clinically indicated. Because of lack of universal routine monitoring for ADV, we cannot accurately estimate the true incidence of ADV viremia in our cohort. Since ADV PCR testing was triggered by symptoms, patients with ADV viremia in our population would be expected to be “sicker”. This ascertainment bias may partially account for the relatively high rates of ADV disease and viral loads in our patients with ADV viremia.
A greater proportion of CONV as compared to TCD recipients were tested by stool culture or blood PCR for ADV. Higher rates of diarrhea, possibly related to GVHD, may have triggered more frequent testing in CONV HSCT. By 1 year post transplant the incidence of ADV in the stool was similar among TCD, unmodified, and cord blood allografts. We had previously reported 9% incidence of ADV enteritis in TCD HCST, suggesting a stable incidence of ADV enteritis at our institution over the last decade (2). In contrast the incidence of ADV viremia was significantly higher in TCD compared to conventional allografts (4% vs 7.9%). Among TCD recipients, pediatric patients had higher incidence of ADV viremia compared to adults.
In agreement with previous studies, young age and acute GVHD were independent risk factors of ADV viremia (11, 23, 25). ADV viremia was a prognostic factor for worse overall survival. Our approach of symptom-based testing is likely to have skewed the viremia population to a sicker group of patients with “significant” ADV viremia and profound immunosuppression.
A striking finding of our study is that while the incidence of ADV viremia was only 2-fold higher in TCD compared to CONV grafts, the risk for invasive ADV disease was roughly ten-fold higher in the TCD cohort, suggesting the critical importance of donor T-cells in controlling viral replication. Immunity against viruses is largely dependent on the recovery of T-lymphocytes and particularly CD4+ T cells. After conventional grafts, such recovery begins at about 2–4 months after transplant (26). Indeed the vast majority of ADV cases among CONV grafts occurred up to 4 months post transplant. Adult recipients of TCD allografts particularly those from unrelated donors have prolonged states of T-cell cytopenia and extreme CD4 lymphopenia. In contrast, reconstitution in pediatric recipients of TCD grafts resembles that reported in children after autologous or matched sibling donor conventional transplants. This suggests that differences in the host environment, such as the lack of a thymus, may underlie limitations of the immune recovery in adults (27). In fact among TCD grafts, pediatric age was not a risk factor for ADV disease in contrast to ADV viremia.
Checking ADV PCR on the basis of clinical suspicion did not lead to earlier diagnosis of ADV disease. The majority of patients had established disease at the time of the first positive ADV PCR. ADV disease was associated with 94% all-cause mortality, and was the primary cause of death in about one-third of all cases. The fatality rates of ADV disease further underscore the need for safe and effective therapy for ADV.
The decision to start antiviral treatment for ADV viremia was based on clinical presentation and degrees of immunosuppression. We examined the outcomes of ADV viremia based on the magnitude of ADV viral load. Eight patients (20%) had low-level ADV viremia (<10,000 copies/mL). Viremia was self-limited in all but 1 patient, and there was no death associated with ADV. Based on our findings, we suggest monitoring low-level viremia by serial ADV PCR. In contrast, the majority of patients with high-level ADV viremia failed to clear viremia despite treatment with cidofovir. Interestingly, 3 patients with ADV disease at autopsy died with negative ADV PCR. In our cohort, one-third of patients with ADV viremia developed invasive disease. Among TCD recipients, 58% patients with viremia developed ADV disease. Our data indicate that preemptive therapy of ADV viremia using a threshold of ≥10,000 copies/mL could be of benefit especially in recipients of TCD allografts. Median viral load at presentation was similar for patients who developed disease and those who did not, while maximum and last available viral loads were higher in patients with disease. The decision to institute antiviral therapy was based on individual patient risk for ADV disease. The last available viral load varied widely in ADV disease reflecting treatment effect or death with a high ADV viral load.
At present there is no approved antiviral treatment for ADV. Cidofovir has in vitro activity against ADV and has been used for the treatment of invasive ADV disease, but its efficacy has not been evaluated in randomized clinical trials (2, 24, 28–32). Preemptive treatment for ADV viremia has not been validated in clinical trials, so there is no established ADV viral load threshold for initiation of antiviral therapy. Erard et al showed that sustained high level viremia was a sensitive indicator of disease (33), while Walls et al reported that ADV viremia cleared spontaneously in 7 of 11 children monitored serially for ADV (3 TCD recipients and 4 CONV recipients) (11). Cidofovir is not an optimal candidate for preemptive therapy because of its toxicity profile, especially in the setting of compromised renal function or concomitant nephrotoxic medications. The novel oral broad-spectrum antiviral (CMX001) has activity against ADV, and is currently in clinical trials for preemptive treatment of ADV viremia in HSCT (34).
Transfer of ex vivo expanded T-cells to restore immunity to multiple viral pathogens could offer long-term restoration of ADV-specific immunity in recipients of T-cell-depleted HSCT and cord blood HSCT (20, 21, 35–37). Logistical constraints limit the broad applicability of this therapy at present. Donor derived antiviral cytotoxic T cells offer the potential of effectively transferring antiviral immunity to viral pathogens such as CMV and EBV without the risk of GVHD (38–40). This approach is currently being developed for ADV (35, 41). The presence of multiple ADV serotypes and low numbers of circulating ADV specific CTLs in healthy donors are some of the hurdles for adoptive immunotherapy for ADV.
Preemptive treatment prompted by a positive ADV PCR in the absence of clinical symptoms could reduce morbidity and potentially improve survival, if a safe and effective antiviral agent was available. Our study did not examine the rates of asymptomatic ADV viremia so we cannot estimate the number of patients needed to treat to prevent one case of ADV disease.
The identification of only a single case of adenovirus disease among 223 conventional transplants (including 92 cord blood transplants) attests to the rarity of this complication in both adults and children who receive T-cell replete grafts. High risk groups such as recipients of TCD allografts could be a good target population for pre-emptive therapy. Randomized clinical trials are needed to optimize management of ADV viremia especially in TCD HSCT and in patients with GVHD.
Acknowledgments
The authors thank to Ms. Carol Pearce for editorial assistance.
Financial disclosure: GAP has received research funding from Chimerix Inc and has been a clinical advisor for Chimerix Inc. Other authors have nothing to disclosure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flomenberg P, Babbitt J, Drobyski WR, et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 2.Symeonidis N, Jakubowski A, Pierre-Louis S, et al. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transplant Infect Dis. 2007;9:108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 3.Howard DS, Phillips IG, Reece DE, et al. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 1999;29:1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 5.Zaia JA. Prevention of cytomegalovirus disease in hematopoietic stem cell transplantation. Clin Infect Dis. 2002;35:999–1004. doi: 10.1086/342883. [DOI] [PubMed] [Google Scholar]
- 6.Zaia J, Baden L, Boeckh MJ, et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44:471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohrmalm L, Lindblom A, Omar H, et al. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. Bone Marrow Transplant. 2011;46:267–272. doi: 10.1038/bmt.2010.86. [DOI] [PubMed] [Google Scholar]
- 8.Bil-Lula I, Ussowicz M, Rybka B, et al. PCR diagnostics and monitoring of adenoviral infections in hematopoietic stem cell transplantation recipients. Arch Virol. 2010;155:2007–2015. doi: 10.1007/s00705-010-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omar H, Yun Z, Lewensohn-Fuchs I, et al. Poor outcome of adenovirus infections in adult hematopoietic stem cell transplant patients with sustained adenovirus viremia. Transplant Infect Dis. 2010;12:465–469. doi: 10.1111/j.1399-3062.2010.00528.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganzenmueller T, Buchholz S, Harste G, Dammann E, Trenschel R, Heim A. High lethality of human adenovirus disease in adult allogeneic stem cell transplant recipients with high adenoviral blood load. J Clin Virol. 2011;52:55–59. doi: 10.1016/j.jcv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Walls T, Hawrami K, Ushiro-Lumb I, Shingadia D, Saha V, Shankar AG. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis. 2005;40:1244–1249. doi: 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 12.Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24:706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 13.Schilham MW, Claas EC, van Zaane W, et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis. 2002;35:526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 14.Grimley MS, Marsh RA, Bleesing JJ, et al. Cmx001 as Therapy for Severe Adenovirus Infections in Immunocompromised Pediatric Patients: Single Experience in 5 Patients. Biol Blood Marrow Transplant. 2012;18:S315–S315. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florescu DF, Pergam SA, Neely MN, et al. Safety and Efficacy of CMX001 as Salvage Therapy for Severe Adenovirus Infections in Immunocompromised Patients. Biol Blood Marrow Transplant. 2012;18:731–738. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 18.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 19.Heller G, Venkatraman ES. Resampling procedures to compare two survival distributions in the presence of right-censored data. Biometrics. 1996;52:1204–1213. [Google Scholar]
- 20.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 21.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 22.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lion T, Baumgartinger R, Watzinger F, et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102:1114–1120. doi: 10.1182/blood-2002-07-2152. [DOI] [PubMed] [Google Scholar]
- 24.Anderson EJ, Guzman-Cottrill JA, Kletzel M, et al. High-risk adenovirus-infected pediatric allogeneic hematopoietic progenitor cell transplant recipients and preemptive cidofovir therapy. Pediatr Transplant. 2008;12:219–227. doi: 10.1111/j.1399-3046.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 26.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–2779. [PubMed] [Google Scholar]
- 27.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 28.Fowler CJ, Dunlap J, Troyer D, Stenzel P, Epner E, Maziarz RT. Life-threatening adenovirus infections in the setting of the immunocompromised allogeneic stem cell transplant patients. Adv Hematol. 2010;2010:601548. doi: 10.1155/2010/601548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 30.Robin M, Marque-Juillet S, Scieux C, et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92:1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf U, Hale GA, Carr J, et al. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81:1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- 32.Bhadri VA, Lee-Horn L, Shaw PJ. Safety and tolerability of cidofovir in high-risk pediatric patients. Transplant Infect Dis. 2009;11:373–379. doi: 10.1111/j.1399-3062.2009.00391.x. [DOI] [PubMed] [Google Scholar]
- 33.Erard V, Huang ML, Ferrenberg J, et al. Quantitative real-time polymerase chain reaction for etection of adenovirus after T cell-replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clin Infect Dis. 2007;45:958–965. doi: 10.1086/521851. [DOI] [PubMed] [Google Scholar]
- 34.National Institute of Health; Chimerix. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2010. A Randomized, Placebo-Controlled Multi-Site Phase 2 Study Evaluation the Safety and Efficacy of Preemptive Treatment With CMX001 for the Prevention of Adenovirus Disease Following Hematopoietic Stem Cell Transplantation in Adults and Children. [cited 2012 May 17]. Available from: http://clinicaltrials.gov/show/NCT01241344 NLM Identifier: NCT012413441. [Google Scholar]
- 35.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sili U, Leen AM, Vera JF, et al. Production of good manufacturing practice-grade cytotoxic T lymphocytes specific for Epstein-Barr virus, cytomegalovirus and adenovirus to prevent or treat viral infections post-allogeneic hematopoietic stem cell transplant. Cytotherapy. 2012;14:7–11. doi: 10.3109/14653249.2011.636963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagliara D, Savoldo B. Cytotoxic T lymphocytes for the treatment of viral infections and posttransplant lymphoproliferative disorders in transplant recipients. Curr Opin InIinfect Dis. 2012;25:431–437. doi: 10.1097/QCO.0b013e3283551dd3. [DOI] [PubMed] [Google Scholar]
- 40.Gerdemann U, Vera JF, Rooney CM, Leen AM. Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J Vis Exp. 2011;(51):2736. doi: 10.3791/2736. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson H, Brewin J, Kinnon C, Veys P, Amrolia PJ. Generation of trispecific cytotoxic T cells recognizing cytomegalovirus, adenovirus, and Epstein-Barr virus: an approach for adoptive immunotherapy of multiple pathogens. J Immunother. 2007;30:544–556. doi: 10.1097/CJI.0b013e3180335b7a. [DOI] [PubMed] [Google Scholar]