Abstract
Over a century ago, Ramon y Cajal first proposed the idea of a directionality involved in nerve conduction and neuronal communication. Decades later, it was discovered that myelin, produced by glial cells, insulated axons with periodic breaks where nodes of Ranvier (nodes) form to allow for saltatory conduction. In the peripheral nervous system (PNS), Schwann cells are the glia that can either individually myelinate the axon from one neuron or ensheath axons of many neurons. In the central nervous system (CNS), oligodendrocytes are the glia that myelinate axons from different neurons. Review of more recent studies revealed that this myelination created polarized domains adjacent to the nodes. However, the molecular mechanisms responsible for the organization of axonal domains are only now beginning to be elucidated. The molecular domains in myelinated axons include the axon initial segment (AIS), where various ion channels are clustered and action potentials are initiated; the node, where sodium channels are clustered and action potentials are propagated; the paranode, where myelin loops contact with the axolemma; the juxtaparanode (JXP), where delayed-rectifier potassium channels are clustered; and the internode, where myelin is compactly wrapped. Each domain contains a unique subset of proteins critical for the domain’s function. However, the roles of these proteins in axonal domain organization are not fully understood. In this review, we highlight recent advances on the molecular nature and functions of some of the components of each axonal domain and their roles in axonal domain organization and maintenance for proper neuronal communication.
Keywords: myelin, axon initial segment, node, paranode, juxtaparanode, caspr, neurofascin, contactin, sodium channels, potassium channels, ankyrin G, band 4.1, PSD93/95
The axon initial segment (AIS) is the 20–40 µm site of the axon proximal to the soma, just after the axon hillock (Hedstrom and Rasband, 2006). All of the excitatory and inhibitory inputs to the neuron are summed at the AIS, and a “decision” is made whether or not to fire an action potential. Clustered at the AIS are ion channels, including sodium (Na+) channels responsible for action potential initiation, NaV1.6, and potassium channels, KV1.1/1.2 and potassium channel voltage-gated KQT-like subfamily (KCNQ2/3), which are important for modulating neuronal excitability and regulating action potential frequency (see Figs. 1, 2A; Pan et al., 2006; Kole et al., 2008). There are also several cell adhesion molecules (CAMs) belonging to the L1 subfamily of immunoglobulin (Ig) CAMs, including the 186-kDa isoform of Neurofascin (NfascNF186), neuron-related cell adhesion molecule (NrCAM), contactin-associated protein-2 (Caspr2), and transiently expressed axonal surface glycoprotein-1 (Tag1; Fig. 2A; Dodd et al., 1988; Davis et al., 1996; Hedstrom et al., 2007; Ogawa et al., 2008; Buttermore et al., 2012). On the intracellular side of the AIS, there are many adaptor and scaffolding proteins, including ankyrinG (AnkG), βIV-spectrin, and postsynaptic density protein 93 (PSD-93; see Fig. 2A; Jenkins and Bennett, 2001; Kole et al., 2008). The adaptor protein AnkG binds transmembrane proteins, including voltage-gated sodium channels and CAMs and links them to the underlying actin/spectrin cytoskeleton through scaffolding proteins, including βIV-spectrin (see Fig. 2A; Zhou et al., 1998; Boiko et al., 2007). NfascNF186 and NrCAM are able to link to AnkG through a conserved motif that is mediated by tyrosine phosphorylation, such that the unphosphorylated motif can bind with AnkG (Davis et al., 1996; Garver et al., 1997; Zhang et al., 1998; Lustig et al., 2001). The accumulation of these complexes is necessary for clustering and stabilization of NaV channels at the AIS (Zhou et al., 1998; Hedstrom et al., 2007; Zonta et al., 2011; Buttermore et al., 2012).
Fig. 1.
Axonal domains in central and peripheral myelinated axons. A: Wild-type cerebellar section immunostained against calbindin (Calb, red), myelin basic protein (MBP, green), and Caspr (blue) highlights the segregation of axonal domains in Purkinje neuron (P) myelinated axons. B: Representative drawing of a Purkinje neuron (P) showing the localization of molecular domains in myelinated axons, including the axon initial segment (AIS), node of Ranvier (node), paranode, and myelinated internode. C: Teased wild-type sciatic nerve fiber immunostained against potassium channels (KV1.2, red), Nfasc186 (NF186, green), and Caspr (blue) highlights the distinct axonal domains flanking the node. D: Representative drawing of the axonal domains flanking the node, which reflects the distinct segregation of each of these domains. Scale bars = 10 µm.
Fig. 2.
Molecular organization of the Purkinje neuron AIS. A: Diagram of molecular components at the AIS. B: Wild-type Purkinje neuron immunostained against Calb (green) and Nfasc (red). C: AnkG−/− Purkinje neuron immunostained against Calb (green) and Nfasc (red) highlights the diffusion of Nfasc distally from the AIS in the absence of AnkG (Ango et al., 2004). D,E: Cultured hippocampal neurons with AnkG shRNA (green cell) or without AnkG shRNA (cell with star) reveal that Nfasc (PAN NF, D) and NaV channels (PAN NaV; E) are absent from the AIS when AnkG is knocked down (Hedstrom et al., 2007). F–I: Cerebellar sections from P10 (F,G) and P20 (H,I) wild-type (F,H) and Pcp2-Cre; NfascFlox/Flox(G,I) mice immunostained against NaV1.6 (a, green), AnkG (b, red), and Calb (c, blue, merged) show that the mature isoform of NaV channels, NaV1.6, clusters to the distal part of the AIS at P10 and fails to localize at the AIS proper at P20 in the absence of Nfasc. Purkinje cell protein 2-Cre (Pcp2-Cre) is expressed specifically in Purkinje neurons in the cerebellum. J–L: Cerebellar sections from wild-type (J), Pcp2-Cre;NfascFlox/Flox(K), and Parv-Cre;NfascFlox/Flox(L) mice immunostained against Calb (red) and KV1.2 (green) reveal severely disorganized pinceau surrounding the Nfasc-deficient Purkinje AIS (Buttermore et al., 2012). Parv-Cre is expressed in parvalbumin-expressing GABAergic neurons throughout the nervous system. Scale bars = 10 µm. (B,C: Reprinted from Cell 2004; 119:257–272, with permission from Elsevier; D,E: © Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN, Neurofascin assembles a specialized extracellular matrix at the axon initial segment; originally published in J Cell Biol 2007; 178:875–886; J–L: Reprinted from J Neurosci 2012; 32:4724–4742, with permission from The Journal of Neuroscience.)
Interestingly, recent reports show that the AIS itself is segregated into proximal and distal domains based on the localization of different sodium channel isoforms with different voltage-sensitivities, such that NaV1.2 clusters at the proximal AIS and NaV1.6 is enriched at the distal AIS (Hu et al., 2009; Buttermore et al., 2012). Furthermore, electrophysiological studies show that sodium channels localized to the distal AIS are activated at a lower voltage threshold than sodium channels at the proximal AIS (Hu et al., 2009). It has been suggested that the distal sodium channels, which have a lower voltage threshold, are responsible for action potential initiation and that the proximal sodium channels, which have a higher voltage threshold, are responsible for action potential back-propagation. Thus, the segregation of these sodium channel isoforms and the precise organization of the AIS are critical for regulating neuronal activity.
FORMATION OF THE AIS
Although the organization of most axonal domains requires intercellular communication, the organization of the AIS occurs intrinsically through the localization of the “AIS master regulator,” AnkG (Bennett and Baines, 2001). Ankyrins are known for their ability to stabilize groups of membrane-bound proteins to specific molecular domains for efficient signaling and interactions between cells (Bennett and Baines, 2001). The critical role for AnkG in AIS organization was discovered in several independent in vivo and in vitro studies (Hedstrom et al., 2007; Zhou et al., 1998). In one study, genetic ablation of AnkG in the cerebellum of mice resulted in loss of clustering of Nfasc and ion channels at the Purkinje AIS (Fig. 2B,C; Zhou et al., 1998; Ango et al., 2004). In addition, knockdown of AnkG using shRNA in cultured hippocampal neurons resulted in failure of all other AIS components to cluster at the AIS (Fig. 2D,E; Hedstrom et al., 2007). However, knockdown of other AIS components, including Nfasc, NrCAM, and βIV-spectrin, did not disrupt the enrichment of the other AIS components. These studies were further supported by recent work showing that the AnkG-binding domain of sodium channels NaV1.6 is required for the localization of ion channels at the AIS (Gasser et al., 2012). Together these results point to a role for AnkG as the master organizer of the AIS.
Importantly, loss of AnkG also resulted in disrupted axonal polarity, with the formation of spines and the mislocalization of dendritic proteins in Purkinje neuron AISs lacking AnkG (Sobotzik et al., 2009). Another study showed that AnkG is also required for AIS stability, insofar as knockdown of AnkG in mature cultured hippocampal neurons with already formed AIS prior to shRNA treatment led to its destabilization (Hedstrom et al., 2008). In these adult AnkG knockdown neurons, AIS markers were no longer clustered at the AIS, and the process that had been the axon contained both axonal and dendritic markers, whereas the other processes contained only dendritic markers. Importantly, in vivo ablation of AnkG from Purkinje neurons did not disrupt the ability of the Purkinje neuron to form an axon, but the axonal projection did contain dendritic spines (Sobotzik et al., 2009). Therefore, in the absence of AnkG, AIS does not form properly, and axonal specification is compromised. The signals responsible for AnkG clustering at the AIS are the focus of many ongoing studies. One study suggested that phosphorylated inhibitor of κBα (κBα) may function as a cofactor in AnkG trafficking to the AIS (Schultz et al., 2006; Sanchez-Ponce et al., 2008; Rasband, 2010). The inhibitor of κBα is known to be important for neurite outgrowth, synaptic plasticity, and neuronal cell survival, and it regulates the transcription factor nuclear factor-κB (Jacobs and Harrison, 1998; Zhang et al., 2005; O’Mahony et al., 2006; Buffington et al., 2012). However, a recent study suggests that phosphorylated inhibitor of κBα is not required for AIS formation (Buffington et al., 2012). Another study suggests that AnkG becomes clustered at the AIS through the formation of a distal axonal cytoskeleton boundary consisting of AnkB, αII-spectrin, and βII-spectrin, which prevents AnkG from localizing to the submembraneous cytoskeleton distal to the AIS (Galiano et al., 2012). However, the in vivo and in vitro results cannot be reconciled, and further studies are required to determine the precise signaling mechanisms responsible for AnkG clustering at the AIS.
AIS ACTS AS SELECTIVE MOLECULAR BARRIER FOR AXONAL TRANSPORT
The loss of axonal polarity and invasion of dendritic markers found in Purkinje neurons that lack AnkG reflects a role of the AIS as a sieve, preventing the diffusion of dendritic or somatic proteins into the axon. It also reflects the idea that dendritic fate of neuronal projections is the default choice and that AnkG and the AIS are needed to maintain the barrier that regulates axonal specification. The role of the AIS as a diffusion barrier was first discovered when it was found that some membrane proteins diffused through the AIS more slowly than others (Winckler et al., 1999). Furthermore, disruption of the F-actin cytoskeleton prevented this decreased mobility, suggesting that the AIS barrier depends on the interaction of membrane proteins with the cytoskeleton. Another study followed individual fluorescently tagged unsaturated phospholipids and showed that their diffusion is blocked in the AIS membrane at the same time in development as when the clustering of AIS proteins occurs (Nakada et al., 2003). Importantly, the barrier function of the AIS restricts both membrane-bound and cytoplasmic proteins from diffusing into the axon. A more recent study found that larger dextrans could not diffuse into the axon after AIS formation, whereas smaller dextrans could (Song et al., 2009). As in previous studies, disruption of the F-actin cytoskeleton disrupted the function of the AIS barrier for cytoplasmic diffusion. It was also observed that axonal entry of kinesin super-family motor proteins was dependent on the cargo that they carried; dendritic cargos were prevented from entering the axon after the AIS was formed (Song et al., 2009). These results show that the AIS is not simply a structure for action potential initiation, it is also critical for maintaining axonal specification.
FUNCTIONAL MATURATION OF THE AIS
Although the initial organization of the AIS requires AnkG localization to this domain, recent data suggest that the maturation of the AIS depends on NfascNF186 expression (Buttermore et al., 2012). The developmental switch of NaV1.2 to NaV1.6 at the AIS is critical for AIS function in the adult myelinated axons (Boiko et al., 2003; Van Wart and Matthews, 2006). Ablation of Nfasc specifically in the Purkinje neurons prevents the maturation of the Purkinje AIS (Buttermore et al., 2012). In the absence of Nfasc, the mature voltage-gated sodium channel isoform, NaV1.6, fails to become enriched at the Purkinje AIS, as it does at the wild-type Purkinje AIS (Fig. 2F – I) suggesting that different mechanisms are responsible for the initial formation and secondary maturation of the AIS. Interestingly, recent studies show that an intact Purkinje AIS is not required for induction of Purkinje neuron firing (Zonta et al., 2011; Buttermore et al., 2012). However, the waveform of the resulting action potential is altered (Zonta et al., 2011). In addition, spontaneous firing of the Purkinje cells is disrupted with loss of the AIS (Zonta et al., 2011; Buttermore et al., 2012). These results suggest that functional maturation of the AIS is required for normal neuronal firing. Fascinating recent studies have also revealed that AIS is a plastic structure that changes in response to neuronal activity (Grubb and Burrone, 2010; Kuba et al., 2010). It is interesting to speculate that the mechanisms responsible for changes in AIS structure during plastic phases are similar to those responsible for the maturation of the AIS.
PLASTICITY OF THE AIS
The discovery that AIS is a plastic structure reinforces the importance of the AIS in the regulation of neuronal activity. Previous authors alluded to the idea that the AIS is not a static structure, because the tuning frequency, AIS size, and location of the AIS differ between neuronal subtypes and brain regions, depending on the requirement of the individual neurons within their circuits (Kuba et al., 2006; Lorincz and Nusser, 2008; Grubb et al., 2011). However, the first direct evidence of AIS plasticity was not revealed until more recently in two separate studies. In one study, auditory deprivation led to an increase in AIS length in auditory neurons of the avian brainstem (Kuba et al., 2010). In another study, evidence for AIS plasticity was uncovered through recent technological advances that allow for live imaging of a functioning AIS through imaging of ion flux (Grubb and Burrone, 2010; Grubb et al., 2011). This study revealed that the AIS is able to shift its position distally along the axon in vitro when it is exposed to increased excitatory activity over a long period (Grubb and Burrone, 2010). This shift in position of the AIS occurred after 2 days of prolonged stimulation and required voltage-gated calcium channel activity. These results suggest that the AIS is able to react to its environment so that it can control its firing rate, in this case decreasing the probability of firing by moving farther down the axon. Thus, AIS is crucial in regulating neuronal activity. However, it remains to be seen whether this AIS plasticity is found in older adult stages as well and whether AIS becomes more stable in adult stages. Interestingly, Nfasc is also critical for the long-term stabilization of the AIS (Zonta et al., 2011). It is likely that distinct mechanisms are in place to maintain the organization of the AIS, because its proper functioning is critical for normal neuronal activity.
REGULATION OF AIS FUNCTION
Whereas cell-autonomous mechanisms may be responsible for the organization and plasticity of the AIS, the AIS is frequently targeted by inhibitory interneurons to aid in modulating neuronal firing. Examples of this can be found in the targeting of chandelier cells to the pyramidal AIS in the cortex and basket cell targeting to the Purkinje AIS in the cerebellum. These interactions have been shown to be critical for proper function of the targeted neuron. For example, the function of the cerebellum is to regulate motor coordination, and the Purkinje cell summates all inputs to the cerebellum and is the sole output source of the cerebellum, so its activity must be tightly regulated (Palay and Palay, 1974). This restriction is achieved, in part, by the basket cell, which is an inhibitory interneuron in the molecular layer that sends projections to the Purkinje AIS (Sotelo, 2008). An extraordinary feature of the basket cell is its axon, which splits into many axonal collaterals that innervate five to seven Purkinje cells. Basket axon collaterals extend around the Purkinje soma, synapsing on the Purkinje AIS and forming the pinceau. Inhibition of the Purkinje AIS is achieved through γ-aminobutyric acid (GABA)-ergic synapses that basket axons form with the Purkinje neuron, releasing the inhibitory neurotransmitter GABA to dampen Purkinje cell excitability (Purves et al., 2004; Huang et al., 2007). A single basket cell innervates several Purkinje cells, allowing for coordinated modulation of a group of Purkinje cells (Ango et al., 2004; Buttermore et al., 2012). Potassium channels (KV1.2) are also enriched at the basket axon terminals of the pinceau (Laube et al., 1996). Importantly, the pinceau has been shown to play a critical role in Purkinje neuron function. One study showed that disruption of pinceau organization results in ataxia, a consequence of Purkinje neuron dysfunction (Xie et al., 2010). The authors utilized a forward genetic screen with mice treated with ENU to identify mouse mutants that displayed motor coordination deficits. This screen uncovered a mouse line that had a missense mutation in the α-subunit of KV1.2 channels. These mice showed a disrupted pinceau structure and cerebellar activity, resulting in ataxia. Interestingly, loss of KV1.2 function in basket interneurons in the cerebellum resulted in hyperactive basket neurons, which resulted in decreased Purkinje neuron firing, so it seems that KV1.2 channels in basket neurons are functioning to dampen neuronal excitability at the pinceau. Upon re-expression of the potassium channels, the ataxia phenotype was partially rescued, reinforcing the importance of the inhibitory balance at the pinceau on Purkinje neuron function.
Although the Purkinje neuron depends on the pinceau for proper function, the Purkinje AIS also plays an important role in the developmental formation and long-term maintenance of the pinceau (Ango et al., 2004; Huang, 2006; Zonta et al., 2011; Buttermore et al., 2012). In one study, the authors found that ablation of AnkG resulted in mislocalization of Nfasc distally down the Purkinje axon, with basket axons following aberrantly localized Nfasc (Ango et al., 2004). In addition, expression of a dominant-negative Nfasc construct in Purkinje neurons prevented the clustering of synaptic marker glutamate decarboxylase-65 (GAD65) at the pinceau (Ango et al., 2004). More recently, it was shown that ablation of Nfasc in adult neurons results in destabilization of the pinceau after 16 weeks (Zonta et al., 2011). Finally, our recent studies utilized cell-specific ablation of Nfasc to show that Nfasc is required in both Purkinje neurons and basket neurons for pinceau organization (Fig. 2J–L; Buttermore et al., 2012). We observed that Nfasc in the Purkinje AIS is required for the stabilization and maintenance of the interaction between the basket axon terminals and the Purkinje axon. We also uncovered that, in the basket axon, Nfasc likely is required for proper basket axon branching and outgrowth toward the Purkinje AIS. Interestingly, a role for Nfasc in GABAergic synapse organization was also found in the developing hippocampus, where Nfasc induces clustering of gephyrin, a postsynaptic scaffolding protein required for recruiting GABA receptors, at the future AIS (Burkarth et al., 2007). In addition, in the adult dentate gyrus, Nfasc stabilizes GABAergic synaptic components (Kriebel et al., 2011). Furthermore, Nfasc has also been shown to cluster the extracellular matrix (ECM) molecule brevican to the extracellular space surrounding the AIS, suggesting that the AIS plays a role in the organization of the ECM (Hedstrom et al., 2007). The ECM surrounding the AIS may be important for buffering ions in the area or for maintaining synaptic contact, inasmuch as perineuronal nets have been shown to function elsewhere in the brain (Celio et al., 1998; Hedstrom et al., 2007). Together these data reveal a conserved mechanism in which AIS organization is critical for targeting of GABAergic synapses for proper regulation of the postsynaptic neuron. Therefore, although the AIS is the one axonal domain that forms independent of other cellular influence, its proper function relies heavily on synaptic input and modulation from the extracellular environment.
AIS IN DISEASE AND INJURY
The importance of in vivo genetic models for elucidating the mechanisms by which the AIS is organized and maintained are underscored by a recent report showing that the AIS is a target for injury during ischemia (Schafer et al., 2009). This study showed that, during stroke, AnkG and bIV-spectrin were proteolyzed by calpain, which disrupted AIS, resulting in loss of neuronal activity. This damage to the AIS resulted independently of axonal degeneration or programmed cell death, revealing the vulnerability of AIS. Dysfunction of the AIS has also been found in diseased brain states. In one study, the AISs of pyramidal neurons in the hippocampal area CA1 of Angelman syndrome mice were found to be elongated, and intrinsic membrane properties were altered (Kaphzan et al., 2011). The initial resting potential of neurons in the Angelman syndrome mice was more hyperpolarized, because of increased expression of sodium/potassium channel pumps. In addition, action potential amplitude and its maximal rate of rise were greater in Angelman syndrome mice, as a result of the increased NaV1.6 and AnkG found at the AIS in the hippocampus, but not the somatosensory cortex. Together these alterations resulted in decreased neuronal excitability (Kaphzan et al., 2011). Therefore, it is critical to maintain the balance of ion channel expression at the AIS for proper neuronal function.
In schizophrenic patients, a decrease in GABA release at the synapses between chandelier neurons and the cortical pyramidal AIS has been observed (Lewis et al., 2005; Rasband, 2010). Although the mechanisms responsible for this decrease in GABA transmission have not been elucidated, another article noted a decrease in AnkG in the superficial cortical layers of schizophrenic patients (Cruz et al., 2009). As previously stated, a decrease in AnkG disrupts localization of AIS components, including Nfasc, and disruption of Nfasc alters GABA receptor clustering and targeting of GABAergic synapses to the AIS (Ango et al., 2004; Burkarth et al., 2007; Cruz et al., 2009; Buttermore et al., 2012). Therefore, it is possible that alterations in AIS stability and function may create an imbalance of neuronal activity that is observed in schizophrenic patients.
Imbalances in excitatory and inhibitory transmission throughout the brain are thought to be at the root of neurological disorders such as epilepsy and seizures. It is well known that sodium channel mutations can cause epilepsy. One study used Drosophila S2 cells to show that mutations in sodium channel β1-subunits disrupts β1-β1 homophilic interactions that are required for proper sodium channel localization (Meadows et al., 2002). It was suggested that the reason for the seizure activity in animals with β1-subunit NaV channel mutations is that the mutation resulted in altered subcellular localization of the channels, resulting in hyperexcitable neurons. Importantly, another study revealed that AnkG and NaV1.6 levels were increased at the AIS in an animal model for epilepsy (Chen et al., 2009). Therefore, a conserved mechanism for seizure activity seems to include an increase in sodium channel activity, possibly at the AIS. Each of these cases reinforces the fact that a fine balance of ion channel function is required within the axon, including the AIS, for proper neuronal function.
THE NODE OF RANVIER (NODE)
Similar to the AIS, nodes are enriched with voltage-gated sodium channels. However, nodes are only about 1 lm long and are critical for action potential propagation (Salzer, 2003; Hedstrom and Rasband, 2006; Rasband, 2006; Thaxton and Bhat, 2009). Action potentials initiated in the AIS move from node to node by way of sodium channel currents generated by the channels clustered at these domains. Therefore, the precise localization of sodium channels to the node is a critical process during the development of myelinated axons. Interestingly, many of the proteins that localize to the AIS are also enriched at the nodes, including NaV1.2, NaV1.6, KCNQ2/3, βIV-spectrin, AnkG, NrCAM, and NfascNF186 (Fig. 3A; Berghs et al., 2000; Boiko et al., 2001; Komada and Soriano, 2002; Salzer, 2003). Similar to the AIS, AnkG interacts with NaV1.6, CAMs, and the underlying cytoskeleton through βIV-spectrin at the node (Bennett and Lambert, 1999). Also similar to the AIS is a developmental switch in NaV channel isoform expression, from NaV1.2 in immature nodes to NaV1.6 in mature nodes (Boiko et al., 2001; Kaplan et al., 2001; Rios et al., 2003). This developmental switch of sodium channel isoforms from NaV1.2 to NaV1.6 is thought to require myelination. One study revealed that, in Shiverer mutant mice, which have a mutation in the myelin basic protein (MBP) gene resulting in disrupted compact myelin, NaV1.2 channels remain localized to axons, with an absence of NaV1.6, suggesting that myelination is important for signaling the developmental switch to NaV1.6 (Boiko et al., 2001). Other in vitro studies showed that NaV1.6 would not cluster at nodes of unmyelinated axons in the presence of oligodendrocytes-conditioned medium, whereas NaV1.2 would, suggesting that the switch from NaV1.2 to NaV1.6 requires myelination (Kaplan et al., 2001). However, other studies have shown that rats with dis-rupted myelin, paranodes, and juxtaparanodes, show normal expression of NaV1.2 and NaV1.6 (Arroyo et al., 2002). Contributing to the debate, another study examined sodium channel isoform expression in the demyelinating experimental allergic encephalomyelitis (EAE) mouse model of multiple sclerosis (MS) and showed that there was a decrease in the developmental switch from NaV1.2 to NaV1.6 (Craner et al., 2003). Interestingly, another study showed that CNS, but not PNS, nodes of Ranvier require intact paranodal junctions for the developmental switch from NaV1.2 to NaV1.6 (Rios et al., 2003). Together these studies reveal that myelination likely plays a critical role in the developmental switch from NaV1.2 to NaV1.6 at the node. However, the precise mechanisms regulating this switch, as well as the mechanisms of nodal organization, are still not well understood and may be quite different from those of the AIS.
Fig. 3.
Organization of the node and formation of a molecular barrier against invading paranodes. A: Diagram of molecular components of the PNS node. B–E: Sciatic nerve (B,C) and spinal cord (D,E) fibers from wild-type (B,D) and Nefl-Cre;NfascFlox (C,E) mice immuno-stained against Caspr (a, green), pan-NaV channels (b, red), and NfascNF186 (c, blue) reveal loss of NaV channel clustering at the node in the absence of NfascNF186 (Thaxton et al., 2011). Nefl-Cre allows neuron-specific expression of Cre under the neurofilament light chain promoter. F–I: Electron micrographs of sciatic nerve (F,G) and spinal cord (H,I) fibers from wild-type (F,H) and Nefl-Cre;NfascFlox (G,I) mice reveal the aberrant overlapping of adjacent paranodal loops when the node has been disrupted by loss of NfascNF186 (Thaxton et al., 2011). Scale bars 5 0.5 µm. (B–I: Reprinted from Neuron 2011; 69:244–257, with permission from Elsevier.)
The spacing of nodes, as well as their assembly, is largely coordinated with the help of myelinating glia (Susuki and Rasband, 2008; Thaxton et al., 2011). In some regions of the nervous system, the spacing of nodes is critical for their function. For example, in the avian brainstem, the spacing between nodes and the diameter of axons are controlled so that interaural time differences can be detected (Seidl et al., 2010). In addition, the spacing of nodes is coordinated to allow for decreased membrane capacitance and increased membrane resistance along the myelinated internode, the necessary ingredients for saltatory conduction (Hille, 2001). For these reasons, many studies have focused on understanding how nodes are organized.
Formation of the Node
Although the AIS forms intrinsically, depending on the localization of AnkG before sodium channels and CAMs become clustered, the node develops with the aid of external signals (Pedraza et al., 2001; Eshed et al., 2005). Interestingly, the mechanisms of node formation in the PNS vs. the CNS are thought to occur differently and likely are due to the differences in glial contribution to the process. In the PNS, Schwann cells produce proteins that localize to Schwann cell microvilli, protrusions that extend from the Schwann cell toward the nodal gap, and proteins that are secreted to become part of the nodal ECM (Eshed et al., 2005, 2007). The nodal ECM contains various glycoproteins that are important for stabilizing Schwann cell microvilli at the node (Saito et al., 2003; Melendez-Vasquez et al., 2005). It is generally agreed that initial binding of the Schwann cell protein gliomedin to neuronal NfascNF186 allows for the recruitment and stabilization of other nodal proteins (Lambert et al., 1997; Eshed et al., 2005; Schafer et al., 2006). Gliomedin interacts with the extracellular domain of NfascNF186, which contains six immunoglobulin domains, three fibronectin type III repeats, and a mucin domain (Thaxton and Bhat, 2009). Knockdown of gliomedin resulted in disrupted clustering of nodal proteins, including NaV channels and NfascNF186 (Eshed et al., 2005). Interestingly, Eshed et al. also revealed that ec-topic gliomedin clusters formed along internodes of myelinating Schwann cell dorsal root ganglion (DRG) neuron cocultures when the extracellular domain of NfascNF186 was added to the media. However, more recent work performed in gliomedin knockout mice showed that loss of gliomedin did not disrupt mature node formation in the PNS of adult mice (Feinberg et al., 2010). Instead, loss of gliomedin only disrupted NaV channel localization at heminodes during early development. These results suggest that gliomedin may be important for initial NaV channel clustering at heminodes, although its role in node formation and stabilization should be further elucidated. Interestingly, Feinberg et al. also showed that Schwann cell expression of NfascNF155 in Nfasc null DRG cocultures allowed for clustering of nodal proteins at mature nodes. In addition, loss of NfascNF186 or NrCAM disrupted heminodal sodium channel clustering. Together these data suggest that gliomedin, NrCAM, and NfascNF186 are required for heminode sodium channel clustering, and, in the absence of heminodal clustering, the paranodes can aid in sodium channel accumulation at nodes. However, nodes form properly in the PNS of paranodal mutants, suggesting that paranodes may play a supportive role but are not necessary for nodal organization (Bhat et al., 2001; Thaxton et al., 2011). Further work is required to elucidate the precise mechanisms that underlie nodal organization and the role that the paranodes play during node formation.
When NfascNF186 becomes localized to the node, it recruits AnkG to the nodal domain through its FIGQY motif (Garver et al., 1997). NaV channels in turn bind to AnkG, and their β-subunit can also bind to Nfasc (Malhotra et al., 2000; Lemaillet et al., 2003; Rasband, 2008). The role for NfascNF186 at the PNS nodes has been further verified by in vitro knockdown studies showing that loss of NfascNF186 disrupts NaV channel clustering at the nodes (Dzhashiashvili et al., 2007). Dzhashiashvili et al. also showed that the external domain of NfascNF186 is required for its role in nodal organization and NaV channel accumulation, reinforcing the likely role of glia in nodal organization. In addition, knockdown of NfascNF186 cannot be rescued by NfascNF186 missing its AnkG-binding domain, suggesting that NfascNF186 recruits AnkG and subsequently NaV channels to the node (Dzhashiashvili et al., 2007). However, NfascNF186 and NaV channels are able to localize to the node, but do not become stabilized, in the absence of AnkG binding (Zhang et al., 2012). Another study revealed that the AnkG binding domain of sodium channels is both necessary and sufficient for channel targeting to node (Gasser et al., 2012). Therefore, further work is required to define precisely the roles of each of these protein interactions in the PNS node formation vs. stabilization.
Although there is general agreement about the organization of the PNS nodes, the formation and stabilization of the CNS nodes is not as well understood. Perinodal astrocytes in the CNS are the proposed equivalent of Schwann cell microvilli in the PNS (Raine, 1984). Perinodal astrocytes fill the extracellular space adjacent to CNS nodes and are thought to interact with nodal proteins (Black and Waxman, 1988). However, a CNS equivalent of gliomedin has yet to be identified. One candidate for this role is brevican, an ECM protein that localizes to the nodal region and is critical for organizing the CNS nodal ECM (Bekku et al., 2009). Ablation of brevican in mice did not reveal major deficits in nodal organization (Brakebusch et al., 2002), but it is possible that other proteins function along with brevican to organize the node and may compensate for the lack of brevican expression in the knockout mice. In addition, oligodendrocytes secrete proteins important for clustering nodal proteins prior to the start of myelination (Kaplan et al., 2001). The extent to which each of these contributions is needed for CNS node formation remains to be determined.
Previous studies have examined the role of several nodal proteins in the organization and stabilization of the CNS node. For example, deficiencies in βIV-spectrin resulted in alterations to axon shape, namely, in vesicle-filled membrane protrusions in the nodal area (Yang et al., 2004). Moreover, loss of βIV-spectrin decreased the levels of AnkG and NaV channels at nodes, resulting in disrupted axonal conduction, which suggests that βIV-spectrin plays an important role in stabilizing this complex (Komada and Soriano, 2002; Lacas-Gervais et al., 2004). On the other hand, ablation of Nfasc in mice resulted in disrupted PNS and CNS nodes, as well as death at P6, when the nervous system was not fully developed (Sherman et al., 2005). These Nfasc null mice lack both major isoforms of Nfasc expressed in the nervous system, namely, the glial-specific NfascNF155 that is expressed at the paranode and the neuronal-specific NfascNF186 that is expressed at the node and AIS. Loss of both isoforms leads to complete disorganization of both paranodes and nodes. To overcome this problem, transgenic NfascNF186 expression in Nfasc null mice revealed that AnkG and NaV1.6 were able to localize to the CNS node, independent of paranode formation (Zonta et al., 2008). Interestingly, it was also observed that transgenic rescue of the glial NfascNF155 to paranodes rescued nodal components in the CNS, suggesting that the paranode can compensate for lack of NfascNF186 expression at the node (Zonta et al., 2008). However, nodes are able to form in paranodal mutant mice, including Caspr, Contactin, and NfascNF155 null mice (Bhat et al., 2001; Boyle et al., 2001; Pillai et al., 2009). This suggests that nodes can form independently of axonal–glial septate junctions (AGSJs) at the paranode. In addition, freeze-fracture electron microscopy (EM) studies showed that early nodal differentiation takes place before paranodal AGSJ formation (Tao-Cheng and Rosenbluth, 1983). Furthering the debate, recent work utilizing Schwann cell–DRG neuron in vitro cocultures from wild-type and Nfasc null mice indicated that paranodes were able to cluster NaV channels at mature nodes, independent of NfascNF186 (Feinberg et al., 2010). Further support for a paranodal role in node stabilization comes from studies showing that loss of AGSJs results in destabilization of NaV channels (Poliak and Peles, 2003; Rios et al., 2003; Rosenbluth et al., 2003). To address definitively the role of paranodes in nodal organization, a study had to be completed in which the node was disorganized while the paranodes remained completely intact.
Recent work addressed this issue in vivo by examining a conditional neuronal knockout of Nfasc, so that the organization of the node could be examined devel-opmentally in the absence of NfascNF186 (Thaxton et al., 2011). In this study, loss of NfascNF186 resulted in the disruption of nodal clustering at every developmental stage observed, whereas paranodal and juxtaparanodal domains formed normally (Fig. 3B – E). This work showed that NfascNF186 is required for the organization of the PNS and CNS nodes. In addition, this work suggests that paranodes are not sufficient for nodal organization, because nodes do not form even though the paranodes are intact. In addition, this study reinforces the idea that NfascNF186 plays a role at the AIS different from that at the node, in that nodal loss of NfascNF186 also disrupts clustering of AnkG, NrCAM, and the PNS-specific proteins gliomedin and ezrin-binding protein 50 (EBP50; Thaxton et al., 2011). This is in accordance with previous studies showing that NfascNF186 can localize to the nodes independently of its interaction with AnkG and that it arrives at the presumptive node prior to other nodal components (Lambert et al., 1997; Lustig et al., 2001; Koticha et al., 2006; Dzhashiashvili et al., 2007; Thaxton et al., 2011). Furthermore, a recent study showed that the fibronectin type-III domain of NfascNF186 is required for its interaction with gliomedin and thus clustering at the node (Labasque et al., 2011). In addition, NfascNF186 can interact directly with the β-subunit of NaV channels, allowing them to be clustered by NfascNF186 at the node (Ratcliffe et al., 2001). It is clear that NfascNF186 is critical for organizing the node, but it is not clear how the nodal components traffic to the node. A few pieces of this mechanistic puzzle have begun to materialize based on recent work in which transected axons were removed from their soma before myelination was induced in vitro (Zhang et al., 2012). After transection and myelination, the study checked which nodal components were able to cluster, thus determining whether each component was already localized to the axonal membrane or had to be transported from the soma. It was observed that CAMs were already expressed on the axolemma surface and just had to be trapped by Schwann cell ligands during myelination to begin to accumulate at the node. In contrast, ion channels and cytoskeletal components required transport from the soma to become targeted to the node. In addition, Zhang et al. found that the recycling of nodal components in mature nodes requires transport from the soma. Together these data suggest that NfascNF186 interacts with the extracellular environment or myelinating glial cells to become clustered at the node, which then signals for the clustering of other components being trafficked along the axon.
The Node Is a Barrier Against Invading Paranodes
A well-known function of the paranode is to act as a barrier to segregate juxtaparanodal potassium channels from sodium channels at nodes. Similarly, the AIS is thought to function as a fence to keep somatodendritic proteins out of the axon. Recent work has revealed that nodes function as a molecular barrier to prevent neighboring paranodes from invading the nodal gap (Thaxton et al., 2011). In the absence of NfascNF186 expression, the node failed to organize and resulted in the invasion of the flanking paranodes into the nodal gap. Electron microscopic analysis revealed that, as these NfascNF186 null mice developed, the paranodal loops would eventually overlap the neighboring paranodal region, resulting in complete obstruction of the node (Fig. 3F–I; Thaxton et al., 2011). Without proper maintenance of the nodal gap and nodal components, a severe disruption in saltatory conduction was observed.
The Node in Disease and Injury
Proper nodal function is critical for action potential propagation. However, not only is the developmental organization of the node essential but so is appropriate maintenance of the nodal components. Nodal disorganization is apparent in multiple disease states, including MS. In MS, sodium channel clusters are no longer stable at nodes (Craner et al., 2004; Coman et al., 2006). This disruption of sodium channels is thought to contribute to the pathology of axonal degeneration, which results in loss of function in MS patients (Craner et al., 2004). The mechanisms responsible for disruption of nodal sodium channels in MS are not well understood. Interestingly, autoantibodies against Nfasc have been discovered in MS patients (Mathey et al., 2007). Studies have also indicated that addition of antibodies that block the function of CAMs results in loss of NaV channels and AnkG accumulation at nodes in Schwann cell–DRG neuron in vitro cocultures (Lustig et al., 2001). From these observations, one can presume that the presence of autoantibodies against Nfasc is preventing the proper maintenance of nodes in MS patients, disrupting NaV channel localization at the node and resulting in disrupted nerve conduction. Other autoimmune disorders, such as acute motor axonal neuropathy (AMAN), show specific alterations at nodes, which leads to disruption in action potential propagation (Susuki et al., 2007; Lonigro and Devaux, 2009). Furthermore, injection of autoantibodies from AMAN patients into rats resulted in disruption of nodal and paranodal proteins, as well as altered axonal conductance, which eventually led to axonal degeneration (Susuki et al., 2011). Therefore, it is important to understand definitively how the node is organized and maintained so that treatments can be developed for these devastating disorders.
The Paranode
As myelinating glia wrap around the axon, the cytoplasm of the glial cell is pushed to the edges, where it fills the loops of the glial membrane that are attached to the axolemma. These loops are known as paranodal loops, and they attach to the axolemma adjacent to the node through the formation of AGSJs (Salzer, 2003; Thaxton and Bhat, 2009). The AGSJs are very similar to the invertebrate septate junctions that form between ensheathing glial cells and axon bundles in the nervous system (Rosenbluth, 1995; Banerjee et al., 2006). AGSJs are electron-dense structures that form a ladder-like distribution between the membrane of the glial paranodal loops and the axonal membrane (Schnapp et al., 1976). The AGSJs comprise three major components, contactin-associated protein (Caspr) and contactin (Cont) on the axonal side, which bind to each other in cis, and the 155-kDa isoform of Nfasc, NfascNF155, on the glial side (Fig. 4A; Menegoz et al., 1997; Peles et al., 1997; Rios et al., 2000; Charles et al., 2002). Loss of any of these three components results in disruption of the AGSJs (Bhat et al., 2001; Boyle et al., 2001; Pillai et al., 2009). Cont is a glycosylphosphatidylinositol (GPI)-anchored protein, and Caspr is a transmembrane protein, so the AGSJs communicate with the axon through interactions within the C-terminus of Caspr (Bhat, 2003). Within the axon at the paranode is an accumulation of several cytoskeletal scaffolding proteins, including αII- and βII-spectrin, protein 4.1B, and the adaptor protein AnkB (Fig. 4A; Ohara et al., 2000; Garcia-Fresco et al., 2006; Ogawa et al., 2006; Thaxton and Bhat, 2009). The C-terminus of Caspr contains a 4.1/ezrin/radixin/moesin (FERM) binding domain, which allows it to interact with protein 4.1B (Menegoz et al., 1997; Peles et al., 1997; Poliak et al., 1999; Gollan et al., 2002; Denisenko-Nehrbass et al., 2003). Caspr has been shown to interact in a complex with 4.1B, spectrin, and actin, suggesting the AGSJs anchor the paranodal loops to the axonal cytoskeleton (Garcia-Fresco et al., 2006; Buttermore et al., 2011).
Fig. 4.
Organization and stabilization of the paranodal domain. A: Diagram of the molecular components of the paranode. B,C: Sciatic nerve fibers from wild-type (B) and Caspr−/− (C) mice immuno-stained against potassium channels (KV1.1, red), Caspr (blue), and sodium channels (NaCh, green) reveal the role of the paranodal AGSJs to segregate potassium channels in the JXP from sodium channels in the node (Bhat et al., 2001). D,E: Electron micrographs of wild-type (D) and Caspr−/− (E) axons showing parallel arrays of axonal cytoskeleton in the wild-type axon and disorganization of the axonal cytoskel-eton in the Caspr−/− (Garcia-Fresco et al., 2006). F,G: Sciatic nerves from wild-type (F) and Act-Cre;4.1BFlox (G) mice immunostained against Nfasc (a, red), Caspr (b, green), AnkG (c, blue), and merged (d) reveal the disruption of paranodes in P30 4.1B mutant PNS axons. Act-Cre allows ubiquitous expression of Cre under β-actin promoter. H,I: Spinal cord sections from wild-type (H) and Act-Cre;4.1BFlox (I) mice immunostained against 4.1B (a, red), Caspr (b, green), AnkG (c, blue), and merged (d) reveal disruption of the paranode in 4-month-old 4.1B mutant CNS axons. J,M: Electron micrographs of sciatic nerve fibers (J,K) and spinal cord fibers (L,M) from wild-type (J,L) and Act-Cre;4.1BFlox (K,M) mice, revealing disrupted AGSJ compaction in the PNS (K) and loss of AGSJs in the CNS (M; Buttermore et al., 2011). Scale bars = 20 µm in C (applies to B,C); 10 µm in F (applies to F,G); 5 µm in H (applies to H,I); 0.2 µm in J–M. (B,C: Reprinted from Neuron 2001; 30:369–383, with permission from Elsevier; D,E: © Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo–glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Originally published in Proc Natl Acad Sci USA 2006; 103:5137–5142; F–M: Reprinted from J Neurosci 2011; 31:8013–8024, with permission from The Journal of Neuroscience.)
The link between AGSJs and the axonal cytoskeleton contributes to the fence function of the paranode, which was first demonstrated in the frog brain and prevents the diffusion of ion channels in the JXP toward ion channels in the node (Rosenbluth, 1976). More recent studies have conclusively shown that the formation of the AGSJs is critical for the maintenance of potassium channel localization to the JXP (Fig. 4B,C; Dupree et al., 1999; Bhat et al., 2001; Boyle et al., 2001; Poliak et al., 2001; Pillai et al., 2009). The para-node is referred to as the “Achilles’ heel” of the myelinated axons because it is an area of transition along the axonal microtubules that can lead to problems with axonal transport and eventually axonal degeneration (Fig. 4D,E; Sousa and Bhat, 2007). Although no functional clustering of ion channels exists at the paranode, the function of the paranode as a fence is a critical determinant of axonal function that must be maintained, so it is important to understand the mechanisms that underlie the organization and the stabilization of the paranodal domain.
Formation of the Paranode
Although the clustering of nodal proteins requires interactions with external cues, and the clustering of AIS proteins does not, the contribution from external sources in the organization of the paranode is a little less clear. An in vitro study revealed that Caspr must form a complex with Cont to be transported from the endoplasmic reticulum to the plasma membrane, suggesting a dependence on Cont for proper localization (Faivre-Sarrailh et al., 2000). On the other hand, ablation of Caspr, or deletion of the Caspr C-terminus, also leads to the loss of Cont from the paranode (Bhat et al., 2001; Gollan et al., 2002). Furthermore, in the absence of Caspr, Cont localizes to CNS nodes (Rios et al., 2000). Thus, an interdependence exists between Caspr and Cont for their stable localization at the paranode. However, the role of NfascNF155 in targeting Caspr/Cont to the para-node is not clear, nor is whether the Caspr/Cont complex stabilizes the paranodal loops. Glial-specific ablation of NfascNF155 also results in loss of AGSJs and paranodal disorganization (Pillai et al., 2009), so Caspr/Cont cannot form paranodes in the absence of NfascNF155. Together these studies reinforce the importance of Caspr/Cont/NfascNF155 in paranode organization; however, questions persist with regard to how this complex establishes a molecular fence at the paranode to segregate the juxtaparanodal components from the nodal components.
Role of Axonal Cytoskeleton in AGSJ Organization
Several mechanisms have been proposed for para-nodal organization. It was previously unclear whether the paranodal cytoskeletal components recruit the AGSJ components to the paranodal membrane or function only to stabilize the complex once formed. The cytoskeletal protein 4.1B becomes diffusely localized within the axon in paranodal mutants, suggesting that enrichment of 4.1B to the paranode requires its interaction with Caspr (Gollan et al., 2002). In addition, recent work showed that the paranodal proteins Caspr and Cont are able to localize to the paranode but do not remain stabilized there in the absence of protein 4.1B (Fig. 4F–I; Buttermore et al., 2011; Cifuentes-Diaz et al., 2011). In the absence of 4.1B expression, the par-anodes became broken, and the electron-dense AGSJs are destabilized and eventually lost (Fig. 4J–M; Buttermore et al., 2011). This destabilization results in the detachment of paranodal loops from the axolemma. These data support earlier findings that Caspr forms a complex with the axonal cytoskeleton through its interactions with protein 4.1B, αII-spectrin, βII-spectrin, AnkB, and actin (Gollan et al., 2002; Denisenko-Nehrbass et al., 2003; Garcia-Fresco et al., 2006; Ogawa et al., 2006). Together, these findings suggest that the AGSJs form in the absence of their interaction with the axonal cytoskeleton, but their continued stabilization relies on the axonal cytoskeleton.
Role of Lipid Rafts in AGSJ Organization
The complex that links Caspr to the axonal cytoskeleton is beginning to be elucidated, but the scaffolding components within the glial paranodal loops that stabilize NfascNF155 at the paranode are not known. It is possible that lipid rafts play a role in this organization during myelination (Simons et al., 2000). In addition to NfascNF155, several proteins are critical for proper AGSJ formation, including ceramide galactosyltransferase (CGT), proteolipid protein (PLP), MBP, myelin-associated glycoprotein (MAG), 2′,3′-cyclic nucleotide 3′-phosphodiesterase, and Nkx6-2 (Rosenbluth, 1981; Trapp, 1990; Coetzee et al., 1996; Klugmann et al., 1997; Lappe-Siefke et al., 2003; Southwood et al., 2004; Garcia-Fresco et al., 2006). CGT is an enzyme required to generate the myelin lipids galactocerebroside and sul-fatide (Dupree et al., 1999; Marcus et al., 2002, 2006). Ablation of CGT resulted in disruption of AGSJs and loss of the segregation of nodal sodium channels and juxtaparanodal potassium channels (Dupree et al., 1999; Marcus et al., 2002). These data, along with subcellular fractionation studies of NfascNF155, help to solidify the idea that lipid rafts form in the paranodal loops and help to organize the glial paranodal components to stabilize NfascNF155 (Schafer et al., 2004). Thus it can be postulated that the paranodal organization and establishment of the AGSJs occur through a coordinated effort of glial and neuronal signals during myelination.
The Paranode Acts as a Fence Against the Juxtaparanodal Complex
As stated above, the role of the paranode is to provide a fence that keeps the potassium channels in the JXP segregated from the sodium channels in the node. Loss of any of the major AGSJ component results in dissolution of the AGSJs (Bhat et al., 2001; Boyle et al., 2001; Pillai et al., 2009). When the AGSJs are lost, the periaxonal space widens. However, the paranodal loops do not completely lift away from the axonal membrane, causing unraveling of the myelin because other adhesive complexes and extracellular support help maintain the myelin. For example, the PNS basal lamina may help to maintain the myelin in the absence of AGSJs (Poliak and Peles, 2003). In addition, molecular interactions between the myelin layers likely contribute to stabilization of the loops in the absence of paranodal AGSJs. However, loss of the AGSJs has grave consequences for axonal conductance and axonal survival. Mice deficient in Caspr do not typically live past 30 days and have severe deficits in both axonal conduction velocity and amplitude (Bhat et al., 2001). These electrophysiological changes are observed in all paranodal mutants and are thought to result from a combination of current leakage and loss of segregation of potassium channels at the JXP and sodium channels at the node (Fig. 4B,C; Dupree et al., 1999; Bhat et al., 2001; Garcia-Fresco et al., 2006; Pillai et al., 2009).
In addition to its role as a fence to separate nodal sodium channels and juxtaparanodal potassium channels, the paranodal complexes are also critical for maintaining the organization of the axonal cytoskeleton. Further examination of the axonal cytoskeleton in Caspr- and NfascNF155-deficient mice revealed the presence of swellings along the axons (Garcia-Fresco et al., 2006; Pillai et al., 2009). Ultrastructural examination of these swellings revealed complete disorganization of the normally parallel arrays of axonal cytoskeleton (Fig. 4D,E). In addition, organelle accumulation was found in the para-nodal region flanking these large swellings, a sign of disrupted axonal transport that eventually leads to axonal degeneration (Garcia-Fresco et al., 2006). The paranodal region is highly susceptible to disrupted axonal transport (Sousa and Bhat, 2007). The swellings that result from disrupted domain organization also contained increased levels of phosphorylated neurofilaments, a hallmark of cytoskeletal disruption (Pillai et al., 2009). Importantly, the presence of swellings and cytoskeletal abnormalities is found prior to axonal degeneration in many neuropathies, suggesting a common mechanism of neuronal destruction in these pathologies (Rodriguez and Scheithauer, 1994; Lappe-Siefke et al., 2003; Fabrizi et al., 2007). Similarly, in Purkinje neurons, the accumulation of organelles is an indicator of axonal degeneration (Palay and Palay, 1974). Thus a functional association between AGSJs and the axonal cytoskeleton is important not only to anchor the paranodal loops but also for the organization and stabilization of the axonal cytoskeleton, which are critical for maintaining long-term axonal health and stability.
The Paranode in Disease
Demyelination is a major contributor to disease progression and axonal degeneration in disorders such as Charcot-Marie Tooth (CMT) disease and MS. These disorders have various causes, including genetic mutations in CMT disease and autoimmune disruption in MS, but both result in axonal domain disorganization (Berger et al., 2006; Nave et al., 2007; Trapp and Nave, 2008). In addition to the disruption of nodes, MS patients also present with disrupted paranodal organization, as shown by loss of Caspr enrichment and disrupted potassium channel localization (Wolswijk and Balesar, 2003; Coman et al., 2006; Howell et al., 2006). NfascNF155 levels are also decreased at the paranodes of MS patients, with decreased lipid raft association (Maier et al., 2007). Destabilization of these paranodal proteins results in disruption of the AGSJs, as shown by the movement of potassium channels into the paranodal region (Howell et al., 2006). As previously mentioned, autoantibodies to Nfasc are found in MS patients and may play a role in altering the localization of this protein at the nodes (NfascNF186) and paranodes (NfascNF155; Mathey et al., 2007). Furthermore, axonal swellings that result from disorganization of axonal domains are also found in neurodegenerative diseases, including amyotro-phic lateral sclerosis (ALS), CMT, Wallerian degeneration, Alzheimer’s disease, and cerebrospinal ataxia (Collard et al., 1995; Brownlees et al., 2002; Stokin et al., 2005). Based on the swellings found in these diseases and the swellings seen at the paranodes of AGSJ mutant mice, this may represent a conserved response to axonal distress and an early sign of axonal degeneration. In the early stages of ALS, the AIS diameter is increased (Sasaki and Maruyama, 1992), so this may be a conserved mechanism of a diseased axon. Each of these studies points to the disruption of axonal domains or structure as part of the pathology that leads to demyelination and axonal degeneration.
The Juxtaparanode
The juxtaparanode (JXP) is the region flanking the paranode that is enriched in delayed rectifier potassium channels, including KV1.1 and KV1.2 (Fig. 5A; Wang et al., 1993; Rhodes et al., 1997). Potassium channels form a complex with the axonal transmembrane CAM Caspr2 at the JXP (Fig. 5A; Poliak et al., 1999). Moreover, the GPI-anchored CAM transient axonal glycoprotein (Tag1) is localized to both the glial and the axonal membranes at the JXP, where it forms a complex with Caspr2 (Fig. 5A; Traka et al., 2002, 2003; Poliak et al., 2003). Importantly, individual loss of either Caspr2 or Tag1 results in loss of potassium channel clustering at the JXP (Poliak et al., 2003). Also, compact myelin is required for potassium channel localization and stabilization at the JXP (Baba et al., 1999). However, the precise mechanisms of JXP organization and how the underlying cytoskeleton might be involved are not known.
Fig. 5.
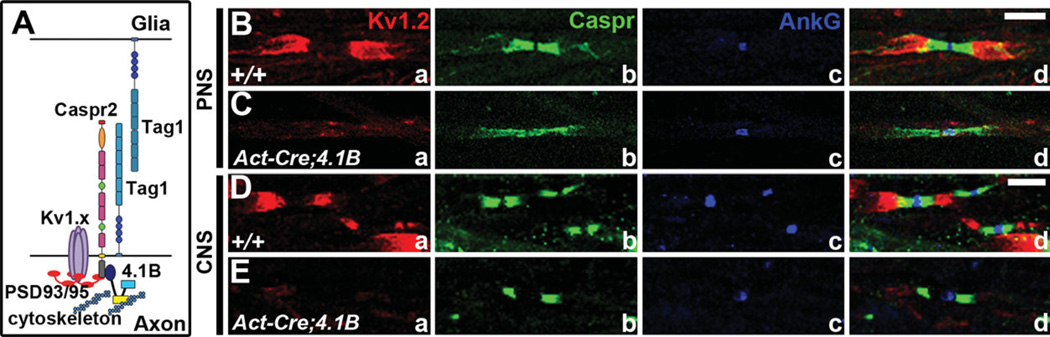
Organization and stabilization of the juxtaparanode. A: Diagram of molecular components of the JXP. B–E: Sciatic nerve (B,C) and spinal cord (D,E) myelinated fibers from wild-type (B,D) and Act-Cre;4.1BFlox (C,E) mice immunostained against potassium channels (a, KV1.2, red), Caspr (b, green), AnkG (c, blue), and merged (d) reveal the importance of the cytoskeletal adaptor protein 4.1B in the organization of the JXP, because loss of 4.1B results is disruption of the JXP in the PNS (C) and CNS (E; Buttermore et al., 2011). Scale bars 5 10 µm in B (applies to B,C); 5 µm in D (applies to D,E). (B–E: Reprinted from J Neurosci 2011; 31:8013–8024, with permission from The Journal of Neuroscience.)
Role of Axonal Cytoskeleton in JXP Organization
Within the axon at the JXP are several postsynaptic density scaffolding proteins, including PSD93 and PSD95 (Fig. 5A; Baba et al., 1999; Horresh et al., 2008). However, ablation of these scaffolding proteins did not disrupt the localization of potassium channels to this domain or disrupt binding of Caspr2 with potassium channels (Rasband et al., 2002; Horresh et al., 2008). Interestingly, this is in contrast to the role of PSD proteins at the AIS, in that PSD93, but not Caspr2 or Tag1, is required for clustering of potassium channels at the AIS (Ogawa et al., 2008). Furthermore, the Caspr2 C-terminus contains a region, just like Caspr, that mediates its binding to protein 4.1B (Menegoz et al., 1997; Peles et al., 1997; Poliak et al., 1999; Gollan et al., 2002; Denisenko-Nehrbass et al., 2003). In one study, mutational analyses using GST-fusion proteins of the Caspr2 C-terminus revealed that Caspr2 lacking its 4.1 binding domain, but not its PDZ binding domain, was unable to interact with the membrane-associated guanylate kinases, suggesting that the interaction with 4.1 may be more important for JXP organization (Horresh et al., 2008). Protein 4.1B is enriched at the JXP, as it is at the paranode, and loss of 4.1B expression results in the diffusion of potassium channels, Caspr2, and PSD95 from the JXP in the PNS and CNS (Fig. 5B–E; Horresh et al., 2010; Buttermore et al., 2011; Cifuentes-Diaz et al., 2011). Although 4.1B is required for the clustering of JXP components, it is not required to maintain the interaction between Caspr2 and KV1 channels (Buttermore et al., 2011).
Role of JXP Potassium Channels in Action Potential Propagation
As previously described, the proper localization and maintenance of potassium channels within the JXP require stable AGSJ formation at the paranode, because disrupted paranodes result in diffusion of potassium channels toward the node (Bhat et al., 2001; Boyle et al., 2001; Garcia-Fresco et al., 2006; Pillai et al., 2009). This suggests that the segregation of potassium channels involves sorting mechanisms at the paranodal axolemma that act to separate the KV1 channels and CAMs at the JXP from paranodal CAMs. After segregation occurs, complexes form within the underlying JXP cytoskeleton to anchor the channels (Poliak and Peles, 2003). The importance of segregating the potassium channels to the JXP lies in the role potassium channels play in the action potential propagation. Delayed rectifier potassium channels are responsible for the repolarization event that equilibrates the membrane potential after it has been depolarized (Purves et al., 2004). Additionally, the potassium channels bring the membrane potential slightly past the resting membrane potential, in a hyperpolarized state that helps prevent back-propagation of action potentials (Purves et al., 2004). Although disrupted potassium channel localization does not disrupt conduction velocity of action potentials, it does result in increased hyperexcitability of nerves (Cifuentes-Diaz et al., 2011). This was evident from the spontaneous and evoked repetitive activity observed in 4.1B null mice, which have mislocalized JXP potassium channels, compared with control nerves (Cifuentes-Diaz et al., 2011). These results are in accordance with previous studies showing that potassium channels help modulate neuronal activity, because disruption of potassium channel function leads to neuronal hyperexcitability and in some cases disorders such as epilepsy (Sutherland et al., 1999; Watanabe et al., 2000; Lopantsev et al., 2003). Together these studies highlight the importance of proper axonal domain organization to ensure that nerve conduction occurs at optimum physiological levels. When any of the domains is disrupted, the normal neuronal conduction properties are altered, with devastating neurological consequences.
The Internode
Although the internode is most well known as the area with tightly wrapped myelin, it is not a region devoid of axon–glial interactions. The myelin is so compact at the internode that the space between the axon and the membrane is only about 12–13 nm (Maurel et al., 2007). Interestingly, the link between the myelin and the axon at the internode can be disrupted by protease treatments, suggesting that there are transmembrane proteins localized to this area that help to stabilize the axon–myelin interaction (Yu and Bunge, 1975; Maurel et al., 2007). Also, along the internode of mature myelinating glial cells in the PNS are cytoplasmic channels known as Schmidt-Lanterman incisures that link the adaxonal membrane (the glial membrane that contacts the axon) and the abaxonal membrane (the glial membrane that contacts the ECM; Poliak and Peles, 2003). The proposed role of the incisures is to aid in promoting/providing transportation of materials across myelin to allow for maintenance and growth of the myelin, if needed (Arroyo et al., 2001). For these structures to compact and organize properly, there must be cell surface proteins that are responsible for communication between the two cell types.
A few proteins have been discovered that are localized to the internode and/or incisures and could play a role in their organization. The CAM known as MAG is localized to Schmidt-Lanterman incisures, as well as along the internode (Trapp, 1990). MAG is an Ig super-family CAM, so it could play a role in axoglial interactions at the internode (Salzer et al., 1987). Studies have shown that MAG mutant mice have normal myelination but have small alterations along the internodal space between the myelin and the axon (Li et al., 1994; Montag et al., 1994). The other proteins localized to the myelin–axon interface at the internode include several transmembrane nectin-like (Necl) proteins (Sakisaka and Takai, 2004; Maurel et al., 2007). Similar to other CAMs involved in axoglial interactions, including Caspr and Caspr2, the Necl proteins belong to the Ig super-family and contain binding domains for 4.1 proteins and PDZ binding motifs (Ogita and Takai, 2006). Interestingly, Necl-2 has been shown to function in adhesion both homophilically and heterophilically and can interact with a truncated form of protein 4.1B (Yageta et al., 2002; Shingai et al., 2003). In the PNS, Necl-1 and Nec1-2 are expressed within the axon, and Necl-4 and Necl-2 are expressed within Schwann cells (Maurel et al., 2007). Immunohistochemical and binding analyses revealed that, along the internode, Necl-4 and Necl-1 interact to help maintain a stable interaction (Maurel et al., 2007). In addition, at the Schmidt-Lanterman incisures, several homophilic and heterophilic interactions occur among the various Necl proteins (Maurel et al., 2007).
Interestingly, recent studies revealed that protein 4.1G is a novel component required for internodal organization (Ivanovic et al., 2012). 4.1G is expressed in Schwann cells and colocalizes with Necl-4 (Horresh et al., 2010; Ivanovic et al., 2012). Interestingly, in 4.1G−/− sciatic nerve fibers, Necl-4 failed to cluster properly at the Schmidt-Lanterman incisures (Ivanovic et al., 2012). In addition, in the absence of 4.1G, para-nodal proteins appeared in broken segments adjacent to the paranode. Furthermore, JXP components, such as KV1.2 channels, accumulated by the broken paranodal segments and were no longer properly localized along the inner mesaxon. These studies suggest that still other molecular components have yet to be discovered that play a critical role in the organization and maintenance of axonal domains, including the internode.
In addition to adhesion between the axon and the myelinating glia, the myelin must sense signals from the axon for proper myelination. For example, during development, the axon diameter must be read by the myelinating glial cell so that the proper number of myelin wraps is achieved (Smith et al., 1982). At the completion of this process, there is a conserved ratio of axon diameter and myelin thickness, known as the “g ratio.” Interestingly, the g ratio is determined differently for Schwann cells and oligodendrocytes. In the PNS, but not CNS, myelin thickness is disrupted in mice with altered levels of phosphorylated neurofilaments, which help to organize the axon cytoskeleton (Elder et al., 2001). Studies have also shown that the g ratio can be disrupted by altering expression of neuregulin 1 (Nrg1), revealing an interaction between the glial protein tyrosine kinase receptor ErbB2 and Nrg1 in myelin wrapping (Michailov et al., 2004; Sousa and Bhat, 2007). These data reveal that, although the myelin is very compactly wrapped around the internode, CAMs and their binding partners still play an important role in axoglial interactions at the internode.
CONCLUSIONS AND EVOLUTIONARY PERSPECTIVE
Myelin proteins and the need to insulate axons evolved separately several times, including in Annelids, Arthropods and Chordates, highlighting the importance of its function for efficient neuronal transmission (Roots, 2008). Interestingly, the mechanisms responsible for clustering ion channels at axonal domains evolved prior to myelination (Hill et al., 2008). For example, the structural amino acid motif in sodium channels that allows them to link to the actin–spectrin cytoskeleton through AnkG evolved in early chordates, allowing for the clustering of sodium channels at the AIS before nodes evolved (Lemaillet et al., 2003; Hill et al., 2008; Rasband, 2008). Furthermore, this AnkG binding motif is also conserved in potassium channels that localize to the AIS and node (Pan et al., 2006; Rasband, 2008). However, the potassium channel motif evolved concurrently with myelination, because potassium channels are needed for proper saltatory conduction (Hill et al., 2008). Importantly, nodes are not found in invertebrate nervous systems, but similar structures do exist for the AIS, as in vertebrates. However, the action potential initiation site in invertebrate axons occurs at variable distances from the soma, and one neurite can split into both axon and dendrite distally from the soma (Meyrand et al., 1992; Rolls et al., 2007; Maniar et al., 2012). Interestingly, axonal domains in C. elegans are analogous to the vertebrate AIS, including the clustering of AnkG and microtubule-stabilizing protein UNC-33, which is the C. elegans protein collapsin response mediator protein-2 (CRMP-2; Maniar et al., 2012). In vertebrates CRMP-2 is important for axon specification (Inagaki et al., 2001; Fukata et al., 2002; Maniar et al., 2012). Thus, the mechanisms responsible for sodium channel clustering and axonal organization appear to be conserved throughout evolution.
In addition to evolutionarily conserved motifs on AIS and nodal ion channels, the paranodal AGSJs are also highly conserved throughout evolution (Banerjee et al., 2006). Septate junctions are prominent in the epithelia and nervous system of invertebrates but are found only at the vertebrate AGSJs (Banerjee et al., 2006). Importantly, the three main components of the AGSJs are highly conserved in invertebrates, such as in Drosophila, in which orthologs for Caspr/Cont/NfascNF155 are neurexin IV/Cont/neuroglian (Banerjee et al., 2006). In addition, neurexin IV contains a 4.1-binding sequence in its C-terminus (Bhat, 2003; Banerjee et al., 2006), and the 4.1 family ortholog coracle colocalizes with neurexin IV at septate junctions (Fehon et al., 1994). Comparison between mutational studies in vertebrates and invertebrates revealed a common mechanism whereby the junctions are stabilized through their interaction with 4.1 proteins to the underlying cytoskeleton (Fehon et al., 1994; Lamb et al., 1998; Ward et al., 1998; Horresh et al., 2010; Buttermore et al., 2011).
The conserved functions of septate junctions and ion channel clustering across evolution reinforce the importance of axonal domain organization for maintenance of neuronal function. Thus, better knowledge of how the molecular processes and interactions lead to organization and maintenance of the AIS, node, paranode, JXP and internode, will open opportunities to design therapeutic strategies to treat and/or prevent the disorders that target these domains. Also, a better understanding of how the molecular fences and barriers are established along the axon and how they are maintained for the entire life of the organism may provide clues for their restoration in demyelinating disorders. This fundamental knowledge could eventually contribute to the prevention of axonal degeneration that often accompanies myelin-related neurological disorders.
ACKNOWLEDGMENTS
The work in our laboratory has been generously supported by the grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the State of North Carolina. We regret that because of space limitations the work of many authors related to this topic could not be cited here.
REFERENCES
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS. Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res. 2001;305:53–66. doi: 10.1007/s004410100403. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Grinspan J, Lambert S, Levinson SR, Brophy PJ, Peles E, Schere SS. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci. 2002;22:1726–1737. doi: 10.1523/JNEUROSCI.22-05-01726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J Neurosci Res. 1999;58:752–764. [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. doi: 10.1111/j.1471-4159.2009.05873.x. [DOI] [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol. 1999;28:303–318. doi: 10.1023/a:1007005528505. [DOI] [PubMed] [Google Scholar]
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, et al. BetaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axoglial junctions. Curr Opin Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St. Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. Axon–glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1:169–183. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci. 2007;27:590–603. doi: 10.1523/JNEUROSCI.4302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Bockers TM, Zhou X, Kreutz MR, et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlees J, Ackerley S, Grierson AJ, Jacobsen NJ, Shea K, Anderton BH, Leigh PN, Shaw CE, Miller CC. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet. 2002;11:2837–2844. doi: 10.1093/hmg/11.23.2837. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Sobotzik JM, Schultz C, Rasband MN. IκBα is not required for axon initial segment assembly. Mol Cell Neurosci. 2012;50:1–9. doi: 10.1016/j.mcn.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkarth N, Kriebel M, Kranz EU, Volkmer H. Neurofascin regulates the formation of gephyrin clusters and their subsequent translocation to the axon hillock of hippocampal neurons. Mol Cell Neurosci. 2007;36:59–70. doi: 10.1016/j.mcn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Buttermore ED, Dupree JL, Cheng J, An X, Tessarollo L, Bhat MA. The cytoskeletal adaptor protein band 4.1B is required for the maintenance of paranodal axoglial septate junctions in myelinated axons. J Neurosci. 2011;31:8013–8024. doi: 10.1523/JNEUROSCI.1015-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttermore ED, Piochon C, Wallace ML, Philpot BD, Hansel C, Bhat MA. Pinceau organization in the cerebellum requires distinct functions of Neurofascin in Purkinje and basket neurons during postnatal development. J Neurosci. 2012;32:4724–4742. doi: 10.1523/JNEUROSCI.5602-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Peri-neuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chen S, Chen L, Zhou J, Dai Q, Yang L, Li X, Zhou L. Long-term increasing co-localization of SCN8A and ankyrin-G in rat hippocampal cornu ammonis 1 after pilocarpine induced status epilepticus. Brain Res. 2009;1270:112–120. doi: 10.1016/j.brainres.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Chareyre F, Garcia M, Devaux J, Carnaud M, Levas-seur G, Niwa-Kawakita M, Harroch S, Girault JA, Giovannini M, et al. Protein 4.1B contributes to the organization of peripheral myelinated axons. PLoS ONE. 2011;6:e25043. doi: 10.1371/journal.pone.0025043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- Coman I, Aigrot MS, Seilhean D, Reynolds R, Girault JA, Zalc B, Lubetzki C. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3186–3195. doi: 10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Lo AC, Black JA, Waxman SG. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain. 2003;126:1552–1561. doi: 10.1093/brain/awg153. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: altered axonal expression of NaV1.2 and NaV1.6 sodium channels and Na+/Ca21 exchanger. Proc Natl Acad Sci U S A. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsy-chopharmacology. 2009;34:2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin1/third FNIII domain2) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-de-pendent domains that assemble by distinct mechanisms. J Cell Biol. 2007;177:857–870. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Friedrich VL, Jr, Lazzarini RA. Schwann cells and oligo-dendrocytes read distinct signals in establishing myelin sheath thickness. J Neurosci Res. 2001;65:493–499. doi: 10.1002/jnr.1179. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell–axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Carey DJ, Peles E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol. 2007;177:551–562. doi: 10.1083/jcb.200612139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130:394–403. doi: 10.1093/brain/awl284. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Gauthier F, Denisenko-Nehrbass N, Le Bivic A, Rou-gon G, Girault JA. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of par-anodin/contactin-associated protein (caspr) J Cell Biol. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homo-logue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Saba-nay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, et al. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neuro-fascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A, Ho TS, Cheng X, Chang KJ, Waxman SG, Rasband MN, Dib-Hajj SD. An ankyrinG-binding motif is necessary and sufficient for targeting NaV1.6 sodium channels to axon initial segments and nodes of ranvier. J Neurosci. 2012;32:7232–7243. doi: 10.1523/JNEUROSCI.5434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol. 2002;157:1247–1256. doi: 10.1083/jcb.200203050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Shu Y, Kuba H, Rasband MN, Wimmer VC, Bender KJ. Short- and long-term plasticity at the axon initial segment. J Neurosci. 2011;31:16049–16055. doi: 10.1523/JNEUROSCI.4064-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Rasband MN. Intrinsic and extrinsic determinants of ion channel localization in neurons. J Neurochem. 2006;98:1345–1352. doi: 10.1111/j.1471-4159.2006.04001.x. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Oka-mura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci. 2008;28:14213–14222. doi: 10.1523/JNEUROSCI.3398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, Vora AJ, Brophy PJ, Reynolds R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3173–3185. doi: 10.1093/brain/awl290. [DOI] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Subcellular organization of GABAergic synapses: role of ankyrins and L1 cell adhesion molecules. Nat Neurosci. 2006;9:163–166. doi: 10.1038/nn1638. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- Ivanovic A, Horresh I, Golan N, Spiegel I, Sabanay H, Frechter S, Ohno S, Terada N, Mobius W, Rosenbluth J, et al. The cytoskeletal adapter protein 4.1G organizes the internodes in peripheral myelinated nerves. J Cell Biol. 2012;196:337–344. doi: 10.1083/jcb.201111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IkappaBa/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome. J Neurosci. 2011;31:17637–17648. doi: 10.1523/JNEUROSCI.4162-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. bIV-Spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koticha D, Maurel P, Zanazzi G, Kane-Goldsmith N, Basak S, Babiarz J, Salzer J, Grumet M. Neurofascin interactions play a critical role in clustering sodium channels, ankyrin G and beta IV spectrin at peripheral nodes of Ranvier. Dev Biol. 2006;293:1–12. doi: 10.1016/j.ydbio.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Kriebel M, Metzger J, Trinks S, Chugh D, Harvey RJ, Harvey K, Volkmer H. The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. J Biol Chem. 2011;286:24385–24393. doi: 10.1074/jbc.M110.212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Labasque M, Devaux JJ, Leveque C, Faivre-Sarrailh C. Fibronectin type III-like domains of neurofascin-186 protein mediate gliomedin binding and its clustering at the developing nodes of Ranvier. J Biol Chem. 2011;286:42426–42434. doi: 10.1074/jbc.M111.266353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, De Camilli P, Moser T, Rasband MN, Solimena M. BetaIVsigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166:983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol Biol Cell. 1998;9:3505–3519. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligoden-droglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Laube G, Roper J, Pitt JC, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar pinceaux. Brain Res Mol Brain Res. 1996;42:51–61. doi: 10.1016/s0169-328x(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Lonigro A, Devaux JJ. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain. 2009;132:260–273. doi: 10.1093/brain/awn281. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Tempel BL, Schwartzkroin PA. Hyperexcitability of CA3 pyramidal cells in mice lacking the potassium channel subunit Kv1.1. Epilepsia. 2003;44:1506–1512. doi: 10.1111/j.0013-9580.2003.44602.x. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig M, Zanazzi G, Sakurai T, Blanco C, Levinson SR, Lambert S, Grumet M, Salzer JL. Nr-CAM and neurofascin interactions regulate ankyrin G and sodium channel clustering at the node of Ranvier. Curr Biol. 2001;11:1864–1869. doi: 10.1016/s0960-9822(01)00586-3. [DOI] [PubMed] [Google Scholar]
- Maier O, Baron W, Hoekstra D. Reduced raft-association of NF155 in active MS-lesions is accompanied by the disruption of the paranodal junction. Glia. 2007;55:885–895. doi: 10.1002/glia.20510. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat Neurosci. 2012;15:48–56. doi: 10.1038/nn.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Dupree JL, Popko B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J Cell Biol. 2002;156:567–577. doi: 10.1083/jcb.200111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, Kriegler S, Isom LL, Ragsdale DS. Functional and biochemical analysis of a sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J Neurosci. 2002;22:10699–10709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia. 2005;52:301–308. doi: 10.1002/glia.20245. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Weimann JM, Marder E. Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci. 1992;12:2803–2812. doi: 10.1523/JNEUROSCI.12-07-02803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthigasan J, Kirschner DA, Wintergerst ES, Nave KA, et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5:626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- Nave KA, Sereda MW, Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies—from basic to clinical research. Nat Clin Pract Neurol. 2007;3:453–464. doi: 10.1038/ncpneuro0583. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life. 2006;58:334–343. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- Ohara R, Yamakawa H, Nakayama M, Ohara O. Type II brain 4.1 (4.1B/KIAA0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Brain Res Mol Brain Res. 2000;85:41–52. doi: 10.1016/s0169-328x(00)00233-3. [DOI] [PubMed] [Google Scholar]
- O’Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, Kwon H, LeFevour A, Chakraborty-Sett S, Greene WC. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Palay VC. Cerebellar Cortex. New York: Springer-Ver-lag; 1974. [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (NfascNF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neu-rexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K1 channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E. Localization of Caspr2 in myelinated nerves depends on axon–glia interactions and the generation of barriers along the axon. J Neurosci. 2001;21:7568–7575. doi: 10.1523/JNEUROSCI.21-19-07568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A-S, McNa-mara JO, Williams SM. Neuroscience. 3. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Raine CS. On the association between perinodal astrocytic processes and the node of Ranvier in the CNS. J Neurocytol. 1984;13:21–27. doi: 10.1007/BF01148316. [DOI] [PubMed] [Google Scholar]
- Ramon y, Cajal S. Histologie du syste`me nerveux de l’hommes et des vertébrés. Paris: Maloine; 1911. [Google Scholar]
- Rasband MN. Neuron-glia interactions at the node of Ranvier. Results Probl Cell Differ. 2006;43:129–149. doi: 10.1007/400_014. [DOI] [PubMed] [Google Scholar]
- Rasband MN. Na+ channels get anchored … with a little help. J Cell Biol. 2008;183:975–977. doi: 10.1083/jcb.200811086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Zhen D, Arbuckle MI, Poliak S, Peles E, Grant SG, Trimmer JS. Clustering of neuronal potassium channels is independent of their interaction with PSD-95. J Cell Biol. 2002;159:663–672. doi: 10.1083/jcb.200206024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol. 2001;154:427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K1 channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St. Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Scheithauer B. Ultrastructure of multiple sclerosis. Ultrastruct Pathol. 1994;18:3–13. doi: 10.3109/01913129409016267. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots BI. The phylogeny of invertebrates and the evolution of myelin. Neuron Glia Biol. 2008;4:101–109. doi: 10.1017/S1740925X0900012X. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976;5:731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Axoglial junctions in the mouse mutant Shiverer. Brain Res. 1981;208:283–297. doi: 10.1016/0006-8993(81)90558-8. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Glial membrane and axoglial junctions. In: Ketten-mann H, Ranson BR, editors. Neuroglia. New York: Oxford University Press; 1995. pp. 613–633. [Google Scholar]
- Rosenbluth J, Dupree JL, Popko B. Nodal sodium channel domain integrity depends on the conformation of the paranodal junction, not on the presence of transverse bands. Glia. 2003;41:318–325. doi: 10.1002/glia.10179. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol. 2004;16:513–521. doi: 10.1016/j.ceb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Holmes WP, Colman DR. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J Cell Biol. 1987;104:957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37:832–844. doi: 10.1016/j.mcn.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Maruyama S. Increase in diameter of the axonal initial segment is an early change in amyotrophic lateral sclerosis. J Neurol Sci. 1992;110:114–120. doi: 10.1016/0022-510x(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neu-rofascin 155 into lipid rafts? J Neurosci. 2004;24:3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Custer AW, Shrager P, Rasband MN. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2006;2:69–79. doi: 10.1017/S1740925X06000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B, Peracchia C, Mugnaini E. The paranodal axo-glial junction in the central nervous system studied with thin sections and freeze-fracture. Neuroscience. 1976;1:181–190. doi: 10.1016/0306-4522(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Schultz C, Konig HG, Del Turco D, Politi C, Eckert GP, Ghebremed-hin E, Prehn JH, Kogel D, Deller T. Coincident enrichment of phosphorylated IkappaBalpha, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol Cell Neurosci. 2006;33:68–80. doi: 10.1016/j.mcn.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Harris DM. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J Neu-rosci. 2010;30:70–80. doi: 10.1523/JNEUROSCI.3464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell–cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- Simons M, Trotter J. Wrapping it up: the cell biology of myelina-tion. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Simons M, Kramer EM, Thiele C, Stoffel W, Trotter J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol. 2000;151:143–154. doi: 10.1083/jcb.151.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, Murray JA, Patterson RC. Internodal myelin volume and axon surface area. A relationship determining myelin thickness? J Neurol Sci. 1982;55:231–246. doi: 10.1016/0022-510x(82)90103-4. [DOI] [PubMed] [Google Scholar]
- Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci U S A. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Development of “Pinceaux” formations and dendritic translocation of climbing fibers during the acquisition of the balance between glutamatergic and gamma-aminobutyric acidergic inputs in developing Purkinje cells. J Comp Neurol. 2008;506:240–262. doi: 10.1002/cne.21501. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles’ heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS myelin paranodes require Nkx6-2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;24:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranv-ier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Rasband MN, Tohyama K, Koibuchi K, Okamoto S, Funa-koshi K, Hirata K, Baba H, Yuki N. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007;27:3956–3967. doi: 10.1523/JNEUROSCI.4401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Raphael AR, Ogawa Y, Stankewich MC, Peles E, Talbot WS, Rasband MN. Schwann cell spectrins modulate peripheral nerve myelination. Proc Natl Acad Sci U S A. 2011;108:8009–8014. doi: 10.1073/pnas.1019600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland ML, Williams SH, Abedi R, Overbeek PA, Pfaffinger PJ, Noebels JL. Overexpression of a Shaker-type potassium channel in mammalian central nervous system dysregulates native potassium channel gene expression. Proc Natl Acad Sci U S A. 1999;96:2451–2455. doi: 10.1073/pnas.96.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Rosenbluth J. Axolemmal differentiation in myelinated fibers of rat peripheral nerves. Brain Res. 1983;285:251–263. doi: 10.1016/0165-3806(83)90023-8. [DOI] [PubMed] [Google Scholar]
- Thaxton C, Bhat MA. Myelination and regional domain differentiation of the axon. Results Probl Cell Differ. 2009;48:1–28. doi: 10.1007/400_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron. 2011;69:244–257. doi: 10.1016/j.neuron.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwa-kura Y, Fukamauchi F, Watanabe K, Soliven B, et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD. Myelin-associated glycoprotein. Location and potential functions. Ann N Y Acad Sci. 1990;605:29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K1 channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Ward RE, Lamb RS, Fehon RG. A conserved functional domain of Drosophila coracle is required for localization at the septate junction and has membrane-organizing activity. J Cell Biol. 1998;140:1463–1473. doi: 10.1083/jcb.140.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, Sasai H. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J Neurochem. 2000;75:28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- Xie G, Harrison J, Clapcote SJ, Huang Y, Zhang JY, Wang LY, Roder JC. A new Kv1.2 channelopathy underlying cerebellar ataxia. J Biol Chem. 2010;285:32160–32173. doi: 10.1074/jbc.M110.153676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yageta M, Kuramochi M, Masuda M, Fukami T, Fukuhara H, Mar-uyama T, Shibuya M, Murakami Y. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res. 2002;62:5129–5133. [PubMed] [Google Scholar]
- Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24:7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RC, Bunge RP. Damage and repair of the peripheral myelin sheath and node of Ranvier after treatment with trypsin. J Cell Biol. 1975;64:1–14. doi: 10.1083/jcb.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Davis JQ, Carpenter S, Bennett V. Structural requirements for association of neurofascin with ankyrin. J Biol Chem. 1998;273:30785–30794. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bekku Y, Dzhashiashvili Y, Armenti S, Meng X, Sasaki Y, Milbrandt J, Salzer JL. Assembly and maintenance of nodes of ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron. 2012;73:92–107. doi: 10.1016/j.neuron.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Desmazieres A, Rinaldi A, Tait S, Sherman DL, Nolan MF, Brophy PJ. A critical role for Neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron. 2011;69:945–956. doi: 10.1016/j.neuron.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]