Abstract
Research has indicated that individuals of Asian descent, relative to other racial groups, demonstrate reduced emotional responding and lower prevalence rates of several anxiety disorders. It is unclear though whether these group differences extend to biomarkers of anxiety disorders and whether genetic differences play a role. The present study compared self-identified Caucasians, Latinos, and Asians (total N = 174) on startle response during a baseline period and while anticipating unpredictable threat–a putative biomarker for certain anxiety disorders–as well as predictable threat. In addition, the association between genetic ancestry and startle response was examined within each racial group to determine potential genetic influences on responding. For the baseline period, Asian participants exhibited a smaller startle response relative to Caucasian and Latino participants, who did not differ. Within each racial group, genetic ancestry was associated with baseline startle. Furthermore, genetic ancestry mediated racial group differences in baseline startle. For the threat conditions, a Race × Condition interaction indicated that Asian participants exhibited reduced startle potentiation to unpredictable, but not predicable, threat relative to Caucasian and Latino participants, who did not differ. However, genetic ancestry was not associated with threat-potentiated startle in any racial group. The present study adds to the growing literature on racial differences in emotional responding and provides preliminary evidence suggesting that genetic ancestry may play an important role. Moreover, reduced sensitivity to unpredictable threat may reflect a mechanism for why individuals of Asian descent are at less risk for particular anxiety disorders relative to other racial groups.
Keywords: race, ancestry informative markers, genetics, unpredictability, startle
Introduction
Fear and anxiety are often used interchangeably to indicate emotional responses to threat. However, one feature that has been proposed to differentiate fear and anxiety in animal and human studies is the predictability of threat (Davis, 2006; Grillon, 2008; Nelson & Shankman, 2011). Specifically, fear is associated with predictable threat and helps prepare an organism for immediate fight, flight, or immobilization. Anxiety is associated with less certain (or present) threat and is associated with sustained vigilance and defensive preparedness.
One of the ways that fear and anxiety have been differentiated physiologically is by measuring startle response while individuals anticipate a predictable or unpredictable aversive stimulus (Grillon & Schmitz, 2012). Interestingly, individuals with particular anxiety disorders, such as panic disorder, exhibit heightened responding to unpredictable aversiveness, while the findings for predictable aversiveness are more mixed (Grillon et al., 2008; 2009; Shankman et al., 2013). These and other findings (Nelson et al., 2013) suggest that heightened startle to unpredictable threat may be a biomarker for anxiety disorders.
Biomarkers are often assumed to be universal, but it is critical to determine whether they are similar across different racial/ethnic groups. This is particularly important for biomarkers of emotional constructs, as prior research has identified important racial/ethnic group differences in emotional responding. For example, individuals of Asian descent have been shown to be less emotionally expressive (Soto, Levenson, & Ebling, 2005) and have reduced startle relative to Caucasians during a baseline period (Swerdlow, Talledo, & Braff, 2005). Furthermore, epidemiological studies have indicated that Asians have lower prevalence rates of certain anxiety disorders (Grant et al., 2006; Smith et al., 2006).1 To date, several cultural explanations for these differences have been proposed (e.g., Asian cultures place greater value on emotional control, Tsai & Levenson, 1997).2 However, no study has examined whether Asians show differences in startle to unpredictable vs. predictable threat, specifically.
The present study compared self-identified Caucasian, Latino, and Asian participants on their startle response during baseline and predictable vs. unpredictable threat-of-shock conditions. We hypothesized that, similar to previous research (Swerdlow et al., 2005), Asians would demonstrated reduced baseline startle relative to Caucasians. In addition, given that heightened startle to unpredictable threat may be a putative biomarker for particular anxiety disorders (Grillon et al., 2008; Nelson et al., 2013) and Asian individuals meet criteria for certain anxiety disorders at lower rates relative to other racial groups (Grant et al., 2006; Smith et al., 2006), we also hypothesized that Asians would demonstrate reduced startle potentiation to unpredictable (but not predictable) threat compared to Caucasian individuals. The inclusion of Latino participants was an exploratory aim and we did not have any a priori hypotheses regarding whether they would be more similar to Asians or Caucasians.
There is also a genetic component to race that may play an important role in emotional responding. Recently, geneticists have identified ancestry-informative markers (AIMs), which are sets of genetic polymorphisms that exhibit substantially different frequencies between populations from various geographical regions (Enoch, Shen, Xu, Hodgkinson, & Goldman, 2006; Kittles & Weiss, 2003). AIMs are useful to estimate what proportion of an individual's genetic ancestry is derived from certain geographical regions and can help address many of the challenges associated with examining racial group differences (e.g., heterogeneity). Thus, as a secondary aim, the present study conducted a preliminary examination of the role of genetic ancestry in population differences in startle. Specifically, we examined whether individual differences in genetic ancestry were associated with startle response within each racial group. If so, results would suggest that genetic background at least partially contributes to group differences in startle. These were also exploratory aims and we did not have any a priori hypotheses regarding the contribution of genetic ancestry to racial differences in startle.
Method
Participants
The sample included 242 introductory psychology students from the University of Illinois - Chicago who could read and write English and participated for course credit. Participants completed one of two larger studies – one examining biomarkers of depression, anxiety, and personality traits (N = 131) and another examining the association between biomarkers of anxiety and attentional threat biases (N = 111). Both studies used identical startle and ancestry marker procedures (described below) and none of the subsequent results differed as a function of study.
Participants self-identified their race by choosing from as many of the following categories as desired: Caucasian, not Latino (n = 83); Latino (n = 58); Asian or Pacific Islander (n = 77); African American, not Latino (n = 23); or Other (n = 17).3 Other participants were able to be re-coded as Caucasian, Latino, or Asian for the purposes of this study (e.g., Arab or Middle Eastern = Caucasian [given their genealogical connection to the South Caucasus region]; Mexican = Latino). Participants who chose more than one race (n = 8) were excluded from the present study to minimize overlap in racial identification. African American participants were excluded from the present study due to the small sample size. Of the remaining participants, participants were excluded from the present study if they were currently taking psychotropic medication (n = 3), missing genetic ancestry data or experienced equipment failure (n = 14), or had more than 50% of startle trials excluded due to blink artifacts (n = 23). Thus, the final sample consisted of 174 participants who self-identified as only Caucasian (n = 69), Latino (n = 51), or Asian (n = 54).
Demographics and Anxiety Symptomatology
Demographics for self-identified racial groups are presented in Table 1. A one-way between-subjects analysis of variance (ANOVA) indicated that racial groups were comparable on age (p = .34) and a chi-square analysis indicated groups were also matched on gender (p = .48).
Table 1.
Demographics and Genetic Ancestry Estimates Across Different Self-Identified Racial Groups
| Caucasians (n = 69) | Latinos (n = 51) | Asians (n = 54) | |
|---|---|---|---|
| Age (SD) | 19.4 (1.6) | 19.0 (1.6) | 19.1 (1.9) |
| Gender (% Female) | 65.2% | 74.5% | 64.8% |
| AIMS | |||
| % European (SD) | 90.5 (5.8) | 46.9 (17.1) | 27.2 (23.8) |
| % West African (SD) | 3.8 (3.2) | 8.0 (8.2) | 8.2 (10.2) |
| % Asian (SD) | 5.7 (4.5) | 45.1 (18.8) | 64.6 (25.5) |
Note. The combination of European, West African, and Asian AIMS totaled 100% for each participant. AIMS = Ancestry Informative Markers; SD = Standard Deviation.
Prior to the experimental task, participants completed self-report measures of anxiety symptomatology. In the first larger study sample (N = 131), participants completed the Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007), which is an 18-item self-report measure of unpleasant sensations experienced in anxiety-related situations. ASI-3 scores for Caucasians (M = 16.36, SD = 9.03), Latinos (M = 19.76, SD = 13.79), and Asians (M = 19.82, SD = 9.63) were comparable to those found in college students and below the mean for various anxiety disorders (Wheaton, Deacon, McGrath, Berman, & Abramowitz, 2012). In the second larger study sample (N = 111), participants completed the Inventory of Depression and Anxiety Symptoms (IDAS; Watson, O'Hara, Simms, Kotov, & Chmielewski, 2007), which is a 64-item self-report measure designed to assess specific symptom dimensions related to major depression and anxiety disorders. The present study examined the three anxiety subscales – social anxiety, panic, and traumatic intrusions. IDAS scores for Caucasians (social anxiety M = 8.85, SD = 4.47; panic M = 12.11, SD = 4.31; traumatic intrusions = 6.52, SD = 3.19), Latinos (social anxiety M = 10.00, SD = 4.79, panic M = 13.28, SD = 5.13, traumatic intrusions M = 8.06, SD = 4.77), and Asians (social anxiety M = 9.63, SD = 3.69, panic M = 11.63, SD = 4.31, traumatic intrusions M = 6.41, SD = 2.58) were comparable to those found in college students and below the means for psychiatric patients (Watson et al., 2007). Importantly, racial groups did not differ on ASI-3 or IDAS measures (all p's > .24).
Genotyping and Ancestry Estimation
Genomic DNA collected with Oragene kits (DNA Genotek, Ontario, Canada) was extracted using standard protocols. A total of 105 ancestry informative markers (AIMs) were genotyped using the Sequenom MassARRAY platform (Giri et al., 2009; Tian et al., 2006). The AIMs used have been published previously (Giri et al., 2009; Hooker et al., 2010; Kupfer et al., 2009, 2010). iPLEX assays were designed using the Sequenom Assay Design software. Primer sequences for polymerase chain reaction (PCR) and single-base extension are available on request. Multiplex PCR was performed to amplify 5–10 ng of genomic DNA. PCR reactions were treated with shrimp alkaline phosphatase to neutralize unincorporated deoxynucleotide triphosphates. A post-PCR single-base extension reaction was performed for each multiplex reaction using concentrations of 0.625 μM for low-mass primers and 1.25 μM for high-mass primers. Reactions were diluted with 16 μL of H2O, and the oligonucleotides were purified with resin, spotted onto Sequenom SpectroCHIP microarrays and separated by MALDI-TOF mass spectrometry. Individual single-nucleotide polymorphism (SNP) genotype calls were generated using Sequenom TYPE software which automatically calls allele-specific peaks according to their expected masses.
Global individual ancestry was determined for each individual using AIMs for European, West African, and Asian/Native American genetic ancestry. Individual ancestry estimates were obtained from the genotype results using the Bayesian Markov Chain Monte Carlo (MCMC) method implemented in the program STRUCTURE 2.1 (Falush, Stephens, & Pritchard, 2003). STRUCTURE 2.1 assumes an admixture model using prior population information and independent allele frequencies. The MCMC model was run using K = 3 populations (60 Europeans, 60 Asians/Native Americans and 131 West Africans) and a burn-in length of 30,000 iterations followed by 70,000 replications.
For each participant, their percentage of individual ancestry for European, West African, and Asian genetic ancestry was determined ranging from 0–100%, with the combination of the three markers totaling 100% (see Table 1 for averages within self-identified racial groups). As expected, Caucasian's genetic ancestry was predominately European (range 72.3% – 98.1%), Asian's genetic ancestry was predominately Asian (although with a bigger range than that of Caucasians; range 10.6% – 91.5%), and Latino's genetic ancestry was nearly evenly split between European (range 5.2% – 82.4%) and Asian (6.4% – 94.1%).
Stimuli Presentation and Physiological Responses
Stimuli were administered using PSYLAB (Contact Precision Instruments, London, UK), and electromyography activity was recorded using Neuroscan 4.4 (Compumedics, Charlotte, NC, USA). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Startle eye blink reflex was measured from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye. Data were collected using a bandpass filter of DC-200-Hz at a sampling rate of 1000-Hz. Electric shocks were 400-ms long and were administered to the wrist of the participant's left (non-dominant) hand. Shock intensity was determined ideographically using a work-up procedure for each subject (see below).
Procedure
After electrode placement, participants were seated in an electrically shielded, sound-attenuated booth approximately 3.5-ft from a 19-in computer monitor. Participants first completed a 2.5-minute baseline task during which nine acoustic startle probes were administered. Previous research within our laboratory has indicated that participants habituate to the startle probe after the third blink (Campbell et al., in press). Therefore, the present study focused on initial baseline startle responding (i.e., average of the first three blinks), which was likely to be more sensitive to group and/or genetic differences than all nine blinks. Next, shock intensity was determined using a work-up procedure where participants received increasing levels of shock, until they reached a level they described as “highly annoying but not painful” (maximum shock level was 5-mA). Racial groups did not differ in mean shock level (p = .72).
The threat-of-shock task was a variant of that used by Grillon and colleagues (Schmitz & Grillon, 2012) and included three within-subjects conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the screen informed participants of the current threat condition by displaying the following information: “no shock” (N), “shock at 1” (P), or “shock at any time” (U). Each condition lasted 90-s, during which a 6-s visual countdown (CD) was presented five times. The interstimulus intervals (ISIs) ranged from 7 to 17-s during which only the text describing the condition was on the screen. In the N condition, no shocks were delivered. In the P condition, participants received a shock every time the countdown reached 1. In the U condition, shocks were administered at any time. Startle probes were presented both during the CD (1-5-s following CD onset) and ISI (5-14-s following ISI onset). The time intervals between shocks and subsequent startle probes were always greater than 10-s to ensure that subsequent startle responses were not affected by prior shocks.
The NPU-threat task consisted of two presentations of each 90-s condition (N, P, U), during which the CD appeared five times. Participants received startle probes during four out of the five CD and ISI presentations. Conditions were presented in one of the following orders (counterbalanced): PNUPNU or UNPUNP. All participants received 20 electric shocks (10 during P, 10 during U), and 48 startle probes (16 during N, 16 during P, and 16 during U) during the CD and ISI (with an equal number of startle probes occurring during the CD and ISI).
Data Processing
Electromyography data were first rectified and then smoothed using a finite impulse response filter with a band-pass of 28-40-Hz. Peak amplitude of the blink reflex was determined in the 20-150-ms time frame following the startle probe onset relative to baseline (average baseline electromyography level for the 50-ms preceding the startle probe onset). Blinks were scored as non-responses if electromyography activity during the 20-150-ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Analyses were conducted using blink magnitude (i.e., averages include values of 0 for non-response trials) as this is a more conservative estimate of blink response (Blumenthal et al., 2005). Startle data were skewed and kurtotic and were therefore square root transformed to achieve normality.
Results
Self-Identified Race
Baseline task
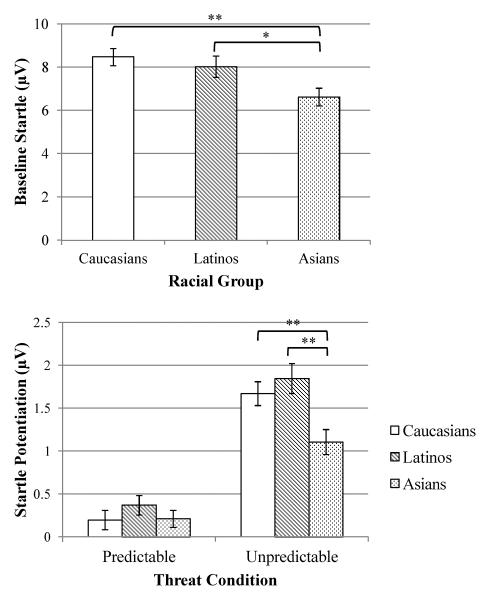
A one-way analysis of variance (ANOVA) indicated that racial groups differed in baseline startle (see top of Figure 1), F(2, 171) = 4.98, p < .01, ηp2= .06, such that Asians (M = 6.62, SD = 3.00) had a smaller startle response relative to Caucasians (M = 8.47, SD = 3.31), F(1, 121) = 10.20, p < .01, ηp2 = .08, and Latinos (M = 8.02, SD = 3.55), F(1, 103) = 4.79, p < .05, ηp2 = .04, who did not differ, F(1, 120) = 0.50, ns.
Figure 1.
Racial group differences in baseline startle (top) and startle potentiation to predictable and unpredictable threat (bottom). Startle potentiation was the difference in startle between the no threat and threat conditions (i.e., P–N, U–N). Error bars represent standard error. N = No Shock; P = Predictable Shock; U = Unpredictable Shock.
* p < .05, ** p < .01
NPU-threat task
A three-way Condition (N, P, U) X Cue (ISI vs. CD) X Race (Caucasian, Latino, Asian) mixed-effects ANOVA was conducted with Condition and Cue as within-subjects factors and Race as the between-subjects factor. Results indicated a main effect for Race, F(2, 171) = 8.25, p < .001, ηp2 = .09, and a Race X Condition interaction, F(4, 342) = 4.28, p < .01, ηp2 = .05. Examination of the Race main effect indicated that, across the entire task, Asians had a smaller startle response relative to Caucasians, F(1, 121) = 17.77, p < .001, ηp2 = .13, and Latinos, F(1, 103) = 8.53, p < .01, ηp2 = .08, who did not differ, F(1, 118) = 0.43, ns.
To follow-up the Race X Condition interaction, potentiation scores from the N condition (i.e., P–N, U–N) were calculated to directly compare whether groups differed in their startle potentiation to P vs. U threat (see bottom of Figure 1). A Race X Condition (with P vs. U potentiation scores as the two levels) ANOVA again indicated a main effect for Race, F(2, 171) = 4.05, p < .05, ηp2 = .05, and a Race X Condition interaction, F(2, 171) = 4.42, p < .05, ηp2 = .05. For the P threat condition, there was no main effect of Race, F(2, 171) = 0.72, ns. In contrast, for potentiation to the U threat condition, there was a main effect of Race, F(2, 171) = 5.98, p < .01, ηp2 = .07, such that Asians had less startle potentiation relative to Caucasians, F(1, 121) = 7.69, p < .01, ηp2 = .06, and Latinos, F(1, 103) = 10.63, p < .01, ηp2 = .09, who did not differ, F(1, 118) = 0.43, ns.
Genetic Ancestry
Baseline task
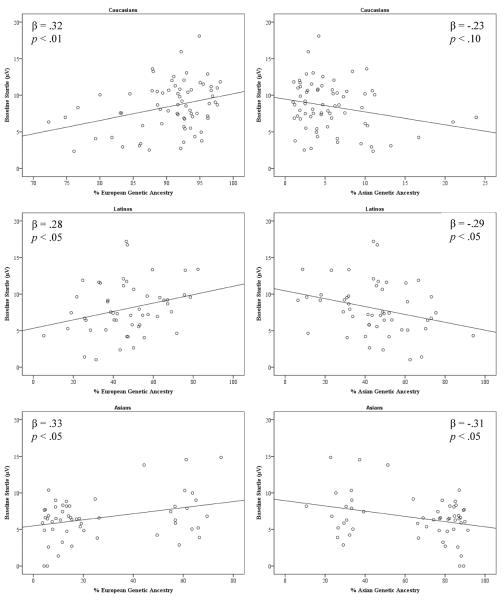
To examine genetic effects, separate linear regressions were conducted within each racial group with baseline startle regressed onto genetic ancestry (see Figure 2). Results indicated that European genetic ancestry was positively associated with baseline startle in Caucasians, β = .32, t(68) = 2.75, p < .01, Latinos, β = .28, t(50) = 2.02, p < .05, and Asians, β = .33, t(53) = 2.52, p < .05. Conversely, Asian genetic ancestry was negatively associated with baseline startle in Latinos, β = −.29, t(50) = −2.08, p < .05 and Asians, β = −.31, t(53) = −2.31, p < .05, and in Caucasians at a trend level, β = −.23, t(68) = −1.97, p < .06.
Figure 2.
Scatterplots of baseline startle and % European (left) and Asian (right) genetic ancestry in Caucasians (top), Latinos (middle), and Asians (bottom).
NPU-threat task
Separate general linear models were conducted within each racial group with Condition (N, P, U) and Cue (CD vs. ISI) entered as within-subjects factors and genetic ancestry entered as a continuous between-subjects variable. Results indicated no main effects or interactions involving genetic ancestry during the NPU-threat task.
Mediational Analyses
Finally, we examined whether genetic ancestry mediated the difference between self-identified race and baseline startle. To test for mediation, we followed MacKinnon, Lockwood, and Williams's (2004) recommendations and used a nonparametric bootstrapping method. This approach has been shown to be statistically more powerful than other tests of mediation (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). Mediational models were tested using the SPSS macro MEDIATE provided by Hayes and Preacher (2013), which provides a bootstrap estimate of the indirect effect between the independent variable and dependent variable and 95% confidence intervals for the population value of the indirect effect. Confidence intervals that do not include zero indicate a significant indirect effect at the p < .05 significance level.
Analysis of 5,000 bootstrap samples indicated that European genetic ancestry mediated the difference between Asians and Caucasians, B = 3.38, 95% CI [1.59, 5.35], and Asians and Latinos, B = 1.05, 95% CI [0.45, 1.95]. Similarly, Asian genetic ancestry mediated the difference between Asians and Caucasians, B = 2.66, 95% CI [1.25, 4.43], and Asians and Latinos, B = 0.88, 95% CI [0.39, 1.75].
Discussion
The present study examined baseline and threat-potentiation startle of three racial groups (Caucasian, Latino, and Asian) and explored whether genetic markers of ancestry were related to group differences. There were several noteworthy findings. Asian participants exhibited smaller baseline startle relative to Caucasians and Latinos, who did not differ. Within each racial group, European and Asian genetic ancestry was associated positively and negatively, respectively, with baseline startle, strongly suggesting genetic influences on baseline startle. Furthermore, genetic ancestry mediated the difference between racial groups on baseline startle. Asian participants also exhibited reduced startle potentiation to unpredictable, but not predicable, threat relative to Caucasians and Latinos (and a direct comparison indicated this effect was larger for unpredictable vs. predictable threat), but there was no association between genetic ancestry and threat-potentiated startle. Results from the present study add to the growing literature on racial differences in emotional responding and provide preliminary evidence suggesting that genetic differences at least partially contribute to these findings.
There are several potential interpretations of these results. First, consistent with prominent cultural theories, individuals of Asian descent may demonstrate greater emotional control, particularly during aversive contexts (Tsai & Levenson, 1997). Prior research has supported this theory using self-report and behavioral measures of emotion (Soto et al., 2005; Tsai, Chentsova-Dutton, Freire-Bebeau, & Przymus, 2002); however, studies have rarely reported group differences in physiological responding. Levenson and colleagues (2007) suggested that physiological measures of emotion may be minimally susceptible to cultural influences – thus, culture may not (exclusively) account for the present findings. One limitation of prior psychophysiological studies is the use of measures that confound valence and arousal (e.g., cardiac, electrodermal responses). In contrast, startle has been shown to be sensitive to valence (Lang, 1995) and can be modulated when engaging in emotion regulation (Jackson, Malmstadt, Larson, & Davidson, 2000). Thus, startle may be an ideal measure to use when examining racial differences in emotional responding.
Second, these findings may reflect a mechanism for why individuals of Asian descent are at less risk for certain anxiety disorders (e.g., panic disorder). Heightened sensitivity to unpredictable threat is a putative mechanism and risk marker for several anxiety disorders (Grillon et al., 2008; Nelson et al., 2013; Shankman et al., 2013), and epidemiological studies have found lower prevalence rates of anxiety disorders among Asians relative to other racial groups (Grant et al., 2006; Smith et al., 2006). Our finding of smaller startle potentiation to unpredictable threat in Asians may reflect a mechanism for these epidemiological findings.
There may be other factors though that could account for these lower prevalence rates, such as differences in symptom presentation or coping abilities (Lewis-Fernández et al., 2009). Heightened sensitivity to unpredictable threat may simply play a smaller etiological role in the development of certain anxiety disorders within Asians. Indeed, Asian individuals exhibited lower startle potentiation to unpredictable threat than the other groups, but comparable levels on anxiety symptomatology. Future studies on risk factors and etiology of anxiety disorders should examine whether Asians have different causal pathways to the illnesses.
Results also provide evidence for genetic influences on startle. Specifically, baseline startle was positively and negatively associated with European and Asian genetic ancestry, respectively, within all three racial groups. In other words, across all racial (and consequently ethnic) identifications, European and Asian genetic ancestry was associated with individual differences in baseline startle. Previous research has indicated that startle magnitude is highly heritable (Anokhin, Heath, Myers, Ralano, & Wood, 2003; Hasenkamp et al., 2010), and results from the present study support the notion that racial differences may be at least partially genetically influenced. Interestingly, neither European nor Asian genetic ancestry was associated with startle potentiation during the NPU-threat task. These results are consistent with a twin study which found that overall startle, but not emotion-modulated startle, was mediated by genetic variance (Anokhin, Golosheykin, & Heath, 2007). Taken together, one interpretation of these findings is that genetic factors (such as genetic ancestry) influences one's basal level of defensive sensitivity, but one's reactivity to threatening stimuli (as indicated by emotion-modulated startle) is mediated more by environmental influences. This hypothesis, however, is quite speculative and further research is needed to determine the mediating factors of emotion-modulated startle.
Another explanation for the genetic ancestry result is that reduced startle in Asians may be due to differences in ocular muscle morphology that are genetically determined. However, this is unlikely as the primary difference between Asian and Caucasian ocular muscle morphology is in the levator aponeurosis that innervates the upper eyelid and not the lower orbicularis oculi muscle from which startle response was measured (Jeong, Lemke, Dortzbach, Park, & Kang, 1999).
The present study provides preliminary evidence that genetic ancestry plays at least a partial role in determining startle response. However, it is important to highlight that this study was not intended to be a definitive comparison of the effects of culture vs. genetics on emotional responding. Race is a construct that confounds social, cultural, and biological factors (Kittles & Weiss, 2003), and the present study was not designed to tease apart the relative contribution of these (and other) factors. Additionally, as genetic ancestry covaries with other biological (e.g., skin pigmentation) and sociocultural variables that play a role in racial identification (Klimentidis, Miller, & Shriver, 2009; Nagel, 1994), it is possible that genetics may not be separable from key cultural variables (Miller, 2010). Therefore, one contribution of this study is that it provides a relatively new `level of analysis' in which cultural differences in emotion (and other aspects of psychology) can be examined. This study also adds to the small, but growing, number of studies combining genetic and cultural psychology research (e.g., Kim et al., 2010; 2011) and more broadly, illustrates the utility of using AIMs in psychology research.
Latino participants responded more similarly to Caucasian than Asian participants. Cultural theories have posited that Latinos often demonstrate enhanced emotional responding (Soto et al., 2005). However, as previously mentioned, most of the supporting literature has utilized self-report and behavioral indices of emotional responding. To our knowledge, the present study is one of the first to examine startle in Latinos, and suggests that the response `channel' (i.e., verbal, behavior, or physiological) is important to consider when examining emotional responding across different racial groups.
The present study had several strengths, including examination of both self-identified race and genetic ancestry, and matching of racial groups on demographics and anxiety symptomatology. There were also several limitations. First, participants were all college students and results may not generalize to all individuals. Second, only self-identified Caucasian, Latino, and Asian participants were examined and more research is needed examining other racial groups as well as subgroups within these populations. Finally, participants were asked to identify as Caucasian, Latino, and/or Asian and racial categories are known to be problematic in psychology research (Helms, Jernigan, & Mascher, 2005). Future research examining racial differences in sensitivity to threat should consider alternative approaches toward quantifying human variation.
In summary, we found that Asian individuals exhibited smaller baseline startle relative to Caucasians and Latinos, who did not differ. Within each racial group, European and Asian genetic ancestry was associated positively and negatively, respectively, with baseline startle, and genetic ancestry mediated the difference between racial groups on baseline startle. Lastly, a Race X Condition interaction indicated that Asian participants also exhibited reduced startle potentiation to unpredictable (but not predicable) threat relative to Caucasians and Latinos, but there was no association between genetic ancestry and threat-potentiated startle. These novel results highlight the importance of examining racial differences in psychiatric biomarkers and suggest that genetic background may play an important role.
Acknowledgments
This study was supported by NIH grants R01MH098093 (PI: S.A.S.) and K08MH083888 (PI: J.R.B), and the UIC Chancellor's Discovery Fund (PIs: S.A.S. and J.R.B).
Footnotes
While there is lower prevalence of certain anxiety disorders in Asian individuals relative to other groups, paradoxically, Asian individuals actually report greater levels of anxiety on some self-report measures (e.g., Norasakkunkit & Kalick, 2002; Okazaki, 1997). However, this discrepancy appears to be only (or largely) for social phobia and not for other anxiety disorders, such as panic disorder and PTSD (Asnaani, Gutner, Hinton, & Hofmann, 2009; Roberts, Gilman, Breslau, Breslau, & Koenen, 2011). For example, Macdonald and colleagues (2013) reported significant concordance rates between clinician-rated and self-reported symptoms of PTSD for Asian individuals and comparable concordance rates between Asian and Caucasian individuals. The findings for panic disorder and PTSD are particularly noteworthy given that they are the two disorders that have been associated with heightened startle to unpredictable threat (Grillon et al., 2008; 2009; Shankman et al., 2013), while, to our knowledge, no study has examined this question in social phobia.
Much of this research was limited to individuals of East Asian descent and results may not necessarily apply to other ethnic groups from Asia (e.g., Filipino, South Asians).
The total number of participants who self-identified as Caucasian, not Latino; Latino; Asian or Pacific Islander; African American, not Latino; or Other (N = 258) was greater than the total sample size (N = 242) because participants were allowed to choose more than one racial group. Thus, these numbers represent the total number of times a given racial group was chosen, not the absolute number of participants in the study.
Participants were asked to self-identify their race but not ethnicity. This likely produced significant ethnic and cultural heterogeneity within each racial group, and limited our ability to compare the role of culture vs. biology to startle response. Despite this limitation, participants in the study on biomarkers of anxiety and attentional threat biases sample (N = 111) were asked to report whether English was their first language and any other language they spoke beyond English. While not an immediate proxy for ethnic background, additional languages spoken may provide some information regarding the ethnic composition within each racial group. Within this sample, 18.5% (n = 5) of Caucasian, 61.1% (n = 11) of Latino, and 59.3% (n = 16) of Asian participants reported that English was not their first language. Furthermore, Caucasian participants reported also speaking Arabic (n = 1), Farsi (n = 2), Greek (n = 1), Polish (n = 5), and Romanian (n = 2); Latino participants also reported speaking Spanish (n = 14); and Asian participants also reported also speaking Burmese (n = 1), Cantonese (n = 2), Chinese (n = 5), Filipino (n = 1), Gujarati (n = 1), Konkani (n = 1), Korean (n = 5), Tagalog (n = 1), Telugu (n = 1), Urdu (n = 3), and Vietnamese (n = 1). We further compared Asian participants for which English was (n = 11) and was not (n = 16) their first language on baseline and threat-potentiated startle. Results indicated that there were no differences in startle between these two groups (p's > .11), although the size of these group likely preclude broader interpretation.
References
- Anokhin AP, Golosheykin S, Heath AC. Genetic and environmental influences on emotion-modulated startle reflect: a twin study. Psychophysiology. 2007;44:106–112. doi: 10.1111/j.1469-8986.2006.00486.x. doi: 10.1111/j.1469-8986.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- Anokhin A, Heath A, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neuroscience Letters. 2003;353:45–48. doi: 10.1016/j.neulet.2003.09.014. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Asnaani A, Gutner CA, Hinton DE, Hofmann SG. Panic disorder, panic attacks and panic attack symptoms across race-ethnic groups: results of the collaborative psychiatric epidemiology studies. CNS Neuroscience & Therapeutics. 2009;15:249–254. doi: 10.1111/j.1755-5949.2009.00092.x. doi: 10.1111/j.1755-5949.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Campbell ML, Gorka SM, McGowan SK, Nelson BD, Sarapas C, Katz AC, Shankman SA. Does anxiety sensitivity correlate with reductions in startle responding? An examination in three separate samples. Cognition & Emotion. doi: 10.1080/02699931.2013.799062. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Xu K, Hodgkinson C, Goldman D. Using ancestry-informative markers to define populations and detect population stratification. Journal of Psychopharmacology. 2006;20:19–26. doi: 10.1177/1359786806066041. doi: 10.1177/1359786806066041. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard J. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri VN, Egleston B, Ruth K, Uzzo RG, Chen DYT, Buyyounouski M, Kittles R. Race, genetic West African ancestry, and prostate cancer prediction by prostate-specific antigen in prospectively screened high-risk men. Cancer Prevention Research. 2009;2:244–250. doi: 10.1158/1940-6207.CAPR-08-0150. doi: 10.1158/1940-6207.CAPR-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, Saha TD. The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:363–374. doi: 10.4088/jcp.v67n0305. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. doi:10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Epstein MP, Green A, Wilcox L, Boshoven W, Lewison B, Duncan E. Heritability of acoustic startle magnitude, prepulse inhibition, and startle latency in schizophrenia and control families. Psychiatry Research. 2010;178:236–243. doi: 10.1016/j.psychres.2009.11.012. doi: 10.1016/j.psychres.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Preacher K,J. Statistical mediation analysis with a multicategorical independent variable. Unpublished white paper. 2013 doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- Helms JE, Jernigan M, Mascher J. The meaning of race in psychology and how to change it: A methodological perspective. American Psychologist. 2005;60:27–36. doi: 10.1037/0003-066X.60.1.27. doi: 10.1037/0003-066X.60.1.27. [DOI] [PubMed] [Google Scholar]
- Hooker S, Hernandez W, Chen H, Robbins C, Torres JB, Ahaghotu C, Kittles RA. Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate. 2010;70:270–275. doi: 10.1002/pros.21061. doi: 10.1002/pros.21061. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. doi: 10.1111/1469-8986.3740515. [PubMed] [Google Scholar]
- Jeong S, Lemke BN, Dortzbach RK, Park YG, Kang HK. The Asian upper eyelid: An anatomical study with comparison to the Caucasian eyelid. Archives of Ophthalmology. 1999;117:907–912. doi: 10.1001/archopht.117.7.907. doi:10.1001/archopht.117.7.907. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Mojaverian T, Sasaki JY, Park J, Suh EM, Taylor SE. Gene-culture interaction: oxytocin receptor polymorphism (OXTR) and emotion regulation. Social Psychological & Personality Science. 2011;2:665–672. doi: 10.1177/1948550611405854. [Google Scholar]
- Kim HS, Sherman DK, Taylor SE, Sasaki JY, Chu TQ, Ryu C, Xu J. Culture, serotonin receptor polymorphism and locus of attention. Social Cognitive and Affective Neuroscience. 2010;5:212–218. doi: 10.1093/scan/nsp040. doi: 10.1093/scan/nsp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittles R, Weiss K. Race, ancestry, and genes: Implications for defining disease risk. Annual Review of Genomics and Human Genetics. 2003;4:33–67. doi: 10.1146/annurev.genom.4.070802.110356. doi: 10.1146/annurev.genom.4.070802.110356. [DOI] [PubMed] [Google Scholar]
- Klimentidis YC, Miller GF, Shriver MD. Genetic admixture, self-reported ethnicity, self-estimated admixture, and skin pigmentation among Hispanics and Native Americans. American Journal of Physical Anthropology. 2009;138:375–383. doi: 10.1002/ajpa.20945. doi: 10.1002/ajpa.20945. [DOI] [PubMed] [Google Scholar]
- Kupfer SS, Anderson JR, Hooker S, Skol A, Kittles RA, Keku TO, Ellis NA. Genetic heterogeneity in colorectal cancer associations between African and European Americans. Gastroenterology. 2010;139:1677–1685. doi: 10.1053/j.gastro.2010.07.038. doi: 10.1053/j.gastro.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer SS, Torres JB, Hooker S, Anderson JR, Skol A, Ellis N, Kittles RA. Novel single nucleotide polymorphism associations with colorectal cancer on chromosome 8q24 in African and European Americans. Carcinogenesis. 2009;30:1353–1357. doi: 10.1093/carcin/bgp123. doi: 10.1093/carcin/bgp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. doi: 10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Soto J, Pole N. Emotion, biology, and culture. Guilford Press, New York, NY; New York, NY, US: 2007. [Google Scholar]
- Lewis-Fernández R, Hinton DE, Laria AJ, Patterson EH, Hofmann SG, Craske MG, Liao B. Culture and the anxiety disorders: recommendations for DSM-V. Depression and Anxiety. 2010;27:212–229. doi: 10.1002/da.20647. doi: 10.1002/da.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A, Greene CJ, Torres JG, Frueh BC. Concordance between clinician-assessed and self-reported symptoms of posttraumatic stress disorder across three ethnoracial groups. Psychological Trauma: Theory, Research, Practice, and Policy. 2013;5:201–208. doi: 10.1037/a0031879. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. doi: 10.1037/1082-989X.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA. Mistreating psychology in the decades of the brain. Perspectives on Psychological Science. 2010;5:716–743. doi: 10.1177/1745691610388774. doi: 10.1177/1745691610388774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Are biomarkers for threat and reward sensitivity associated with family history of psychopathology? Journal of Abnormal Psychology. 2013;122:662–671. doi: 10.1037/a0033982. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J. Constructing ethnicity: Creating and recreating ethnic-identity and culture. Social Problems. 1994;41:152–176. doi: 10.1525/sp.1994.41.1.03x0430n. [Google Scholar]
- Norasakkunkit V, Kalick SM. Culture, ethnicity, and emotional distress measures: The role of self-construal and self-enhancement. Journal of Cross-Cultural Psychology. 2002;33:56–70. doi: 10.1177/0022022102033001004. [Google Scholar]
- Okazaki S. Sources of ethnic differences between Asian American and White American college students on measures of depression and social anxiety. Journal of Abnormal Psychology. 1997;106:52–60. doi: 10.1037//0021-843x.106.1.52. doi: 10.1037/0021-843X.106.1.52. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological Medicine. 2011;41:71–83. doi: 10.1017/S0033291710000401. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SA, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Stinson FS, Dawson DA, Goldstein R, Huang B, Grant BF. Race/ethnic differences in the prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Psychological Medicine. 2006;36:987–998. doi: 10.1017/S0033291706007690. doi: 10.1017/S0033291706007690. [DOI] [PubMed] [Google Scholar]
- Soto JA, Levenson RW, Ebling R. Cultures of moderation and expression: Emotional experience, behavior, and physiology in Chinese Americans and Mexican Americans. Emotion. 2005;5:154–165. doi: 10.1037/1528-3542.5.2.154. doi: 10.1037/1528-3542.5.2.154. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo JA, Braff DL. Startle modulation in Caucasian-Americans and Asian-Americans: A prelude to genetic/endophenotypic studies across the `Pacific Rim'. Psychiatric Genetics. 2005;15:61–65. doi: 10.1097/00041444-200503000-00010. doi: 10.1097/00041444-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Cardenas SJ. Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19:176–188. doi: 10.1037/1040-3590.19.2.176. doi: 10.1037/1040-3590.19.2.176.supp. [DOI] [PubMed] [Google Scholar]
- Tian C, Hinds DA, Shigeta R, Kittles R, Ballinger DG, Seldin MF. A genomewide single-nucleotide-polymorphism panel with high ancestry information for African-American admixture mapping. American Journal of Human Genetics. 2006;79:640–649. doi: 10.1086/507954. doi: 10.1086/507954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JL, Chentsova-Dutton Y, Freire-Bebeau L, Przymus DE. Emotional expression and physiology in European Americans and Hmong Americans. Emotion. 2002;2:380–397. doi: 10.1037/1528-3542.2.4.380. doi: 10.1037/1528-3542.2.4.380. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW. Cultural influences of emotional responding: Chinese American and European American dating couples during interpersonal conflict. Journal of Cross-Cultural Psychology. 1997;28:600–625. doi: 10.1177/0022022197285006. [Google Scholar]
- Watson D, O'Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychological Assessment. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Wheaton MG, Deacon BJ, McGrath PB, Berman NC, Abramowitz JS. Dimensions of anxiety sensitivity in the anxiety disorders: Evaluation of the Anxiety Sensitivity Index-Third Version. Journal of Anxiety Disorders. 2012;26:401–408. doi: 10.1016/j.janxdis.2012.01.002. doi: 10.1016/j.janxdis.2012.01.002. [DOI] [PubMed] [Google Scholar]