Abstract
The effects of selective PI3K and AKT inhibitors were compared in human tumor cell lines in which the pathway is dysregulated. Both caused inhibition of AKT, relief of feedback inhibition of RTKs, and growth arrest. However, only the PI3K inhibitors caused rapid induction of cell death. In seeking a mechanism for this phenomenon, we found that PI3K inhibition, but not AKT inhibition, causes rapid inhibition of wild type RAS and of RAF/MEK/ERK signaling. Inhibition of RAS-ERK signaling is transient, rebounding a few hours after drug addition, and is required for rapid induction of apoptosis. Combined MEK and AKT inhibition also promotes cell death and in murine models of HER2+ cancer, either pulsatile PI3K inhibition or combined MEK and AKT inhibition causes tumor regressions. We conclude that PI3K is upstream of RAS and AKT and that pulsatile inhibition of both pathways is sufficient for effective antitumor activity.
Introduction
The PI3K/AKT/mTOR signaling pathway is frequently activated in cancer, deregulates control of metabolism, cell proliferation and apoptosis and is required for the initiation and maintenance of transformation in model systems. Hyperactivation of this pathway is associated with exaggerated physiologic feedback inhibition of many components of the signaling network, the consequences of which include marked downregulation of multiple receptors and their ability to signal.
Many components of this pathway have been shown to be mutated or otherwise dysregulated in tumors (1–5). Mechanisms of activation include amplification or mutation of receptors that entrain PI3K signaling, especially HER2 and HER3, mutation or amplification of the genes encoding the catalytic or regulatory subunits of class-I PI3 kinases, prominently PIK3CA, and loss of function mutations of genes that encode negative regulators of the pathway, such as PTEN, INPP4B, TSC, and LKB. Such mutations are very common in endometrial, prostate, breast, colorectal, and other cancers. In some cancers (colorectal, melanoma), they often coexist with mutations in RAS or RAF that activate the RAS/ERK signaling pathway; in other cancers (breast, prostate), they do not.
The prevalence of activation of PI3K signaling in tumors has led to the development of inhibitors of several components of the pathway, including the PI3K, AKT, mTOR kinases and Rapamycin-analogs that inhibit mTORC1. Experimental models of tumors with dysregulated activation of the pathway, especially those with PIK3CA mutation or HER2 amplification, tend to be selectively sensitive to inhibitors of AKT or PI3Kα if they do not have coexisting mutations in RAS or RAF (6). In contrast, sensitivity to mTOR inhibitors is less genotype specific and most tumor cell lines tend to be at least somewhat sensitive to these drugs.
Despite the sensitivity of tumor models to both genetic and pharmacologic inhibition of the pathway, the therapeutic efficacy of these inhibitors has been marginal. This may be due in part to the use of unselective drugs that do not inhibit the pathway effectively because off-target toxicities limit dosing. In addition, mTOR and AKT inhibitors relieve feedback inhibition of receptor signaling and activate PI3K, ERK, and other effectors (7–10). Reactivation of upstream signaling may attenuate or even prevent the antitumor activity of these drugs. Inhibition of AKT reactivates receptor signaling (by inhibiting mTOR/S6 kinase) and receptor expression (by activating FoxO-dependent expression of HER3 and IGF/Insulin receptors), thereby inducing PI3K and ERK. Inhibition of mTORC1 similarly reactivates receptor, PI3K, and ERK signaling, but also activates AKT, thus enforcing FoxO inhibition, and receptor expression is not induced. Thus, the effects of inhibitors of different components of the pathway differ both in the spectrum of substrates they suppress and in the details of their effects of feedback. However, both mTOR and AKT inhibitors activate receptor signaling, PI3K activity, and ERK signaling.
Since mTOR and AKT inhibitors reactivate PI3K signaling, we asked whether PI3K inhibitors have more significant antitumor activity, perhaps by inhibiting other PI3K targets in addition to AKT/mTOR. Selective PI3K and AKT inhibitors were compared in tumors with activation of PI3K pathway signaling in order to assess differences in the biochemical and biologic consequences of their inhibition. Both inhibitors effectively inhibited downstream targets of AKT, relieved feedback inhibition of growth factor receptors, and inhibited cell growth. However, in HER2-dependent breast cancers, PI3K inhibitors, but not AKT inhibitors, caused the rapid induction of a significant degree of apoptosis. We find that, whereas AKT inhibitors inhibit AKT/mTOR and activate ERK signaling, PI3K inhibitors inhibit both. They cause durable inhibition of AKT signaling but also transient inhibition of RAS activation and ERK signaling, both of which are required for induction of apoptosis. Moreover, induction of apoptosis by an AKT inhibitor is significantly enhanced when combined with a MEK inhibitor. Our results show that PI3K is upstream of wild type RAS as well as AKT/mTOR, and this causes the therapeutic consequences of PI3K inhibition to be significantly greater than those of AKT inhibition.
Results
AKT and PI3K inhibitors both inhibit AKT/mTOR signaling but AKT and mTOR inhibitors activate (8, 10), whereas PI3K inhibitors inhibit, PI3K activity. To examine the different biochemical and biologic consequences of inhibiting these two nodes of the pathway, we compared the effects of the AKT inhibitor MK2206 with those of the PI3K inhibitor BAY 80-6946. MK2206 is a highly selective, allosteric inhibitor of AKT 1, 2, and 3 that inhibits the phosphorylation of these kinases by preventing their association with the membrane (6, 11). BAY 80-6946 (chemical structure depicted in Supplementary Fig. S1A) is an ATP-competitive selective inhibitor of class-I PI3 kinases, more potent against PI3Kα (IC50=0.5nM) and PI3Kδ (IC50=0.7nM) than PI3Kβ (IC50=3.7nM) and PI3Kγ (IC50=6.4nM), and much less active against mTOR (IC50=45nM) and other PIKs (no inhibition at 1μM) (12). After one hour of exposure of the HER2-amplified breast cancer cell line BT-474 to 50nM BAY 80-6946, the phosphorylation of AKT, its substrates such as PRAS40 and MDM2, as well as downstream effectors such as S6 and 4E-BP1 are potently inhibited by the compound (Supplementary Fig. S1B). To identify a dose-range in which BAY 80-6946 inhibits PI3K but not mTOR, we utilized LAM TSC2−/−, a cell line in which mTOR signaling is not dependent upon AKT activation (Supplementary Fig. S1C). In this model, after three hours of treatment, the mTOR kinase inhibitor AZD8055 blocked the phosphorylation of mTOR substrates and their targets (4E-BP1 and S6), whereas MK2206 blocked the phosphorylation of AKT but not that of mTOR substrates. Treatment with doses of BAY 80-6946 ranging from 10nM to 250nM blocked the phosphorylation of AKT S473 and PRAS40 T246, but did not affect the phosphorylation of mTOR targets. Above 500nM, BAY 80-6946 blocked both AKT and mTOR substrates. Thus, at doses of 250nM and below, BAY 80-6946 selectively inhibits PI3Ks and not mTOR in cells.
Selective doses of BAY 80-6946 (50nM) and MK2206 (2μM) were used to compare the effects of PI3K and AKT inhibition in BT-474 cells, a breast tumor model with HER2 amplification and PI3K mutation that is dependent on AKT for proliferation (6, 8). Both drugs rapidly inhibited the phosphorylation of AKT (S473, T308) as well as its direct substrates PRAS40 (T246) and GSK3β (S9), and inhibition was sustained for up to 24 hours (Fig. 1A). Despite these similarities in modulating AKT signaling, there was a marked difference in induction of apoptosis, as assessed by the appearance of cleaved caspases 3 and 7. Compared to AKT inhibition, PI3K inhibition led to significantly more cleaved caspase beginning at 30 minutes after drug exposure and persisting for up to 24 hours. Similar results were obtained with another HER2-amplified, PI3K-mutant tumor model, MDA-MB-361 (Supplementary Fig. S1D). These findings suggested a major difference in the biologic consequences of PI3K and AKT inhibition.
Figure 1. PI3K inhibition induces more apoptosis than AKT inhibition.
(A) BT-474 cells were treated with BAY 80-6946 (50nM) or MK2206 (2μM) and collected at indicated times. Immunoblots of AKT, AKT substrates, and surrogates for apoptosis show similar AKT inhibition but greater apoptosis with PI3K inhibition.
(B) Cell cycle distribution of BT-474 cells treated with DMSO, MK2206 (2μM), or BAY 80-6946 (50nM) for 24, 48, or 72 hours as determined by FACS analysis show drugs causing similar suppression of S phase distribution. Apoptosis results were reported as mean percent SubG1 fraction with standard errors and show greater %SubG1 with PI3K inhibition.
(C) BT-474 cells were treated with DMSO, MK2206 (2μM), or BAY 80-6946 (50nM) and viable cells were counted at indicated times, demonstrating loss in viable cell number with PI3K inhibition. Results were reported as a mean of triplicate with standard errors.
(D) BT-474 cells untransfected or stably transfected with either pQCXIP vector or pQCXIP-BCL-XL were treated with BAY 80-6946 (50nM) and collected at indicated times. Immunoblots show BCL-XL overexpression abolishes the induction of cleaved caspase-3, cleaved-caspase-7 and cleaved PARP.
(E) BT-474 cells stably transfected with pQCXIP vector or pQCXIP-BCL-XL and treated with DMSO or BAY 80-6946 (50nM) show that the decrease in viable cells by BAY 80-6946 is blocked by BCL-XL overexpression.
We have previously reported that breast cancer models with PI3K mutation or HER2 amplification are sensitive to AKT inhibition. In these tumors, inhibiting AKT reduces D-cyclin expression and causes growth arrest in the G1 phase of the cell cycle (6). Apoptosis is modest and occurs later, 48–72 hours after drug addition. BAY 80-6946 had similar effects on cell proliferation and the accumulation of cells in G1 (Fig. 1B, Supplementary Fig. S1E and F). However, whereas AKT inhibition was associated with a slight increase in cell number followed by cessation of growth (Fig. 1C), PI3K inhibition caused significant apoptosis (Fig. 1B, Supplementary Fig. S1E and F) and a 33.5% reduction in cell number by 72 hours after drug exposure (Fig. 1C). The apoptotic process was induced rapidly after PI3K inhibition. Cleaved caspase-3, caspase-7, and PARP were detected within 30 minutes of PI3K inhibition (Fig. 1A and D), reached a peak by two hours, and persisted for at least 24 hours after drug exposure. By contrast, AKT inhibition caused only a modest induction of caspase cleavage two hours after drug exposure, which fell to trace levels at four hours, and modest induction in the fraction of subG1 DNA at 72 hours after MK2206 addition (Fig. 1A and B). Enhanced induction of apoptosis with PI3K inhibition as compared to AKT inhibition was also noted in two other HER2-amplified breast cancer models, SK-BR-3 and MDA-MB-361 (Supplementary Fig. S1E and F).
To determine whether induction of caspase cleavage was due to activation of the intrinsic apoptotic pathway, BT-474 cells were engineered to express high levels of BCL-XL. BCL-XL overexpression did not alter inhibition of PI3K signaling by BAY 80-6946 (Fig. 1D). However, the accumulation of cleaved caspase-3, cleaved caspase-7 and cleaved PARP that was evident in untransfected and vector-transfected BT-474 cells was completely suppressed by overexpression of BCL-XL. PI3K inhibition did suppress the proliferation of these cells, but it did not reduce their viability, as it did in control cells (Fig. 1E). In fact, the response of BCL-XL-overexpressing cells to BAY 80-6946 resembled the response of untransfected BT-474 to the AKT inhibitor (Fig. 1C and E). We conclude that, although PI3K and AKT inhibitors inhibit AKT signaling to an equivalent degree, PI3K inhibition induces significantly more apoptosis and does so very rapidly.
We attempted to determine the basis for the differential effects of PI3K and AKT inhibition on cell viability (Fig. 2A, Supplementary Fig. S2A). Substrates of AKT kinases such as PRAS40, GSK3β, and FoxO were inhibited similarly by both drugs. Targets of mTOR such as P-S6 and P-4E-BP1 were also inhibited by both, although PI3K inhibition blocked 4E-BP1 phosphorylation more potently and longer than AKT inhibition (Fig. 2A, Supplementary Fig. S2A). Activation of PI3K/AKT/mTOR signaling has been shown to cause feedback inhibition of multiple components of the signaling network, including the expression and activity of receptors that are important activators of PI3K (8–10). AKT and mTOR inhibitors relieve this feedback and reactivate receptor signaling. Differential induction of any of these receptors could result in altered cell survival. We found that the expression and phosphorylation of HER3, IGF1R, and IR were induced to a similar degree and with similar kinetics by both drugs. Induction of HER3 expression and phosphorylation in cells treated with AKT inhibitors has also been shown to be associated with induction of HER3 heterodimerization with other HER kinases and a reciprocal decrease in HER2 and EGFR phosphorylation (8). This occurred with similar kinetics in cells treated with the PI3K or the AKT inhibitor.
Figure 2. PI3K, but not AKT or mTOR, is upstream of the ERK signaling pathway.
(A) BT-474 cells were treated with BAY 80-6946 (50nM) or MK2206 (2μM) and collected at indicated times. Immunoblots of AKT and mTOR substrates, RTKs, and parallel pathways like STAT and ERK.
(B, C, D) BT-474 cells were treated with BAY 80-6946 (50nM) (B), or MK2206 (2μM) (C), or AZD8055 (250nM) (D), and collected at indicated times. Immunoblots show downregulation of P-ERK by PI3K, but not AKT or mTOR, inhibition.
Activation of receptor signaling in cells exposed to AKT or mTOR inhibitors activates PI3K as well as parallel pathways (13). We found that STAT3 phosphorylation was induced by both drugs with the same kinetics, albeit to a slightly greater degree by PI3K inhibition (Fig. 2A). We and others have also shown that AKT or mTOR inhibition causes activation of ERK signaling (7, 14) as shown in Fig. 2C. By contrast, we found that selective inhibition of PI3K with BAY 80-6946 inhibited the phosphorylation of ERK in BT-474 cells (Fig. 2A). Inhibition was rapid and began within 10 minutes after drug exposure, concomitant with inhibition of AKT phosphorylation (Fig. 2B). ERK phosphorylation reached a nadir in 30 minutes and began to rebound in 50 minutes (Fig. 2B). By contrast, there was no decline in P-ERK in DMSO-treated control cells (Supplementary Fig. S2B), in cells treated with the AKT inhibitor (MK2206) (Fig. 2A and C), a selective mTOR kinase inhibitor (AZD8055) (Fig. 2D), the mTORC1 inhibitor Rapamycin (Supplementary Fig. S2C). These results were not peculiar to BAY 80-6946 or to the BT-474 model. Another selective, class-I PI3K inhibitor (GDC-0941) also rapidly inhibited ERK signaling, in this case in the PTEN-null, triple negative breast cancer cell line MDA-MB-468 (Supplementary Fig. S3). Thus, the data suggest that in some cells, ERK activation is dependent on PI3K, but not AKT or mTOR activity.
Recently, it has become clear that PI3K signaling is PI3K isoform dominant in many neoplasms, as a function of both mechanism of pathway activation (PI3Kα when the pathway is activated by RTKs; PI3Kβ in PTEN mutant tumors (15–17)) and lineage (PI3Kδ in B-cell lymphomas (18, 19)). We found that downregulation of P-ERK by PI3K inhibition was not specific for a particular class-I isoform (Fig. 3A). BYL-719, a PI3Kα selective inhibitor; GSK2311418A, a PI3Kβ specific inhibitor; and CAL101, a PI3Kδ specific inhibitor, all rapidly reduced P-ERK in models driven by the isoforms they target (for PI3Kα, BT-474, a HER2-amplified, PIK3CA-mutant breast cancer; for PI3Kβ, MDA-MB-468, a PTEN-deficient breast cancer; for PI3Kδ, CCRF-SB, an acute lymphoblastic leukemia cell line). In all three tumor models, the isoform selective inhibitors caused rapid loss of both AKT and ERK phosphorylation within 15 minutes of drug administration. In each case, there were concomitant decreases of MEK phosphorylation and CRAF S338 phosphorylation. These data suggest that PI3K affects ERK activation by inhibiting RAF activity.
Figure 3. PI3K isoform selective inhibitors and PTEN induction decrease ERK phosphorylation.
(A) BT-474 cells were treated with the PI3Kα specific inhibitor BYL-719 (5μM) (left); MDA-MB-468 cells were treated with the PI3Kβ specific inhibitor GSK2311418A (1μM) (center); CCRF-SB cells were treated with the PI3Kδ specific inhibitor CAL101 (1μM) (right).
(B) PTEN expression was induced in MDA-MB-468TR-PTEN cells by doxycycline (100ng/mL).
(A, B) Cells were collected at indicated times. Immunoblots show loss of phosphorylation of RAF/MEK/ERK with inhibition of PI3K isoforms or induction of PTEN.
N.S. denotes non-specific band.
Thus, a variety of selective inhibitors of class I PI3Ks suppress ERK signaling. In order to determine whether selective genetic inhibition of the pathway had a similar effect, we utilized an MDA-MB-468 cell line engineered to express wild type PTEN when treated with doxycycline (20). In these cells, doxycycline induced PTEN protein expression and reduced AKT phosphorylation in approximately one hour (Fig. 3B). Induction was associated with decreased phosphorylation of ERK, MEK, and CRAF. These data, together with our finding that selective inhibition of mTOR or AKT does not inhibit ERK, suggest that inhibition of PI3K reduces ERK signaling by reducing PIP3, and via an AKT/mTOR independent pathway.
We surveyed the ability of BAY 80-6946 to inhibit ERK phosphorylation in a panel of tumor cell lines in order to assess the generality of this phenomenon (Fig. 4). Downregulation occurred in the majority (16/23) of cell lines, including those with HER amplification, PI3K mutation, or PTEN or INPP4B deficiency, as well as some cell lines without known mechanisms for activation of the pathway. Inhibition of ERK phosphorylation was rapid and rebounded quickly in most tumors, but was more long-lasting in some, including the B-cell malignancies. By contrast, in seven cell lines, ERK phosphorylation was insensitive to PI3K inhibition, despite marked inhibition of AKT. These include all four cell lines with RAS mutations. In HCT-116, a cell line with a catalytic domain mutation in PIK3CA and an activating mutation in KRAS, PI3K inhibition downregulated P-AKT without decreasing ERK, MEK, or CRAF phosphorylation (Supplementary Fig. S4).
Figure 4. PI3K regulates ERK signaling in many tumor models.
Cell lines with indicated tumor genotype were treated with BAY 80-6946 (50nM) and collected at indicated times. Immunoblots show downregulation of P-AKT in all cell lines and loss of P-ERK in the majority of RAS wild type cells.
The observation that the reduction in P-ERK after PI3K inhibition occurs in a majority of tumors with wild type RAS, but not in those with mutant RAS, suggests that it may be due to direct inhibition of wild type RAS activity. This possibility is supported by the rapidity with which it occurs and the coincident decline in MEK and RAF phosphorylation in all tested cells, including the HER2-amplified breast tumors SK-BR-3 and BT-474 (Fig. 5A). We therefore utilized pull-downs of the RAF1 RAS-binding domain (RBD) to assess levels of the active GTP-bound form of RAS. We found that, in four of four cell lines tested, SK-BR-3, BT-474 (Fig. 5A), the lung cancer cell line NCI-H292 (Supplementary Fig. S5A) and the lymphoma cell line CCRF-SB (Supplementary Fig. S5B), the PI3K inhibitor caused a rapid and marked decline in RAS-GTP within 15 minutes of drug addition. The decline occurred in all three RAS isoforms and was coincident with the decline in P-AKT as well as the decline in P-ERK, P-MEK and P-338 CRAF (Fig. 5A). RAS-GTP was also inhibited by another PI3K inhibitor (BYL-719) in BT-474 (Supplementary Fig. S5C). By contrast, the AKT inhibitor reduced AKT phosphorylation, but not the level of RAS-GTP (Fig. 5B). Stimulation with EGF for 5 minutes induced P-ERK, P-MEK, P-CRAF, and RAS-GTP levels, whereas inhibition of HER receptor signaling with lapatinib potently blocked P-ERK, P-MEK, P-CRAF, and also inhibited RAS-GTP (Fig. 5B).
Figure 5. PI3K regulates the level of active RAS in RAS wild type cells.
(A) SK-BR-3 cells (left) or BT-474 cells (right) were treated with BAY 80-6946 (50nM) and collected at indicated times. RAS-GTP level was assessed by RAF1-RBD pull down (PD) and lysates from the same samples were immunoblotted. A control for the PD (Neg. Ctrl.) was performed in the same manner as the other samples but without the addition of RAF1-RBD. Immunoblots demonstrate that PI3K inhibition blocks AKT phosphorylation and causes a loss of RAS-GTP level (total and individual isoforms) corresponding with changes in RAF-MEK-ERK activity.
(B) SK-BR-3 cells (left) or BT-474 cells (right) were treated with BAY 80-6946 (50nM) or MK2206 (2μM) for 30 minutes, EGF (100ng/mL) for 5 minutes, Lapatinib (2μM) for 1h, or left untreated, and collected for RAS-GTP analysis (as in (A)) or immunoblotting. Treatment with EGF activates RAS and RAF/MEK/ERK, while RAS-ERK signaling is inhibited by RTK or PI3K inhibition but not AKT inhibition.
(C) SK-BR-3 cells stably transfected with TTIGp-MLUEX-NRAS Q61K were pretreated for 24h with doxycycline (1μg/mL) to induce the expression of exogenous NRAS Q61K (right) or not pretreated (left) before the addition of the PI3K inhibitor BAY 80-6946 (50nM) for indicated times, EGF (100ng/mL) for 5 minutes, or Lapatinib (2μM) for 1h. RAS-GTP analysis was performed as in (A), and lysates were immunoblotted. PI3K inhibition decreases the GTP-bound level of the endogenous wild type, but not the exogenous mutant, RAS protein. Accordingly, P-ERK was only decreased in cells without the induction of NRAS Q61K expression.
N.S. denotes non-specific band.
RAS-GTP levels rebounded with variable kinetics in the four cell lines, 30–240 minutes after exposure of cells to the PI3K inhibitor (Fig. 5A, Supplementary Fig. S5A and B). In each of the four cell lines, ERK phosphorylation fell at the same time and then rebounded with the same kinetics as RAS-GTP. These results strongly suggest that PI3K inhibitors transiently suppress the activation of wild type RAS in tumors in a PIP3-dependent, AKT-independent manner and that this is responsible for the transient ERK inhibition caused by these drugs. Consistent with this model, in tumors with mutant RAS (HCT116, A549), in which the PI3K inhibitor had no effect on ERK phosphorylation, it also had no effect on RAS-GTP levels, but did inhibit AKT phosphorylation (Supplementary Fig. S5D and E).
In order to determine whether mutant RAS is insensitive to PI3K inhibition, we expressed a FLAG-tagged Q61K mutant NRAS in SK-BR-3 cells in a doxycycline-inducible manner (Fig. 5C). In the absence of doxycycline, the PI3K inhibitor BAY 80-6946 potently and rapidly inhibited RAS-GTP and P-ERK in these cells. After doxycycline induction, mutant NRAS was expressed in these cells and both mutant and wild type RAS were bound to GTP. Addition of BAY 80-6946 had no effect on levels of GTP bound to mutant N-RAS or levels of P-ERK, although it still decreased levels of GTP bound to wild type RAS. This finding supports the conclusions that PI3K regulates wild type RAS activation and that PI3K inhibitors suppress ERK signaling by inhibiting activation of wild type RAS.
We have identified two early consequences of PI3K inhibition that do not occur with AKT inhibitors: suppression of RAS/RAF/MEK/ERK signaling and significant induction of apoptosis. Both occur within two hours of drug administration. We sought to determine if inhibition of ERK signaling was required for induction of apoptosis. To date, PI3K inhibitors administered alone have had significant antitumor activity in clinic in only a limited subset of tumors: B-cell lymphomas, tumors with HER2 amplification, and breast cancers with PI3K mutations. We asked which members of a small set of breast cancer cell lines underwent significant apoptosis when exposed to BAY 80-6946, as measured by induction of subG1 DNA-content. In our panel, significant apoptosis at 72 hours was essentially limited to those tumors with HER2 amplification (Supplementary Fig. S6).
We used BT-474, a model that underwent significant apoptosis, to ask whether the downregulation of P-ERK in tumor cells exposed to PI3K inhibitors was required for induction of early apoptosis. Given the rapid and transient inhibition of ERK, we took a physiologic and pharmacologic approach to the question. In HER2-amplified cells, RAS activation is under the control of HER kinases. We treated BT-474 cells with BAY 80-6946 alone, or together with EGF (Fig. 6A). As expected, PI3K inhibition alone led to a decline in phosphorylation of AKT, the AKT substrate PRAS40, and ERK, while inducing apoptosis, as demonstrated by an increase in the cleaved forms of caspase-3, caspase-7 and PARP (lane 3). When coadministered with EGF, BAY-80-6946 still potently decreased P-AKT and its substrate P-PRAS40, but P-ERK was no longer downregulated (lanes 4, 5). Under this condition, apoptosis was significantly abrogated as well. To ensure that the rescue of apoptosis was due to lack of ERK inhibition and not EGF activation of other signaling pathways, we utilized a highly selective inhibitor of the MEK kinases, PD0325901 (PD901). PD901 alone inhibited ERK, but not AKT or PRAS40 phosphorylation and did not induce caspase or PARP cleavage (lanes 8, 9). When PD901 was co-administered with BAY 80-6946 and EGF, phosphorylation of AKT and PRAS40 remained inhibited and P-ERK was now inhibited as well (lanes 6, 7). Restoration of ERK inhibition was associated with restoration of apoptosis, as demonstrated by accumulation of cleaved caspases, and PARP. We conclude from these data that the transient inhibition of ERK caused by PI3K inhibition is required for the rapid induction of apoptosis in these cells.
Figure 6. Rapid induction of apoptosis by PI3K inhibitor is dependent on its downregulation of P-ERK.
(A) BT-474 cells were left untreated (lane 1), treated with BAY 80-6946 (50nM) alone (lanes 2, 3), or BAY 80-6946 (50nM) and EGF (100ng/mL) (lanes 4, 5), or BAY 80-6946 (50nM), EGF (100ng/mL) and PD901 (50nM) (lanes 6, 7), or PD901 (50nM) alone (lanes 8, 9), and collected at indicated times. Immunoblots showing greatest induction of cleaved caspase-3, cleaved caspase-7 and cleaved-PARP when both P-ERK and P-AKT are inhibited.
(B) BT-474 cells were treated with MK2206 (2μM) alone, PD901 (50nM) alone, or a combination of MK2206 (2μM) and PD901 (50nM), and collected at indicated times. Immunoblots showing greatest induction of cleaved caspase-3 and cleaved caspase-7 by the combination of AKT and MEK inhibition.
(C) BT-474 cells (top), SK-BR-3 cells (middle) or MDA-MB-361 cells (bottom) were treated in triplicate with DMSO, MK2206 (2μM), PD901 (50nM), BAY 80-6946 (50nM), a combination of MK2206 (2μM) and PD901 (50nM), or a combination of BAY 80-6946 (50nM) and PD901 (50nM), and collected at 72h. Samples were analyzed for induction of apoptosis by FACS, and results were reported as mean percent SubG1 fraction with standard errors (n=3/group).
PI3K and AKT inhibitors both inhibit the growth of HER2-dependent breast cancers, but the latter causes much less cell death. Our model suggests that this is because they elevate rather than inhibit ERK signaling and predicts that combined inhibition of AKT and MEK will cause enhanced apoptosis and have greater antitumor effects compared to AKT inhibition alone. We treated BT-474 cells with the AKT inhibitor MK2206, the MEK inhibitor PD901, or a combination of the two. MK2206 downregulated phosphorylation of AKT and its substrate PRAS40, while PD901 downregulated phosphorylation of ERK, and the combination effectively blocked both pathways (Fig. 6B). Two hours after drug administration, combined AKT and MEK inhibition caused a greater degree of induction of cleaved caspase-3 and cleaved caspase-7 than either AKT or MEK inhibition alone.
PI3K inhibitors cause rapid inhibition of ERK in breast cancer cells with HER2 amplification, but P-ERK levels rebound fairly quickly. Even so, this transient inhibition is required for significant induction of apoptosis by these drugs. We asked whether more complete and sustained inhibition of ERK might enhance induction of cell death by the PI3K inhibitor. We found that, in BT-474, SK-BR-3 or MDA-MB-361 cells, addition of PD901 could further enhance the apoptotic effects of the PI3K inhibitor, as quantitated by subG1 DNA content (Fig. 6C). In BT-474 and MDA-MB-361 cells, MEK inhibition enhanced the induction of apoptosis caused by AKT inhibition alone, but the combination was not nearly as potent as PI3K inhibition alone. In SK-BR-3 cells, combined MEK and AKT inhibition appeared nearly equivalent to PI3K inhibition alone. These results suggest that, in some cells, inhibition of other non-AKT targets of PI3K contribute to induction of apoptosis, or that stronger MEK inhibition is required to fully activate apoptosis. Combined inhibition of MEK and PI3K caused more apoptosis than any of the other treatments in all three models.
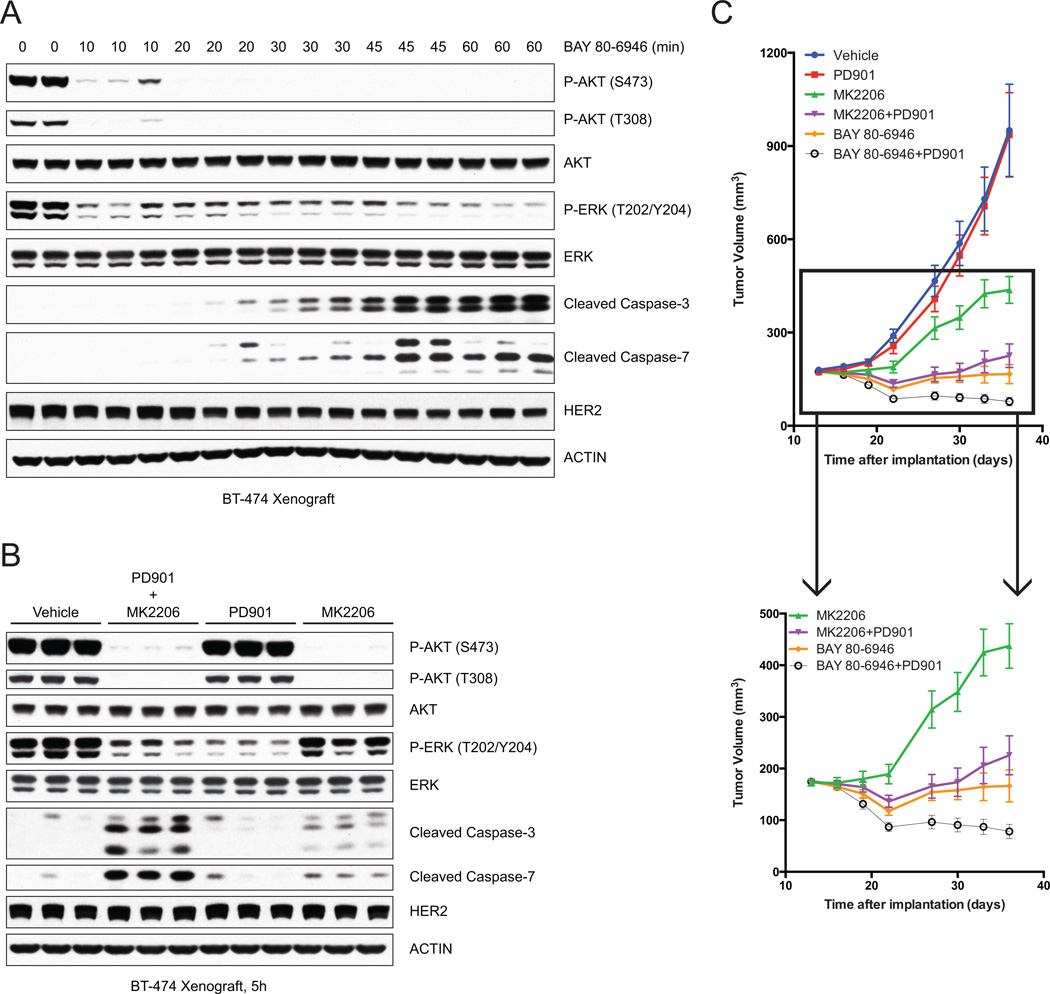
We wished to determine whether these findings were relevant in vivo. For this purpose, we utilized the BT-474 model grown as xenografts in immunocompromised mice. Intravenous administration of BAY 80-6946 caused rapid (within 10 minutes) inhibition of PI3K activity, as measured by decreased phosphorylation of AKT S473 and T308, and of ERK phosphorylation (Fig. 7A). Both were sustained for at least 60 minutes. Induction of caspase-3 and caspase-7 cleavage was detectable within 20 minutes of drug administration and markedly increased at 45 and 60 minutes after dosing. This experiment was performed three separate times with similar results. Thus, BAY 80-6946 causes both rapid inhibition of ERK signaling and rapid induction of apoptosis in vivo.
Figure 7. Combined MEK and AKT inhibition improves antitumor efficacy in vivo.
(A) Mice bearing BT-474 xenograft were treated with a single dose of BAY 80-6946 (14mg/kg) and tumors were collected at indicated times post-treatment. Immunoblots of tumor lysates demonstrate downregulation of P-AKT and P-ERK, as well as induction of cleaved caspase-3 and cleaved caspase-7.
(B) Mice bearing BT-474 xenograft were treated with vehicle, or a combination of MK2206 (100mg/kg and PD901 (5mg/kg), or either agent alone, and tumors were collected at 5h post-treatment. Immunoblots of tumor lysates demonstrate greatest induction of cleaved caspase-3 and cleaved caspase-7 with the combination of MK2206 and PD901.
(C) Mice bearing BT-474 xenograft were randomized to (1) Vehicle five times/week, (2) PD901 5mg/kg five times/week, (3) MK2206 100mg/kg five times/week, (4) BAY 80-6946 14mg/kg three times/week, (5) combination of (2) and (3), or (6) combination of (2) and (4), and tumor size was measured by vernier caliper two times/week. The results were reported as mean tumor volume with standard errors (n=10/group). Inset excludes group (1) and (2).
We asked whether inhibition of ERK with PD901 enhanced the induction of apoptosis by the AKT inhibitor MK2206 in vivo. As reported in previous work, AKT inhibition induced moderate apoptosis in HER2-amplified breast cancer xenografts ((8), Fig. 7B and Supplementary Fig. S7A). MEK inhibition with PD901 was associated with minimal caspase cleavage not noticeably different from that observed with vehicle controls, 5 and 7 hours after drug addition (Fig. 7B and Supplementary Fig. S7A). By contrast, combined inhibition of MEK and AKT synergized to cause marked induction of apoptosis.
BAY 80-6946 has an extremely short half-life in mice (T1/2=0.7 hours) and maximal inhibition of the target only persists for 4 hours (data not shown), so we determined whether its therapeutic efficacy required continuous dosing by infusion. In the BT-474 xenograft model, we compared the effects of pulsatile BAY 80-6946 treatment (14mg/kg three times/week) and continuous administration of BAY 80-6946 via a mini-pump (42mg/kg/week). Pulsatile administration of BAY 80-6946 suppressed tumor growth to a much greater extent than continuous dosing (Supplementary Fig. S7B). Thus, relatively brief inhibition of the pathway three times per week was sufficient to significantly inhibit tumor growth. We speculate that transient inhibition of PI3K was more effective because of more complete inhibition of the target for a short time and limited relief of feedback and reactivation of upstream signaling.
Having established the pulsatile schedule and dosing for BAY 80-6946 in vivo, we compared it with AKT inhibition by MK2206 (QDx5 at 100mg/kg) in the BT-474 breast cancer xenograft model (Fig. 7C). AKT inhibition slowed the growth of tumors, but they still grew significantly. In contrast, pulsatile PI3K inhibition caused initial tumor regression and significantly suppressed tumor growth. The effectiveness of intermittent administration of the PI3K inhibitor and its superior antitumor activity compared to AKT inhibition were confirmed in another HER2 amplified, PI3K mutant breast cancer model, MDA-MB-361 (Supplementary Fig. S7C).
We hypothesized that the effectiveness of PI3K inhibition was due in part to its combined inhibition of ERK and AKT. As previously reported, MEK inhibition with PD901 (QDx5 at 5mg/kg) had no effect on BT-474 tumor growth (Fig. 7C, (21)). However, the combined inhibition of MEK and AKT was clearly synergistic, as tumors regressed for approximately 10 days and then began to grow. Taken together, the data support the idea that induction of significant apoptosis in HER2+ tumors requires the inhibition of both AKT and MEK signaling that is achieved with PI3K inhibitors. Combining MEK and AKT inhibition with two separate drugs worked almost as well as PI3K inhibition in this model. However, combining MEK and PI3K inhibition (three times/week of 14mg/kg BAY 80-6946 plus five times/week of 5mg/kg PD901) caused greater and more sustained tumor regression than the latter alone, suggesting that deeper and longer ERK pathway inhibition is required to maximize antitumor activity.
DISCUSSION
The PI3K signaling pathway is dysregulated in a large proportion of tumors, most commonly by activating mutations of PIK3CA, activation of RTKs such as HER2, and loss of activity of the PTEN and INPP4B tumor suppressors that negatively regulate the pathway (4, 22). Despite the powerful transforming activity of these alterations, clinical activity of inhibitors of PI3K, AKT and mTOR has been disappointing and significantly less than that obtained with inhibitors of other oncogene activated pathways (e.g., BRAF, EGFR, ALK) (23–25). We and others have found that activation of the PI3K pathway causes feedback inhibition of receptor signaling (8–10, 14). PI3K pathway inhibitors relieve this feedback and reactivate receptor signaling. It has been posited that this reactivation attenuates the therapeutic effects of pathway inhibitors. Inhibition of mTOR has been shown to activate receptor signaling and reactivate PI3K/AKT (9, 10), whereas AKT inhibition inhibits AKT but still reactivates PI3K (8). These data predict that PI3K inhibitors would inhibit AKT/mTOR signaling and reactivate receptor signaling, but would inhibit, rather that reactivate, PI3K itself. In this paper, we asked whether this difference would have biologic or therapeutic consequences. Our ability to pursue this question was dependent on determining the dose-range over which selective PI3K inhibitors do not affect mTOR activity.
We compared the effects of selective inhibition of PI3K and AKT and found that they were significantly different. In HER2-amplified breast cancer cells, the effects of the drugs on AKT signaling and reactivation of receptor tyrosine kinases (RTK) are very similar. PI3K inhibition, however, causes a rapid and significant induction of apoptosis, whereas AKT inhibition does not. The differential induction of apoptosis induced by PI3K turns out to be due to inhibition of PI3K targets other than AKT/mTOR. Remarkably, inhibition of PI3K, but not AKT, leads to the rapid inhibition of RAS activity in these tumors, which is accompanied by inhibition of CRAF, MEK and ERK phosphorylation. Thus, inhibiting PI3K causes the rapid inhibition of both AKT-mTOR and RAS-ERK signaling, whereas AKT inhibitors suppress only the former and, in fact, activate the latter. The effects of the PI3K inhibitor on the two pathways were observed in cultured cells and in vivo in tumor xenografts, suggesting their potential clinical significance.
In a large panel of tumor models, PI3K inhibitors were shown to inhibit AKT signaling in all cells and inhibit RAS-ERK signaling in most cells but not those harboring a mutant allele of RAS. To confirm that mutant RAS signals independently of PI3K, we induced mutant RAS expression in a model with wild type RAS and found that both mutant RAS-GTP levels and downstream CRAF-MEK-ERK activation were unaffected by the PI3K inhibitor. The data together suggest that PI3K regulates wild type, but not mutant, RAS.
Although PI3K inhibitors caused a rapid decline in both AKT and RAS in the RAS wild type tumors, the duration of inhibition was quite different. Inhibition of AKT was sustained in most cases beyond 24 hours, whereas inhibition of RAS was transient, typically lasting 60–240 minutes. Changes in CRAF-MEK-ERK activity closely followed those in RAS activation, further implying that the changes in ERK are due to the changes in RAS. The transient changes in RAS-ERK activation led us to wonder if they were necessary for the rapid induction of apoptosis we observed in these cells. We and others had previously shown that MEK-ERK inhibition alone in these cells had little to no effect (21), so we speculated that inhibition of both AKT and ERK was necessary for induction of apoptosis. We found that addition of EGF could prevent the downregulation of ERK (but not AKT) caused by the PI3K inhibitor and prevent apoptosis. Apoptosis could be restored in these cells by adding a selective inhibitor of MEK so that once again both AKT and ERK are downregulated. This then clarified the mechanism underlying the difference in induction of apoptosis by PI3K and AKT inhibitors. PI3K inhibitors downregulate both AKT and ERK, while AKT inhibitors downregulate AKT but relieve RTK feedback and activate PI3K and ERK. Indeed, we find that combining MEK and AKT inhibitors causes synergistic apoptosis, though it does not rise to the level induced by PI3K inhibition.
The finding that PI3K is upstream of wild type RAS is surprising though not novel. Although PI3K is almost always thought of as an effector rather than an activator of RAS, there are several descriptions of the latter (26–30). In most of these, pretreatment with a drug that inhibits PI3K prevented or reduced ligand-induced activation of RAS and/or ERK. For instance, Wennstrom and Downward showed that when serum-starved COS7 cells were pretreated with LY294002 or wortmannin, induction of RAS-GTP after 5-minute-stimulation with EGF was blunted (26). Similarly, pretreatment of cells with wortmannin blocked low dose PDGF activation of ERK, but not when higher concentrations of PDGF were used or in cells with high levels of PDGFR (27). These findings are consistent with our data that EGF stimulation causes PI3K-independent activation of ERK in BT-474. It is not clear why the idea that PI3K is upstream of RAS has not been incorporated into standard models of signaling. It may be due to the complexity of the systems, the reliance on ligand activation of serum-starved cells and transfection studies, the use of less specific drugs like LY294002 and wortmannin, and most likely, the absence of a defined biologic consequence of PI3K regulation of RAS activation.
We have now used selective compounds to show that PI3K is upstream of both RAS and AKT, that inhibition of PI3K downregulates both ERK signaling (transiently) and AKT/mTOR signaling (durably), and that the transient inhibition of ERK is necessary for the potent induction of apoptosis by these drugs. Our data strongly suggest that PI3K inhibitors induce a greater degree of cell death than AKT inhibitors because they inhibit ERK signaling. MEK inhibition by itself has no effect, so the question becomes why transient ERK inhibition synergizes with PI3K inhibition to cause cell death. We and others have previously shown that the ERK and PI3K pathways converge on key targets that regulate cell survival and proliferation. These include the BH3 domain protein BAD (20), the regulator of cap-dependent translation 4E-BP1 (31), and Bcl2 (32). In tumors with coexistent activation of both ERK and AKT signaling, both pathways must be inhibited in order to suppress these targets, inhibit tumor growth, and induce cell death.
The transience of this phenomenon may provide a clue for understanding the complexity of regulation of RAS/ERK signaling by PI3K. Our previous data and that of others show that PI3K activation will downregulate receptor signaling and ERK signaling via AKT/mTOR-dependent feedback mechanisms (7, 8, 14). AKT or mTOR inhibitors relieve this feedback and activate ERK signaling (7). Our data here show that PI3K is also required to maintain tonic levels of RAS activation in tumors with wild type RAS. Thus, PI3K activation has two opposing effects on RAS signaling: at modest levels of PI3K signaling, ERK output may integrate PI3K output and feedback; in tumors with dysregulated activation of AKT and mTOR, feedback may predominate and result in low levels of ERK. In this model, selective inhibition of PI3K will inhibit RAS directly but also relieve feedback inhibition of receptors that would ultimately lead to RAS activation. We believe that the former occurs more quickly than the latter and that this accounts for the initial decline and subsequent rebound in ERK phosphorylation. The model also accounts for why inhibition of ERK signaling has not, for the most part, been recognized as a prominent property of PI3K inhibitors. First, most inhibitors used in the past have had multiple other targets, including, prominently, mTOR inhibition, which would be expected to blunt or negate the phenomenon. Second, the effect is rapid and obscured by relief of feedback and other adaptations over time.
These results have important implications for the treatment of patients with PI3K-dependent tumors, especially those with HER2-dependent cancers. We show that PI3K inhibitors ought to be more effective than AKT inhibitors, if they are selective and can be administered at doses that effectively inhibit the pathway. Selective AKT inhibitors and mTOR inhibitors that can potently inhibit AKT/mTOR signaling are expected to activate ERK, via receptor and PI3K activation, and this might blunt their therapeutic effects.
Recently, treatment with more selective PI3K inhibitors has led to greater therapeutic efficacy in lymphomas and in breast cancer with PI3K mutation or HER2 amplification. The ability of any PI3K inhibitor to inhibit signaling adequately is limited by physiologic toxicity. Moreover, attempts to combine MEK inhibitors with `dual specificity’ PI3K or AKT inhibitors have been complicated by severe toxicity at modest doses of these drugs. The idea that the pathway must be inhibited continuously dominates the clinical development of these drugs. Our finding that transient inhibition of PI3K is effective in in vivo models suggests that periodic rather than continuous target inhibition is an alternative strategy that would allow adequate pathway inhibition without causing inordinate toxicity or chronic feedback reactivation of receptors. Thus, combining PI3K inhibitors, MEK inhibitors and, perhaps, inhibitors of key reactivated RTKs, and administering them at high dose on intermittent schedules may be a more effective therapeutic strategy for these tumors.
MATERIALS AND METHODS
Cell lines
SKMEL cell lines were from A. Houghton (MSKCC). 293T packaging cell line was from Clontech. All other cell lines were from the American Type Culture Collection. BT-474, SK-BR-3, LAM TSC2−/−, UACC-893, HCC1954, MDA-MB-453, MDA-MB-361, MCF7, HCC1806, MDA-MB-468, MDA-MB-468TRPTEN were grown in DMEM-F12 medium; T-47D, NCI-H292, NCI-H1650, CCRF-SB, U937, SU-DHL-4, SU-DHL-5, NCI-H1703, NCI-H661, PC9 were grown in RPMI1640 medium; BT-20 was grown in MEM medium; A549 was grown in F-12K medium; HCT116 was grown in McCoy’s medium; SK-MEL-30, SK-MEL-2, and 293T were grown in DMEM medium. All media were supplemented with 100μg/mL penicillin, 100mg/mL streptomycin, 4mM glutamine, and 10% fetal bovine syndrome. All cells were maintained at 37°C in 5% CO2. Breast cancer cell lines previously described as HER2-amplified (BT-474, SK-BR-3, UACC-893, HCC1954, MDA-MB-453, MDA-MB-361) were confirmed to overexpress HER2 by immunoblotting for HER2. Breast cancer cell lines that were previously described as expressing estrogen receptor (MCF-7, T-47D, BT-474, MDA-MB-361) were confirmed to express ER by immunoblotting for ER. Lung cancer cell lines previously described to have mutation in EGFR (NCI-H1650, PC9) were validated by Sanger sequencing of EGFR. SK-MEL cell lines were validated by genetic fingerprinting. Lymphoma cell lines (CCRF-SB, U937, SU-DHL-4, SU-DHL-5) were obtained from ATCC less than 6 months before performing the experiments in this paper. Remaining cell lines were not independently validated in our laboratory.
Ras-GTP Assay
Cells were harvested on ice, lysed immediately in either Igepal lysis buffer (50mM Tris-HCL pH 7.5, 10mM MgCl2, 0.5M NaCl, 2% Igepal), or Ras-GTP Lysis Buffer (Pierce) before centrifugation at 16,000×g for 2 minutes at 4°C. BT-474, SK-BR-3, and NCI-H292 experiments were performed with both lysis buffers and yielded similar results. The supernatant was collected and an aliquot was removed for BCA assay (Pierce) to determine protein concentration, while the rest of the lysate was snap-frozen using dry ice and ethanol. Equal amount of protein (500–700μg) from each sample was loaded onto a column with purified GST-RAF1-Ras-Binding-Domain and glutathione agarose resin (Pierce), except for the negative control sample, which does not contain the purified GST1-RAF1-Ras-Binding-Domain. The mixture is incubated for 1h at 4°C before washing five times with Wash Buffer (25mM Tris-HCL pH7.5, 30mM MgCl2, 40mM NaCl). Samples were eluted in 2X LDS Sample Buffer (diluted with water from 4X LDS Sample Buffer by Invitrogen) with 200mM dithiothreitol. Samples were subsequently electrophoresed on a 4–12% or a 12% Bis-Tris mini gels (Invitrogen) and GTP-bound level of total RAS or RAS isoforms were detected by immunoblotting.
Further details can be found in Supplemental Materials and Methods.
Supplementary Material
Statement of Significance.
We show that the RAS-ERK pathway is a key downstream effector pathway of oncogenic PI3K. Coordinate downregulation of AKT and ERK is necessary for induction of apoptosis and antitumor activity and can be accomplished with pulsatile dosing, which will likely decrease toxicity and allow administration of therapeutic doses.
ACKNOWLEDGEMENTS
FINANCIAL SUPPORT
This work is supported by NIH Program Grant 5P01CA094060 (NR), Arlene Taub and the Breast Cancer Research Foundation (NR), Damon Runyon Cancer Research Foundation (SC), Geoffrey Beene Cancer Research Center (SC and NR). M.W. was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number: T32GM007739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank D. Domingo for assistance with FACS; Qing Bai She for use of the doxycycline inducible PTEN MDA-MB-468 model; and Alan Hall and Poulikos Poulikakos for helpful discussions.
Footnotes
CONFLICT OF INTEREST
Claudia Schneider and Ningshu Liu are employees of Bayer Research Labs
No other author has a conflict of interest to disclose.
REFERENCES
- 1.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 2.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer research. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 3.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.The Cancer Genome Atlas N, Genome sequencing centres: Washington University in St L. Koboldt DC, Fulton RS, McLellan MD, Schmidt H, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012 doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrik-Outmezguine V, Chandarlapaty S, Pagano N, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR Kinase Inhibition causes Feedback-Dependent Biphasic Regulation of AKT Signaling. Cancer Discovery. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Molecular cancer therapeutics. 2005;4:271–279. [PubMed] [Google Scholar]
- 12.Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Molecular cancer therapeutics. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 13.Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Muller U, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22:796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011 doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee S, Wiederschain D, Maira S-M, Loo A, Miller C, deBeaumont R, et al. PTEN-deficient cancers depend on PIK3CB. Proceedings of the National Academy of Sciences. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torbett NE, Luna-Moran A, Knight ZA, Houk A, Moasser M, Weiss W, et al. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. The Biochemical journal. 2008;415:97–110. doi: 10.1042/BJ20080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Molecular and cellular biology. 2002;22:8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. The Journal of experimental medicine. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 23.Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:670–678. doi: 10.6004/jnccn.2013.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nature reviews Clinical oncology. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 25.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 26.Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Molecular and cellular biology. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duckworth BC, Cantley LC. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. The Journal of biological chemistry. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, et al. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. The Journal of cell biology. 2007;178:185–191. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahedi B, Goo H-j, Beaulieu N, Tazmini G, Kay RJ, Cornell RB. Phosphoinositide 3-Kinase Regulates Plasma Membrane Targeting of the Ras-specific Exchange Factor RasGRP1. Journal of Biological Chemistry. 2011;286:12712–12723. doi: 10.1074/jbc.M110.189605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yart A, Laffargue M, Mayeux P, Chretien S, Peres C, Tonks N, et al. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. The Journal of biological chemistry. 2001;276:8856–8864. doi: 10.1074/jbc.M006966200. [DOI] [PubMed] [Google Scholar]
- 31.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR Leads to Adaptive Resistance in Matrix-Attached Cancer Cells. Cancer cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.