Abstract
The hormone hepcidin promotes iron sequestration by macrophages. A recent study by Kim et al (2014) implicates the orphan receptor ERRγ in the regulation of hepcidin production and suggests that targeting the ERRγ-hepcidin axis may be beneficial during infection with the facultative intracellular pathogen Salmonella.
Macrophages play a central role in scavenging and recycling iron from senescent red blood cells. This process is controlled by the master regulatory hormone hepcidin, which is primarily synthesized in the liver.During inflammation,the interleukin-6 (IL-6)-inducible peptide hepcidin promotes the degradation of its receptor, the iron exporter ferroportin, resulting in iron retention by macrophages, reduced intestinal iron absorption, and hypoferremia. While this iron-withholding response is a central component of innate nutritional immunity that restricts iron availability to circulating microbes (Weiss and Schett, 2013; Cassat and Skaar, 2013), intracellular bacteria such as Salmonella have been shown to benefit from the iron retained within macrophages.
Adding to our understanding of iron regulation during infection, anew study by Kim et al. has implicatedERRγ (Estrogen-related receptor γ, also called NR3B3), a nuclear hormone receptor with no known activating ligand, in the control of iron withholding (Kim et al., 2014). In this studySalmonella entericasv.Typhimurium was shown to induce hepcidin expression, hypoferremia and ERRγexpression in mice.IL-6 KO mice failed to exhibit these responses and were observed to have lower bacterial burdens, indicating that ERRγacts downstream of IL-6 and has a detrimental effect on host defense against Salmonella. ERRγ expression was shown to result fromtransactivation by the transcription factor STAT3. The researchers further showedthat overexpression of ERRγ induceshepcidin production and hypoferremia, and were able to identify an ERRγ response element in the hepcidin promoter.ERRγ did not affect expression of NOS2 (inducible nitric oxide synthase), which can also alter iron trafficking in macrophages by stimulating iron export via ferroportin(Nairz et al., 2013).A reverse agonistdesignated GSK5182,which binds theERRγ receptor and decreases its activity, was able to ameliorate Salmonella-induced hepcidin expression, hypoferremia and iron accumulation in the liver and spleen, along with a reduction in bacterial burden, macrophage iron content and proinflammatory cytokine production. The authors attributed the detrimentaleffects of hepcidin to increased iron availability in the macrophages where Salmonella resides (figure 1). They suggested that ERRγ and hepcidin might be therapeutically targeted to treat infections with intracellular pathogens.
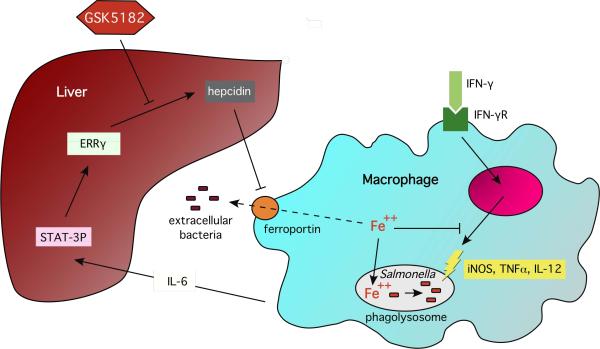
Figure 1. Estrogen-Related Receptor γregulates host iron trafficking by inducing hepcidin formation in the liver.
ERRγ transcription is stimulated by interleukin-6 (IL-6) and the transcription factor STAT3. Hepcidin is released into the circulation and binds to the iron export protein ferroportin 1 (FP1), resulting in FP1 degradation and macrophage iron retention. This results in systemic hypoferremia butincreases access of the intracellular pathogen Salmonella to iron. The increase in macrophage iron levels alsoinhibitsinterferon-γ-dependent anti-bacterial immune responses. ERRγinhibition by the reverse agonist GSK5182 counteracts the effects of hepcidin on intra-macrophage iron availability and anti-microbial immune effector pathways but may increase the availability of iron to extracellular pathogens.
This study represents a significant advance in our understanding of the regulatory linkage between innate immune responses and iron homeostasis. Previous work by the same group has shown that ERRγ controls hepatic glucose production in response to glucagon (Kim et al., 2012), andit willbe interesting to see whetherERRγcan account for the observed association between IL-6 and hyperglycemia in septic patients.
In considering the pharmacological targeting of this pathway to treat infection with intracellular pathogens, a few caveats are in order. First, observations regarding the effects of hepcidinduringSalmonella infection have beensomewhat conflicting. Kim et al. found that antagonism of hepcidin by the inhibition ofERRγ signaling is beneficial during systemic Salmonella infection. This is consistent with earlier observations showing that hepcidin antagonism can ameliorate Salmonella-induced inflammation (Wang et al., 2009) and that HFE mice, which have deficient hepcidin expression, are able to restrict Salmonella replication (Nairz et al., 2009). However, in contrast,hepcidin-deficient mice on a different genetic background are reportedly more susceptible to Salmonella challenge, and hepcidin administration can enhancethe survivalthese mice during Salmonella infection(Yuki et al., 2013). Differences in inoculum size, mouse strain, route of infection, iron distribution in tissues and effects of partial versus complete ERRγ/hepcidin inhibition are among the variables that should be further explored to explain these discrepant findings.
Second, in addition to its regulation of ferroportin, hepcidincan modulatehost inflammatory responses(Pagani et al., 2011). Intracellular iron levelsper seare immunomodulatory, and thedisruption of iron homeostasis affects pro-inflammatory cytokine production, either directly via regulation of interferon-gamma (IFN-γ) activity or by modulating the production of nitric oxide or reactive oxygen species, which in turn affect redox-sensitive signaling pathways(Nairz et al., 2013; Pagani et al., 2011; Weiss and Schett, 2013). In view of the relatively modest effects of GSK5182 on bacterial burden, particularly in the spleen, it is conceivable that the survival benefit observed by Kim et al. following GSK5182 treatmentmight be attributable in part to reduced immunopathology rather than to antimicrobial effects resulting from lessened iron availability. Previous studies have suggested that hepcidin antagonists can ameliorate inflammation in Salmonella enterocolitis(Wang et al., 2009),and effects of iron homeostasis on T-lymphocyte and macrophage polarization have been described(Weiss and Schett, 2013).Immunomodulatory actions of ERRγ and hepcidinwill have to be carefully considered when targeting these proteins during infection, as such actions may be either protective or detrimental depending on the nature and primary localization of the microbe and time course of the specific host-pathogen interaction(Cassat and Skaar, 2013; Drakesmith and Prentice, 2012).
Finally, it must be remembered that the changes in iron compartmentalization mediated by hepcidin can restrictthe growth of pathogens at some tissue sitesand in the circulation while having the opposite effect on intracellular pathogens. Accordingly, hepcidin plays a protective anti-microbial role in host resistance to Vibrio vulnificus, Yersinia and Plasmodiumspp. despitepromoting the growth of Salmonella, Leishmania and mycobacteria in macrophages(Cassat and Skaar, 2013; Drakesmith and Prentice, 2012; Nairz et al., 2013). Therapeutic targeting of the ERRγ-hepcidin-ferroportin axis during infection will therefore requirea detailed knowledge of the cellular localization of the specific pathogen involved. Co-infection with malaria and Salmonella or tuberculosis, a not infrequent scenario in Africa, would pose a therapeutic dilemma from an iron standpoint. Depriving one pathogen only to benefit another would be, one might say, ironic.
ACKNOWLEDGMENTS
F.C.F. is supported by NIH grants AI39557, AI55396 and AI101084. G.W. is supported by a grant from the Austrian Research Fund (TRP-188).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim HS, et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella Typhimurium infection by modulating host iron homeostasis. Nat Med. 2014;20:419–424. doi: 10.1038/nm.3483. [DOI] [PubMed] [Google Scholar]
- Kim DK, Ryu D, Koh M, Lee MW, Lim D, Kim MJ, Kim YH, Cho WJ, Lee CH, Park SB, et al. Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J Biol Chem. 2012;287:21628–21639. doi: 10.1074/jbc.M111.315168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 2013;210:855–873. doi: 10.1084/jem.20121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–3651. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118:736–746. doi: 10.1182/blood-2011-02-337212. [DOI] [PubMed] [Google Scholar]
- Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, Lin HY, Babitt JL, Cherayil BJ. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119:3322–3328. doi: 10.1172/JCI39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215. doi: 10.1038/nrrheum.2012.183. [DOI] [PubMed] [Google Scholar]
- Yuki KE, Eva MM, Richer E, Chung D, Paquet M, Cellier M, Canonne-Hergaux F, Vaulont S, Vidal SM, Malo D. Suppression of hepcidin expression and iron overload mediate Salmonella susceptibility in ankyrin 1 ENU-induced mutant. PLoS One. 2013;8:e55331. doi: 10.1371/journal.pone.0055331. [DOI] [PMC free article] [PubMed] [Google Scholar]