Abstract
More than 98% of eukaryotic transcriptomes are composed of non-coding RNAs with no functional protein-coding capacity. Those transcripts also include tens of thousands of long non-coding RNAs (lncRNAs) which are emerging as key elements of cellular homeostasis, essentially tumorigenesis steps. However, we are only beginning to understand the nature and extent of the involvement of lncRNAs on tumorigeneis. Here, we highlight recent progresses that have identified a myriad of molecular functions on tumorigenesis for several lncRNAs including metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), prostate cancer associated non-coding RNA 1 (PRNCR1), prostate cancer gene expression marker 1 (PCGEM1), H19, and homeobox transcript antisense intergenic RNA (HOTAIR), and several new lncRNAs for glioma development. Potential therapeutic approaches for the lncRNAs in various human diseases are also discussed.
Keywords: Non-coding RNA, lncRNA, Tumorigenesis, Glioma
INTRODUCTION
The global human genome project was launched in the 1990's and researchers expect to understand information of the whole human genome, especially genes which encode functional proteins. However, they recognized that the human genome contains only about 20000 protein-coding genes [1,2]. It is not surprising that the numbers of protein-coding genes are pretty similar in number to those of Caenorhabditis elegans. Because the protein-coding genes could not properly explain the human gene expression complexity in view of physiological and evolutional aspects, investigators have suspected that the potential non-protein coding but transcriptionally active genes (>98% of transcribed genes) may be fully accountable for gene expression complexity of human [3,4,5].
Recently, investigators categorized the non-coding RNAs (ncRNAs) as short ncRNA, mid-size ncRNA, and long non-coding RNA (lncRNA) by their lengths, and sometimes ncRNAs are subdivided by function, loci, and post-transcriptional modification. Among these, most onco-molecular biologists are deeply interested in the short ncRNAs [e.g., microRNAs (miRNAs)] [6] and very recently lncRNAs for their research projects as an aspect of gene expression regulation [7,8,9,10,11]. This review, we essentially highlight and introduce our recent understanding of roles of lncRNAs in tumorigenesis, including glioma development.
LNCRNAS: MODE OF ACTION
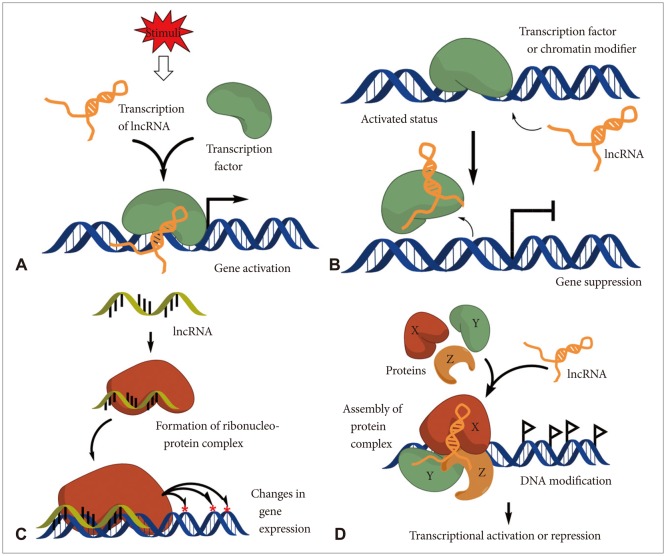
Recent reports showed that lncRNAs have multifunctional roles on modulating embryonic pluripotency, differentiation, development, and various diseases, essentially in cancers [7,8,9,10,11]. Therefore, dysregulation of lncRNAs has been shown to be associated with a broad range of defects on those physiological phenomena. lncRNAs may be classified according to their mode of action and functions in cells such as, 1) mediators on signaling pathway, 2) serving as molecular decoys, 3) work as molecular guides for the ribonucleoprotein complexes to certain specific chromatin site, and also have 4) scaffold function for the proper complex formation (Fig. 1) [11].
Fig. 1.
Schematic diagram of lncRNA action mechanisms. A: Mediators on signaling pathway: lncRNAs can serve as molecular signaling mediators which modulate certain set of gene expression in conjunction with specific transcription factors or chromatin modifiers. B: Molecular decoys: lncRNAs can serve as the molecular decoy which takes away proteins or RNAs from the specific location. C: Work as molecular guides: lncRNAs can serve as the molecular guides by locating certain ribonucleoprotein complexes to specific target site on chromatin. D: Scaffold function: lncRNAs can support the assembly of protein complexes which link the factors together to generate brand new functions. IncRNA: long non-coding RNA.
lncRNAs: mediators on signaling pathway
In most cases, transcription of lncRNAs is temporally regulated and the expression has tissue specificity. Moreover, their expression is modulated in response to the internal and external stimuli [12]. Therefore, lncRNAs may serve as molecular signaling mediators which modulate a certain set of gene expression temporally and even spatially (Fig. 1A).
lncRNAs: molecular decoys
lncRNAs may serve as molecular decoys which take proteins or RNAs away from a specific location. Sometimes decoy lncRNAs can serve as "sponge" to proteins (e.g., transcription factors and chromatin modifiers) and small ncRNAs (e.g., miRNAs) (Fig. 1B) [13]. This event can lead to overall transcriptome change of cells.
lncRNAs: work as molecular guides
lncRNAs can serve as the molecular guides by locating certain ribonucleoprotein complexes to a specific target site on the chromatin (Fig. 1C) [14]. The gene expression can be altered either in neighboring genes (in cis) or distantly located ones (in trans).
lncRNAs: scaffold function
lncRNAs can support the assembly of protein complexes which link the factors together to generate brand new functions (Fig. 1D). Some lncRNAs possess distinct protein-binding domains that combine each molecule together. This event may have impact on action of transcription or repression.
LNCRNAS ON TUMORIGENESIS
Generally the lncRNAs designate ncRNAs which is more than 200 nt long [7,8,9,10,11]. 1) Usually those modulate DNA methylation which is closely related with genome imprinting (e.g., X-chromosome inactivation by lncRNA XIST) [15]. 2) Several large intergenic non-coding RNAs (lincRNAs) which locate intergenic (gene to gene) region induced by tumor suppressor p53 in response to DNA damage (e.g., lincRNA-p21) [16]. 3) The ultra-conserved region (UCR) is extremely conserved DNA sequences among species which is more than 200 nt long [17,18]. From this region the lncRNA T-UCR is transcribed actively. Although the exact physiological roles of T-UCR have not been reported yet, however investigators assumed that T-UCRs have existed from early evolution stage, and have special roles for interacting with miRNA or different distribution in tissues.
In most cases lncRNAs are involved in transcriptional regulation [19]. The lncRNAs can be classified by their action mechanisms, 1) acting in cis; the lncRNA transcription affects the surrounding coding gene expression, 2) modulating histone modification [14,20], and 3) acting in trans; XIST induces X-chromosome inactivation of female [15].
Recent reports showed that the dysfunction of lncRNAs is closely associated with tumor formation, proliferation, invasion, and metastasis [3,8,9,11]. In this review, we highlight several essential lncRNAs which are closely related with tumorigeneis. As the lncRNAs 1) metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and 2) prostate cancer associated non-coding RNA 1 (PRNCR1), prostate cancer gene expression marker 1 (PCGEM1), and as the lincRNA 3) H19 and 4) homeobox transcript antisense intergenic RNA (HOTAIR) are discussed.
lncRNA MALAT1
The lncRNA MALAT1 is a 7-kb long, spliced non-coding RNA, also known as non-coding nuclear-enriched abundant transcript 2 (NEAT2), which is highly conserved amongst mammals and dominantly expressed in the nucleus [21]. Interestingly, a conserved tRNA-like sequence at the 3' end is cleaved off and processed to generate a short tRNA-like ncRNA, MALAT1-associated small cytoplasmic RNA (mascRNA) [22]. Moreover, MALAT1 modulates the speckle association of a subset of pre-mRNA splicing factors [23,24,25].
MALAT1 RNA is usually overexpressed in cancer tissues, and overexpression of MALAT1 is associated with cell hyper-proliferation and metastasis [21,26]. Some reports showed that MALAT1 regulates the expression of metastasis-associated genes and MALAT1 regulates expression of cell cycle genes and performs key roles in G1/S and mitotic progression [27].
It also known that MALAT1 controls cell cycle progression by modulating the oncogenic transcription factor B-MYB (Mybl2) [28]. Tripathi et al. [27] showed that specific deletion of MALAT1 in cells leads to activation of p53 and its target genes. MALAT1-depleted cells show cell cycle defects which are sensitive to the p53 levels, indicating that p53 is a main downstream mediator for the MALAT1 activity. In MALAT1-depleted cells, replication of the S-phase is reduced, and rather cell population of G1 and G2/M increased. Also, expression of genes associated with cell cycle arrest (e.g., tumor suppressor p53, cdk inhibitor p21) also increases. In addition, reduced expression of oncogenic transcription factor B-MYB which is related with G2/M progression, was observed in MALAT1-depleted cells [27].
lncRNAs PRNCR1 and PCGEM1
Two lncRNAs, PRNCR1 (also known as PCAT8) and PCGEM1, are usually overexpressed in aggressive prostate cancers and have a close relationship with castration resistance and proliferation of cancer cells [29,30,31]. PRNCR1 and PCGEM1, specifically interact with androgen receptor (AR) and strongly enhance androgen receptor-mediated gene activation in both ligand-dependent and -independent manner [14].
After the interaction between PRNCR1 and AR, acetylation occurs at the C-terminal of AR protein, which accelerates the association of DOT1L (disruptor of telomeric silencing 1 like histone H3 methyltransferase) to the PRNCR1-AR complex. Consequently, DOT1L mediates N-terminal acetylation of AR protein which enhances recruitment of lncRNA PCGEM1. In the castration-resistant prostate cancer cells, expression for short hairpin RNA targeting these two lncRNAs strongly suppressed proliferation of cancer cells and xenograft tumor growth in mice [14]. Taken together, the data indicate that lncRNAs are potentially essential for the castration resistance in prostate cancers. However, the specific mechanistic roles of these two lncRNAs in castration resistance during prostate cancer development are still under investigation.
lincRNA H19
The lincRNA H19 gene encodes around 2.3-kb long transcript which does not contain any known open reading frames [32,33,34]. It is normally transcribed by RNA polymerase II likewise general mRNA transcripts and transported to the cytoplasm after sequential capping-polyadenylation-splicing steps. Furthermore, H19 lincRNA is one of the most highly expressed in the placenta and evolutionarily conserved at the nucleotide level between human and rodent [34]. Interestingly, H19 is the imprinted gene which is only transcribed from the maternally inherited allele; the paternal H19 allele dose not expressed. H19 gene is located on downstream of growth-promoting insulin-like growth factor 2 (igf2) and H19 and igf2 share common imprinting mechanism. Recent reports show that H19 is closely associated with tumorigenesis and fetal growth syndrome. However, the exact physiological functions of H19 are largely unknown yet [35,36,37,38,39,40].
Intriguingly, recent reports showed that H19 serves as miRNA precursor. miR-675 is located in the first exon of H19, and expressed through the excision from full H19 lincRNA transcript in the placenta of gestational time point which shows to stop normal growth [41]. The overexpression of miR-675 in embryonic cell and extra-embryonic cell caused decrease of cell proliferation. The target gene of miR-675 (ex. lgf1r) is derepressed in H19 null placenta. Furthermore, processing of miR-675 from H19 is known to be regulated by stress-response RNA-binding protein HuR. HuR inhibits miR-675 processing by binding to H19 transcript. Release of miR-675 from H19 transcript inhibits cell proliferation rapidly in response to cellular stress or oncogenic signals [34]. miR-675 processing mechanism from H19 seems to be associated with molecular pathology in fetal growth and tumorigenesis.
lincRNA HOTAIR
HOTAIR usually shows overexpressed patterns in early and metastatic breast cancer cells [20]. HOTAIR regulates the gene expression by interacting with polycomb repressive complex 2 (PRC2) and lysine-specific demethylase 1A. Together with these two enzymes, HOTAIR can control methylation and demethylation status of histones [20]. PRC2 seems to be more important related with cancer because cancer cells which contain lysine-27 methylated histone H3 showed similar gene expression of embryonic fibroblasts. Generally, PRC2 expression induces metastasis of cancer cells. However metastasis can be suppressed when PRC2 is too overexpressed.
LNCRNAS ON GLIOMA DEVELOPMENT
A glioma is a type of tumor that arises from glial cells mostly in the human brain. High-grade gliomas usually have a tendency to infiltrate into the extracellular matrix of the brain and this trait makes it difficult to perform surgery and radio-therapy [42]. Therefore the understanding of the molecular mechanism of infiltrative phenotype of gliomas and identification of key regulator(s) for invasion are essential for the efficient treatment of this hardly curable disease [43,44].
Recent reports showed that several lncRNAs have close relationship with glioma development. Some lncRNAs that may contribute to brain development and certain specific differentially expressed lncRNAs may play an important role in the pathogenesis of glioblastoma multiforme [45]. The most highly upregulated lncRNA is Colorectal Neoplasia Differentially Expressed (CRNDE) [46]. The importance of CRNDE and its roles in specialized processes such as brain function have not been addressed yet.
Another lncRNA, maternally expressed gene 3 (MEG3), has been found that it markedly decreased in glioma tissues compared with adjacent normal tissues [47]. Moreover, overexpression of the lncRNA MEG3 in human glioma cell lines inhibits cell proliferation and promotes cell apoptosis. Therefore MEG3 might have inhibitory role in glioma development and can serve as potential drug in anti-glioma therapy.
The lincRNA H19 and its derivative miR-675 were positively correlated with glioma grades. Moreover, H19-derived miR-675 regulated cadherin 13 which is the directly target of miR-675, thereby modulating glioma cell invasion [48]. The potential oncogenic role of lincRNA H19/miR-675 may serve as development of anti-glioma therapy.
THERAPEUTIC APPROACHES FOR TARGETING LNCRNAS
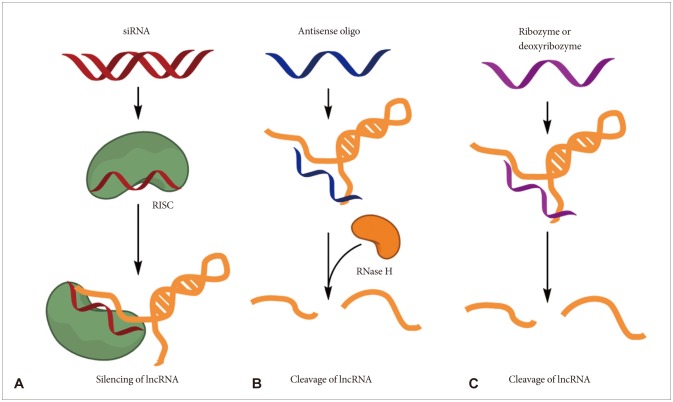
Various therapeutic approaches for the lncRNAs have been developed and several pharmaceutical companies are also actively developing lncRNA-targeting therapeutics [49,50]. First, the small interfering RNAs (siRNAs) against the specific lncRNA can be used for the strategies to regulate lncRNA function (Fig. 2A). In most cases, predominant localization of lncRNAs is in the nucleus, and thus siRNAs may be less accessible to lncRNAs than mRNAs. However, successful knockdown of lncRNAs have been reported by many researchers irrespective of their subcellular localization.
Fig. 2.
Potential therapeutic approaches for targeting lncRNAs. Several methods, including small interfering RNAs (siRNAs), antisense oligonucleotides (ASOs) and ribozymes or deoxyribozymes, can be used to block the function of lncRNAs. A: Synthetic double-stranded short RNA can be delivered to cells and the antisense strand of the siRNA duplex loads on to the RNA-induced silencing complex (RISC) and degrades the targeted lncRNA. B: ASOs are single-stranded, chemically modified DNA oligomers (less than 25 nt in length) that are designed to be complementary to a target lncRNA. ASOs form a heteroduplex with the target lncRNA, and RNase H recognizes the lncRNA-DNA heteroduplex and cleaves the RNA strand. C: However, ribozymes or deoxyribozymes do not dependent on the RISC, which mediates siRNA-induced degradation, or on RNase H. IncRNA: long non-coding RNA.
Other strategies [e.g., antisense oligonucleotides (ASOs) as well as ribozymes or deoxyribozymes] can be adapted to directly target lncRNAs when an overall secondary structure or the nucleotide sequence is not favorable for the optimized design of siRNAs (Fig. 2B). Antisense oligonucleotides have advantages over siRNAs including independence of RNA-induced silencing complex (RISC) machinery, high specificity, and low off-target effects. Recent reports showed that MALAT1 function on lung cancer cells in mouse successfully inhibited by using ASOs [22,23,27].
Ribozymes or deoxyribozymes (e.g., hammerhead ribozyme) bind to a target sequence complementarily and promote the cleavage of the flanking RNA region (Fig. 2C). These may be useful for the targeting of lncRNAs that are not favorable for optimal siRNA design [49,50].
CONCLUSION
Recent global analysis showed that cancer transcriptome is more complex than previously expected. Dysregulated expression of lncRNAs, including protein-coding genes and miRNAs have potential pervasive roles as the driver of human cancers and development and progression of the cancers [3,8,11]. The epigenomic reprogramming by lncRNAs can be applicable to many other human diseases characterized by aberrant lncRNA expression [51]. Therefore, in the context of cancer cells, ectopic expression or specific knockdown of lncRNAs such as PRNCR1, PCGEM1, and HOTAIR seems to re-impose that chromatin state, thereby enabling gene expressions are more favorable or unfavorable to the mobilization and matrix invasion of cancer cells.
As the non-epigenomic regulation, such as regulation of alternative splicing (e.g., MALAT1) [23] and generation of miRNA precursor (e.g., lincRNA H19) [41] lncRNAs can modulate gene expression more favorable to tumor development.
Therefore, understanding the precise molecular mechanisms of lncRNAs to the various biological processes will be a critical step in exploring new strategies in future cancer therapy.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2011-0024692) and a grant from the National Cancer Center, Republic of Korea (1410080).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 2.International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 4.Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 5.Kapranov P, Willingham AT, Gingeras TR. Genome-wide transcription and the implications for genomic organization. Nat Rev Genet. 2007;8:413–423. doi: 10.1038/nrg2083. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 8.Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34:613–620. doi: 10.1007/s13277-013-0658-6. [DOI] [PubMed] [Google Scholar]
- 9.Gibb EA, Vucic EA, Enfield KS, et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6:e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallen AN, Zhou XB, Xu J, et al. The imprinted H19 lncRNA antago nizes let-7 microRNAs. Mol Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Lin C, Jin C, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plath K, Fang J, Mlynarczyk-Evans SK, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 16.Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bejerano G, Pheasant M, Makunin I, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 18.Lujambio A, Portela A, Liz J, et al. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene. 2010;29:6390–6401. doi: 10.1038/onc.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 22.Wilusz JE, Freier SM, Spector DL. 3' end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–932. doi: 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 25.Manley JL, Krainer AR. A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins) Genes Dev. 2010;24:1073–1074. doi: 10.1101/gad.1934910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutschner T, Hämmerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathi V, Shen Z, Chakraborty A, et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joaquin M, Watson RJ. Cell cycle regulation by the B-Myb transcription factor. Cell Mol Life Sci. 2003;60:2389–2401. doi: 10.1007/s00018-003-3037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovics G, Zhang W, Makarem M, et al. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–611. doi: 10.1038/sj.onc.1207069. [DOI] [PubMed] [Google Scholar]
- 30.Chung S, Nakagawa H, Uemura M, et al. Association of a novel long non-coding RNA in 8q24 with prostate cancer susceptibility. Cancer Sci. 2011;102:245–252. doi: 10.1111/j.1349-7006.2010.01737.x. [DOI] [PubMed] [Google Scholar]
- 31.Komiya A, Yasuda K, Watanabe A, Fujiuchi Y, Tsuzuki T, Fuse H. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Mol Clin Oncol. 2013;1:257–262. doi: 10.3892/mco.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rachmilewitz J, Goshen R, Ariel I, Schneider T, de Groot N, Hochberg A. Parental imprinting of the human H19 gene. FEBS Lett. 1992;309:25–28. doi: 10.1016/0014-5793(92)80731-u. [DOI] [PubMed] [Google Scholar]
- 34.Keniry A, Oxley D, Monnier P, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J. 2013;280:1709–1716. doi: 10.1111/febs.12185. [DOI] [PubMed] [Google Scholar]
- 37.Matouk IJ, DeGroot N, Mezan S, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matouk IJ, Mezan S, Mizrahi A, et al. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim Biophys Acta. 2010;1803:443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Tsang WP, Ng EK, Ng SS, et al. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Yang F, Yuan JH, et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- 41.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwak HS, Kim TH, Jo GH, et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449. doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwak HJ, Kim YJ, Chun KR, et al. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene. 2011;30:2433–2442. doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 44.Kim YJ, Park SJ, Choi EY, et al. PTEN modulates miR-21 processing via RNA-regulatory protein RNH1. PLoS One. 2011;6:e28308. doi: 10.1371/journal.pone.0028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellis BC, Molloy PL, Graham LD. CRNDE: A Long Non-Coding RNA Involved in CanceR, Neurobiology, and DEvelopment. Front Genet. 2012;3:270. doi: 10.3389/fgene.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham LD, Pedersen SK, Brown GS, et al. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer. 2011;2:829–840. doi: 10.1177/1947601911431081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113:1868–1874. doi: 10.1002/jcb.24055. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Wang Y, Luan W, et al. Long Non-Coding RNA H19 Promotes Glioma Cell Invasion by Deriving miR-675. PLoS One. 2014;9:e86295. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 50.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]