Abstract
Many eukaryotes, including plants, produce a large number of long noncoding RNAs (lncRNAs). Growing number of lncRNAs are being reported to have regulatory roles in various developmental processes. Emerging mechanisms underlying the function of lncRNAs indicate that lncRNAs are versatile regulatory molecules. They function as potent cis- and trans-regulators of gene expression, including the formation of modular scaffolds that recruit chromatin-modifying complexes to target chromatin. LncRNAs have also been reported in plants. Here, we describe our current understanding on potential roles of lncRNA in plants.
Keywords: Long noncoding RNA (lncRNA), Chromatin modification, RNA-seq, Gene silencing, Gene activation
Introduction
Extensive transcriptome analyses have revealed that up to 90 % of eukaryotic genomes are transcribed (Wilhelm et al. 2008), whereas only 1–2 % of the genome encodes proteins (Birney et al. 2007). This suggests that a large proportion of the eukaryotic genome produces an unexpected plethora of RNA molecules that have no protein-coding potential. These are collectively called noncoding RNAs (ncRNAs).
NcRNAs can be grouped into two classes according to the size of transcripts. NcRNAs with more than 200 nucleotides are considered as long ncRNAs (lncRNAs) whereas short ncRNAs are less than 200 nucleotides. Short ncRNAs include micro RNA (miRNAs), small interfering RNAs (siRNAs), and Piwi-interacting RNAs (piRNAs) (Ghildiyal and Zamore 2009). Mechanistic details on the roles of short ncRNAs in transcriptional and post-transcriptional regulation in eukaryotes are fairly well characterized (Chitwood and Timmermans 2010). In contrast, the molecular mechanism of gene regulation by lncRNAs is not well understood. Most lncRNAs that have been characterized participate in the regulation of gene expression. One prevailing regulatory theme for those lncRNAs is that they modulate transcriptional activity through the interaction with regulatory protein complexes (Chaumeil et al. 2006; Rinn et al. 2007; Wang et al. 2011). LncRNAs can act as a decoy for splicing factors (Tripathi et al. 2010) and as a competitor for miRNA binding sites (Cesana et al. 2011;Karreth et al. 2011), indicating that lncRNAs potentially play roles in various regulatory circuitries in eukaryotes. In plants, a few lncRNAs have been identified and functionally characterized (Ben Amor et al. 2009; Heo and Sung 2011; Swiezewski et al. 2009). In this review, we provide an overview on epigenetic regulation of gene expression by plant lncRNAs and discuss the current and future research that could shed further light on understanding the functional role of plant lncRNAs.
Identification of long ncRNA
A large-scale sequencing of full-length cDNA library unexpectedly identified a number of lncRNAs in mammals (Okazaki et al. 2002). Some lncRNAs are transcribed from intergenic regions or introns, and others overlap with protein-coding regions. They are transcribed in either sense or antisense direction compared to neighboring protein-coding transcripts. Some are polyadenylated and others are not polyadenylated. Currently, in silico identification differentiates lncRNAs from protein-coding transcripts based on the absence of discernible open-reading frames. Full-length targeted cDNA sequencing may be the best method to determine the coding potential of transcripts, although this approach is time-consuming and expensive. Alternatively, tiling DNA microarray for genome-wide transcriptome analysis has been used to detect and to determine expression of lncRNAs in plants. For example, tiling DNA microarray analyses identified novel stress-induced lncRNAs in Arabidopsis (Matsui et al. 2010; Rehrauer et al. 2010) and intergenic lncRNAs in rice (Li et al. 2006). Using a similar approach, a group of antisense lncRNAs, collectively known as COOLAIR, was identified from FLOWERING LOCUS C (FLC) in Arabidopsis (Swiezewski et al. 2009). High-throughput deep RNA sequencing can be used to identify missing or incomplete transcripts of lncRNA and to determine the levels of lncRNA expression. A similar approach also identified rare alternative splicing variants of an lncRNA, HOTAIR (Mercer et al. 2011). Genome-wide histone modification profiles indicate that a number of long intergenic ncRNAs (lincRNAs) are transcribed from a K4-K36 domain, which marks active promoters with trimethylation of lysine 4 of histone H3 (H3K4me3) and trimethylation of lysine 36 of histone H3 (H3K36me3), suggesting that most lncRNAs are transcribed from independent promoters (Zhang et al. 2009). Unlike short RNAs and proteins, function of lncRNAs cannot simply be inferred from their sequence or structure. In this review, we focus on a few lncRNAs whose function is relatively well characterized in plants. In particular, we describe examples of lncRNAs that function to regulate gene expression at the level of chromatin modification and in the recruitment of chromatin-modifying complexes.
Role of lncRNAs in the recruitment of polycomb repression complex 2 in animals
It has been known that purified chromatin contains both RNA and DNA, suggesting that RNA may affect chromatin structure and gene regulation (Paul and Duerksen 1975). Earlier genetic studies showed that a few lncRNAs are associated with heterochromatin formation and genomic imprinting (Barlow et al. 1991; Brown et al. 1991). Functional analyses of identified lncRNAs demonstrate that lncRNAs are required for proper chromatin structure and recruitment of the chromatin-modifying complexes to DNA (Bernstein and Allis 2005). One well-known function of lncRNAs is to mediate epigenetic changes by recruiting chromatin-remodeling complex to specific genomic loci. For example, Xist lncRNA is expressed from the inactive X chromosome and “coats” the X chromosome, leading to the recruitment of polycomb repressive complex 2 (PRC2), which trimethylates histone H3 at lysine 27 to silence transcription of local genes as a cis-acting lncRNA (Chaumeil et al. 2006). A small internal non-coding transcript from the Xist locus, RepA, recruits PRC2 to silence one X chromosome, whereas PRC2 is titrated from the remaining active X chromosome by the antisense transcript Tsix (Zhao et al. 2008). Another study showed that Xist and Tsix form an RNA duplex that is processed by Dicer to generate siRNAs that are required for the repressive chromatin modification on the inactive X chromosome (Lander et al. 2001). Other lncRNAs, Air and Kcnq1ot1, also interact with chromatin and target repressive histone modifiers to specific cis-linked gene loci (Nagano et al. 2008). In humans, the antisense lncRNA HOTAIR, which is transcribed from the HOXC locus, regulates epigenetic changes at the HOXD locus in trans by recruiting PRC2 (Rinn et al. 2007). HOTAIR physically associates with the PRC2 and modulates PRC2 activity to deposit H3K27me3 marks at target chromatin throughout the genome (Rinn et al. 2007; Tsai et al. 2010). Additional studies of both HOTAIR and Xist revealed that the methyltransferase subunit EZH2 of the PRC2 complex physically associates with both lncRNAs (Kaneko et al. 2010; Zhao et al. 2008). Although molecular nature of the interaction between lncRNAs and PRC2 is yet to be determined, the interaction between lncRNAs and chromatin-modifying complexes appears to be a general mechanism for epigenetic repression in animals.
Polycomb-mediated repression by vernalization in plants
Plants respond to environmental cues to trigger developmental changes (i.e., flowering) only during a certain period of the year. One example of such environmental cues is prolonged cold of winter known as vernalization (Sung and Amasino 2004b). Vernalization results in epigenetic silencing of FLC, and the repression of FLC is stably maintained even after winter cold. Molecular studies have revealed that both activation and repression of chromatin-remodeling complexes are involved in the regulation of FLC expression (Kim et al. 2009). A high expression level of FLC results in delayed flowering, whereas flowering is promoted when FLC is repressed by vernalization. Genetic approaches identified that several protein components are necessary for establishing the stable repression of FLC by vernalization (Sung and Amasino 2004a, Kim and Sung 2012). VERNALIZATION INSENSITIVE 3 (VIN3) was identified as an essential gene to trigger repression of FLC by vernalization (Sung and Amasino 2004b). VIN3 encodes a plant homeodomain (PHD) finger protein that is induced only during the cold. The PHD finger motif in VIN3 is often found in various components of chromatin-remodeling complexes (Sung et al. 2006, Kim and Sung 2013). VIN3 was biochemically co-purified with PRC2 (De Lucia et al. 2008). This result suggests that the PHD-PRC2 association is required for the function of PRC2. Vernalization results in increased association of PHD-PRC2 with FLC chromatin and increased deposition of H3K27me3 marks at the FLC chromatin (De Lucia et al. 2008; Wood et al. 2006; Kim and Sung 2013). Increased enrichments of PRC2 and H3K27me3 are hallmarks for the stable repression of FLC by vernalization.
PRC2 is a conserved repressive chromatin-remodeling complex in higher eukaryotes. In mammals, PRC2 contains four core subunits, including SUPPRESSOR OF ZESTE-12 [Su(Z)12], enhancer of zeste [E(Z)], ESC, and P55. Arabidopsis VRN2 encodes a homolog of Su(Z)12, which is a core component of PRC2 (Wood et al. 2006; De Lucia et al. 2008). There are at least three homologs of E(Z) (i.e., CLF, SWN, and MEDEA) and five homologs of p55 (MSI1 ∼5). CLF, SWN, MSI1, and FIE are unambiguously identified as components of the vernalization PRC2 complex (De Lucia et al. 2008; Wood et al. 2006). A homolog of ESC, FIE, is the only PRC2 component that is represented by a single member in Arabidopsis (De Lucia et al. 2008).
Roles of long noncoding RNAs derived from FLC locus
Two classes of lncRNAs were identified to be involved in vernalization-mediated FLC repression (Fig. 1). A group of lncRNAs, COOLAIR, are transcribed from the 3′ end of FLC in an antisense direction compared to FLC mRNA. Six different COOLAIR transcripts can be grouped into two groups based on two alternative polyadenylation sites (Swiezewski et al. 2009). The expression levels of COOLAIR increase transiently at an early stage of vernalization, and distally polyadenylated variants of COOLAIR partly overlap with the 5′ end of FLC mRNA (Fig. 1). It has been suggested that COOLAIR plays a role in transcriptional interference of FLC (Swiezewski et al. 2009). However, insertional mutants (in which COOLAIR transcription is impaired) show reduced FLC expression by vernalization (Helliwell et al. 2011). Alternatively, it has been proposed that COOLAIR may function in “co-transcriptional” regulation during the early phase of vernalization (De Lucia and Dean 2010). In this model, pervasive transcription of antisense lncRNAs would affect sense FLC transcription. Molecular details on the function of COOLAIR in vernalization-mediated FLC repression still remain to be addressed.
Fig. 1.
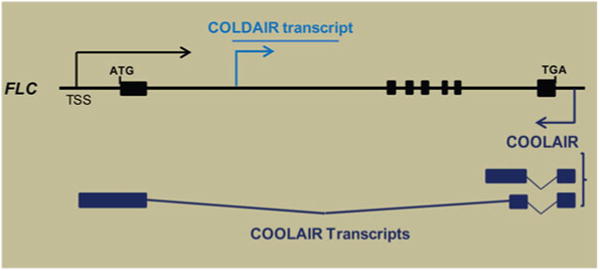
Noncoding RNAs from FLC. From FLC, both sense and antisense nonprotein coding transcripts are detected. COLDAIR is transcribed from the first intron of FLC in a sense direction compared to FLC mRNA. Two classes of antisense ncRNAs are transcribed. COOLAIR is largely classified based on their polyadenylations (Proximal pA and Distal pA). Splicing variants are also detected for both classes of COOLAIR
The second lncRNA is another cold-inducible intronic lncRNA, COLDAIR (Fig. 1), which is transcribed from the first intron of FLC in a sense direction compared to FLC mRNA (Heo and Sung 2011). COLDAIR is 1.1 kb long. COLDAIR contains 5′-capped structure but no apparent polyadenylation tail, although it is transcribed by RNA polymerase II. COLDAIR is transiently induced by vernalization, and its induction is controlled by a cryptic cold-inducible promoter located within the vernalization response element (Heo and Sung 2011). In vitro transcription and pull-down assays showed that COLDAIR binds to CLF protein, a homolog of E(z) component of the PRC2 chromatin-modifying complex. RNA immunoprecipitation assays verified that COLDAIR associates with PRC2 in vivo. A genetic study of COLDAIR using RNA-interference system demonstrates that COLDAIR is a necessary component for the vernalization response in Arabidopsis. COLDAIR knockdown lines do not display a decrease in the level of FLC in response to vernalization compared to that in parental lines, and FLC is derepressed in the knockdown lines when the plants are returned to warm condition (Heo and Sung 2011). The enrichment of CLF and H3K27me3 at the FLC locus chromatin in response to vernalization is largely impaired in COLDAIR knockdown lines, which indicates that COLDAIR is necessary for recruiting PRC2 to FLC chromatin. Therefore, COLDAIR is similar to HOTAIR and Xist ncRNAs in that they act to recruit PRC2 to target chromatin. Taken together, lncRNA-mediated epigenetic gene silencing by PRC2 appears to be an evolutionarily conserved mechanism between plants and animals.
Role of lncRNAs in the recruitment of polycomb repression complex 1
The primary research focus has been on PRC2-mediated epigenetic gene silencing by lncRNAs. However, there is increasing evidence that lncRNAs also play role in the recruitment of the PRC1 complex. PRC1 recognizes the H3K27me3 mark and executes stable transcriptional repression (Zhang et al. 2007). In Drosophila, the PRC1 complex contains the two RING finger proteins posterior sex combs (Psc) and dRING, which are orthologs of the mammalian PRC1 subunits BMI1 and RING1A/RING1B, respectively (Scheuermann et al. 2010). Mammalian PRC1 is an E3 ubiquitin ligase complex that performs mono-ubiquitination on histone H2A lysine 119, which results in stable repression of the target locus (Cao et al. 2005). RNA immunoprecipitation followed by sequencing or RT-PCR analysis identified thousands of lncRNAs associated with the PRC2 and other histone-modifying complexes (Zhao et al. 2008; Guttman et al. 2011). Therefore, lncRNA-mediated chromatin modification is not restricted to PRC2. For example, HOTAIR functions as a modular scaffold for both PRC2 and LSD1/coREST complex (Tsai et al. 2010). Another example of an lncRNA as a modular scaffold is ANRIL, which associates with both PRC2 and PRC1 (Yap et al. 2010). ANRIL appears to recruit PRC1 together with PRC2 to repress specific target genes. A tumor suppressor locus, INK4b/ARF/INK4a, is repressed by PRC1 in a ANRIL lncRNA-dependent manner (Yap et al. 2010). CBX7, which is a component of the core PRC1 complex in mammals, is localized to Xi domains. RNase treatment disrupted CBX7 association with Xi domains (Yap et al. 2010), suggesting that the lXist lncRNA has a direct role in localizing PRC1 to target chromatin.
PRC1 subunits in plants have been diverged. For example, polycomb protein does not exist in plants. Instead, LIKE HETEROCHROMATIN PROTEIN1 (LHP1) binds to H3K27me3 and functions similarly to polycomb (Mylne et al. 2006; Sung et al. 2006, Turck et al. 2007; Zhang et al. 2007). Molecular genetic studies suggest that EMF1 and VRN1, which are non-sequence-specific DNA-binding proteins, also exhibit PRC1-like function (Aubert et al. 2001; Bastow et al. 2004). Apparent functional homologs of PRC1 RING finger proteins have been identified in Arabidopsis. They are associated with LHP1 and function to repress PcG target genes in planta (Xu and Shen 2008). AtBMI1A and AtBMI1B are RING finger proteins that function similarly as mammalian BMI1 (a component of PRC1); they interact with LHP1 and EMF1 and repress PcG target genes (Bratzel et al. 2010). It will be interesting to determine whether Arabidopsis PRC1-like complex includes lncRNA components for its function in gene repression (Fig. 2).
Fig. 2.
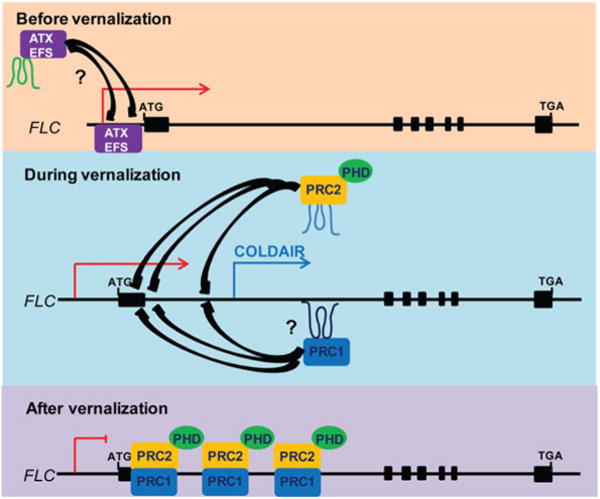
Vernalization-mediated FLC repression by polycomb group protein complexes. FLC chromatin undergoes dynamic changes from Trithorax-dominant chromatin to polycomb-dominant chromatin. COLDAIR is required for the recruitment of PRC2 during the cold exposure
Role of lncRNAs in activating chromatin-remodeling complexes
Some lncRNAs are implicated in establishing active chromatin states. Chromatin immunoprecipitation followed by sequencing analysis showed that H3K4me1 and H3K27ac histone marks are associated with known enhancer loci. Interestingly, these loci produced lncRNAs, implicating their function in gene activation (De Santa et al. 2010; Kim et al. 2010). The brain-specific lncRNA Evf-2 in human is transcribed within an enhancer between Dlx5 and Dlx6, which are members of the DLX/dll homeodomain-containing protein family. Evf-2 specifically cooperates with Dlx-2 to increase the transcriptional activity of Dlx5 and Dlx6 (Feng et al. 2006). In XCL, Jpx RNA is involved in the induction of Xist expression. Two lncRNAs were characterized in the HoxA locus. The first was an enhancerlike lncRNA termed HOTTIP (Wang et al. 2011). HOTTIP is transcribed from the distal 5′ end of the HOXA gene cluster and plays a role in the maintenance of H3K4me3 at HOXA locus (Wang et al. 2011). HOTTIP physically interacts with WDR5 protein, which is a key component of the mixed-lineage leukemia (MLL)/Trx complex that catalyzes the deposition of the H3K4me3 mark on target genes (Wang et al. 2011). The second lncRNA, Mistral, also interacted with MLL and was considered to activate neighboring HoxA6 and HoxA7 (Bezhani et al. 2007). Therefore, lncRNAs may also be an important component in activating chromatin-remodeling complexes.
In plants, there is no evidence to support the role of lncRNAs in activating chromatin-remodeling complexes up to date. In Arabidopsis, five ARABIDOPSIS TRITHORAX (ATX1–ATX5) and two ATX-RELATED (ATXR3 and ATXR7) proteins have been proposed as H3K4 methyltransferases (Ng et al. 2007). ATX1–ATX5 are TRX-group H3K4 methyltransferases. ATXR7 is an ortholog of yeast Set1, and ATXR3 is considered to be a different class of H3K4 methyltransferase (Alvarez-Venegas and Avramova 2001). Among these Arabidopsis H3K4 methyltransferases, ATX1, ATX2, ATXR3, and ATXR7 are involved in FLC activation through H3K4 methylation (Berr et al. 2009; Pien et al. 2008; Saleh et al. 2008a, 2008b; Tamada et al. 2009; Yun et al. 2012). Another TRX-like set domain protein, EARLY FLOWERING IN SHORT DAYS (EFS), is required for di- and trimethylation of histone H3 Lys36 (H3K36) at target chromatin (Xu and Shen 2008). A knockout mutant of EFS exhibits reduced FLC expression and early flowering due to decreased H3K4me3 at FLC chromatin (Kim et al. 2005; Ko et al. 2010). This suggests that EFS is necessary for the enrichment of H3K4me3, H3K36me2, and H3K36me3 at its target chromatin. It will be interesting to determine whether lncRNAs function in these active chromatin-modifying complexes (Fig. 2).
Other classes of lncRNAs in plants
Besides lncRNAs that clearly function in chromatin modifications, a few other ncRNAs also play roles in other biological processes in plants. The soybean early nodulin gene, Enod40, is involved in the regulation of symbiotic interactions between leguminous plants and soil bacteria (Fujita et al. 1993). Enod40 transcript contains only small predicted open-reading frames for two small peptides (12 and 24 amino acid residues). These peptides interact with sucrose synthase, suggesting that Enod40 plays a role in regulation of sucrose utilization in nodules (Rohrig et al. 2002). Interestingly, Enod40 transcripts themselves interact with MtRBP1 (Medicago truncatula RNA-binding protein1) and function to re-localize MtRBP1 from nuclear speckles to cytoplasmic granules during the nodulation in M. truncatula (Rohrig et al. 2002). These results suggest that Enod40 transcripts function as a lncRNA for the MtRBP1 re-localization.
Another lncRNA, INDUCED BY PHOSPHATE STARVATION 1 (IPS1), sequesters miRNA-399, which regulates the expression of PHO2. IPS1 contains complementary sequences to phosphate (Pi) starvation-induced miRNA399. IPS1 competes with PHO2 for the binding of miRNA-399, resulting in attenuating miR399-mediated repression of PHO2 (Franco-Zorrilla et al. 2007). The association of IPS1 with miRNA399 does not affect cleavage of the IPS1 transcript because of a mismatched loop at the cleavage site (Franco-Zorrilla et al. 2007; Liu et al. 1997). Therefore, IPS1 plays a role as a mimic of a miRNA target.
Perspectives
A large number of lncRNAs have been identified in animals, particularly through high-throughput transcriptome sequencing. Further high-throughput sequencing with various alterations will enable identification and characterization of numerous lncRNAs in plants in the next few years. Indeed, such approach has been employed to identify lncRNAs in plants and revealed that there are correlative expressions between lncRNAs and their neighboring protein-coding genes, suggesting that a majority of identified lncRNAs are cis-acting (Liu et al. 2012). Although there are correlative expression patterns, it still remains to be determined how lncRNAs affect expression of neighboring genes. It is also expected that more targeted approaches (i.e., RNA immunoprecipitation followed by sequencing) will identify different classes of lncRNAs. It is also noteworthy that regulation of transcription and processing of lncRNAs are poorly understood. Given that lncRNAs can be transcribed in various loci, it will be interesting to address how these transcripts are regulated and processed. For example, the homeodomain protein AtNDX has been identified to influence the transcription of COOLAIR, the antisense lncRNA transcribed from the 3′ end of FLC (Sun et al. 2013). AtNDX associates with the COOLAIR promoter and inhibits COOLAIR transcription through the R-loop stabilization (Sun et al. 2013). Although our understanding of biological function and regulation of plant lncRNAs is still in its infancy, unraveling function of plant lncRNAs will provide fertile ground for exciting discoveries in understanding the fundamental principles of lncRNA-mediated regulation of gene expression.
Acknowledgments
J.B. Heo is supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant no.: PJ00951601) and by the National Research Foundation of Korea (NRF-MEST no. 2011–0013137). S. Sung is supported by the University of Texas at Austin, National Science Foundation (IOS-0950785) and National Institute of Health (R01GM100108).
Abbreviations
- ncRNA
Noncoding RNA
- lncRNA
Long noncoding RNA
- miRNA
Micro RNA
- siRNA
Small interfering RNA
- piRNA
Piwi-interacting RNA
- RNA-seq
RNA sequencing
- lincRNA
Long intergenic ncRNA
- PHD
Plant homeodomain
- PRC2
Polycomb repressive complex 2
Footnotes
Responsible editors: Brian P. Chadwick, Kristin C. Scott, and Beth A. Sullivan
Contributor Information
Jae Bok Heo, Department of Molecular Biotechnology, Dong-A University, Busan 604-714, South Korea.
Yong-Suk Lee, Department of Biotechnology, Dong-A University, Busan 604-714, South Korea.
Sibum Sung, Email: sbsung@austin.utexas.edu, Department of Molecular Biosciences and Institute for Cellular and Molecular Biology, University of Texas, Austin, TX 78712, USA.
References
- Alvarez-Venegas R, Avramova Z. Two Arabidopsis homologs of the animal trithorax genes: a new structural domain is a signature feature of the trithorax gene family. Gene. 2001;271:215–221. doi: 10.1016/s0378-1119(01)00524-8. [DOI] [PubMed] [Google Scholar]
- Aubert D, Chen L, Moon YH, Martin D, Castle LA, Yang CH, Sung ZR. EMF1, a novel protein involved in the control of shoot architecture and flowering in Arabidopsis. Plant Cell. 2001;13:1865–1875. doi: 10.1105/TPC.010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DP, Stoger R, Herrmann BG, Saito K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349:84–87. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- Ben Amor B, Wirth S, Merchan F, Laporte P, d'Aubenton-Carafa Y, et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009;19:57–69. doi: 10.1101/gr.080275.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–1655. doi: 10.1101/gad.1324305. [DOI] [PubMed] [Google Scholar]
- Berr A, Xu L, Gao J, Cognat V, Steinmetz A, Dong A, Shen WH. SET DOMAIN GROUP25 encodes a histone methyltransferase and is involved in FLOWERING LOCUS C activation and repression of flowering. Plant Physiol. 2009;151:1476–1485. doi: 10.1104/pp.109.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezhani S, Winter C, Hershman S, Wagner JD, Kennedy JF, Kwon CS, Pfluger J, Su Y, Wagner D. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell. 2007;19:403–416. doi: 10.1105/tpc.106.048272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, et al. Identification and analysis of functional elements in 1 % of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratzel F, Lopez-Torrejon G, Koch M, Del Pozo JC, Calonje M. Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol. 2010;20:1853–1859. doi: 10.1016/j.cub.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long non-coding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil J, Le Baccon P, Wutz A, Heard E. A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 2006;20:2223–2237. doi: 10.1101/gad.380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Timmermans MC. Small RNAs are on the move. Nature. 2010;467:415–419. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- De Lucia F, Dean C. Long non-coding RNAs and chromatin regulation. Curr Opin Plant Biol. 2010;14:168–173. doi: 10.1016/j.pbi.2010.11.006. [DOI] [PubMed] [Google Scholar]
- De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- Fujita T, Kouchi H, Ichikawa T, Syono K. Isolation and characterization of a cDNA that encodes a novel proteinase inhibitor I from a tobacco genetic tumor. Plant Cell Physiol. 1993;34:137–142. [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Robertson M, Finnegan EJ, Buzas DM, Dennis ES. Vernalization-repression of Arabidopsis FLC requires promoter sequences but not antisense transcripts. PLoS One. 2011;6:e21513. doi: 10.1371/journal.pone.0021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JB, Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Li G, Son J, Xu CF, Margueron R, Neubert TA, Reinberg D. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24:2615–2620. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreth FA, Tay Y, Perna D, Ala U, Tan SM, et al. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol. 2009;25:277–299. doi: 10.1146/annurev.cellbio.042308.113411. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sung S. Environmentally coordinated epigenetic silencing of FLC by protein and long noncoding RNA components. Curr Opin Plant Biol. 2012;15:51–56. doi: 10.1016/j.pbi.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sung S. Coordination of the vernalization response through a VIN3 and FLC gene family regulatory network in Arabidopsis. Plant Cell. 2013;25:454–469. doi: 10.1105/tpc.112.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, He Y, Jacob Y, Noh YS, Michaels S, Amasino R. Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell. 2005;17:3301–3310. doi: 10.1105/tpc.105.034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Mitina I, Tamada Y, Hyun Y, Choi Y, Amasino RM, Noh B, Noh YS. Growth habit determination by the balance of histone methylation activities in Arabidopsis. EMBO J. 2010;29:3208–3215. doi: 10.1038/emboj.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Li L, Wang X, Stolc V, Li X, Zhang D, Su N, Tongprasit W, Li S, Cheng Z, Wang J, Deng XW. Genome-wide transcription analyses in rice using tiling microarrays. Nat Genet. 2006;38:124–129. doi: 10.1038/ng1704. [DOI] [PubMed] [Google Scholar]
- Liu C, Muchhal US, Raghothama KG. Differential expression of TPS11, a phosphate starvation-induced gene in tomato. Plant Mol Biol. 1997;33:867–874. doi: 10.1023/a:1005729309569. [DOI] [PubMed] [Google Scholar]
- Liu J, Jung C, Xu J, Wang H, Deng S, Bernad L, Arenas-Huertero C, Chua NH. Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell. 2012;24:4333–4345. doi: 10.1105/tpc.112.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylne JS, Barrett L, Tessadori F, Mesnage S, Johnson L, Bernatavichute YV, Jacobsen SE, Fransz P, Dean C. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc Natl Acad Sci U S A. 2006;103:5012–5017. doi: 10.1073/pnas.0507427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Ishida J, Morosawa T, Okamoto M, Kim JM, et al. Arabidopsis tiling array analysis to identify the stress-responsive genes. Methods Mol Biol. 2010;639:141–155. doi: 10.1007/978-1-60761-702-0_8. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, Mattick JS, Rinn JL. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol. 2011;30:99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Ng DW, Wang T, Chandrasekharan MB, Aramayo R, Kertbundit S, Hall TC. Plant SET domain-containing proteins: structure, function and regulation. Biochim Biophys Acta. 2007;1769:316–329. doi: 10.1016/j.bbaexp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- Paul J, Duerksen JD. Chromatin-associated RNA content of heterochromatin and euchromatin. Mol Cell Biochem. 1975;9:9–16. doi: 10.1007/BF01731728. [DOI] [PubMed] [Google Scholar]
- Pien S, Fleury D, Mylne JS, Crevillen P, Inze D, Avramova Z, Dean C, Grossniklaus U. ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell. 2008;20:580–588. doi: 10.1105/tpc.108.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehrauer H, Aquino C, Gruissem W, Henz SR, Hilson P, et al. AGRONOMICS1: a new resource for Arabidopsis transcriptome profiling. Plant Physiol. 2010;152:487–499. doi: 10.1104/pp.109.150185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrig H, Schmidt J, Miklashevichs E, Schell J, John M. Soybean ENOD40 encodes two peptides that bind to sucrose synthase. Proc Natl Acad Sci U S A. 2002;99:1915–1920. doi: 10.1073/pnas.022664799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z. Dynamic and stable histone H3 methylation patterns at the Arabidopsis FLC and AP1 loci. Gene. 2008a;423:43–47. doi: 10.1016/j.gene.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Yilmaz M, Le O, Hou G, Sadder M, Al-Abdallat A, Xia Y, Lu G, Ladunga I, Avramova Z. The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell. 2008b;20:568–579. doi: 10.1105/tpc.107.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Csorba T, Skourti-Stathaki K, Proudfoot NJ, Dean C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science. 2013;340:619–621. doi: 10.1126/science.1234848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004a;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- Sung S, Amasino RM. Vernalization and epigenetics: how plants remember winter. Curr Opin Plant Biol. 2004b;7:4–10. doi: 10.1016/j.pbi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Sung S, He Y, Eshoo TW, Tamada Y, Johnson L, Nakahigashi K, Goto K, Jacobsen SE, Amasino RM. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat Genet. 2006;38:706–710. doi: 10.1038/ng1795. [DOI] [PubMed] [Google Scholar]
- Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. [DOI] [PubMed] [Google Scholar]
- Tamada Y, Yun JY, Woo SC, Amasino RM. ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell. 2009;21:3257–3269. doi: 10.1105/tpc.109.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BT, Marguerat S, Watt S, Schubert F, Wood V, Goodhead I, Penkett CJ, Rogers J, Bahler J. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453:1239–1243. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci U S A. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Shen WH. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JY, Tamada Y, Kang YE, Amasino RM. Arabidopsis trithorax-related3/SET domain GROUP2 is required for the winter-annual habit of Arabidopsis thaliana. Plant Cell Physiol. 2012;53:834–846. doi: 10.1093/pcp/pcs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Germann S, Blus BJ, Khorasanizadeh S, Gaudin V, Jacobsen SE. The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat Struct Mol Biol. 2007;14:869–871. doi: 10.1038/nsmb1283. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009;10:R62. doi: 10.1186/gb-2009-10-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]


